Circulating levels of C-reactive protein do not differentiate individuals at higher mortality risk due to excess adiposity.
Abstract
Context:
It has been proposed that adiposity is a protective response to excess caloric supply, but it is cardiometabolically harmful once adipocytes become inflamed.
Objective:
The objective of the study was to assess whether elevated C-reactive protein (CRP), a measure of systemic inflammation, can differentiate individuals at higher mortality risk due to excess adiposity.
Design, Setting, and Participants:
We conducted an observational study of 16,486 white and 11,168 black men and women in the Reasons for Geographic and Racial Differences in Stroke study, a U.S. national cohort.
Main Outcome:
The main outcome was all-cause mortality.
Results:
The mean age of the cohort was 64 ± 9 yr. Over a 6-yr period, 927 whites and 669 blacks died. The absolute risk of death was highest among underweight whites and blacks (9.2 and 14%, respectively), not the obese (4.7% whites; 4.0% blacks) or severely obese (5.9% whites; and 4.6% blacks). Among those with elevated CRP (≥3 vs. <1 mg/liter), underweight [hazard ratio (HR) 2.08, 95% confidence interval (CI) 1.03–4.21] and normal-weight (HR 2.62, 95% CI 1.87–3.67) whites were at significantly higher mortality risk but not severely obese whites (HR 1.55, 95% CI 0.77–2.96), resulting in a statistical interaction (P = 0.01). Similar results were also seen for blacks, although a higher mortality risk among severely obese blacks with CRP 3 or greater vs. less than 1 mg/liter was also demonstrated (HR 2.58, 95% CI 1.04–6.41). Among whites and black women, higher waist circumference was associated with an increased mortality risk, although this relationship was not modified by CRP levels (P = 0.47 for whites and P = 0.25 for blacks).
Conclusion:
Among middle-aged and older adults, the addition of CRP was most informative among underweight and normal-weight individuals, not the obese. This negated our hypothesis that increased levels of CRP would differentiate individuals at higher mortality risk due to excess adiposity.
The prevalence of obesity in the U.S. population has doubled over the past 3 decades and in 2007–2008 was greater than 30% (1). Consequently, there is a major public health initiative to fight obesity and target prevention efforts toward the obese at highest risk for adverse events (2, 3). This is especially important among blacks, who have the highest rates of obesity in the United States and a 50% greater prevalence than whites (4). Mortality risk across the body mass index (BMI) distribution is highest among the underweight and the extremely obese (U shaped mortality), not the overweight and class I obese (5–9). An important unanswered question is whether there are specific strategies to identify and target individuals whose health is adversely impacted by excess adiposity, and second, whether ethnic differences exist within these relationships.
One approach to targeting the at-risk obese may be to identify those who also manifest a proinflammatory milieu. Metabolically active adipose tissue produces inflammatory cytokines and chemokines, resulting in elevated systemic levels of inflammation (10, 11). Subsequently, inflammatory signaling cascades influence multiple biological pathways, (12–16) including alterations in insulin signaling within liver and muscle (17) and production of reactive oxygen species leading to oxidative stress and premature aging (18). Clinically, C-reactive protein (CRP) is a widely used blood marker of systemic inflammation (19), correlated with measures of obesity, (20) and a predictor of future cardiovascular risk (21–23). To this end, biological and epidemiological studies demonstrate that obesity and inflammation are interrelated in the disease process, raising the hypothesis whether a proinflammatory state for a given level of adiposity increases mortality risk.
Ethnic differences between adiposity and subsequent mortality risk have been studied within several population-based cohorts. The relationship between obesity and mortality has been less robust among blacks, most notably older blacks (24). To date, there have been no studies to assess whether an elevated CRP in the setting of excess adiposity is a better predictor of death and how these variables relate to ethnicity.
The Reasons for Geographic and Racial Differences in Stroke (REGARDS) study is a national population-based cohort that began collecting medical history, body composition, and mortality outcomes in 2003 (25). Among 16,486 white and 11,168 black individuals, we determined the risk of death across the BMI and waist circumference distribution. Second, we assessed for a given level of adiposity, whether elevated CRP predicted higher mortality risk among whites and blacks.
