The endometrium of women with PCOS is dysfunctional, exhibiting signs of progesterone resistance, consistent with the elevated risk of endometrial cancer and poor reproductive performance.
Abstract
Context:
Polycystic ovary syndrome (PCOS), the most common endocrinopathy of reproductive-aged women, is characterized by ovulatory dysfunction and hyperandrogenism.
Objective:
The aim was to compare gene expression between endometrial samples of normal fertile controls and women with PCOS.
Design and Setting:
We conducted a case control study at university teaching hospitals.
Patients:
Normal fertile controls and women with PCOS participated in the study.
Interventions:
Endometrial samples were obtained from normal fertile controls and from women with PCOS, either induced to ovulate with clomiphene citrate or from a modeled secretory phase using daily administration of progesterone.
Main Outcome Measure:
Total RNA was isolated from samples and processed for array hybridization with Affymetrix HG U133 Plus 2 arrays. Data were analyzed using GeneSpring GX11 and Ingenuity Pathways Analysis. Selected gene expression differences were validated using RT-PCR and/or immunohistochemistry in separately obtained PCOS and normal endometrium.
Results:
ANOVA analysis revealed 5160 significantly different genes among the three conditions. Of these, 466 were differentially regulated between fertile controls and PCOS. Progesterone-regulated genes, including mitogen-inducible gene 6 (MIG6), leukemia inhibitory factor (LIF), GRB2-associated binding protein 1 (GAB1), S100P, and claudin-4 were significantly lower in PCOS endometrium; whereas cell proliferation genes, such as Anillin and cyclin B1, were up-regulated.
Conclusions:
Differences in gene expression provide evidence of progesterone resistance in midsecretory PCOS endometrium, independent of clomiphene citrate and corresponding to the observed phenotypes of hyperplasia, cancer, and poor reproductive outcomes in this group of women.
Polycystic ovary syndrome (PCOS) is a common endocrine disorder, characterized by ovulatory dysfunction and hyperandrogenism, and is associated with increased risk for endometrial hyperplasia and cancer (1). Early studies demonstrated that PCOS patients also exhibit poor reproductive outcomes, suggesting possible diminished endometrial receptivity (2), in part due to anovulation and reduced exposure to progesterone with elevated levels of androgens or insulin. Estrogen receptor (ER) α and steroid receptor coactivators are also overexpressed in PCOS endometrium, signifying a reduced effectiveness of progesterone (3, 4).
Progesterone, acting through its cognate receptors PR-A and PR-B, is absolutely required for successful embryo implantation and postimplantation embryo survival in all placental mammals, including humans (5–7). Changes in endometrial steroid receptor and coactivator expression in PCOS are associated with reduced endometrial receptivity (4) and enhanced actions of estrogen (8), contributing to the elevated risk for hyperplasia and cancer noted in these women (9).
A resistance to the actions of progesterone has been described in some women with infertility (10, 11). The possible mechanisms involved in this progesterone resistance may reside in PR itself, which was first reported by Chrousos et al. (12) and is supported by recent data (13). Alterations in the expression or function of PR coactivators, chaperones, and co-chaperones that are bound to PR before activation have been implicated in progesterone resistance (14, 15). Similarly, the overexpression of co-chaperone FKBP51 (16) or lack of co-chaperone FKBP52 causes progesterone resistance in experimental murine models (17).
Clomiphene citrate (CC) has been used to induce follicle maturation and ovulation in PCOS. However, CC has been associated with lower pregnancy rates (15%) and a higher likelihood of miscarriage (18), suggesting that CC may adversely affect the endometrial receptivity (19). High-throughput DNA microarray techniques have demonstrated that human endometrium undergoes programmatic changes in gene expression throughout the menstrual cycle that can become dysfunctional in certain conditions such as endometriosis (11). However, there are few reports using microarray analysis to demonstrate the endometrial changes associated with PCOS (20) and none that examine anovulatory PCOS endometrium in women treated with CC. Here, we investigate differences in gene expression in PCOS endometrium that might account for poor reproductive outcome and higher risk for endometrial hyperplasia and cancer, compared with normal women. A secondary objective is to determine whether these changes are dependent or independent of CC, a commonly used fertility treatment for women with PCOS.
Patients and Methods
Sample collection and processing
Endometrium was obtained by timed endometrial biopsy using approved Institutional Review Board protocols from the University of California (San Francisco, CA), the University of North Carolina (Chapel Hill, NC), and Greenville Hospital System (Greenville, SC). Patients diagnosed with PCOS according to the Rotterdam criteria (1) were included in the study. Secretory endometrium was obtained in urinary LH-timed cycles after ovulation induction with CC [100 mg/d from d 5–9; n = 3 PCOS patients treated with CC (PCOScc)] or after daily 10-mg progesterone oil injections for 10 d in anovulatory women with PCOS, based on published methods that mimic midsecretory endometrium [n = 3 PCOS patients treated with progesterone (PCOSp)] (21). In the PCOSp group, serum levels of progesterone were obtained to validate the in vivo model, and all were above 10 ng/ml (17 ± 4.68 ng/ml; mean ± sd). Histological changes were appropriate for the midsecretory phase in all cases.
The results using DNA microarray were compared with normal endometrium previously obtained by LH-timed endometrial biopsy from proven fertile controls [n = 3 normal mid-secretory endometrium (MSEn)] and characterized by DNA microarray (22). None of the subjects had taken any other hormone-altering medications for the past 3 months. Endometrial tissue obtained by Pipelle (CooperSurgical, Inc., Trumbull, CT) sampling was divided and used for histological examination in formalin-fixed, paraffin-embedded blocks or snap-frozen in liquid nitrogen for preparation of RNA. For validation of gene differences, additional samples of endometrium were obtained from 18 women with PCOS based on Rotterdam criteria, and normal fertile controls were used for validation of gene expression differences using immunohistochemistry and/or real-time PCR.
RNA preparation, target preparation, array hybridization, and scanning
Total RNA extraction, purification, quality analysis, cDNA and cRNA generation and hybridization with high-density human genome (HG) U133 Plus 2.0 Arrays (Affymetrix, Inc., Santa Clara, CA), scanning, and microarray gene expression data analysis were performed as previously described (22, 23).
