Premature adrenarche is often associated with insulin resistance, metabolic syndrome, and PCOS; evaluation and management during childhood and adolescence are described.
Abstract
Premature pubarche, or the development of pubic hair before the age of 8 in girls or 9 in boys, is most commonly caused by premature adrenarche. Adrenarche is the maturation of the adrenal zona reticularis in both boys and girls, resulting in the development of pubic hair, axillary hair, and adult apocrine body odor. Although originally thought to be a benign variant of normal development, premature adrenarche has been associated with insulin resistance and the later development of metabolic syndrome and polycystic ovary syndrome. Although further studies are needed to confirm these relationships, the case presented herein argues for periodic assessment of children at risk. Indeed, recognition of these associations may allow for early preventive measures.
Accreditation and Credit Designation Statements.
The Endocrine Society is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians. The Endocrine Society has achieved Accreditation with Commendation.
The Endocrine Society designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Learning Objectives
Upon completion of this educational activity, participants should be able to
Evaluate a young girl who presents with early onset of pubic hair
Manage the clinical care of a child with premature adrenarche
Describe the progression of metabolic abnormalities in some girls with premature adrenarche
Target Audience
This Journal-based CME activity should be of substantial interest to endocrinologists.
Disclosure Policy
Authors, editors, and Endocrine Society staff involved in planning this CME activity are required to disclose to learners any relevant financial relationship(s) that have occurred within the last 12 months with any commercial interest(s) whose products or services are discussed in the CME content. The Endocrine Society has reviewed all disclosures and resolved or managed all identified conflicts of interest, as applicable.
Disclosures for JCEM Editors are found at http://www.endo-society.org/journals/Other/faculty_jcem.cfm.
The following individuals reported NO relevant financial relationships:
Sharon E. Oberfield, M.D., Aviva B. Sopher, M.D., Adrienne T. Gerken, A.B., and Leonard Wartofsky, M.D., reported no relevant financial relationships.
Endocrine Society staff associated with the development of content for this activity reported no relevant financial relationships.
Acknowledgement of Commercial Support
This activity is not supported by grants, other funds, or in-kind contributions from commercial supporters.
Privacy and Confidentiality Statement
The Endocrine Society will record learner's personal information as provided on CME evaluations to allow for issuance and tracking of CME certificates. No individual performance data or any other personal information collected from evaluations will be shared with third parties.
Method of Participation
This Journal-based CME activity is available in print and online as full text HTML and as a PDF that can be viewed and/or printed using Adobe Acrobat Reader. To receive CME credit, participants should review the learning objectives and disclosure information; read the article and reflect on its content; then go to http://jcem.endojournals.org and find the article, click on CME for Readers, and follow the instructions to access and complete the post-activity test questions and evaluation. The estimated time to complete this activity, including review of material, is 1 hour. If you have questions about this CME activity, please direct them to education@endo-society.org.
Activity release date: June 2011
Activity expiration date: June 2012
Adrenarche is the normal maturation of the zona reticularis (ZR) in both boys and girls, resulting in the development of pubic hair, axillary hair, and adult apocrine body odor. These physical changes are preceded by biochemical adrenarche, which has been described to begin physiologically as early as ages 5–6 yr (1) and consists of increased ZR production of Δ5 steroids, principally dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS).
Premature adrenarche (PA) occurs when signs of adrenarche begin before the age of 8 yr in girls or 9 in boys. The majority of children with PA have idiopathic premature adrenal androgen secretion. Idiopathic PA occurs more frequently in girls than boys by a ratio of about 9:1 (2–5).
The clinical presentation of idiopathic PA is similar to normally timed adrenarche, and adrenal androgens—more correctly termed androgen precursors (6)—principally DHEA, as well as DHEAS, are elevated for chronologic age but are usually consistent with early puberty or Tanner II-III pubic hair. Smaller elevations are noted for testosterone and Δ4-androstenedione (Δ4-A). There may be modestly increased growth velocity and skeletal age (consistent with height age), but pubertal timing and adult height are usually unaltered (4, 5, 7–9).
Although PA was formerly thought to be a variant of normally timed adrenarche, studies support an association between PA and a history of being small for gestational age and having low birth weight (10), as well as an association with obesity (11). In girls with PA and a history of low birth weight, a 3-fold increase in menarche before age 12 yr has been reported (12).
PA is also associated with a risk of polycystic ovary syndrome (PCOS) and metabolic syndrome (MeS). Metabolic abnormalities reported in prepubertal children with PA include insulin resistance (IR) (13–15), hyperinsulinism (16), increased free IGF-1 (15, 17) and plasminogen activator inhibitor 1 (18), and lower IGF binding protein-1 (IGFBP-1) (15, 16). It has been suggested that in prepubertal girls with PA, plasminogen activator inhibitor 1 levels can predict progression to PCOS (18).
Despite these preliminary data, it is not known which children with PA are most at risk of developing MeS or PCOS. Thus, although PA is frequently a benign process, the diagnosis must carry a heightened suspicion for future metabolic and endocrine abnormalities.
The following patient description will serve to outline the evaluation of the young girl with early onset of pubic hair. We will describe clinical, biochemical, anthropometric, and body composition measures over the period of time from early childhood to late adolescence in a girl with PA. We will also describe management issues, including current controversies regarding treatment modalities.
Case History
Age 5 yr
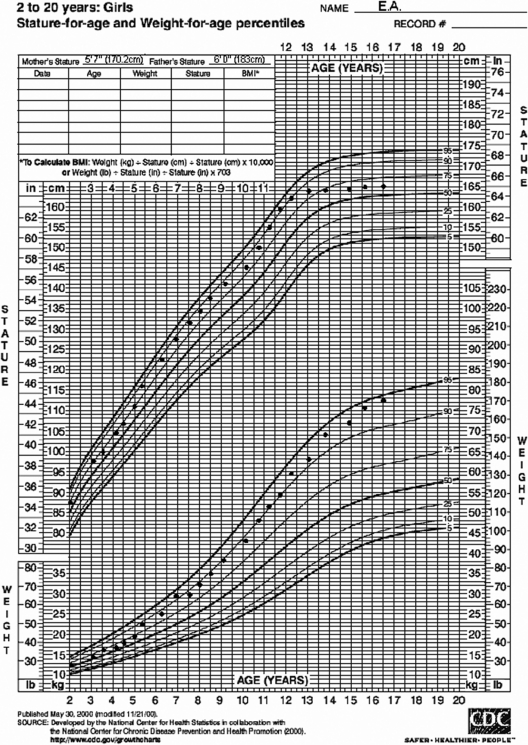
EA (“Early Adrenarche”) is a 5 -yr-old Caucasian girl referred for evaluation of apocrine body odor. She was born full-term and weighed 7 pounds [3175 g; ∼30th percentile for sex (19)] at birth. There were no pregnancy complications, including gestational diabetes, and the 17-hydroxyprogesterone (17OHP) newborn screen for congenital adrenal hyperplasia (CAH) was normal. She has grown consistently along the 75th percentile for height, corresponding to the 40th percentile for midparental sex-adjusted target height, but has recently increased her growth velocity to 3.2 cm in 4 months. She has previously plotted in the 50th-75th percentile for weight, but in the past 4 months has plotted in the 75th-90th percentile (Fig. 1). She has not been exposed to lice treatments (tea tree oils), lavender oil, or estrogen sprays or creams and has not increased her intake of soy.
Fig. 1.
Standardized growth curve for E.A.
EA's parents are both of Ashkenazi Jewish heritage. Her mother is 5 feet, 7 inches tall (170.2 cm) and her father is 6 feet tall (183 cm). Midparental sex-adjusted target height is 5 feet, 7 inches. Her mother had menarche at age 12 yr. The patient's father was treated for severe acne at age 14 yr, had his growth spurt at 15 yr, and developed frontal balding at 30 yr. EA's 12-yr-old sister has just reached menarche, and her 9-yr-old brother is prepubertal. The patient's review of systems is negative for excessive thirst or urination, history of diabetes, or headaches.
On examination, EA's height is 116 cm (≈90th percentile), weight is 22.5 kg (≈90th percentile), and body mass index (BMI) is 16.7 kg/m2 (83rd percentile). Her blood pressure is 106/70 mm Hg. Funduscopic and thyroid exams are normal. She has no glandular breast tissue (Tanner I). She has faint pubic down on the mons pubis, with sparse curly hairs along the labia majora (Tanner II) and perirectal hairs. Her clitoris is 0.5 cm by 0.3 cm, her posterior labia minora are not fused, and her vagina mucosa is shiny, pink-red, and nonestrogenized. She has no axillary hair, but does have adult-like apocrine odor. Her neurological exam is grossly normal, and she has no areas of skin hyper- or hypopigmentation.
The results of the patient's initial evaluation (Table 1) include a bone age of 5 to 6 yr, consistent with her height age. Blood work drawn at 0730 h reveals 17OHP of 90 ng/dl, Δ4-A of 66 ng/dl, DHEA of 120 ng/dl, DHEAS of 80 μg/dl, testosterone of 8 ng/dl, FSH of 1 mIU/ml, and LH of 0.12 mIU/ml. Fasting serum lipids demonstrate elevated triglycerides, a fasting glucose-to-insulin ratio (FGIR) of 6.5 [normal, < 7 (20)], fasting glucose of 98 mg/dl, and fasting insulin of 15 μIU/ml.