Materials and Methods
The REGARDS study enrolled 30,239 white and black men and women 45 yr old and older between February 2003 and October 2007 (25). Of those enrolled, 16,486 whites and 11,168 blacks were included in the present study after excluding subjects with missing BMI (n = 184), CRP (n = 1726), both (n = 151), or follow-up data (n = 524). Fifty-six percent of participants resided in the stroke belt (North Carolina, South Carolina, Georgia, Alabama, Mississippi, Tennessee, Arkansas, and Louisiana), and the remaining were from the other 40 contiguous states. Individuals were recruited from a commercial list using mail and telephone. Demographic and medical history was obtained by telephone interview. At an in-home examination, blood pressure, anthropomorphic measures, blood samples, electrocardiogram, and medication inventory were assessed. Participants are followed up by telephone every 6 months for surveillance off medical events. Study methods were reviewed and approved by the institutional review boards of each study institution.
Anthropomorphic measures were obtained using standard protocols by trained staff. BMI was calculated as weight in kilograms divided by the square of height in meters and waist circumference reported in centimeters. Resting blood pressure was measured two times in the seated position and the average two readings were used. Hypertension was defined as systolic blood pressure 140 mm Hg or greater, diastolic pressure 90 mm Hg or greater or self-reported physician diagnosis of hypertension with use of antihypertensive medications. Diabetes was defined by self-reported physician diagnosis with use of antidiabetic medications, fasting glucose 126 mg/dl or greater or nonfasting glucose 200 mg/dl or greater; impaired fasting glucose was defined as fasting glucose 110 mg/dl or greater. Medication use was determined based on clinical staff pill bottle review of prescribed medications. Smoking status (never, former, current), educational level (less than high school, high school, some college, college and above), and physical activity (none, one to three times a week, more than three times weekly) of study participants were obtained by questionnaire. Region of residence was defined as the stroke belt or the remainder of the states.
Laboratory methods
Phlebotomy was performed by trained personnel using standardized procedures. Participants were asked to fast 10–12 h before their blood collection. Samples were centrifuged an average of 97 min after collection and serum or plasma separated and shipped overnight on ice packs to the University of Vermont as previously described (26). On arrival, samples were centrifuged at 30,000 g at 4 C and either analyzed (general chemistries) or stored at less than 80 C. CRP was analyzed in batches by particle enhanced immunonephelometry using the BNII nephelometer (N High Sensitivity CRP; Dade Behring, Deerfield, IL) with interassay coefficients of variation of 2.1–5.7%. Cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and glucose were measured by colorimetric reflectance spectrophotometry using the Ortho Vitros Clinical Chemistry System 950IRC instrument (Johnson & Johnson Clinical Diagnostics, New Brunswick, NJ).
Mortality tracking
Participants are contacted every 6 months. If a proxy reports a participant has died, an interview is conducted with the next of kin listed on study forms. REGARDS used social security death index, next of kin, and a combination of both to obtain dates of death. Time to death is recorded as the time from baseline interview to date of death.
Statistical analysis
All statistical analyses were assessed at a level of significance of P = 0.05. Student's t tests were used for comparing means of normally distributed continuous variables, Wilcoxon rank sum test for skewed continuous variables, and χ2 tests for proportions. Log transformations and standardization of skewed continuous variables were used if necessary in statistical models. BMI and waist circumference were as analyzed as both continuous and categorical variables. BMI categories were defined as follows: underweight (BMI <20 kg/m2); normal weight (BMI 20–24.9 kg/m2); overweight (BMI 25–29.9 kg/m2); obese (BMI 30–34.9 kg/m2); severely obese (≥35 kg/m2). The underweight category was expanded from standard definition (<18.5 kg/m2) to allow a sufficient sample size. Because there is no standard cut point for waist circumference, sex-race specific quartiles were used based on International Diabetes Federation guidelines (27). The categories of CRP were determined by American Heart Association guidelines (<1 mg/liter, low risk, 1–3 mg/liter, ≥3 mg/liter, high risk) (19). Age- and sex-specific quartiles of CRP and extreme cut point greater than 6 mg/liter were also explored.
Hazard ratios (HR) for all-cause mortality rates were obtained from Cox proportional hazard models in which all ties, if any, were corrected using the exact method. The relationships of BMI and waist circumference, separately, with all-cause mortality were assessed by nonparametric restricted age-adjusted quadratic splines stratified by sex and race. The association between measures of adiposity (BMI, waist circumference) and CRP on mortality risk among blacks and whites was adjusted for age, sex, hypertension, diabetes, smoking, alcohol use, and physical activity. Interaction terms between BMI-CRP and waist-CRP were used as continuous variables in age-adjusted models. Analyses were conducted using SAS software 9.1.3 (Cary, NC) and R software 2.9.2 (Vienna, Austria).