Statistical analysis, sample size calculation, principal component analysis (PCA), and Venn diagram
Sample size was calculated according to the literature (24), considering the median of a sd value of 0.2 for HG U133 Plus 2 microarray data set, 2.5-fold change or greater for a true effect size, a proportion of nondifferentially expressed genes of 0.9, a power of 0.8, and a false discovery rate of 0.05, yielding three samples per group. That was the number of samples included in each group (PCOScc, PCOSp, and MSEn). Gene significance was performed through ANOVA using the following parameters: P < 0.05, Benjamini-Hochberg false discovery rate as multiple testing correction, and Tukey as a post hoc test (GeneSpring GX 11; Agilent, Santa Clara, CA). Genes derived from ANOVA were plotted in a Venn diagram, and the intersection among the three possible comparisons of the three groups was submitted to PCA and filtered using the Volcano plot, having the 2.5-fold change and a P < 0.05. This list of genes represented the common genes related to PCOS (PCOScc + PCOSp) vs. MSEn.
Ingenuity pathways analysis (IPA)/canonical pathway analysis: entire data set
Preliminary data obtained from ANOVA were uploaded to IPA 5.5 (Ingenuity Systems, www.ingenuity.com) having a 2.0-fold change cutoff among the conditions and a P < 0.05. A 2.0-fold change was chosen instead of a 2.5-fold change because the former yielded a more robust IPA analysis based on larger numbers of gene differences. Explanation of the graphical representation of the pathways and the molecules can be accessed at http://www.ingenuity.com/company/pdf/Citation_Guidelines_2005-09-13.pdf. To control for possible confounding factors, such as age and ethnicity, two genes from a list of genes submitted to validation were analyzed using analysis of covariance (MedCalc v. 11.5.0.0; MedCalc Software, Mariakerke, Belgium), having gene expression as the dependent variable, age and ethnicity as covariables, and treatment groups as factors.
Validation of microarray data by real-time PCR
Generation of the first stand cDNA was done with iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) from 1 μg of total RNA of all samples. Genes with different expression fold changes in MSEn, PCOScc, and PCOSp were selected for validation by real-time PCR. Selection was based on genes related to endometrium and infertility (22). Primer performance and efficiencies, cycle threshold (Ct) value calculations, and normalization with ribosomal protein L19 (RPL19) were performed as previously described (23). Primers are listed in Table 1. Primers for anillin (ANLN), cyclin B1 (CCNB1), and claudin-4 (CLDN4) were done with predesigned TaqMan probe-primer sets from Applied Biosystems (Foster City, CA), and amplification was done according to the manufacturer's instructions. Statistical analysis of the RT-PCR data was performed using the ANOVA or Kruskal-Wallis test of the relative expression of each group, having as post hoc test Tukey and Dunn test, respectively, and P < 0.05 (GraphPad InStat; GraphPad Software, Inc., La Jolla, CA).
Table 1.
Primers used for microarray gene expression validation
| Gene | Forward primer (5′-3′) | Reverse primer (5′-3′) | Amplicon length (bp) | Annealing temperature (C) |
|---|---|---|---|---|
| LIF | CTGTTGGTTCTGCACTGGAA | GCCACATAGCTTGTCCAGGT | 131 | 60 |
| GAB1 | GATGGAGGAAGCAGCCCTAT | GAGCCAAGGTCTTCTGTTGG | 152 | 60 |
| S100P | AAGGTGCTGATGGAGAAGGA | ACTTGTGACAGGCAGACGTG | 167 | 58 |
| Gastrin (GAST) | CTTAGGTACAGGGGCCAACA | TCAGTTTTTCAGGGGACAGG | 154 | 58 |
| ANXA4 | CACCGATGATAACACCCTCA | GAATCCTCCTGTCCTTCTGG | 176 | 58 |
| Cyclin B1 | CCAACTCTACAACATTACCTGTCA | AGTGCAGAATTCAGCTGTGG | 162 | 58 |
| Tetraspanin 8 | TCATTTGGACTGGCAGTTATTG | GGGGTTTGACTGACGATAGGT | 119 | 56 |
| MIG6 | TTGCTGCTCAGGAGATCAGA | TTCAGACTGTAGGCCATGGTT | 154 | 58 |
| SWS | TCTACCCTGATATTCCAAATCCA | GAAATGCTGATCGAGTTTCCA | 151 | 56 |
| Cyclin E2 | TGGATGGTACCTTTTGTCAATG | CAACTGTCCCCCTTTTCTGA | 196 | 56 |
| SLC1A1 | ATGGTGATTGTGCTGAGTGC | TCGTTGTCAAGGATTGTGGA | 234 | 56 |
Immunohistochemistry
Protein validation of specific gene expression was performed by immunohistochemistry using sections of endometrial biopsies from normal and PCOS women using specific antibodies [mitogen-inducible gene 6 (MIG6), R3028 at 1:400 (Sigma, St. Louis, MO); and GRB2-associated binding protein 1 (GAB1), antiphospho-GAB1 04-355 at 1:100 (Upstate Cell Signaling Solutions, Temecula, CA)]. Sections were deparaffinized in toluene and rehydrated in graded concentrations of ethanol (100 to 70%). Antigen retrieval was achieved by heating sections in a 750-watt microwave at 80% power in 0.01 m citrate buffer before treatment. After a PBS rinse, endogenous peroxidase was quenched with a 30-min incubation with 0.3% H2O2 in absolute ethanol and incubated with blocking serum for 15 min at 22 C (1:100 dilution of nonimmune goat serum). The primary antibody was serially diluted in a solution of PBS containing 1% normal goat serum and 0.1% sodium azide.
Tissue sections were incubated with the primary antibody at 4 C overnight and washed with PBS twice and then with 1% normal goat serum for 30 min. Negative control sections were treated with nonimmune serum diluted in the same manner. Sections were incubated with Biotin-SP-conjugated goat antirat IgM (Jackson ImmunoResearch Labs, West Grove, PA) for 30 min at room temperature. After rinsing with PBS, the immunoreactive antigen was visualized using avidin-biotin peroxidase complex (Vectastain Elite ABC Kit; Vector Laboratories, Inc., Burlingame, CA) and 3,3-diaminobenzidine as the chromogen. Slides were counterstained with Mayer's hematoxylin blue/toluidine blue, dehydrated in a graded dilution of ethanol, cleared in xylene, and mounted. Staining was evaluated microscopically by a single blinded observer (B.A.L.) using a semiquantitative histological score (HSCORE) (25).