Table 1.
Clinical and laboratory data by age for EA
| Age (yr) |
|||
|---|---|---|---|
| 5 | 10 | 16 | |
| Height (cm) | 116 | 150 | 165.2 |
| Weight (kg) | 22.5 | 48.0 | 77.5 |
| BMI, kg/m2 (percentile for age) | 16.7 (83rd %) | 21.3 (88th %) | 28.4 (93rd %) |
| Bone age (yr) | 5 to 6 | 12 ½ | |
| Height age (yr) | 6 ¼ | 11 ¾ | |
| Predicted height (cm) | 164 | ||
| Waist circumference (cm) | 54 (50–75th %) | 70 (75th %) | 84 (75th %) |
| Breasts | Tanner I | Tanner IV | Tanner IV |
| Pubic hair | Tanner II | Tanner IV | Tanner IV |
| Ferriman-Gallwey score | 16 | ||
| Blood pressure (mm Hg) | 106/70 | 110/70 | 118/76 |
| Testosterone (ng/dl) | 8 (7–28) | 28 (13–32) | 41 (13–32) |
| Δ4 (ng/dl) | 66 (<10–72) | 194 (47–208) | 306 (47–208) |
| 17OHP (ng/dl) | 90 (11–98) | 114 (18–230) | 121 (18–230) |
| DHEAS (μg/dl) | 80 (34–129) | 237 (58–260) | 282 (58–260) |
| LH (mIU/ml) | 0.12 (0.02–4.7) | 4.4 (0.4–11.7) | 19.0 (0.4–11.7) |
| FSH (mIU/ml) | 1.0 (1.0–10.8) | 2.1 (1.5–11.7) | 5.4 (1.5–11.7) |
| Total cholesterol (mg/dl) | 140 (126–205)a | 165 (124–201)a | 174 (120–200)a |
| LDL (mg/dl) | 89 (68–140)a | 112 (68–136)a | 126 (60–135)a |
| HDL (mg/dl) | 45 (36–73)a | 36 (37–70)a | 34 (35–73)a |
| Triglycerides (mg/dl) | 144 (32–105)a | 162 (37–131)a | 190 (39–124)a |
| Fasting glucose (mg/dl) | 98 | 100 | 94 |
| Fasting insulin (μIU/ml) | 15 | 22 | 18 |
| FGIR | 6.5 (<7)b | 4.5 (<7)b | 5.2 (<7)b |
| oGTTc | |||
| 0 min | 98/15 | 100/22 | 94/18 |
| 30 min | 138/54 | 165/92 | 156/70 |
| 60 min | 102/18 | 141/86 | 144/62 |
| 90 min | 108/40 | 117/58 | 138/52 |
| 120 min | 112/38 | 118/66 | 116/36 |
Age 10 yr
EA has been seen every 6 months by her pediatrician and has continued to grow along the 75th-90th percentile for height and 90th percentile for weight. Her pubic hair was Tanner stage III–IV by age 9 yr. Tanner stage II breast tissue was noted at age 9 yr. She had menarche this week and has again been referred to you.
At this visit, EA's height is 150 cm (≈90th percentile), weight is 48 kg (≈90th percentile), BMI is 21.3 kg/m2 (≈90th percentile), waist circumference is 70 cm [≈75th percentile (21)], and blood pressure is 110/70 mm Hg. She has a fine moustache, greasy facial skin with comedones, and two pustules on her chin. She has Tanner IV breasts and pubic hair, with mild periareolar, periumbilical, and perirectal hair. The examination is otherwise normal.
EA's height age is 11 yr, her bone age is 12 yr, and her estimated adult height is 64.5 ± 2 inches according to the tables of Bailey and Pinneau, which is the 15th percentile for midparental sex-adjusted target height. Her fasting lipid profile demonstrates dyslipidemia (Table 1). Her FGIR has decreased to 4.5, and her oral glucose tolerance test (oGTT) reflects moderate hyperinsulinemia (22). She has high-normal adrenal and ovarian androgens for Tanner stage with normal gonadotropins.
The patient chooses to participate in an Institutional Review Board-approved body composition protocol. Dual-energy x-ray absorptiometry (DEXA) reveals 35% body fat [≈98th percentile for age, sex, and ethnicity (23)] with increased trunk fat. Measures obtained by magnetic resonance spectroscopy for intramyocellular lipid content (IMCL) and intrahepatic lipid (IHL) content are increased compared with her age-matched peers.
Age 16 yr
EA has been seen two times per year for the past 6 yr. After menarche, she had irregular cycles (every 2–4 months). Transabdominal ultrasound of the pelvis showed 4-cc ovaries with multiple peripheral follicles. At the age of 12 yr, after 2 yr of persistently irregular cycles and increasing hyperinsulinemia, she was started on extended release metformin at a dose of 500 mg/d for 1 wk and increased to 1000 mg/d thereafter. Menses now occur every 4–5 wk.
EA has followed a Mediterranean diet (low glycemic index, low carbohydrates, high grain and fiber content). She does aerobic and resistance exercises three times per week. She reports continued acne, for which she uses topical drying agents. She is upset about her body and facial hair and regularly undergoes laser hair removal with significant improvement.
Her height is 165.2 cm (50th-75th percentile), weight is 77.5 kg (≈95th percentile), BMI is 28.4 kg/m2 (>90th percentile), waist circumference is 84 cm [75th percentile (21)], and blood pressure is 118/76 mm Hg. She has significant facial hair, periumbilical and perirectal hairs, and mild acne. Her Ferriman-Gallwey score is elevated at 16. She has Tanner IV breasts and pubic hair and faint acanthosis nigricans of the neck and periaxillary areas. Laboratory tests show elevated Δ4-A, DHEAS, and LH and borderline-high fasting lipids. oGTT shows an FGIR of 5.2. She now has 40% body fat on DEXA [>99th percentile for age, sex, and ethnicity (23)].
Dietary and exercise interventions are reviewed and weight loss of 10–15 pounds over the next year is recommended. In preparation for possible initiation of a low-dose oral contraceptive pill containing ethinyl estradiol and norgestimate or norgestrel, which may improve her acne and hirsutism, a negative family history for clotting disorders is confirmed.
Discussion
EA's course from childhood through midadolescence is representative of a child who presents with PA. Her clinical findings will be discussed in detail after a review of the pathophysiology, evaluation, and clinical spectrum of adrenarche.
Adrenal development
The early adrenal cortex consists of the fetal zone, which involutes completely by the end of the first year of life (1), and the definitive zone, which persists and compartmentalizes to become the mature adrenal cortex.
The fetal zone is ACTH-dependent (24, 25) and becomes the primary site of fetal steroidogenesis at around 16–20 wk of gestational age. The outer portion of the fetal zone, termed the transitional zone, is the site of fetal cortisol synthesis. The fetal zone also produces DHEA, a precursor for the fetal estrogens required to sustain pregnancy, and DHEAS. The fetal zone involutes rapidly after birth (24, 26), and DHEA and DHEAS levels decline with postnatal regression of the fetal zone and remain low until adrenarche (27).
The definitive zone, or neocortex, begins as a narrow band of cells outside the fetal zone and continues to grow into the postnatal period, when it is remodeled to become the adult adrenal cortex (25). The adrenal medulla, meanwhile, is populated by neural crest cells concurrent with encapsulation of the adrenal. The neural crest cells differentiate into catecholamine-producing chromaffin cells that persist as medullary islands until birth, when they coalesce to form the immature medulla (27).
Postnatally, the adrenal cortex is remodeled to consist of continuous bands of zona glomerulosa and fasciculata cells. Islands of reticularis cells appear around the age of 3 yr and eventually coalesce to form a continuous ZR at 6–8 yr of age, concurrent with the beginning of biochemical adrenarche. The ZR continues to expand from adrenarche through early adolescence, peaking at around age 13 (1).
Classical steroid pathways in DHEA and DHEAS synthesis
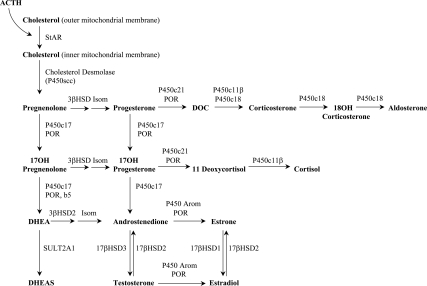
The biochemical hallmark of adrenarche is increased production of DHEA and DHEAS by the ZR without a concomitant rise in cortisol. DHEA and DHEAS are produced in the ZR via the Δ5 pathway of steroidogenesis, and DHEA may be converted to Δ4-A (Fig. 2).
Fig. 2.