Results
The average age of participants in the current analysis was 64 ± 9 yr. The BMI distribution for each sex-race group closely resembled the BMI distribution of the U.S. population (28). Black men and women had a mean (sd) BMI of 28.9 (5.4) and 31.9 (7.1), respectively, whereas both white men and women had a mean BMI of 28.2 (5.5). Among the 27,654 individuals, 669 blacks and 927 whites died over a mean 6-yr period. Three percent were white women, 8% were white men, 4% were black women, and 9% black men died.
Table 1 demonstrates the relationship between participant characteristics and CRP 3 mg/liter or greater across the BMI distribution for whites and blacks, separately. In general, age was inversely associated with higher BMI. The severely obese with CRP 3 mg/liter or greater were younger compared with the severely obese with CRP less than 3 mg/liter (61 vs. 63 yr, P < 0.0001 for both blacks and whites). As expected, overweight, obese, and severely obese women were more likely to have CRP 3 mg/liter or greater compared with men (P < 0.0001 for each BMI category). Smoking was more common among those with elevated CRP (≥3 mg/liter) in all BMI categories. For both whites and blacks, diabetes was more prevalent with increasing BMI category. The percent of individuals with diabetes with CRP 3 mg/liter or greater varied within each BMI category, although it was similar for both whites and blacks. Other cardiovascular risk factors such as systolic blood pressure, low-density lipoprotein (LDL) cholesterol, and triglycerides increased with higher BMI category for both race groups. Those with elevated CRP (≥3 mg/liter) among the severely obese had higher levels of LDL cholesterol, triglycerides, and lower levels of HDL compared with those severely obese with CRP less than 3 mg/liter, but there was no difference in systolic blood pressure between CRP categories for the severely obese. Lastly, statin use was more common with increasing BMI category, although the percent with CRP 3 mg/liter or greater who were statin users was significantly lower among the obese or severely obese.
Table 1.
Baseline characteristics by BMI and CRP categories for whites and blacks in REGARDS
| BMI <20 kg/m2 CRP (mg/liter) |
BMI 20-24.9 kg/m2 CRP (mg/liter) |
BMI 25–29.9 kg/m2 CRP (mg/liter) |
BMI 30–34.9 kg/m2 CRP (mg/liter) |
BMI ≥35 kg/m2 CRP (mg/liter) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <3 | ≥3 | <3 | ≥3 | <3 | ≥3 | <3 | ≥3 | <3 | ≥3 | |
| Whites | ||||||||||
| Age (yr) (n,%) | 66.1 (10.78) | 68.9 (10.00)a | 66.1 (10.19) | 68.4 (9.73)b | 65.7 (9.32) | 66.6 (9.29)a | 64.5 (8.83) | 64.3 (8.69) | 63.0 (8.06) | 61.4 (8.01)b |
| Sex | ||||||||||
| Men | 96 (23%) | 35 (32%)a | 1444 (44%) | 442 (42%) | 2836 (63%) | 969 (47%)b | 1138 (60%) | 583 (42%)b | 373 (53%) | 382 (34%)b |
| Women | 319 (77%) | 73 (68%) | 1813 (56%) | 618 (58%) | 1643 (37%) | 1075 (53%) | 752 (40%) | 812 (58%) | 337 (47%) | 746 (66%) |
| Region | ||||||||||
| Stroke Belt | 246 (59%) | 64 (59%) | 1880 (58%) | 671 (63%)a | 2519 (56%) | 1236 (60%)a | 1070 (57%) | 885 (63%)a | 412 (58%) | 677 (60%) |