Results
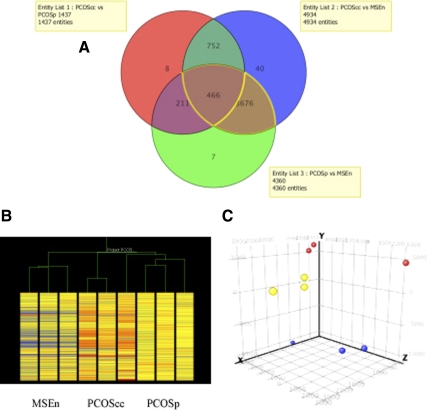
Characteristics of the studied population are shown in Table 2. A Venn diagram of overall differences in gene expression for the three groups (PCOScc, PCOSp, and MSEn) is shown in Fig. 1A. The ANOVA analysis among the three groups, using a 2.5-fold change, yielded 5160 genes (a full list of gene changes is found in Supplemental Data, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Relationships between the three groups are visually apparent in both the unsupervised hierarchical cluster analysis (heat map; Fig. 1B) and PCA (Fig. 1C). The PCA analysis is a tool to characterize the predominant gene expression patterns, derived from a matrix of the measurements of the 54,675 genes in different subjects. Using PCA, it is possible to identify whether samples from the same conditions have similar patterns of gene expression. The patterns that are grouped in similar areas within the three-dimensional condition scatter plot demonstrate similarity between members of each sample group as well as differences between groups (PCOScc, PCOSp, and MSEn).
Table 2.
Characteristics of the samples
| Patient ID | Cycle phase | Age (yr) | Indication/diagnosis | Ethnicity | Medications |
|---|---|---|---|---|---|
| M153 | MSEa | 34 | Endometrial biopsy –volunteer | Caucasian | |
| M158 | 23 | Endometrial biopsy –volunteer | Black | ||
| M163 | 33 | Endometrial biopsy –volunteer | Caucasian | ||
| U927 | PCOScca | 25 | Infertility–PCOS | Caucasian | CC |
| U858 | 30 | Infertility–PCOS | Black | CC | |
| U874 | 28 | Infertility–PCOS | Caucasian | CC | |
| LE-01 | PCOSpa | 25 | Study volunteer–PCOS | Hispanic | Progesterone in oil |
| LE-02 | 33 | Study volunteer–PCOS | Caucasian | Progesterone in oil | |
| LE-04 | 32 | Study volunteer–PCOS | Caucasian | Progesterone in oil | |
| U58b | PCOS | 32 | Infertility–PCOS | Caucasian | CC |
| U504b | PCOS | 31 | Infertility–PCOS | Caucasian | CC |
| U699b | PCOS | 28 | Infertility–PCOS | Black | CC |
| U575b | PCOS | 27 | Infertility–PCOS | Caucasian | CC |
| U75b | PCOS | 33 | Infertility–PCOS | Caucasian | CC |
| U502b | PCOS | 32 | Infertility–PCOS | Black | CC |
| T1188b | PCOS | 24 | Infertility–PCOS | Caucasian | No meds |
| U858b | PCOS | 31 | Infertility–PCOS | Black | Pioglitazone |
| U838b | PCOS | 32 | Infertility–PCOS | Caucasian | No meds |
| T1396b | PCOS | 32 | Infertility–PCOS | Caucasian | No meds |
| U116b | PCOS | 27 | Infertility–PCOS | Caucasian | No meds |
| U779b | PCOS | 34 | Infertility–PCOS | Black | No meds |
| U999b | PCOS | 32 | Infertility–PCOS | Asian | No meds |
| T1728b | PCOS | 24 | Infertility–PCOS | Black | Proliferative phase |
| U109b | PCOS | 40 | Infertility–PCOS | Caucasian | Proliferative phase |
| T1426b | PCOS | 40 | Infertility–PCOS | Caucasian | Proliferative phase |
| U869b | PCOS | 30 | Infertility–PCOS | Caucasian | CC |
| U425b | PCOS | 25 | Infertility–PCOS | Caucasian | No meds |
Cycle phase MSE is from Ref. 22.
Sample used for array analysis and RT-PCR validation.
Sample used for immunohistochemistry.
Fig. 1.
A, Venn diagram showing gene expression differences within each group of patients tested, including PCOScc, PCOSp, and MSE samples. B and C, Relationships between groups of subjects is apparent by hierarchical cluster analysis (B) and PCA (C). In the former, unsupervised grouping of each sample based on gene expression differences correctly sorts the nine samples into their appropriate groups. Using PCA, applied to nine endometrium samples that were characterized by the gene expression of all probes on the Affymetrix chip HG U133 Plus 2.0, there are three groupings, including normal controls (blue), PCOScc (yellow), and anovulatory PCOSp (red).
To explore the pertinent differences between normal midsecretory endometrium and the two PCOS groups, we used the gene list from the intersection among the three groups depicted in the Venn diagram (466 genes). These 466 genes represent common genes that were statistically different across the three possible comparisons between PCOS cases (PCOScc + PCOSp) and MSEn. With this approach, the analysis was adjusted for confounding factors (i.e. CC and progesterone). From these 466 genes, a 2.5-fold change filter was applied. This filtering between PCOS and MSEn yielded 107 genes down-regulated (Supplemental Table 1a) and 37 genes up-regulated (Supplemental Table 1b).
Gene differences common to both PCOS groups (PCOScc and PCOSp)
Based on the analysis of 466 genes that were uniformly down- or up-regulated in both PCOS groups, compared with MSEn, several patterns of gene expression were found that are relevant to the window of implantation and establishment of uterine receptivity. Two of the most down-regulated genes in the PCOS groups were S100P and claudin-4, followed by prostaglandin E receptor 3 (subtype EP3) and thereafter by fucosyltransferase 7 (α (1,3) fucosyltransferase) (FUT7). Another well-characterized implantation gene that was significantly down-regulated in one PCOS group (PCOScc) compared with MSEn was leukemia inhibitory factor (LIF). Validation of claudin-4, LIF, and S100P and selected nonimplantation genes by RT-PCR was confirmed as shown in Table 3.
Table 3.