Adrenal steroidogenesis. 17βHSD1, 17β hydroxysteroid dehydrogenase, type 1; 17βHSD2, 17β hydroxysteroid dehydrogenase, type 2; 17βHSD3, 17β hydroxysteroid dehydrogenase, type 3; 3βHSD, 3β hydroxysteroid dehydrogenase; Arom, aromatase; b5, cytochrome b5; DOC, 11-deoxycorticosterone; Isom, isomerase; P450c11β, 11β-hydroxylase; P450c17, 17α-hydroxylase and 17,20 lyase; P450c18, 18-hydroxylase; P450c21, 21α-hydroxylase; P450scc, cholesterol side-chain cleavage enzyme (or desmolase); POR, cytochrome P450 oxidoreductase; StAR, steroidogenic acute regulatory protein; SULT2A1, dehydroepiandrosterone sulfotransferase.
CYP11A1 (P450scc)
The first committed step in adrenal steroidogenesis is the conversion of cholesterol to pregnenolone by the cholesterol side-chain cleavage enzyme, CYP11A1 (P450scc), which is expressed throughout the adrenal cortex. CYP11A1 is regulated acutely by the steroidogenic acute regulatory protein, which mediates the transfer of cholesterol into the inner mitochondrial membrane (28, 29) and chronically via transcriptional regulation (29). CYP11A1 expression does not change with adrenarche (30).
CYP17 (P450c17)
Qualitative regulation of steroidogenesis occurs via CYP17 (P450c17), which has a dual enzymatic role in the adrenal: 17α-hydroxylation of pregnenolone and progesterone, and 17,20-lyase activity on their 17-hydroxy derivatives (31). Both the 17α-hydroxylase and 17,20-lyase actions of CYP17 are required to produce adrenal androgens, and the relative efficiency of these two reactions helps to determine the types of steroids produced. In young children, the 17α-hydroxylase activity of CYP17 produces cortisol, but 17,20-lyase activity (and thus androgen production) is low. During adrenarche, an increase in 17,20-lyase activity leads to increased DHEA and DHEAS production (32).
Several factors influence the balance of 17α-hydroxylase and 17,20-lyase activity of CYP17. The flavoprotein P450 oxidoreductase (POR) transfers electrons from reduced NADPH (nicotinamide adenine dinucleotide phosphate) and is required for both functions of CYP17 (33). In vitro, as the ratio of POR to CYP17 increases, 17,20-lyase activity is preferentially enhanced, leading to DHEA production (34). The 17,20-lyase activity of CYP17 is also augmented by cytochrome b5 (b5, CYB5), an allosteric effector of the CYP17-POR interaction (35–37).
Finally, the 17,20-lyase activity of CYP17 is enhanced by phosphorylation of serine and threonine residues by a cAMP-dependent protein kinase (38) and decreased by dephosphorylation via protein phosphatase 2A (39). CYP17 phosphorylation has been suggested as a possible link between the adrenal hyperandrogenism and IR in PA and PCOS (38) because serine phosphorylation of the insulin receptor has been shown in vitro to cause IR (40, 41).
3βHSD2
The balance of adrenal androgen production is also affected by 3β-hydroxysteroid dehydrogenase type 2 (3βHSD2), which catalyzes the conversion of DHEA to Δ4-A (31, 42). This conversion diverts adrenal androgens from the Δ5 pathway, which produces DHEA and DHEAS, to the Δ4 pathway, which produces Δ4-A. Recent in vitro evidence has shown that 3βHSD2 is inhibited by cortisol, raising the possibility that rising intraadrenal cortisol levels may play a role in the initiation of adrenarche (43). Peripheral conversion of Δ4-A produces the potent androgens testosterone and dihydrotestosterone. During adrenarche, expression of 3βHSD2 in the ZR decreases (37, 44), increasing adrenal production of DHEA and DHEAS relative to Δ4-A.
SULT2A1
The final step in the Δ5 pathway is the conversion of DHEA to DHEAS via DHEA sulfotransferase (SULT2A1) (31, 45). SULT2A1 expression increases during adrenarche in association with maturation of the ZR (37). Sulfation limits the pool of DHEA available for conversion to more potent androgens, and defects in sulfation have been associated with PA (45).
Clinical findings
Hyperinsulinemia and IGF-1
Decreased insulin sensitivity is a key component of MeS (46, 47). Numerous studies have demonstrated diminished insulin sensitivity in children with PA (13, 14, 16, 20, 48). Of note, Ibáñez et al. (49) have also reported that, in a population of Spanish girls, first-degree relatives of girls with PA were at greater risk for impaired glucose tolerance and type 2 diabetes.
In a small group of prepubertal girls with PA, Ibáñez et al. (48) reported that girls with PA had increased mean serum insulin levels and increased early insulin response to oGTT; however, PA subjects had a higher average BMI than controls. No comparison was made between obese and nonobese PA girls (16, 48). Denburg et al. (13) demonstrated decreased insulin sensitivity on oGTT and elevated IGF-1 levels independent of obesity in prepubertal boys with PA, although this was not seen in a cohort of lean Spanish boys (10).
In vitro, both IGF-1 and insulin have been shown to potentiate LH-stimulated androgen synthesis in rat (50) and human (51) theca-interstitial cells, suppress SHBG production in hepatoma cell lines (52), and induce steroidogenic enzymes in cultured human adrenocortical cells (25, 53). Additionally, both women with PCOS and patients with PA have decreased levels of IGFBP-1, which are inversely correlated with fasting insulin levels and ACTH-stimulated adrenal steroid levels (16).
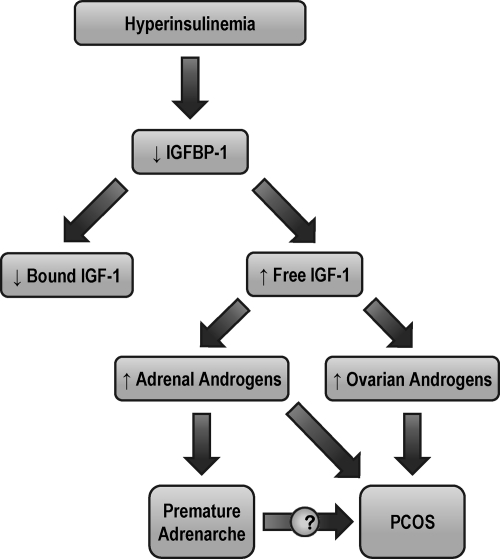
Taken together, these findings suggest a link between IR, IGF-1, and the hyperandrogenemia of PA and PCOS (Fig. 3) (17). Some authors have proposed that hyperinsulinemia is the inciting event in a cascade leading to hyperstimulation of ovarian and/or adrenal androgen synthesis, such that in some populations, IR and hyperandrogenism are causally related (53, 54).
Fig. 3.
Proposed mechanism for the development of PCOS or MeS in patients with PA. [Adapted from M. E. Silfen et al., J Clin Endocrinol Metab 87:398–403 2002 (17). © The Endocrine Society.]
Obesity
Although there appears to be an association between obesity and PA (11), the exact nature of this relationship is unclear. A small study found that obese African-American and Caribbean Hispanic adolescent females with a history of both childhood obesity and PA were hyperandrogenic and hyperinsulinemic (55, 56). The emerging picture based on limited studies of prepubertal Hispanic and African-American girls with PA is that most obese girls with PA have decreased insulin sensitivity compared with nonobese girls with PA (15, 17, 20, 57). Additional data are needed regarding other ethnic groups. Recently, Marshall's group (58) has demonstrated that obesity in the prepubertal and early pubertal child may enhance LH secretion and ovarian androgen production, providing additional evidence linking obesity and hyperandrogenism.
PCOS and/or functional ovarian hyperandrogenism
In a cohort of Catalan girls with a history of PA, nearly half of the postpubertal females with a history of PA had hirsutism, oligomenorrhea, and/or elevated androgen levels (16, 59, 60). Patients with a history of PA and low birth weight also had an increased prevalence of anovulatory cycles (10, 61). However, none of these results were analyzed with respect to the impact of obesity. In contrast, in a cohort of pubertal Finnish females with a history of childhood PA, no hyperandrogenism was observed (9).
It is difficult to assess what proportion of girls with PA progress to PCOS, in part due to multiple consensus statements for the diagnosis of PCOS (62, 63). We have suggested that a definitive diagnosis of PCOS in adolescents should not be made until at least 2 yr after menarche and should require the presence of hyperandrogenism, chronic anovulation, and polycystic ovaries. In addition, we have suggested that hyperandrogenism be defined as hyperandrogenemia and that clinical findings such as acne or alopecia should be discounted, with the exception of documented progressive hirsutism (64).
Lipid abnormalities
Dyslipidemia, including increased triglycerides and low high-density lipoprotein, is a component of MeS in both adults and children and portends future risk of type 2 diabetes (65, 66). Dyslipidemia has been demonstrated in girls with PA and a history of PA, primarily manifested by hypertriglyceridemia (48). Güven et al. (67) demonstrated higher mean total cholesterol, low-density lipoprotein (LDL), VLDL (very low-density lipoprotein), and atherogenic index in prepubertal Turkish girls with PA than in controls, and Denburg et al. (13) have shown a trend toward higher triglycerides in a BMI-adjusted analysis of prepubertal boys with PA. Although another study showed no difference in lipoprotein (a) levels between prepubertal girls with PA and controls, pubertal girls with a history of PA had significantly higher lipoprotein (a) levels than their age-matched peers (68).