| Other states | 415 (41%) | 44 (41%) | 1377 (42%) | 389 (37%) | 1960 (44%) | 808 (40%) | 820 (43%) | 510 (37%) | 298 (42%) | 451 (40%) |
| Education | ||||||||||
| Less than high school | 27 (6%) | 12 (11%)a | 157 (5%) | 112 (10%)b | 258 (6%) | 190 (9%)b | 143 (7%) | 110 (8%)a | 58 (8%) | 121 (11%) |
| High school | 85 (21%) | 38 (35%) | 707 (22%) | 283 (27%) | 1025 (23%) | 541 (27%) | 489 (26%) | 405 (29%) | 187 (26%) | 285 (25%) |
| Some college | 108 (26%) | 25 (23%) | 793 (24%) | 262 (25%) | 1136 (25%) | 601 (29%) | 523 (28%) | 411 (29%) | 219 (31%) | 336 (30%) |
| College | 194 (47%) | 33 (31%) | 1600 (49%) | 402 (38%) | 2059 (46%) | 708 (35%) | 735 (39%) | 467 (34%) | 246 (35%) | 386 (34%) |
| Smoking status | ||||||||||
| Never | 203 (49%) | 38 (36%)a | 1657 (51%) | 420 (40%)b | 2006 (45%) | 804 (39%)b | 849 (45%) | 565 (41%)b | 339 (48%) | 515 (46%)b |
| Former | 117 (28%) | 25 (23%) | 1206 (37%) | 369 (45%) | 2076 (46%) | 891 (44%) | 865 (46%) | 631 (45%) | 321 (45%) | 471 (42%) |
| Current | 94 (23%) | 44 (41%) | 379 (12%) | 267 (25%) | 384 (9%) | 345 (17%) | 170 (9%) | 196 (14%) | 48 (7%) | 136 (12%) |
| Diabetes | ||||||||||
| None | 377 (96%) | 95 (94%) | 2886 (93%) | 888 (89%)b | 3564 (85%) | 1555 (84%)a | 1274 (75%) | 904 (74%)a | 355 (57%) | 592 (62%)a |
| Impaired fasting | 7 (2%) | 4 (4%) | 87 (3%) | 54 (5%) | 220 (5%) | 126 (7%) | 114 (7%) | 116 (10%) | 54 (9%) | 105 (11%) |
| Diabetes | 8 (2%) | 2 (2%) | 136 (4%) | 55 (6%) | 411 (10%) | 170 (9%) | 318 (18%) | 200 (16%) | 215 (34%) | 265 (28%) |
| Statin use | ||||||||||
| Yes | 80 (19%) | 22 (20%) | 868 (27%) | 258 (24%) | 1684 (38%) | 613 (30%)b | 787 (42%) | 444 (32%)b | 337 (47%) | 361 (32%)b |
| No | 335 (81%) | 86 (80%) | 2389 (73%) | 802 (76%) | 2795 (62%) | 1431 (70%) | 1103 (58%) | 951 (68%) | 373 (53%) | 767 (68%) |
| SBP (mm Hg) (mean/sd) | 117.4 (17.66) | 125 (20.43)a | 121.1 (15.96) | 125 (17.47)b | 125.1 (14.98) | 126 (16.10)a | 127.7 (14.71) | 128.2 (15.62) | 129.1 (15.52) | 129.9 (15.41) |
| DBP (mm Hg) (mean/sd) | 70.4 (9.41) | 71.2 (11.04) | 72.9 (8.93) | 73.0 (9.33) | 75.2 (8.80) | 75.1 (9.29) | 76.9 (8.88) | 77.0 (9.42) | 77.9 (9.87) | 78.1 (9.34) |
| LDL cholesterol (mg/dl) (mean/sd) | 105.1 (29.34) | 103.2 (30.49) | 113.4 (32.32) | 111 (34.86)a | 112.0 (32.93) | 114.9 (35.1)a | 109.6 (34.39) | 114 (33.78)a | 103.1 (32.62) | 111 (33.38)b |
| HDL cholesterol (mg/dl) (mean/sd) | 67.4 (17.78) | 60.6 (18.87)a | 57.7 (17.50) | 54.6 (18.52)b | 49.2 (14.18) | 49.4 (15.92) | 45.8 (13.71) | 46.3 (13.69) | 43.9 (12.95) | 45.4 (12.97)a |
| TG (median/IQR) | 87 (68–113) | 104 (78–134)a | 97 (73–134) | 117 (86–165)b | 119 (88–169) | 134 (97–188)b | 139 (101–198) | 149 (110–210)b | 151 (110–212) | 154 (113–210) |
| Blacks | ||||||||||
| Age (yr) (n, %) | 66.9 (11.35) | 66.9 (11.21) | 65.4 (10.02) | 66.3 (9.80) | 64.8 (9.56) | 65.0 (8.98) | 63.6 (8.78) | 63.7 (8.78) | 62.8 (8.96) | 61.4 (8.00)b |
| Sex | ||||||||||
| Men | 80 (44%) | 36 (53%) | 604 (51%) | 290 (49%) | 1148 (51%) | 555 (37%)b | 594 (45%) | 426 (28%)b | 233 (31%) | 316 (18%)b |