Real-time relative quantification of fluorescence of ΔCt gene of interest/ΔCt RPL19 transcript for MSEn, PCOScc, and PCOSp samples
| Gene of interest | MSEn (A) | PCOScc (B) | PCOSp (C) | P value | Post hoc test |
|---|---|---|---|---|---|
| ANXA4 | 2047 ± 86 | 160.4 ± 62.1 | 931.8 ± 3.3 | <0.0001 | A vs. B; A vs. C; B vs. C |
| GAB1 | 17.4 ± 1.4 | 4 ± 0.7 | 1.6 ± 0.03 | <0.0001 | A vs. B; A vs. C |
| GAST | 33.1 ± 3.6 | 0.3 ± 0.2 | 19.1 ± 1.2 | 0.001 | A vs. B; B vs. C |
| TSPAN8 | 20.6 ± 3.8 | 0.59 ± 0.2 | 39.8 ± 3.2 | 0.006 | B vs. C |
| Cyclin B1 | 1.01 ± 0.1 | 3.49 ± 0.9 | 1.14 ± 0.05 | 0.001 | A vs. B; B vs. C |
| Claudin-4 | 12.54 ± 2.4 | 2.64 ± 0.9 | 7.47 ± 1.9 | 0.001 | A vs. B; A vs. C |
| Anillin | 1.03 ± 0.2 | 3.97 ± 1.7 | 1.84 ± 0.3 | 0.01 | A vs. B |
| CYCE2 | 2.2 ± 0.3 | 3.4 ± 0.3 | 1.6 ± 0.3 | 0.02 | B vs. C |
| LIF | 30.7 ± 10.2 | 1.7 ± 1.3 | 12.1 ± 0.4 | 0.03 | A vs. B |
| SLC1A1 | 388.6 ± 107.5 | 15.7 ± 9.7 | 482.1 ± 9.6 | 0.03 | B vs. C |
| SWS | 13.2 ± 1.4 | 7.7 ± 1.8 | 5.8 ± 0.6 | 0.03 | A vs. C |
| MIG6 | 11.6 ± 2.0 | 10.5 ± 2.3 | 4.4 ± 0.8 | 0.03 | A vs. C |
| S100P | 5.1 (0.84–38.6) | 0.004 (0.002–0.1) | 0.03 (0.006–0.05) | 0.03 | A vs. B; A vs. Ca |
TSPAN8, Tetraspanin 8; CYCE2, cyclin E2; SCL1A1, solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system Xag), member 1; SWS, leukemia inhibitory factor receptor α. See text for other genes.
Kruskal-Wallis test; values are expressed as median (range).
Ingenuity pathways analysis
The 5160 genes obtained from ANOVA analysis were uploaded to IPA, which evaluated the changes of the genes over the three conditions (PCOScc, PCOSp, MSEn). After using a 2.0-fold cutoff, the analysis identified 400 genes that were eligible for generating network maps. The comparison analysis performed by IPA among the three conditions identified 45 main canonical pathways that reached the 2-fold cutoff in at least one of the conditions (Supplement Data). From these pathways, two were chosen for further analysis—epidermal growth factor (EGF), and estrogen signaling pathways—because of their importance in endometrial receptivity and function. Excessive growth leading to hyperplasia or cancer as well as progesterone resistance associated with infertility and pregnancy loss are related to the balance between estrogen and progesterone action.
EGF signaling pathway
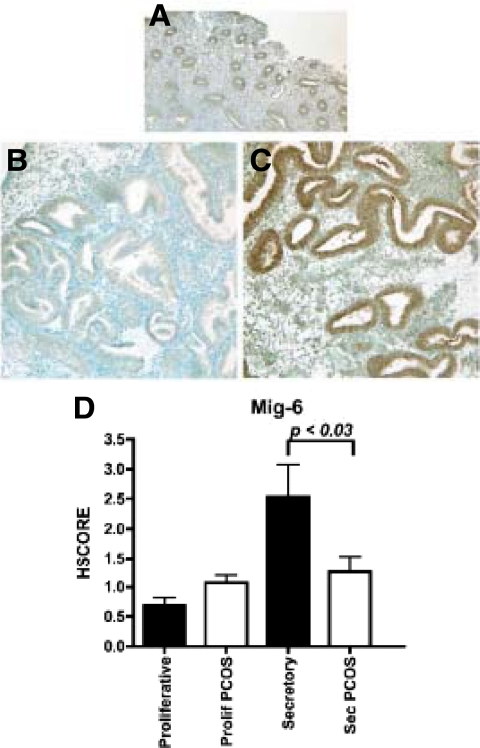
The EGF signaling pathway demonstrated key genes of interest. When the EGF pathway was analyzed in detail, the down-regulation of MIG6 (also known as ERRFI1) was noted in PCOSp, and validation was performed using RT-PCR (Table 3) and immunohistochemistry (Fig. 2, A–D). The expression of MIG6 was higher in the epithelium of normal samples in the midsecretory phase compared with proliferative endometrium in normal women (Fig. 2D). However, MIG6 was significantly down-regulated (P < 0.05) in the midsecretory phase of the additional PCOS patients studied, compared with the midsecretory endometrium from controls (Fig. 2D).
Fig. 2.
A–C, Immunohistochemistry of MIG6 in proliferative endometrium (A) and secretory endometrium from PCOS (B) and normal (C) subjects. D, HSCORE, as described in Patients and Methods, was used as a semiquantitative measure of tissue expression. Comparison by ANOVA revealed significant difference in MIG6 expression between normal and PCOS endometrium (P < 0.03).
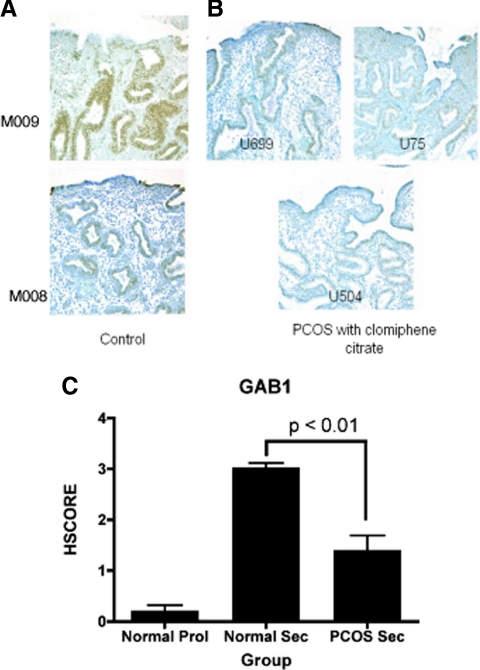
A second gene of interest derived from IPA analysis was GAB1, which was down-regulated in both PCOSp and PCOScc compared with MSEn. Validation of the reduced levels of GAB1 expression in PCOS endometrium (both PCOScc and PCOSp) was performed by both RT-PCR and immunohistochemistry. RT-PCR values showed a reduced expression of this gene in PCOS patients, compared with normal secretory controls (Table 3). Confirmation of GAB1 immunohistochemistry expression between PCOS endometrial biopsies and normal fertile controls was performed with anti-phospho GAB1 (pGAB1) antibodies. A decrease in pGAB1 levels in PCOS endometrium compared with fertile controls (Fig. 3, A and B) was identified. The HSCORE results show that the active form of GAB1 (pGAB1) expression is regulated during the menstrual cycle with low expression in the proliferative phase and an increased expression in the secretory phase of normal controls. In PCOS endometrium, there was a significant reduction in midsecretory GAB1 expression compared with controls (Fig. 3C).
