Abstract
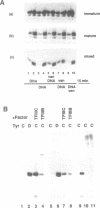
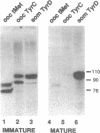
The Xenopus tyrosine tRNAtyrC (TyrC) genes are developmentally regulated. These multicopy genes are expressed in early oocytes and inactivated as oocytes reach maturity. As shown here, this developmental regulation can be reproduced in vitro in extracts of early and late stage oocytes: the TyrC gene is transcribed in early oocyte extracts but is virtually inactive in mature oocyte extracts. The inability to transcribe the TyrC gene is not due to the lack of functional pol III transcriptional components, since the somatic-type TyrD gene is fully active in mature oocyte extracts. Instead, the loss of TyrC transcription appears to be due to a change in the template specificity of transcription factor TFIIIC: addition of TFIIIC from immature extracts restores TyrC transcription in mature extracts. In mixtures of immature and mature extracts, the transcriptional activity of the TyrC gene is reduced. The presence of sodium vanadate, an inhibitor of tyrosine phosphatases, increases the level of TyrC transcription in the extract mixtures. Also, cdc25 phosphatase treatment of immature extracts causes a decrease in TyrC transcription which is reversed by addition of exogenous TFIIIC. These findings indicate that changes in phosphorylation state alters the template specificity of TFIIIC leading to the selective inactivation of oocyte type TyrC genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews M. T., Loo S., Wilson L. R. Coordinate inactivation of class III genes during the Gastrula-Neurula Transition in Xenopus. Dev Biol. 1991 Jul;146(1):250–254. doi: 10.1016/0012-1606(91)90466-g. [DOI] [PubMed] [Google Scholar]
- Bartholomew B., Kassavetis G. A., Braun B. R., Geiduschek E. P. The subunit structure of Saccharomyces cerevisiae transcription factor IIIC probed with a novel photocrosslinking reagent. EMBO J. 1990 Jul;9(7):2197–2205. doi: 10.1002/j.1460-2075.1990.tb07389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew B., Kassavetis G. A., Geiduschek E. P. Two components of Saccharomyces cerevisiae transcription factor IIIB (TFIIIB) are stereospecifically located upstream of a tRNA gene and interact with the second-largest subunit of TFIIIC. Mol Cell Biol. 1991 Oct;11(10):5181–5189. doi: 10.1128/mcb.11.10.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Clarkson S. G., Kurer V., Smith H. O. Sequence organization of a cloned tDNA met fragment from Xenopus laevis. Cell. 1978 Jul;14(3):713–724. doi: 10.1016/0092-8674(78)90253-2. [DOI] [PubMed] [Google Scholar]
- Cohen I., Reynolds W. F. The Xenopus YB3 protein binds the B box element of the class III promoter. Nucleic Acids Res. 1991 Sep 11;19(17):4753–4759. doi: 10.1093/nar/19.17.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Brizuela L., Beach D., Newport J. The Xenopus cdc2 protein is a component of MPF, a cytoplasmic regulator of mitosis. Cell. 1988 Jul 29;54(3):423–431. doi: 10.1016/0092-8674(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991 Oct 4;67(1):189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Engelke D. R., Ng S. Y., Shastry B. S., Roeder R. G. Specific interaction of a purified transcription factor with an internal control region of 5S RNA genes. Cell. 1980 Mar;19(3):717–728. doi: 10.1016/s0092-8674(80)80048-1. [DOI] [PubMed] [Google Scholar]
- Gabrielli B. G., Lee M. S., Walker D. H., Piwnica-Worms H., Maller J. L. Cdc25 regulates the phosphorylation and activity of the Xenopus cdk2 protein kinase complex. J Biol Chem. 1992 Sep 5;267(25):18040–18046. [PubMed] [Google Scholar]
- Gabrielsen O. S., Sentenac A. RNA polymerase III (C) and its transcription factors. Trends Biochem Sci. 1991 Nov;16(11):412–416. doi: 10.1016/0968-0004(91)90166-s. [DOI] [PubMed] [Google Scholar]
- Geiduschek E. P., Tocchini-Valentini G. P. Transcription by RNA polymerase III. Annu Rev Biochem. 1988;57:873–914. doi: 10.1146/annurev.bi.57.070188.004301. [DOI] [PubMed] [Google Scholar]
- Glikin G. C., Ruberti I., Worcel A. Chromatin assembly in Xenopus oocytes: in vitro studies. Cell. 1984 May;37(1):33–41. doi: 10.1016/0092-8674(84)90298-8. [DOI] [PubMed] [Google Scholar]
- Gouilloud E., Clarkson S. G. A dispersed tyrosine tRNA gene from Xenopus laevis with high transcriptional activity in vitro. J Biol Chem. 1986 Jan 5;261(1):486–494. [PubMed] [Google Scholar]
- Hartl P., Gottesfeld J., Forbes D. J. Mitotic repression of transcription in vitro. J Cell Biol. 1993 Feb;120(3):613–624. doi: 10.1083/jcb.120.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffler W. K., Kovelman R., Roeder R. G. Activation of transcription factor IIIC by the adenovirus E1A protein. Cell. 1988 Jun 17;53(6):907–920. doi: 10.1016/s0092-8674(88)90409-6. [DOI] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Izumi T., Walker D. H., Maller J. L. Periodic changes in phosphorylation of the Xenopus cdc25 phosphatase regulate its activity. Mol Biol Cell. 1992 Aug;3(8):927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. L., Fan R. S., Treinies M. L. Analysis of the molecular mechanisms for the species-specific transcription of Drosophila and human tRNA gene transcription components. J Biol Chem. 1991 Aug 25;266(24):16037–16043. [PubMed] [Google Scholar]