Although dyslipidemia represents an independent risk factor for cardiovascular disease, an additional question is the extent to which abnormal lipid deposition within the tissues may contribute to IR and type 2 diabetes. Although the relationship between IR and body composition, particularly abdominal fat, IMCL, and IHL, is just beginning to be investigated, preliminary results have yielded interesting hypotheses regarding the origin of IR in these patients. Indeed, the “adipose-tissue expandability hypothesis” argues that IR in obesity and other disorders, including PCOS, is attributable to lipotoxicity due to the inability to expand adipose tissue in a metabolically safe manner (69). The relationship between IR and PA is currently being investigated.
Bone mineral density and body composition
To date, three reports have addressed body composition in exclusively prepubertal populations with PA (70–72). Our group has found increased bone mineral content and bone mineral density using DEXA in prepubertal girls with PA, compared with control subjects (71).
We have also found increased IMCL in PA girls compared with controls, but no difference in visceral adipose tissue (VAT), abdominal SAT (subcutaneous adipose tissue), or VAT:SAT ratio. Of note, however, this population consisted of a very small group of six girls with PA and eight controls (70).
The critical question with respect to body composition in PA is its relationship with IR and risk for MeS. Ibáñez et al. (73) have evaluated body composition by DEXA in prepubertal and adolescent girls (aged 6–18 yr) with a history of PA. Although BMI did not differ between PA and control girls, those with PA had greater total and trunk fat mass in the prepubertal and pubertal periods. Furthermore, trunk fat mass showed an independent and significant positive relationship with fasting insulin and free androgen index (73). In a group of adolescent girls and young women with varying degrees of hyperandrogenism, hyperinsulinism, and anovulation, Ibáñez et al. (74–77) found that short-term therapy with metformin or a metformin-flutamide combination resulted in decreased fat mass, increased lean body mass, and improved androgens, insulin sensitivity, and serum lipids without a change in overall body mass.
Differential diagnosis
Premature adrenarche must be distinguished from a number of other benign and pathological conditions (Table 2).
Table 2.
Differential diagnosis of early onset of pubic hair
| Pubic hair | Breast development, testicular enlargement, other physical findings | Androgens | LH and FSH | Bone age | Growth velocity | Additional notes | |
|---|---|---|---|---|---|---|---|
| Isolated PP | Present | Absent | Prepubertal, DHEAS not elevated | Prepubertal | ↔ | ↔ | Possibly related to increased androgen receptor sensitivity |
| PA | Present | Absent | ↑ but appropriate for Tanner II-III | Prepubertal | Slight ↑ correlated with height age | Slight ↑ | Most common cause of PP |
| Precocious puberty | Present | + Breast development, + testicular enlargement | ↑↑ for males, estradiol can be ↑ for females | Early pubertal | ↑ | ↑↑ | More common in girls; more often pathological in boys |
| NC-CAH | Present | +/− Penile/clitoral enlargement, absent breast development or testicular enlargement | ↑↑ May be elevated for Tanner stage, with ↑ adrenal androgen precursors | Prepubertal | ↑ | ↑ | ↑ 17OHP in 21-hydroxylase deficiency (most common form) |
| Virilizing tumors | Present | +/− Breast development, + penile/clitoral enlargement, infantile testes | Usually ↑ but variable, depending on tumor expression | Prepubertal or ↓ | ↑ | ↑ | May have ↑ DHEA, DHEAS, T, Δ4-A, or βhCG |
| Exogenous hormone exposure | Present | Variable; may have breast development, small testes, and/or penile/clitoral enlargement | Variable, depends on specific exposure | Prepubertal | ↑ | ↑↑ | Take careful medication history from all caretakers |
T, Testosterone; ↑, increased; ↓, decreased; ↑↑, markedly increased; ↓↓, markedly decreased; ↔, normal for age.
Premature pubarche without elevated androgens
Although PA is the most common cause of premature (or precocious) pubarche (PP), the latter occasionally occurs in isolation, without a concomitant rise in adrenal androgens. Bone age is not advanced in isolated PP, and growth velocity is not accelerated for age. It has been speculated that these individuals have an increased receptor sensitivity to low circulating levels of androgens (78).
Precocious puberty
Precocious puberty may be central (also called gonadotropin-dependent precocious puberty) or peripheral (also termed gonadotropin-independent). Central precocious puberty (CPP) is generally complete and may be idiopathic, while peripheral precocious puberty is often incomplete and always pathological in nature. Precocious puberty is more common in girls by a ratio of 5:1, and idiopathic CPP by 9:1 (79). However, in girls 80% of precocious puberty is idiopathic, while approximately two thirds of males with precocious puberty have central nervous system pathology (79, 80).
Idiopathic CPP may present with pubic or axillary hair but is differentiated clinically from PA by the presence of breast or testicular development. It is caused by early maturation of the hypothalamic-pituitary-gonadal axis and is more common in African-American children compared with their Caucasian or Hispanic peers (81, 82). Androgens (and sometimes estradiol) are elevated for age, and gonadotropins are in the early pubertal range. Bone age and growth velocity are accelerated, which may lead to reduced adult height (83, 84). Of note, prior exposure to elevated serum androgens (e.g. virilizing tumors, untreated CAH) may result in CPP due to early maturation of the hypothalamus (85, 86). For a review of precocious puberty, please refer to Carel and Léger's article (87).
Nonclassical CAH (NC-CAH)
NC-CAH may also present with PP. Estimates of the proportion of cases of PP due to NC-CAH vary widely, from 0 to 40% (53), although these differences may be due to ethnic differences between study populations. NC-CAH is the result of mild deficiencies in the same enzymes responsible for classical CAH, namely 21-hydroxylase, 11-hydroxylase, or 3βHSD2. These deficiencies are typically not sufficient to cause an abnormal newborn screening for CAH; i.e. in nonclassic 21-hydroxylase deficiency, basal 17OHP levels shortly after birth are within normal limits. The diagnosis is suspected when androgens are elevated in the presence of prepubertal gonadotropins. Accelerated height velocity and a moderate increase in bone age raise suspicion for NC-CAH, as do marked virilization (clitoral or penile growth) or early development of cystic acne (88). Clinical variables, however, have poor predictive value for distinguishing NC-CAH from idiopathic PA (89).
The diagnosis of NC-CAH is confirmed when elevated levels of the appropriate precursor steroids are noted after ACTH stimulation. However, there is ongoing controversy regarding which children with PP should receive ACTH stimulation testing. Those for whom there is a heightened index of suspicion, and therefore greater indication for testing, include children with elevated basal androgens, bone age advancement greater than 1–2 yr above chronological age, or virilization in the absence of true puberty. Early morning basal 17OHP above 200 ng/dl is 100% sensitive and 99% specific for NC-CAH (89).
Virilizing tumors
Virilizing adrenal or gonadal tumors are a rare cause of PP. Clinically, these tumors may present with rapidly progressive and/or exaggerated symptoms of virilization, such as clitoral enlargement or rapid penile growth. Growth velocity is notably accelerated, and bone age is advanced and rapidly progressive. Serum androgens are markedly increased, while gonadotropins are prepubertal or suppressed (79). Serum β-human chorionic gonadotropin (hCG), which acts at gonadal LH receptors to stimulate testosterone production, may be elevated in hCG-producing tumors of the testis, brain, or liver.
Exogenous steroid exposure
PP with feminizing signs may be attributable to estrogen exposure from creams, oils, or sprays (e.g. EvaMist) (90) prescribed for caretakers, or from exposure to lavender or tea tree oil, which have estrogenic activity (91). Synthetic environmental endocrine disruptors such as bisphenol A also appear to have estrogenic effects that may alter the course of normal pubertal development (92), although the mechanisms and extent of these effects are still being explored.
Exogenous androgen exposure from creams or gels containing testosterone or Δ4-A may precipitate PP with other androgenic signs, usually in the absence of elevation of androgen precursors, thus mimicking PA (93). Importantly, it should be noted that exogenous steroids need not be applied directly to the patient but may be transferred via skin-to-skin contact (90, 93).
Laboratory and imaging assessment
A definitive algorithm for the evaluation of PA cannot be recommended because some variance is due to the clinical concern of the physician. In our practice, all patients with PA receive baseline blood tests for androgens (testosterone, Δ4, DHEAS), gonadotropins (LH and FSH), and 17OHP. Thyroid studies (TSH and free T4) are ordered if hypothyroidism is suspected. If early morning 17OHP is above 200 ng/dl, an ACTH-stimulation test to evaluate for NC-CAH is indicated (89). In boys, β-hCG should be obtained to rule out an hCG-secreting tumor.