Fig. 3.
GAB1 expression in secretory phase endometrium from controls (A) and PCOScc (B). C, HSCORE comparisons in cycling and PCOS women demonstrated increased expression between the proliferative and normal midsecretory phase and significantly more GAB1 in normal midsecretory samples compared with PCOS endometrium in the midsecretory phase.
Estrogen pathway
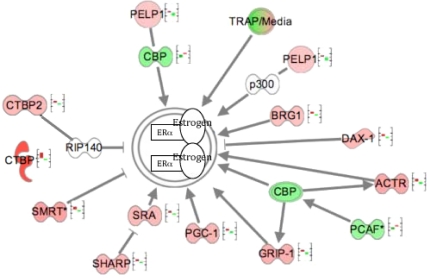
The ER signaling pathway, derived from IPA analysis, is depicted in Fig. 4. The genes belonging to this pathway and represented in Fig. 4 show an inverted expression in MSEn compared with PCOScc (all P < 0.05). Multiple signaling pathway genes down-regulated in the PCOScc group were not reduced in the PCOSp group (e.g. GRIP1, CPB/p300, PGC-1), but an end-point of estrogen action (proliferation) was validated by real-time RT-PCR using cyclin B1 and cyclin E2 (Table 3).
Fig. 4.
Regulation of the estrogen signaling pathway inside the nucleus. The figure displays gene expression of cofactors that modulate the ER + estrogen complex inside the nucleus. The first bar, from left to right, represents the fluorescent expression of a particular gene in MSEn, and the second bar in the graph represents PCOScc. Genes represented in red are up-regulated, and genes represented in green are down-regulated in PCOScc relative to MSEn. These values were obtained from the microarray analysis and exported to IPA. Note that each gene represented in the MSEn has the opposite expression in the PCOScc. The genes represented with a graph bar are those that reached a P < 0.05. Among the coactivators are: glucocorticoid receptor-interacting protein 1 (GRIP1); cAMP-response element binding protein (CBP/p300); thyroid hormone receptor-associated protein complex component (TRAP220); peroxisome proliferator-activated receptor γ coactivator-1 (PGC-1); nuclear receptor coactivator 3 (ACTR); SWItch/Sucrose NonFermentable-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 4 (BRG1); E1A binding protein p300 (p300) and K (lysine) acetyltransferase 2B (PCAF); proline glutamate and leucine rich protein 1 (PELP1); and steroid receptor RNA activator (SRA). Several corepressors regulate the activity of ERα either directly or via inhibiting a coactivator. Examples of direct inhibition include receptor-interacting protein (RIP140), silencing mediator of retinoid and thyroid receptors (SMRT), and nuclear receptor subfamily 0, group B, member 1 (DAX-1). Corepressors of ER that inhibit indirectly include SMRT/HDAC1 associated repressor protein (SHARP) and C-terminal binding protein 2 (CTBP2).
Gene expression and ethnicity
Noting that there are ethnic differences between groups and that ethnicity could potentially influence the patterns of gene expression (26), we applied analysis of covariance, having age and ethnicity as covariates to the gene expression data, either by microarray analysis (MIG6) or by RT-PCR results (GAB1) and found that the validation of two genes, MIG6 and GAB1, was not influenced by the differences in ethnicity or age (data not shown).
Discussion
Assessment of the secretory phase endometrium in anovulatory PCOS women has been difficult to study without using medications such as CC to induce ovulation. This selective ER modulator has long been suspected of causing an abnormal endometrial phenotype (19, 27). To examine PCOS endometrium without the effects of CC, we developed a model of midsecretory phase endometrium in anovulatory PCOS women that relies on exogenously administered progesterone (21).
The changes in gene expression that were coordinately down- or up-regulated in both PCOS groups suggest that PCOS endometrium is inherently different in terms of its response to progesterone. These data support the use of a transcriptome approach to detect endometrial abnormalities that are independent of histology. The use of hierarchical cluster analysis (Fig. 1B) and PCA (Fig. 1C) demonstrates that the gene expression patterns are clustered uniquely within each group of patients. Many genes of interest related to implantation are found in the list of changes in PCOS endometrium (Fig. 1 and Supplemental Tables 1a and 1b). Only one study examined the endometrium in women with PCOS. Qiao et al. (20) performed a microarray study on ovulatory PCOS patients and reported 105 genes that were differentially expressed. Interestingly, only one gene (Synuclein, α non-A4 component of amyloid precursor— SNCA) was found in common with our gene list.
In our study, claudin-4 was up-regulated in MSEn, as previously reported (28); its expression was reduced in both PCOS groups compared with MSEn controls (Table 3). Likewise, LIF, a critical factor for implantation, is down-regulated in PCOScc compared with MSEn (Supplemental Data), confirmed by RT-PCR (Table 3). Low expression of LIF and claudin-4 in PCOS patients is consistent with previous reports of low expression of LIF and claudin-4 associated with poor in vitro fertilization outcomes (29). Decreased LIF expression in PCOS endometrium may contribute to the well-documented impairment in other implantation-specific biomarkers in PCOS endometrium (3, 4). Importantly, CC by itself does not adversely alter LIF expression (30), suggesting that decreased LIF expression is likely due to intrinsic endometrial dysfunction in PCOS.
S100P, a small calcium binding protein family member, was the most down-regulated gene (23-fold) in the PCOS subjects. The function of S100P has yet to be defined, but our results are in accordance with those published by Tong et al. (31), who reported high expression of this gene during the window of implantation in normal human endometrium.
There was reduced expression of two glycotransferases thought to be involved in embryo implantation, FUT7 and MGAT5B [mannosyl (α-1,6-)-glycoprotein β-1,6-N-acetyl-glucosaminyltransferase] in PCOS endometrium. FUT7 is a key enzyme for Lewis X antigen synthesis, and the Lewis X antigen motif is a key component of L-selectin ligand, implicated in embryo-endometrial attachment (32). MGAT5B is a key enzyme for addition of N-glycans to cell surface proteins, including integrins (33), which are related to endometrial receptivity (34).