| Women | 103 (56%) | 32 (47%) | 591 (49%) | 296 (51%) | 1114 (49%) | 930 (63%) | 734 (55%) | 1099 (72%) | 522 (69%) | 1465 (82%) |
| Region | ||||||||||
| Stroke Belt | 93 (51%) | 35 (51%) | 571 (48%) | 308 (53%) | 1080 (48%) | 747 (50%) | 675 (51%) | 795 (52%) | 408 (54%) | 990 (56%) |
| Other states | 90 (49%) | 33 (49%) | 624 (52%) | 278 (47%) | 1182 (52%) | 738 (50%) | 653 (49%) | 730 (48%) | 347 (46%) | 791 (44%) |
| Education | ||||||||||
| Less than high school | 51 (28%) | 20 (29%) | 190 (16%) | 147 (25%)b | 394 (18%) | 303 (20%)a | 230 (17%) | 312 (20%)a | 158 (21%) | 379 (21%) |
| High school | 36 (20%) | 23 (34%) | 304 (25%) | 186 (32%) | 618 (27%) | 398 (27%) | 351 (27%) | 414 (27%) | 227 (30%) | 539 (30%) |
| Some college | 46 (25%) | 15 (22%) | 329 (28%) | 140 (24%) | 586 (26%) | 415 (28%) | 356 (27%) | 425 (28%) | 199 (26%) | 478 (27%) |
| College and above | 49 (27%) | 10 (15%) | 371 (31%) | 113 (19%) | 663 (29%) | 367 (25%) | 388 (29%) | 373 (25%) | 170 (23%) | 382 (21%) |
| Smoking status | ||||||||||
| Never | 67 (37%) | 14 (21%) | 526 (44%) | 185 (32%)b | 1054 (47%) | 590 (40%)b | 639 (48%) | 673 (44%)b | 390 (52%) | 910 (51%) |
| Former | 38 (21%) | 19 (28%) | 408 (35%) | 176 (30%) | 882 (39%) | 573 (39%) | 551 (42%) | 577 (38%) | 288 (38%) | 646 (37%) |
| Current | 78 (43%) | 35 (51%) | 252 (21%) | 225 (38%) | 313 (14%) | 319 (21%) | 129 (10%) | 271 (18%) | 76 (10%) | 216 (12%) |
| Diabetes | ||||||||||
| None | 151 (88%) | 53 (85%) | 920 (83%) | 399 (78%)a | 1498 (73%) | 934 (72%) | 760 (64%) | 845 (65%) | 373 (56%) | 861 (58%)a |
| Impaired fasting | 11 (6%) | 3 (5%) | 62 (6%) | 29 (7%) | 129 (6%) | 109 (8%) | 90 (7%) | 123 (9%) | 45 (7%) | 164 (11%) |
| Diabetes | 10 (6%) | 6 (10%) | 125 (11%) | 75 (15%) | 419 (21%) | 266 (20%) | 343 (29%) | 332 (26%) | 252 (37%) | 466 (31%) |
| Statin use | ||||||||||
| Yes | 25 (14%) | 11 (16%) | 253 (21%) | 131 (22%) | 686 (30%) | 419 (28%) | 467 (35%) | 443 (29%)a | 288 (38%) | 530 (30%)b |
| No | 158 (86%) | 57 (84%) | 942 (79%) | 455 (78%) | 1576 (70%) | 1066 (72%) | 861 (65%) | 1082 (71%) | 467 (62%) | 1251 (70%) |
| SBP (mm Hg) (mean/sd) | 125.1 (19.90) | 124.0 (16.81) | 127.1 (17.82) | 130 (19.58)a | 129.7 (17.22) | 131 (16.46)a | 130.6 (16.09) | 132 (17.24)a | 133.4 (16.75) | 133.6 (17.44) |
| DBP (mm Hg) (mean/sd) | 75.1 (9.77) | 73.0 (9.16) | 76.1 (9.96) | 76.9 (10.62) | 77.9 (9.55) | 77.5 (9.83) | 79.1 (9.99) | 78.9 (9.52) | 80.3 (10.32) | 80.9 (10.56) |
| LDL cholesterol (mg/dl) (mean/sd) | 108.2 (42.22) | 106.7 (40.76) | 114.2 (36.39) | 117.4 (36.25) | 116.5 (35.78) | 120 (38.24)a | 115.2 (34.89) | 120 (36.14)a | 110.2 (35.12) | 119 (35.76)b |
| HDL cholesterol (mg/dl) (mean/sd) | 67.8 (20.14) | 56.5 (15.32)b | 60.2 (17.89) | 54.2 (16.07)b | 54.0 (16.04) | 52.3 (15.72)a | 52.1 (14.94) | 52.0 (15.41) | 52.2 (15.22) | 50.6 (13.69)a |
| TG (median/IQR) | 75 (60–101) | 86 (68–122)a | 83.0 (63–111) | 98 (74–135)b | 92 (70–126) | 102 (76–139)b | 96 (70–133) | 107 (81–146)a | 94 (75–127) | 104 (82–140)b |
IQR, Interquartile range; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides (mg/dL).