The decision to order imaging studies is based on clinical impression and results of initial laboratory evaluation. Rapid virilization or physical signs of precocious puberty are indications to obtain an x-ray of the left hand to determine bone age. If initial studies show very elevated DHEAS, abdominal ultrasound may be indicated as an initial screen to evaluate for adrenal neoplasm. Abdominal computed tomography or magnetic resonance imaging is indicated if a mass is noted, although controversy still exists regarding which of these is preferred. Similarly, ultrasonographic evaluation of the ovaries or testes may be indicated if significantly elevated testosterone or Δ4-A is found, if a testicular mass is palpated, or if there is significant testicular asymmetry. Elevated β-hCG in boys should prompt an evaluation for the presence of a tumor of the testis, liver, or brain. Finally, brain magnetic resonance imaging with contrast may be indicated in precocious puberty (especially in boys), or if there is any suspicion of central nervous system pathology.
Management
Once the diagnosis of PA is made, families should be counseled regarding the increased risk of early puberty and should be given instructions to return promptly if marked growth acceleration or progressive virilization occur. In the majority of cases, when clinical and laboratory findings are consistent with typical PA, these children may be followed by a general pediatrician, family physician, or pediatric endocrinologist at approximately 6-month intervals, with some variation in the frequency of follow-up depending on the preference of the physician. Careful assessment of growth velocity, weight gain, and progression of signs of androgen excess are indicated. Yearly fasting measures of IR (such as FGIR) and lipid levels are recommended, particularly if there has been significant weight gain. Some clinicians follow adrenal androgen levels.
Most children do not demonstrate rapid pubertal progress or bone age advancement and do well with lifestyle interventions. However, should the rate of pubertal changes suddenly accelerate, a bone age and androgen levels (testosterone, Δ4, DHEAS) should be repeated to reassess the child's status. If not previously performed, an ACTH-stimulation test should be conducted to exclude defects of adrenal steroidogenesis.
In our practice, we continue to follow girls with a history of PA at least yearly after menarche. We encourage continued adherence to a low-fat, low-glycemic index diet and regular exercise. If menstrual regularity has not been established 2 yr after menarche, additional evaluation is undertaken to assess ovarian steroids, and a pelvic sonogram is obtained. At that point, we may recommend metformin, particularly if there is evidence of IR, and/or an oral contraceptive pill.
Areas of current controversy
Pharmacotherapy
In pediatrics, medications are usually employed to treat a disease. However, it is not clear whether PA, per se, qualifies as a disease, although it often is an antecedent to adolescent and adult metabolic disease, androgen excess, or PCOS. Because PA does not always progress to a state of metabolic dysregulation, the question of how to identify which young children require pharmacotherapy remains. If we view PA as a potential antecedent of adult disease, earnest thought must be given to the benefit of early intervention with altered lifestyle and/or medical therapy. Because treatment may be continued for years, the long-term risk of pharmacological intervention must also be carefully weighed.
A common factor in the progression of metabolic disturbances associated with PA is IR, a feature shared with PCOS. In PCOS, metformin has been reported to have salutary effects on menstrual irregularity, fertility, weight, and hyperandrogenism (94, 95). However, studies of the effects of metformin treatment on metabolic changes in young patients and populations at risk for PCOS have been limited. Metformin acts through AMP kinase, a regulator of energy metabolism that restores ATP levels by switching on catabolic pathways (glycolysis, fatty acid oxidation) and switching off gluconeogenesis. AMP kinase may also affect reproductive functions including granulosa cell steroidogenesis, nuclear oocyte maturation, and hypothalamic LH secretion and may be involved in adipokine signaling. Other effects of metformin include increased glucose uptake through facilitation of glucose transporter translocation to the plasma membrane, increased insulin receptor tyrosine kinase activity, and reduced availability of IGF-1 through increased IGFBP-1 (95).
Although formal guidelines are not presently available for pharmacotherapy in PA, preliminary studies suggest that in girls with PA and a history of low birth weight and rapidly progressive puberty, metformin may reverse the trends of advancing weight gain, increasing truncal fat mass, and lipid abnormalities (75). Ibáñez et al. (96) also used metformin in an effort to halt the advancement of rapidly progressive puberty in girls as young as 8 yr of age. After 4 yr, girls in the treated group were thinner, less insulin resistant, and less hyperandrogenic; had less visceral fat; and had lower IGF-1 levels than untreated girls (96).
A recent 6-yr, randomized, controlled, longitudinal study of metformin treatment in 38 girls with PA and a history of being small for gestational age, who were thus at high risk for development of early menarche and PCOS, demonstrated the lasting beneficial effects of metformin for at least 1 yr after termination of treatment. Adolescents who were previously treated with metformin had later menarche compared with controls, accompanied by a more normal distribution of age at menarche. More than 2 yr after menarche, treated adolescents had less IHL and VAT, greater lean mass, and lower testosterone and fasting insulin levels compared with controls (97). During treatment, IGF-1 levels were significantly lower in the treatment group (98), but treated adolescents were closer to their target heights at 2 yr after menarche (97).
Despite promising results of metformin therapy, longer-term studies in larger populations are needed before universal recommendations for drug therapy can be made.
Back to the Patient
Age 5 yr
At the time of presentation, EA had signs of androgen excess, i.e. growth acceleration and onset of body odor and pubic hair. Although she had a normal newborn screen for 21-hydroxylase deficiency, it is not usually positive for patients with NC-CAH. Additionally, EA's family history may suggest NC-CAH, given her Ashkenazi ancestry and her father's history of acne and early frontal balding. However, EA's normal 17OHP on initial evaluation was not suggestive of NC-CAH. Her negative history of exposure to steroid-containing compounds excludes an exogenous source of androgens or estrogens.
On physical examination, the absence of clitoromegaly supported the diagnosis of PA rather than a pathological virilizing process. Lack of breast tissue and prepubertal gonadotropins were not consistent with early puberty. Although her parents were tall, her increase in growth velocity was suspicious. However, her bone age was not significantly advanced, and her adrenal androgens were concordant with her Tanner II stage of pubic hair development. These findings exclude an adrenal tumor as the cause of her PP. It is noteworthy that even at this young age EA demonstrated elevated triglycerides and a slight decrease in her FGIR suggestive of early IR, which could be consistent with the adipose-tissue expandability hypothesis (69).
Age 10 yr
EA had onset of breast tissue at a normal age but had relatively early menarche. Mean age of menarche for Caucasian females is close to 12 yr (81, 99). At this time, she was overweight and had excess facial and body hair and comedones. Hirsutism may be associated with adrenal hyperandrogenism or may be an early manifestation of PCOS.
With bone age advancement, EA's height prediction was in the lower range for her midparental sex-adjusted target height, although she was still within the normal range for adult female height. Idiopathic PA is usually not associated with a significantly diminished adult height (4, 5).
EA's elevated trunk fat, percentage body fat, IMCL, and IHL are all consistent with a picture of developing PCOS and MeS. Ectopic fat deposition refers to accumulation of fat in the skeletal muscle (IMCL), liver (IHL), and trunk (VAT). The progression of IR likely involves ectopic fat deposition in the following sequence: IMCL leads to skeletal muscle IR, followed by dyslipidemia, IHL deposition, hepatic IR, VAT accumulation, and intrapancreatic lipid deposition. This causes the abnormal release of adipokines, resulting in the development of MeS and type 2 diabetes (100). Our preliminary data from nonobese adolescents with PCOS and controls show that IMCL correlates with measures of IR, while IHL correlates with dyslipidemia, supporting this proposed sequence (101).
Age 16 yr
EA was counseled on diet and exercise to decrease her relative IR. Two years after the onset of menses, she was started on metformin for persistently irregular cycles and hyperinsulinemia. Although limited long-term data are available on outcomes of girls with PA treated with metformin, this appears to be a reasonable intervention in an attempt to decrease IR. Despite metformin use, she continued to have borderline elevation of her lipids and blood glucose.
During the progression of puberty, her weight percentile stabilized and she did not become hypertensive, although her hormone levels were consistent with ovarian hyperandrogenism or PCOS. The issue of use of oral contraceptive pills in this population is somewhat controversial because they can aggravate low-grade inflammation and adiposity (102), but oral contraceptive pills can serve to decrease free circulating androgens by increasing SHBG levels and thus result in improvement of acne.
Conclusion
EA's history underscores the need for careful clinical and laboratory follow-up of children with PA. Although many children who present with PA will not have substantial metabolic risk, some indeed are at risk for adult MeS and PCOS. The task of the future will be to identify those at greatest risk and discern who might benefit from both lifestyle changes and early pharmacological intervention. If the inciting event in the cascade leading to PA is, in fact, hyperinsulinemia, efforts to reverse this process with agents such as metformin may prove reasonable.