Platelet-activating factor receptor (PTAFR) is another down-regulated gene implicated in embryo implantation. PTAFR expression is increased in the porcine endometrium at implantation (35), and its ligand, platelet-activating factor, is essential for implantation in the mouse model (36). The reduced expression of multiple receptivity-associated genes, claudin-4, FUT7, MGAT5B, and PTAFR in PCOS endometrium may account for the observed reduction in cycle fecundability in these women.
Aside from fertility problems, PCOS patients also have a higher risk of endometrial hyperplasia and cancer (9). A number of genes associated with cellular proliferation including tumor repressors and DNA synthesis-related genes were altered in PCOS endometrium. Annexin IV (ANXA4) is highly expressed in endometrial tissue in the presence of progesterone (37), and its expression is significantly reduced in the midsecretory endometrium of PCOS women, despite the presence of progesterone (PCOSp; Table 3). Likewise, anillin, cyclin B1, and cyclin E2 were related to cell proliferation, and we validated their increase in PCOS endometrium. In the study by Qiao et al. (20), annexin A1 was differentially expressed in PCOS endometrium.
The estrogen signaling pathway was aberrant in PCOS endometrium based on microarray analysis supported by earlier reports. ERα regulates cell proliferation in the endometrium (38) and has enhanced activity in PCOS endometrium (3, 8). Changes in gene expression related to cell proliferation/mitosis include PELP1, ACTR, DAX-1, and CTBP1, each present in the IPA analysis (Fig. 4). A reduction in DAX-1 could lead to increased local estrogen production, further augmenting the proliferative phenotype. Topoisomerase 2α is an enzyme that contributes to DNA relaxation during transcription and other cellular processes (39). High expression of this enzyme is found in hyperplastic endometrium (40). Kinesin-like protein, KIF11, is related to hyperplasia and cancer (41) and is up-regulated in PCOScc (Supplemental Data).
Expression of tumor suppressor genes was reduced in both PCOS groups compared with controls, including discoidin, CUB, and LCCL domain contain 2 (DCBLD2); glioma tumor suppressor candidate region gene 2 (GLTSCR2); PDZ and LIM domain 4 (PDLIM4); RAS-like, family 10, member B (RASL10B); runt-related transcription factor 3 (RUNX3); and SRY (sex determining region Y)-box 15 (SOX15). A negative regulator of nuclear factor kB, nuclear factor of κ light polypeptide gene enhancer in B-cells inhibitor-like 2 (NFKBIL2), previously thought to be a biomarker of endometrial cancer (42), was lower in PCOS. Genes involved in the WNT signaling pathway, such as T-BOX1 (43) and DACT3 (44), were disrupted in both PCOS groups, corroborating the hyperplastic phenotype of the endometrium. TTK is a protein kinase found to be up-regulated in both PCOS groups. This gene is associated with chromosomal instability and neoplastic metastasis, including cervical cancers (45).
Of special interest, CTBP1, down-regulated in PCOScc compared with MSEn (Fig. 4), has been shown to mediate estrogen-induced transcriptional repression (46). The differential pattern of gene expression between PCOScc and PCOSp may be related to the antiestrogenic effects of CC rather than an intrinsic defect of PCOS endometrium itself. However, cyclins, proteins known for their essential role in the cell cycle, were increased in PCOS (cyclins B1 and E2 are up-regulated in PCOS, compared with MSEn) and significantly higher in PCOScc compared with PCOSp (Table 3). It is possible that other pathways (such as EGF) contribute to the proliferative phenotype observed in PCOS endometrium.
The EGF pathways are important for both implantation and differentiation of the uterus (47). Neuregulin-1, a member of the EGF family, is down-regulated in PCOS endometrium, compared with midsecretory control endometrium, and was shown to be down-regulated in endometrial adenocarcinomas (48). MIG6 is an early response gene that can be induced by various mitogens and acts as an inhibitor of the EGF receptor, abrogating cell proliferation (49). Null mutation of this gene is associated with endometrial hyperplasia in mice (50), making this gene of particular interest in PCOS. We previously reported that MIG6 was reduced in the endometrium of women with endometriosis (11), and this supports the finding of progesterone resistance in that disorder. Reduction of MIG6 in PCOS endometrium could contribute to the elevated risk of hyperplasia or cancer observed in the uterus from women with PCOS (51) and is consistent with the finding of a proliferative phenotype during the secretory phase and histological evidence for progesterone resistance (52).
GRB2-associated binding protein 1
During our validation of gene changes associated with the EGF signaling pathway, we found alterations in GAB1 through the menstrual cycle, as shown in Fig. 3. GAB1 is a direct substrate of both EGF and the insulin receptor and activates phosphatidylinositol-3 kinase and other effector proteins in response to the activation of many receptor tyrosine kinases (53). Regulated expression of GAB1 in human endometrium has not been reported heretofore. Regulation of GAB1 is under investigation, but the altered expression of this scaffolding protein during the midsecretory phase is a novel finding that will require further investigation.
In summary, we present a list of significant differences in gene expression and have validated important examples in PCOS endometrium that demonstrate progesterone resistance and elevated estrogen activity resulting in reduced endometrial receptivity and increased risk for hyperplasia and cancer. The observed differences in gene expression provide a roadmap for future studies to examine the effects of insulin resistance and type II diabetes on the PCOS endometrium and also the efficacies of various targeted therapies for PCOS that could improve endometrial function, improve fertility, and reduce the risk for subsequent gynecological disease.
Supplementary Material
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (NIH) through cooperative agreement U54-HD35041-12 (to B.A.L.) and U54HD055764-03 (to L.C.G.), as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior BEX3656-06-3 (to R.F.S.); and NIH Grant R01HD057873 (to J.-W.J.).
Disclosure Summary: All authors declare that they have no potential conflict of interest.
Footnotes
- ANXA4
- Annexin IV
- CC
- clomiphene citrate
- Ct
- cycle threshold
- EGF
- epidermal growth factor
- ER
- estrogen receptor
- FUT7
- fucosyltransferase 7
- GAB1
- GRB2-associated binding protein 1
- HG
- human genome
- HSCORE
- histological score
- IPA
- ingenuity pathways analysis
- LIF
- leukemia inhibitory factor
- MIG6
- mitogen-inducible gene 6
- MSEn
- normal mid-secretory endometrium
- PCA
- principal component analysis
- PCOS
- polycystic ovary syndrome
- PCOScc
- PCOS patients treated with CC
- PCOSp
- PCOS patients treated with progesterone
- PR
- progesterone receptor
- PTAFR
- platelet-activating factor receptor.