P < 0.05.
P < 0.0001.
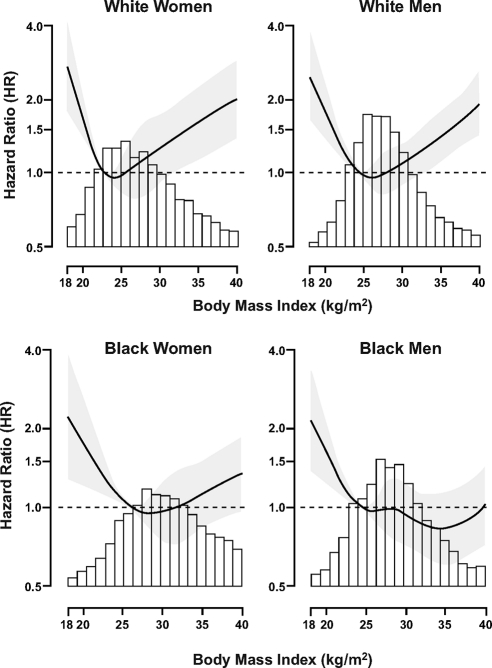
Figure 1 provides the age-adjusted HR and 95% confidence intervals (CI) for mortality across the distribution of BMI by race-sex grouping. Similar to previous studies of whites (7), there was a U-shaped curve for mortality, such that underweight and severely obese white women and men were at higher risk for death, whereas overweight and obese subjects were not at higher risk. For example, compared with the normal weight, overweight white men and women had an age-adjusted HR for mortality of 0.86 [95% CI 0.71–1.04] and 0.99 (95% CI 0.73–1.36), respectively, whereas the severely obese had a 1.5- to 2.0-fold higher risk of death (HR 2.11, 95% CI 1.46–3.06, white women; HR 1.53, 95% CI 1.13–2.07, white men). Mortality risk among the overweight and obese was also not significantly higher among blacks. Importantly, unlike whites, black men and women did not have a higher risk for mortality if severely obese (HR 0.84, 95% CI 0.57–1.24 black men; HR 0.91, 95% CI 0.68–1.40, black women).
Fig. 1.
HR for mortality associated with BMI by sex-race group. Gray shadow represents the 95th CI for the HR. The reference group for each spline is the 20th percentile for the each respective BMI distribution.
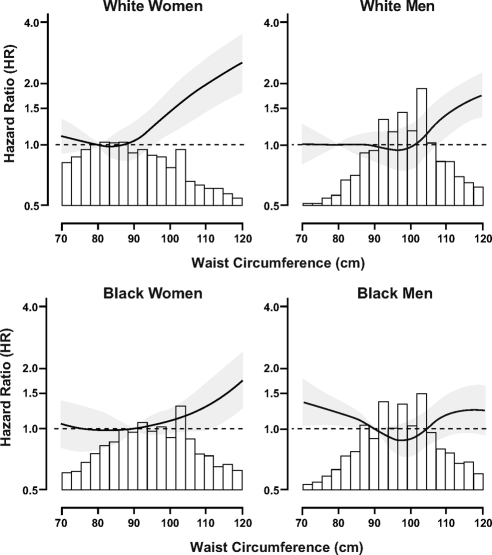
Among white men and women, higher waist circumference was associated with an increased age-adjusted HR for mortality (Fig. 2). Waist circumference above the cut point of 90 cm for women and 100 cm for men, based on National Cholesterol Education Program guidelines, (29) was associated with a HR of 1.49 (95% CI 1.18–1.88) for white women and 1.32 (95% CI 1.13–1.54) for white men in age-adjusted models. The same cut points for blacks resulted in a 29% higher risk for death among black women but only a 3% increased risk for black men (HR 1.03, 95% CI 0.84–1.26). When exploring a higher waist circumference cut point of 110 cm for blacks, we detected a significantly higher risk for death among black women (HR 1.75, 95% CI 1.34–2.28), which was not present among black men (HR 1.12, 95% CI 0.87–1.45).