Acknowledgments
This work was supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Training Grant in Pediatric Endocrinology T32 DK065522 (Principal Investigator, S.E.O.); NIH/NIDDK Medical Student Research Training Program Grant 3T32DK065522-06S1 (to A.T.G.); and a Doris Duke Clinical Research Foundation grant to Columbia University College of Physicians and Surgeons (to A.T.G.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Δ4-A
- Δ4-Androstenedione
- BMI
- body mass index
- CAH
- congenital adrenal hyperplasia
- CPP
- central precocious puberty
- DEXA
- dual-energy x-ray absorptiometry
- DHEA
- dehydroepiandrosterone
- DHEAS
- DHEA sulfate
- FGIR
- fasting glucose-to-insulin ratio
- hCG
- human chorionic gonadotropin
- 3βHSD2
- 3β-hydroxysteroid dehydrogenase type 2
- IGFBP-1
- IGF binding protein-1
- IHL
- intrahepatic lipid
- IMCL
- intramyocellular lipid content
- IR
- insulin resistance
- LDL
- low-density lipoprotein
- MeS
- metabolic syndrome
- NC-CAH
- nonclassical CAH
- oGTT
- oral glucose tolerance test
- 17OHP
- 17-hydroxyprogesterone
- PA
- premature adrenarche
- PCOS
- polycystic ovary syndrome
- POR
- P450 oxidoreductase
- PP
- premature pubarche
- VAT
- visceral adipose tissue
- ZR
- zona reticularis.
References
- 1. Dhom G. 1973. The prepubertal and pubertal growth of the adrenal (adrenarche). Beitr Pathol 150:357–377 [DOI] [PubMed] [Google Scholar]
- 2. Silverman SH, Migeon C, Rosemberg E, Wilkins L. 1952. Precocious growth of sexual hair without other secondary sexual development: “premature pubarche,” a constitutional variation of adolescence. Pediatrics 10:426–432 [PubMed] [Google Scholar]
- 3. Sigurjonsdottir TJ, Hayles AB. 1968. Premature pubarche. Clin Pediatr (Phila) 7:29–33 [DOI] [PubMed] [Google Scholar]
- 4. Pang S. 1981. Precocious thelarche and premature adrenarche predictors. Pediatr Ann 10:340–345 [Google Scholar]
- 5. Pang S. 1984. Premature adrenarche. Pediatr Adolesc Endocrinol 13:173–184 [Google Scholar]
- 6. Miller WL, Auchus RJ. 2011. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 32:81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghizzoni L, Milani S. 2000. The natural history of premature adrenarche. J Pediatr Endocrinol Metab 13:1247–1251 [PubMed] [Google Scholar]
- 8. Ibañez L, Virdis R, Potau N, Zampolli M, Ghizzoni L, Albisu MA, Carrascosa A, Bernasconi S, Vicens-Calvet E. 1992. Natural history of premature pubarche: an auxological study. J Clin Endocrinol Metab 74:254–257 [DOI] [PubMed] [Google Scholar]
- 9. Pere A, Perheentupa J, Peter M, Voutilainen R. 1995. Follow up of growth and steroids in premature adrenarche. Eur J Pediatr 154:346–352 [PubMed] [Google Scholar]
- 10. Ibáñez L, Potau N, Marcos MV, de Zegher F. 1999. Exaggerated adrenarche and hyperinsulinism in adolescent girls born small for gestational age. J Clin Endocrinol Metab 84:4739–4741 [DOI] [PubMed] [Google Scholar]
- 11. Diaz A, Bhandari S, Sison C, Vogiatzi M. 2008. Characteristics of children with premature pubarche in the New York metropolitan area. Horm Res 70:150–154 [DOI] [PubMed] [Google Scholar]
- 12. Ibáñez L, Jiménez R, de Zegher F. 2006. Early puberty-menarche after precocious pubarche: relation to prenatal growth. Pediatrics 117:117–121 [DOI] [PubMed] [Google Scholar]
- 13. Denburg MR, Silfen ME, Manibo AM, Chin D, Levine LS, Ferin M, McMahon DJ, Go C, Oberfield SE. 2002. Insulin sensitivity and the insulin-like growth factor system in prepubertal boys with premature adrenarche. J Clin Endocrinol Metab 87:5604–5609 [DOI] [PubMed] [Google Scholar]
- 14. Oppenheimer E, Linder B, DiMartino-Nardi J. 1995. Decreased insulin sensitivity in prepubertal girls with premature adrenarche and acanthosis nigricans. J Clin Endocrinol Metab 80:614–618 [DOI] [PubMed] [Google Scholar]
- 15. Vuguin P, Linder B, Rosenfeld RG, Saenger P, DiMartino-Nardi J. 1999. The roles of insulin sensitivity, insulin-like growth factor I (IGF-1), and IGF-binding protein-1 and -3 in the hyperandrogenism of African-American and Caribbean Hispanic girls with premature adrenarche. J Clin Endocrinol Metab 84:2037–2042 [DOI] [PubMed] [Google Scholar]
- 16. Ibáñez L, Potau N, Zampolli M, Riqué S, Saenger P, Carrascosa A. 1997. Hyperinsulinemia and decreased insulin-like growth factor-binding protein-1 are common features in prepubertal and pubertal girls with a history of premature pubarche. J Clin Endocrinol Metab 82:2283–2288 [DOI] [PubMed] [Google Scholar]
- 17. Silfen ME, Manibo AM, Ferin M, McMahon DJ, Levine LS, Oberfield SE. 2002. Elevated free IGF-1 levels in prepubertal Hispanic girls with premature adrenarche: relationship with hyperandrogenism and insulin sensitivity. J Clin Endocrinol Metab 87:398–403 [DOI] [PubMed] [Google Scholar]
- 18. Ibáñez L, Aulesa C, Potau N, Ong K, Dunger DB, de Zegher F. 2002. Plasminogen activator inhibitor-1 in girls with precocious pubarche: a premenarcheal marker for polycystic ovary syndrome? Pediatr Res 51:244–248 [DOI] [PubMed] [Google Scholar]
- 19. Centers for Disease Control and Prevention National Center for Health Statistics 2008. National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; http://www.cdc.gov/growthcharts/ [Google Scholar]
- 20. Silfen ME, Manibo AM, McMahon DJ, Levine LS, Murphy AR, Oberfield SE. 2001. Comparison of simple measures of insulin sensitivity in young girls with premature adrenarche: the fasting glucose to insulin ratio may be a simple and useful measure. J Clin Endocrinol Metab 86:2863–2868 [DOI] [PubMed] [Google Scholar]
- 21. Fernández JR, Redden DT, Pietrobelli A, Allison DB. 2004. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr 145:439–444 [DOI] [PubMed] [Google Scholar]
- 22. Ohki Y, Orimo H, Kishi M, Ohkawa T. 2004. Indexes of insulin resistance using the oral glucose tolerance test (O-GTT) in Japanese children and adolescents. J Nippon Med Sch 71:84–87 [DOI] [PubMed] [Google Scholar]
- 23. McCarthy HD, Cole TJ, Fry T, Jebb SA, Prentice AM. 2006. Body fat reference curves for children. Int J Obes (Lond) 30:598–602 [DOI] [PubMed] [Google Scholar]
- 24. Lanman JT. 1953. The fetal zone of the adrenal gland: its developmental course, comparative anatomy, and possible physiologic functions. Medicine (Baltimore) 32:389–430 [DOI] [PubMed] [Google Scholar]
- 25. Mesiano S, Katz SL, Lee JY, Jaffe RB. 1997. Insulin-like growth factors augment steroid production and expression of steroidogenic enzymes in human fetal adrenal cortical cells: implications for adrenal androgen regulation. J Clin Endocrinol Metab 82:1390–1396 [DOI] [PubMed] [Google Scholar]
- 26. Spencer SJ, Mesiano S, Lee JY, Jaffe RB. 1999. Proliferation and apoptosis in the human adrenal cortex during the fetal and perinatal periods: implications for growth and remodeling. J Clin Endocrinol Metab 84:1110–1115 [DOI] [PubMed] [Google Scholar]
- 27. Hammer GD, Parker KL, Schimmer BP. 2005. Minireview: transcriptional regulation of adrenocortical development. Endocrinology 146:1018–1024 [DOI] [PubMed] [Google Scholar]
- 28. Stocco DM, Clark BJ. 1996. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev 17:221–244 [DOI] [PubMed] [Google Scholar]
- 29. Miller WL. 2007. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim Biophys Acta 1771:663–676 [DOI] [PubMed] [Google Scholar]
- 30. Nakamura Y, Gang HX, Suzuki T, Sasano H, Rainey WE. 2009. Adrenal changes associated with adrenarche. Rev Endocr Metab Disord 10:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Auchus RJ, Rainey WE. 2004. Adrenarche—physiology, biochemistry and human disease. Clin Endocrinol (Oxf) 60:288–296 [DOI] [PubMed] [Google Scholar]
- 32. Miller WL, Auchus RJ, Geller DH. 1997. The regulation of 17,20 lyase activity. Steroids 62:133–142 [DOI] [PubMed] [Google Scholar]
- 33. Miller WL, Huang N, Pandey AV, Flück CE, Agrawal V. 2005. P450 oxidoreductase deficiency: a new disorder of steroidogenesis. Ann NY Acad Sci 1061:100–108 [DOI] [PubMed] [Google Scholar]
- 34. Lin D, Black SM, Nagahama Y, Miller WL. 1993. Steroid 17 α-hydroxylase and 17,20-lyase activities of P450c17: contributions of serine106 and P450 reductase. Endocrinology 132:2498–2506 [DOI] [PubMed] [Google Scholar]