- Kakizuka A., Sebastian B., Borgmeyer U., Hermans-Borgmeyer I., Bolado J., Hunter T., Hoekstra M. F., Evans R. M. A mouse cdc25 homolog is differentially and developmentally expressed. Genes Dev. 1992 Apr;6(4):578–590. doi: 10.1101/gad.6.4.578. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Joazeiro C. A., Pisano M., Geiduschek E. P., Colbert T., Hahn S., Blanco J. A. The role of the TATA-binding protein in the assembly and function of the multisubunit yeast RNA polymerase III transcription factor, TFIIIB. Cell. 1992 Dec 11;71(6):1055–1064. doi: 10.1016/0092-8674(92)90399-w. [DOI] [PubMed] [Google Scholar]
- Kovelman R., Roeder R. G. Purification and characterization of two forms of human transcription factor IIIC. J Biol Chem. 1992 Dec 5;267(34):24446–24456. [PubMed] [Google Scholar]
- Kumagai A., Dunphy W. G. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992 Jul 10;70(1):139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- Kumagai A., Dunphy W. G. The cdc25 protein controls tyrosine dephosphorylation of the cdc2 protein in a cell-free system. Cell. 1991 Mar 8;64(5):903–914. doi: 10.1016/0092-8674(91)90315-p. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Martin P. L., Roeder R. G. Transcription of class III genes: formation of preinitiation complexes. Science. 1983 Nov 18;222(4625):740–748. doi: 10.1126/science.6356356. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Christenson E., Litchfield D. W., Krebs E. G., Eisenman R. N. Myb DNA binding inhibited by phosphorylation at a site deleted during oncogenic activation. Nature. 1990 Apr 5;344(6266):517–522. doi: 10.1038/344517a0. [DOI] [PubMed] [Google Scholar]
- Manak J. R., Prywes R. Mutation of serum response factor phosphorylation sites and the mechanism by which its DNA-binding activity is increased by casein kinase II. Mol Cell Biol. 1991 Jul;11(7):3652–3659. doi: 10.1128/mcb.11.7.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miake-Lye R., Newport J., Kirschner M. Maturation-promoting factor induces nuclear envelope breakdown in cycloheximide-arrested embryos of Xenopus laevis. J Cell Biol. 1983 Jul;97(1):81–91. doi: 10.1083/jcb.97.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F., Clarkson S. G., Galas D. J. Sequence of a 3.18 kb tandem repeat of Xenopus laevis DNA containing 8 tRNA genes. Nucleic Acids Res. 1987 Sep 11;15(17):7191–7191. doi: 10.1093/nar/15.17.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. M., Folk W. R. Unraveling the complexities of transcription by RNA polymerase III. Trends Biochem Sci. 1990 Aug;15(8):300–304. doi: 10.1016/0968-0004(90)90018-7. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. p34cdc2: the S and M kinase? New Biol. 1990 May;2(5):389–401. [PubMed] [Google Scholar]
- Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991 Oct 17;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Reynolds W. F. Effect of sequence differences between somatic and oocyte 5S RNA genes on transcriptional efficiency in an oocyte S150 extract. Mol Cell Biol. 1988 Nov;8(11):5056–5058. doi: 10.1128/mcb.8.11.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds W. F., Johnson D. L. Differential expression of oocyte-type class III genes with fraction TFIIIC from immature or mature oocytes. Mol Cell Biol. 1992 Mar;12(3):946–953. doi: 10.1128/mcb.12.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds W. F. Sequences preceding the minimal promoter of the Xenopus somatic 5S RNA gene increase binding efficiency for transcription factors. Nucleic Acids Res. 1989 Nov 25;17(22):9381–9394. [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W. Three in one and one in three: it all depends on TBP. Cell. 1993 Jan 15;72(1):7–10. doi: 10.1016/0092-8674(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Segall J., Matsui T., Roeder R. G. Multiple factors are required for the accurate transcription of purified genes by RNA polymerase III. J Biol Chem. 1980 Dec 25;255(24):11986–11991. [PubMed] [Google Scholar]
- Seidel C. W., Peck L. J. Kinetic control of 5 S RNA gene transcription. J Mol Biol. 1992 Oct 20;227(4):1009–1018. doi: 10.1016/0022-2836(92)90517-n. [DOI] [PubMed] [Google Scholar]
- Setzer D. R., Brown D. D. Formation and stability of the 5 S RNA transcription complex. J Biol Chem. 1985 Feb 25;260(4):2483–2492. [PubMed] [Google Scholar]
- Stutz F., Gouilloud E., Clarkson S. G. Oocyte and somatic tyrosine tRNA genes in Xenopus laevis. Genes Dev. 1989 Aug;3(8):1190–1198. doi: 10.1101/gad.3.8.1190. [DOI] [PubMed] [Google Scholar]
- Wolffe A. P. Transcription fraction TFIIIC can regulate differential Xenopus 5S RNA gene transcription in vitro. EMBO J. 1988 Apr;7(4):1071–1079. doi: 10.1002/j.1460-2075.1988.tb02915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga S. K., L'Etoile N. D., Berk A. J. Purification and characterization of transcription factor IIIC2. J Biol Chem. 1989 Jun 25;264(18):10726–10731. [PubMed] [Google Scholar]
- Young L. S., Takahashi N., Sprague K. U. Upstream sequences confer distinctive transcriptional properties on genes encoding silkgland-specific tRNAAla. Proc Natl Acad Sci U S A. 1986 Jan;83(2):374–378. doi: 10.1073/pnas.83.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]