References
- 1. 2004. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19:41–47 [DOI] [PubMed] [Google Scholar]
- 2. Dor J, Itzkowic DJ, Mashiach S, Lunenfeld B, Serr DM. 1980. Cumulative conception rates following gonadotropin therapy. Am J Obstet Gynecol 136:102–105 [DOI] [PubMed] [Google Scholar]
- 3. Gregory CW, Wilson EM, Apparao KB, Lininger RA, Meyer WR, Kowalik A, Fritz MA, Lessey BA. 2002. Steroid receptor coactivator expression throughout the menstrual cycle in normal and abnormal endometrium. J Clin Endocrinol Metab 87:2960–2966 [DOI] [PubMed] [Google Scholar]
- 4. Quezada S, Avellaira C, Johnson MC, Gabler F, Fuentes A, Vega M. 2006. Evaluation of steroid receptors, coregulators, and molecules associated with uterine receptivity in secretory endometria from untreated women with polycystic ovary syndrome. Fertil Steril 85:1017–1026 [DOI] [PubMed] [Google Scholar]
- 5. Baulieu EE. 1989. Contragestion and other clinical applications of RU-486, an antiprogesterone at the receptor. Science 245:1351–1357 [DOI] [PubMed] [Google Scholar]
- 6. Lessey BA, Alexander PS, Horwitz KB. 1983. The subunit structure of human breast cancer progesterone receptors: characterization by chromatography and photoaffinity labeling. Endocrinology 112:1267–1274 [DOI] [PubMed] [Google Scholar]
- 7. Yang Z, Wolf IM, Chen H, Periyasamy S, Chen Z, Yong W, Shi S, Zhao W, Xu J, Srivastava A, Sánchez ER, Shou W. 2006. FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform. Mol Endocrinol 20:2682–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. MacLaughlan SD, Palomino WA, Mo B, Lewis TD, Lininger RA, Lessey BA. 2007. Endometrial expression of Cyr61: a marker of estrogenic activity in normal and abnormal endometrium. Obstet Gynecol 110:146–154 [DOI] [PubMed] [Google Scholar]
- 9. Navaratnarajah R, Pillay OC, Hardiman P. 2008. Polycystic ovary syndrome and endometrial cancer. Semin Reprod Med 26:62–71 [DOI] [PubMed] [Google Scholar]
- 10. Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, Julie Kim J. 2006. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol 248:94–103 [DOI] [PubMed] [Google Scholar]
- 11. Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. 2007. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148:3814–3826 [DOI] [PubMed] [Google Scholar]
- 12. Chrousos GP, MacLusky NJ, Brandon DD, Tomita M, Renquist DM, Loriaux DL, Lipsett MB. 1986. Progesterone resistance. Adv Exp Med Biol 196:317–328 [DOI] [PubMed] [Google Scholar]
- 13. Igarashi TM, Bruner-Tran KL, Yeaman GR, Lessey BA, Edwards DP, Eisenberg E, Osteen KG. 2005. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil Steril 84:67–74 [DOI] [PubMed] [Google Scholar]
- 14. Lonard DM, Kumar R, O'Malley BW. 2010. Minireview: the SRC family of coactivators: an entree to understanding a subset of polygenic diseases? Mol Endocrinol 24:279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rowan BG, O'Malley BW. 2000. Progesterone receptor coactivators. Steroids 65:545–549 [DOI] [PubMed] [Google Scholar]
- 16. Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. 2003. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology 144:2380–2387 [DOI] [PubMed] [Google Scholar]
- 17. Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF, Dey SK. 2005. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci USA 102:14326–14331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Homburg R. 2008. Polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol 22:261–274 [DOI] [PubMed] [Google Scholar]
- 19. Bonhoff AJ, Naether OG, Johannisson E. 1996. Effects of clomiphene citrate stimulation on endometrial structure in infertile women. Hum Reprod 11:844–849 [DOI] [PubMed] [Google Scholar]
- 20. Qiao J, Wang L, Li R, Zhang X. 2008. Microarray evaluation of endometrial receptivity in Chinese women with polycystic ovary syndrome. Reprod Biomed Online 17:425–435 [DOI] [PubMed] [Google Scholar]
- 21. Usadi RS, Groll JM, Lessey BA, Lininger RA, Zaino RJ, Fritz MA, Young SL. 2008. Endometrial development and function in experimentally induced luteal phase deficiency. J Clin Endocrinol Metab 93:4058–4064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC. 2006. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 147:1097–1121 [DOI] [PubMed] [Google Scholar]
- 23. Savaris RF, Hamilton AE, Lessey BA, Giudice LC. 2008. Endometrial gene expression in early pregnancy: lessons from human ectopic pregnancy. Reprod Sci 15:797–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu P, Hwang JT. 2007. Quick calculation for sample size while controlling false discovery rate with application to microarray analysis. Bioinformatics 23:739–746 [DOI] [PubMed] [Google Scholar]
- 25. Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS., Jr 1988. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab 67:334–340 [DOI] [PubMed] [Google Scholar]
- 26. Storey JD, Madeoy J, Strout JL, Wurfel M, Ronald J, Akey JM. 2007. Gene-expression variation within and among human populations. Am J Hum Genet 80:502–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thatcher SS, Donachie KM, Glasier A, Hillier SG, Baird DT. 1988. The effects of clomiphene citrate on the histology of human endometrium in regularly cycling women undergoing in vitro fertilization. Fertil Steril 49:296–301 [DOI] [PubMed] [Google Scholar]
- 28. Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B. 2002. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod 8:871–879 [DOI] [PubMed] [Google Scholar]
- 29. Serafini PC, Silva ID, Smith GD, Motta EL, Rocha AM, Baracat EC. 2009. Endometrial claudin-4 and leukemia inhibitory factor are associated with assisted reproduction outcome. Reprod Biol Endocrinol 7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ku°cu NK, Koyuncu FM, Var A, Laçin S, Uyanik BS, Ceylan E. 2002. Clomiphene citrate does not adversely affect endometrial leukemia inhibitory factor levels. Gynecol Endocrinol 16:151–154 [DOI] [PubMed] [Google Scholar]