- 35. Auchus RJ, Geller DH, Lee TC, Miller WL. 1998. The regulation of human P450c17 activity: relationship to premature adrenarche, insulin resistance and the polycystic ovary syndrome. Trends Endocrinol Metab 9:47–50 [DOI] [PubMed] [Google Scholar]
- 36. Mapes S, Corbin CJ, Tarantal A, Conley A. 1999. The primate adrenal zona reticularis is defined by expression of cytochrome b5, 17α-hydroxylase/17,20-lyase cytochrome P450 (P450c17) and NADPH-cytochrome P450 reductase (reductase) but not 3β-hydroxysteroid dehydrogenase/Δ5–4 isomerase (3β-HSD). J Clin Endocrinol Metab 84:3382–3385 [DOI] [PubMed] [Google Scholar]
- 37. Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE. 2000. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 53:739–747 [DOI] [PubMed] [Google Scholar]
- 38. Zhang LH, Rodriguez H, Ohno S, Miller WL. 1995. Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proc Natl Acad Sci USA 92:10619–10623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pandey AV, Mellon SH, Miller WL. 2003. Protein phosphatase 2A and phosphoprotein SET regulate androgen production by P450c17. J Biol Chem 278:2837–2844 [DOI] [PubMed] [Google Scholar]
- 40. Chin JE, Dickens M, Tavare JM, Roth RA. 1993. Overexpression of protein kinase C isoenzymes α, βI, γ, and ε in cells overexpressing the insulin receptor. Effects on receptor phosphorylation and signaling. J Biol Chem 268:6338–6347 [PubMed] [Google Scholar]
- 41. Takayama S, White MF, Kahn CR. 1988. Phorbol ester-induced serine phosphorylation of the insulin receptor decreases its tyrosine kinase activity. J Biol Chem 263:3440–3447 [PubMed] [Google Scholar]
- 42. Lorence MC, Murry BA, Trant JM, Mason JI. 1990. Human 3 β-hydroxysteroid dehydrogenase/Δ5–4 isomerase from placenta: expression in nonsteroidogenic cells of a protein that catalyzes the dehydrogenation/isomerization of C21 and C19 steroids. Endocrinology 126:2493–2498 [DOI] [PubMed] [Google Scholar]
- 43. Topor LS, Asai M, Dunn J, Majzoub JA. 2011. Cortisol stimulates secretion of dehydroepiandrosterone in human adrenocortical cells through inhibition of 3βHSD2. J Clin Endocrinol Metab 96:E31–E39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gell JS, Carr BR, Sasano H, Atkins B, Margraf L, Mason JI, Rainey WE. 1998. Adrenarche results from development of a 3β-hydroxysteroid dehydrogenase-deficient adrenal reticularis. J Clin Endocrinol Metab 83:3695–3701 [DOI] [PubMed] [Google Scholar]
- 45. Noordam C, Dhir V, McNelis JC, Schlereth F, Hanley NA, Krone N, Smeitink JA, Smeets R, Sweep FC, Claahsen-van der Grinten HL, Arlt W. 2009. Inactivating PAPSS2 mutations in a patient with premature pubarche. N Engl J Med 360:2310–2318 [DOI] [PubMed] [Google Scholar]
- 46. Duncan GE, Li SM, Zhou XH. 2004. Prevalence and trends of a metabolic syndrome phenotype among US adolescents, 1999–2000. Diabetes Care 27:2438–2443 [DOI] [PubMed] [Google Scholar]
- 47. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. 2004. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350:2362–2374 [DOI] [PubMed] [Google Scholar]
- 48. Ibáñez L, Potau N, Chacon P, Pascual C, Carrascosa A. 1998. Hyperinsulinaemia, dyslipaemia and cardiovascular risk in girls with a history of premature pubarche. Diabetologia 41:1057–1063 [DOI] [PubMed] [Google Scholar]
- 49. Ibáñez L, Castell C, Tresserras R, Potau N. 1999. Increased prevalence of unknown type 2 diabetes mellitus and impaired glucose tolerance in first-degree relatives of girls with a history of precocious pubarche. Clin Endocrinol (Oxf) 51:395–401 [DOI] [PubMed] [Google Scholar]
- 50. Cara JF, Rosenfield RL. 1988. Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology 123:733–739 [DOI] [PubMed] [Google Scholar]
- 51. Duleba AJ, Spaczynski RZ, Olive DL. 1998. Insulin and insulin-like growth factor I stimulate the proliferation of human ovarian theca-interstitial cells. Fertil Steril 69:335–340 [DOI] [PubMed] [Google Scholar]
- 52. Plymate SR, Hoop RC, Jones RE, Matej LA. 1990. Regulation of sex hormone-binding globulin production by growth factors. Metabolism 39:967–970 [DOI] [PubMed] [Google Scholar]
- 53. Ibáñez L, Dimartino-Nardi J, Potau N, Saenger P. 2000. Premature adrenarche—normal variant or forerunner of adult disease? Endocr Rev 21:671–696 [DOI] [PubMed] [Google Scholar]
- 54. Rosenfield RL. 1996. Editorial: evidence that idiopathic functional adrenal hyperandrogenism is caused by dysregulation of adrenal steroidogenesis and that hyperinsulinemia may be involved. J Clin Endocrinol Metab 81:878–880 [DOI] [PubMed] [Google Scholar]
- 55. Dimartino-Nardi J. 1999. Premature adrenarche: findings in prepubertal African-American and Caribbean-Hispanic girls. Acta Paediatr Suppl 88:67–72 [DOI] [PubMed] [Google Scholar]
- 56. DiMartino-Nardi J. 2000. Pre- and postpubertal findings in premature adrenarche. J Pediatr Endocrinol Metab 13(Suppl 5):1265–1269 [PubMed] [Google Scholar]
- 57. Vuguin P, Saenger P, Dimartino-Nardi J. 2001. Fasting glucose insulin ratio: a useful measure of insulin resistance in girls with premature adrenarche. J Clin Endocrinol Metab 86:4618–4621 [DOI] [PubMed] [Google Scholar]
- 58. McCartney CR, Prendergast KA, Chhabra S, Eagleson CA, Yoo R, Chang RJ, Foster CM, Marshall JC. 2006. The association of obesity and hyperandrogenemia during the pubertal transition in girls: obesity as a potential factor in the genesis of postpubertal hyperandrogenism. J Clin Endocrinol Metab 91:1714–1722 [DOI] [PubMed] [Google Scholar]
- 59. Ibañez L, Potau N, Virdis R, Zampolli M, Terzi C, Gussinyé M, Carrascosa A, Vicens-Calvet E. 1993. Postpubertal outcome in girls diagnosed of premature pubarche during childhood: increased frequency of functional ovarian hyperandrogenism. J Clin Endocrinol Metab 76:1599–1603 [DOI] [PubMed] [Google Scholar]
- 60. Ibáñez L, Díaz R, López-Bermejo A, Marcos MV. 2009. Clinical spectrum of premature pubarche: links to metabolic syndrome and ovarian hyperandrogenism. Rev Endocr Metab Disord 10:63–76 [DOI] [PubMed] [Google Scholar]
- 61. Ibáñez L, Valls C, Potau N, Marcos MV, de Zegher F. 2001. Polycystic ovary syndrome after precocious pubarche: ontogeny of the low-birthweight effect. Clin Endocrinol (Oxf) 55:667–672 [DOI] [PubMed] [Google Scholar]
- 62. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group 2004. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81:19–25 [DOI] [PubMed] [Google Scholar]
- 63. Zawadzki JK, Dunaif A. 1992. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR. eds. Polycystic ovary syndrome. Oxford, UK: Blackwell; 59–69 [Google Scholar]
- 64. Carmina E, Oberfield SE, Lobo RA. 2010. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol 203:201.e1–5 [DOI] [PubMed] [Google Scholar]
- 65. Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. 2003. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med 157:821–827 [DOI] [PubMed] [Google Scholar]
- 66. de Ferranti SD, Gauvreau K, Ludwig DS, Neufeld EJ, Newburger JW, Rifai N. 2004. Prevalence of the metabolic syndrome in American adolescents: findings from the Third National Health and Nutrition Examination Survey. Circulation 110:2494–2497 [DOI] [PubMed] [Google Scholar]
- 67. Güven A, Cinaz P, Bideci A. 2005. Is premature adrenarche a risk factor for atherogenesis? Pediatr Int 47:20–25 [DOI] [PubMed] [Google Scholar]
- 68. Andiran N, Yordam N. 2008. Lipoprotein(a) levels in girls with premature adrenarche. J Paediatr Child Health 44:138–142 [DOI] [PubMed] [Google Scholar]
- 69. de Zegher F, Lopez-Bermejo A, Ibáñez L. 2009. Adipose tissue expandability and the early origins of PCOS. Trends Endocrinol Metab 20:418–423 [DOI] [PubMed] [Google Scholar]