- 31. Tong XM, Lin XN, Song T, Liu L, Zhang SY. 2010. Calcium-binding protein S100P is highly expressed during the implantation window in human endometrium. Fertil Steril 94:1510–1518 [DOI] [PubMed] [Google Scholar]
- 32. Wang B, Sheng JZ, He RH, Qian YL, Jin F, Huang HF. 2008. High expression of L-selectin ligand in secretory endometrium is associated with better endometrial receptivity and facilitates embryo implantation in human being. Am J Reprod Immunol 60:127–134 [DOI] [PubMed] [Google Scholar]
- 33. Saravanan C, Liu FT, Gipson IK, Panjwani N. 2009. Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on α3β1 integrin. J Cell Sci 122:3684–3693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lessey BA. 1998. Endometrial integrins and the establishment of uterine receptivity. Hum Reprod 13(Suppl 3):247–258 [DOI] [PubMed] [Google Scholar]
- 35. Yang W, Diehl JR, Yerle M, Ford JJ, Christenson RK, Roudebush WE, Plummer WE. 2003. Chromosomal location, structure, and temporal expression of the platelet-activating factor receptor (PAFr) gene in porcine endometrium and embryos relative to estrogen receptor α gene expression. Mol Reprod Dev 64:4–12 [DOI] [PubMed] [Google Scholar]
- 36. Nishi O, Tominaga T, Goto Y, Hayashi K, Mori T. 1995. Effects of platelet activating factor on mouse embryo implantation in vitro. J Assist Reprod Genet 12:330–334 [DOI] [PubMed] [Google Scholar]
- 37. Ponnampalam AP, Rogers PA. 2006. Cyclic changes and hormonal regulation of annexin IV mRNA and protein in human endometrium. Mol Hum Reprod 12:661–669 [DOI] [PubMed] [Google Scholar]
- 38. Okulicz WC, Balsamo M. 1993. A double immunofluorescent method for simultaneous analysis of progesterone-dependent changes in proliferation and the oestrogen receptor in endometrium of rhesus monkeys. J Reprod Fertil 99:545–549 [DOI] [PubMed] [Google Scholar]
- 39. Lynch BJ, Guinee DG, Jr, Holden JA. 1997. Human DNA topoisomerase II-alpha; a new marker of cell proliferation in invasive breast cancer. Hum Pathol 28:1180–1188 [DOI] [PubMed] [Google Scholar]
- 40. Ito K, Sasano H, Yabuki N, Matsunaga G, Sato S, Kikuchi A, Yajima A, Nagura H. 1997. Immunohistochemical study of Ki-67 and DNA topoisomerase II in human endometrium. Mod Pathol 10:289–294 [PubMed] [Google Scholar]
- 41. Valensin S, Ghiron C, Lamanna C, Kremer A, Rossi M, Ferruzzi P, Nievo M, Bakker A. 2009. A KIF11 inhibition for glioblastoma treatment: reason to hope or a struggle with the brain? BMC Cancer 9:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Domenyuk VP, Litovkin KV, Verbitskaya TG, Dubinina VG, Bubnov VV. 2007. Identification of new DNA markers of endometrial cancer in patients from the Ukrainian population. Exp Oncol 29:152–155 [PubMed] [Google Scholar]
- 43. Freyer L, Morrow BE. 2010. Canonical Wnt signaling modulates Tbx1, Eya1, and Six1 expression, restricting neurogenesis in the otic vesicle. Dev Dyn 239:1708–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiang X, Tan J, Li J, Kivimäe S, Yang X, Zhuang L, Lee PL, Chan MT, Stanton LW, Liu ET, Cheyette BN, Yu Q. 2008. DACT3 is an epigenetic regulator of Wnt/β-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer Cell 13:529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harima Y, Ikeda K, Utsunomiya K, Shiga T, Komemushi A, Kojima H, Nomura M, Kamata M, Sawada S. 2009. Identification of genes associated with progression and metastasis of advanced cervical cancers after radiotherapy by cDNA microarray analysis. Int J Radiat Oncol Biol Phys 75:1232–1239 [DOI] [PubMed] [Google Scholar]
- 46. Stossi F, Madak-Erdogan Z, Katzenellenbogen BS. 2009. Estrogen receptor α represses transcription of early target genes via p300 and CtBP1. Mol Cell Biol 29:1749–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ejskjaer K, Sørensen BS, Poulsen SS, Mogensen O, Forman A, Nexø E. 2005. Expression of the epidermal growth factor system in human endometrium during the menstrual cycle. Mol Hum Reprod 11:543–551 [DOI] [PubMed] [Google Scholar]
- 48. Srinivasan R, Benton E, McCormick F, Thomas H, Gullick WJ. 1999. Expression of the c-erbB-3/HER-3 and c-erbB-4/HER-4 growth factor receptors and their ligands, neuregulin-1 α, neuregulin-1 β, and betacellulin, in normal endometrium and endometrial cancer. Clin Cancer Res 5:2877–2883 [PubMed] [Google Scholar]
- 49. Jeong JW, Lee HS, Lee KY, White LD, Broaddus RR, Zhang YW, Vande Woude GF, Giudice LC, Young SL, Lessey BA, Tsai SY, Lydon JP, DeMayo FJ. 2009. Mig-6 modulates uterine steroid receptor responsiveness and exhibits altered expression in endometrial disease. Proc Natl Acad Sci USA 106:8677–8682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jin N, Gilbert JL, Broaddus RR, Demayo FJ, Jeong JW. 2007. Generation of a Mig-6 conditional null allele. Genesis 45:716–721 [DOI] [PubMed] [Google Scholar]
- 51. Pillay OC, Te Fong LF, Crow JC, Benjamin E, Mould T, Atiomo W, Menon PA, Leonard AJ, Hardiman P. 2006. The association between polycystic ovaries and endometrial cancer. Hum Reprod 21:924–929 [DOI] [PubMed] [Google Scholar]
- 52. Palomino WA, Fuentes A, González RR, Gabler F, Boric MA, Vega M, Devoto L. 2005. Differential expression of endometrial integrins and progesterone receptor during the window of implantation in normo-ovulatory women treated with clomiphene citrate. Fertil Steril 83:587–593 [DOI] [PubMed] [Google Scholar]
- 53. Mattoon DR, Lamothe B, Lax I, Schlessinger J. 2004. The docking protein Gab1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC Biol 2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.