- 70. Leibel N, Shen W, Mao X, Punyanitya M, Gallagher D, Horlick M, Shungu DC, Oberfield SE. 2009. Body composition in premature adrenarche by structural MRI, 1H MRS and DXA. J Pediatr Endocrinol Metab 22:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sopher AB, Thornton JC, Silfen ME, Manibo A, Oberfield SE, Wang J, Pierson RN, Jr, Levine LS, Horlick M. 2001. Prepubertal girls with premature adrenarche have greater bone mineral content and density than controls. J Clin Endocrinol Metab 86:5269–5272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Utriainen P, Jääskeläinen J, Saarinen A, Vanninen E, Mäkitie O, Voutilainen R. 2009. Body composition and bone mineral density in children with premature adrenarche and the association of LRP5 gene polymorphisms with bone mineral density. J Clin Endocrinol Metab 94:4144–4151 [DOI] [PubMed] [Google Scholar]
- 73. Ibáñez L, Ong K, de Zegher F, Marcos MV, del Rio L, Dunger DB. 2003. Fat distribution in non-obese girls with and without precocious pubarche: central adiposity related to insulinaemia and androgenaemia from prepuberty to postmenarche. Clin Endocrinol (Oxf) 58:372–379 [DOI] [PubMed] [Google Scholar]
- 74. Ibáñez L, De Zegher F. 2003. Flutamide-metformin therapy to reduce fat mass in hyperinsulinemic ovarian hyperandrogenism: effects in adolescents and in women on third-generation oral contraceptives. J Clin Endocrinol Metab 88:4720–4724 [DOI] [PubMed] [Google Scholar]
- 75. Ibáñez L, Ferrer A, Ong K, Amin R, Dunger D, de Zegher F. 2004. Insulin sensitization early post-menarche prevents progression from precocious pubarche to polycystic ovary syndrome. J Pediatr 144:23–29 [DOI] [PubMed] [Google Scholar]
- 76. Ibáñez L, Ong K, Ferrer A, Amin R, Dunger D, de Zegher F. 2003. Low-dose flutamide-metformin therapy reverses insulin resistance and reduces fat mass in nonobese adolescents with ovarian hyperandrogenism. J Clin Endocrinol Metab 88:2600–2606 [DOI] [PubMed] [Google Scholar]
- 77. Ibáñez L, Potau N, Ferrer A, Rodriguez-Hierro F, Marcos MV, De Zegher F. 2002. Anovulation in eumenorrheic, nonobese adolescent girls born small for gestational age: insulin sensitization induces ovulation, increases lean body mass, and reduces abdominal fat excess, dyslipidemia, and subclinical hyperandrogenism. J Clin Endocrinol Metab 87:5702–5705 [DOI] [PubMed] [Google Scholar]
- 78. Vottero A, Capelletti M, Giuliodori S, Viani I, Ziveri M, Neri TM, Bernasconi S, Ghizzoni L. 2006. Decreased androgen receptor gene methylation in premature pubarche: a novel pathogenetic mechanism? J Clin Endocrinol Metab 91:968–972 [DOI] [PubMed] [Google Scholar]
- 79. Kaplan SL, Grumbach MM. 1998. Pathogenesis of sexual precocity. In: Grumbach MM, Sizonenko PC, Aubert ML. eds. Williams textbook of endocrinology. Philadelphia: WB Saunders; 1509–1625 [Google Scholar]
- 80. Chalumeau M, Chemaitilly W, Trivin C, Adan L, Bréart G, Brauner R. 2002. Central precocious puberty in girls: an evidence-based diagnosis tree to predict central nervous system abnormalities. Pediatrics 109:61–67 [DOI] [PubMed] [Google Scholar]
- 81. Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, Hasemeier CM. 1997. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the Pediatrics Research in Office Settings network. Pediatrics 99:505–512 [DOI] [PubMed] [Google Scholar]
- 82. Wu T, Mendola P, Buck GM. 2002. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the third National Health and Nutrition Examination Survey, 1988–1994. Pediatrics 110:752–757 [DOI] [PubMed] [Google Scholar]
- 83. Thamdrup E. 1961. Precocious sexual development: a clinical study of 100 patients. Springfield, IL: Charles C. Thomas Publisher Ltd.; 50 [Google Scholar]
- 84. Paul D, Conte FA, Grumbach MM, Kaplan SL. 1995. Long-term effect of gonadotropin-releasing hormone agonist therapy on final and near-final height in 26 children with true precocious puberty treated at a median age of less than 5 years. J Clin Endocrinol Metab 80:546–551 [DOI] [PubMed] [Google Scholar]
- 85. Pescovitz OH, Cassorla F, Comite F, Loriaux DL, Cutler GB., Jr 1985. LHRH analog treatment of central precocious puberty complicating congenital adrenal hyperplasia. Ann NY Acad Sci 458:174–181 [DOI] [PubMed] [Google Scholar]
- 86. Pescovitz OH, Hench K, Green O, Comite F, Loriaux DL, Cutler GB., Jr 1985. Central precocious puberty complicating a virilizing adrenal tumor: treatment with a long-acting LHRH analog. J Pediatr 106:612–614 [DOI] [PubMed] [Google Scholar]
- 87. Carel JC, Léger J. 2008. Precocious puberty. N Engl J Med 358:2366–2377 [DOI] [PubMed] [Google Scholar]
- 88. Kohn B, Levine LS, Pollack MS, Pang S, Lorenzen F, Levy D, Lerner AJ, Rondanini GF, Dupont B, New MI. 1982. Late-onset steroid 21-hydroxylase deficiency: a variant of classical congenital adrenal hyperplasia. J Clin Endocrinol Metab 55:817–827 [DOI] [PubMed] [Google Scholar]
- 89. Armengaud JB, Charkaluk ML, Trivin C, Tardy V, Bréart G, Brauner R, Chalumeau M. 2009. Precocious pubarche: distinguishing late-onset congenital adrenal hyperplasia from premature adrenarche. J Clin Endocrinol Metab 94:2835–2840 [DOI] [PubMed] [Google Scholar]
- 90. Voelker R. 2010. Estrogen spray poses risks to children, pets through contact with treated skin. JAMA 304:953. [DOI] [PubMed] [Google Scholar]
- 91. Henley DV, Lipson N, Korach KS, Bloch CA. 2007. Prepubertal gynecomastia linked to lavender and tea tree oils. N Engl J Med 356:479–485 [DOI] [PubMed] [Google Scholar]
- 92. Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. 2009. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev 30:75–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kunz GJ, Klein KO, Clemons RD, Gottschalk ME, Jones KL. 2004. Virilization of young children after topical androgen use by their parents. Pediatrics 114:282–284 [DOI] [PubMed] [Google Scholar]
- 94. Diamanti-Kandarakis E, Economou F, Palimeri S, Christakou C. 2010. Metformin in polycystic ovary syndrome. Ann NY Acad Sci 1205:192–198 [DOI] [PubMed] [Google Scholar]
- 95. Palomba S, Falbo A, Zullo F, Orio F., Jr 2009. Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: a comprehensive review. Endocr Rev 30:1–50 [DOI] [PubMed] [Google Scholar]
- 96. Ibáñez L, López-Bermejo A, Díaz M, Marcos MV, de Zegher F. 2008. Metformin treatment for four years to reduce total and visceral fat in low birth weight girls with precocious pubarche. J Clin Endocrinol Metab 93:1841–1845 [DOI] [PubMed] [Google Scholar]
- 97. Ibáñez L, Lopez-Bermejo A, Diaz M, Marcos MV, de Zegher F. 2011. Early metformin therapy to delay menarche and augment height in girls with precocious pubarche. Fertil Steril 95:727–730 [DOI] [PubMed] [Google Scholar]
- 98. Ibáñez L, Lopez-Bermejo A, Diaz M, Marcos MV, de Zegher F. 2010. Pubertal metformin therapy to reduce total, visceral, and hepatic adiposity. J Pediatr 156:98–102.e1 [DOI] [PubMed] [Google Scholar]
- 99. Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sørensen TI, Dunkel L, Himes JH, Teilmann G, Swan SH. 2008. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics 121:S172–191 [DOI] [PubMed] [Google Scholar]
- 100. Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, Papademetris X, Rothman DL, Shulman GI. 2007. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA 104:12587–12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sopher AB, Lee E, Root J, Castle RH, Frisse EA, Gallagher D, Hirsch J, Mao X, Shungu DK, Hassoun A, McMahon DJ, Oberfield SE, Intramyocellular lipid (IMCL) deposition is an early marker of insulin resistance (IR) in adolescent girls with polycystic ovary syndrome (PCOS) and controls. Program of the Annual Meeting of the Pediatric Academic Societies, Vancouver, British Columbia, 2010 (Abstract 2855.402) [Google Scholar]
- 102. Ibáñez L, de Zegher F. 2004. Ethinylestradiol-drospirenone, flutamide-metformin, or both for adolescents and women with hyperinsulinemic hyperandrogenism: opposite effects on adipocytokines and body adiposity. J Clin Endocrinol Metab 89:1592–1597 [DOI] [PubMed] [Google Scholar]
- 103. National Heart, Lung, and Blood Institute 1980. The Lipid Research Clinics Population Studies Data Book. Vol. I. The prevalence study. Bethesda, MD: U.S. Department of Heart and Human Services, Public Health Service, National Institutes of Health, NIH pub. no. 80-1527 [Google Scholar]