Fig. 2.
HR for mortality associated with waist circumference by sex-race group. Gray shadow represents the 95th CI for the HR. The reference group for each spline is the 20th percentile for the each respective waist circumference distribution.
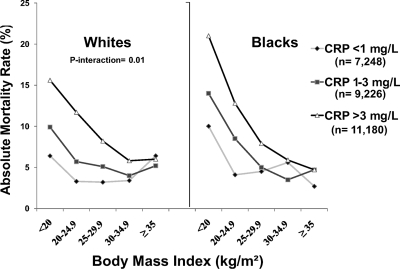
Underweight whites and blacks with CRP 3 mg/liter or greater had the highest absolute risk of death (16% for whites; 21% for blacks) (Fig. 3). As BMI category increased, the absolute risk of death decreased and differences in risk by CRP category also decreased. When assessing adjusted relative risk among those with CRP greater than 3 vs. less than 1 mg/liter, underweight (HR 2.08, 95% CI 1.03–4.21), and normal weight (HR 2.62, 95% CI 1.87–3.67), whites were at significantly higher mortality risk but not severely obese whites (HR 1.55, 95% CI 0.77–2.96) (Table 2). This resulted in a statistical interaction between BMI and CRP on mortality risk among whites (P for interaction = 0.01). Similar results were also seen for blacks (Table 3), although a higher mortality risk among severely obese blacks with CRP 3 or greater vs. less than 1 mg/liter was also demonstrated (HR 2.58, 95% CI 1.04–6.41), resulting in no statistical interaction (P = 0.26). Using alternate cut points for CRP (top 25th percentile of CRP for sex-race or absolute cut point >6 mg/liter) or severe obesity (≥40 kg/m2) did not alter these results nor did additional adjustment for statin use.
Fig. 3.
Absolute mortality rates (percentage) by BMI and CRP categories.
Table 2.
Relationship between CRP and mortality within BMI categories in whites (n = 16,486)
| n = dead | Absolute risk (%) | HRa | 95% CI | P value | ||
|---|---|---|---|---|---|---|
| Underweight CRP <1 (n = 283) | 18 | 6.4 | 1 | |||
| Underweight CRP 1–3 (n = 132) | 13 | 9.9 | 1.56 | 0.73 | 3.33 | 0.25 |
| Underweight CRP ≥3 (n = 108) | 17 | 15.6 | 2.08 | 1.03 | 4.21 | 0.04 |
| 48/523 = 9.2% | ||||||
| Normal CRP <1 (n = 1853) | 59 | 3.3 | 1 | |||
| Normal CRP1–3 (n = 1404) | 80 | 5.7 | 1.36 | 0.95 | 1.94 | 0.09 |
| Normal CRP ≥3 (n = 1060) | 124 | 11.7 | 2.62 | 1.87 | 3.67 | <0.0001 |
| 263/2647 = 10.0% | ||||||
| Overweight CRP1 (n = 2032) | 65 | 3.2 | 1 | |||
| Overweight CRP1–3 (n = 2447) | 124 | 5.1 | 2.30 | 1.70 | 3.11 | 0.01 |
| Overweight CRP ≥3 (n = 2044) | 167 | 8.2 | 1.52 | 1.11 | 2.08 | <0.0001 |
| 356/6523 = 5.5% | ||||||
| Obese CRP1 (n = 612) | 21 | 3.4 | 1 | |||
| Obese CRP1–3 (n = 1278) | 51 | 4.0 | 1.33 | 0.77 | 2.29 | 0.31 |
| Obese CRP ≥3 (n = 1395) | 81 | 5.8 | 1.88 | 1.12 | 3.17 | 0.012 |
| 153/3285 = 4.7% | ||||||
| Severe Obese CRP1 (n = 173) | 11 | 6.4 | 1 | |||
| Severe Obese CRP1–3 (n = 537) | 28 | 5.2 | 1.12 | 0.54 | 2.34 | 0.76 |
| Severe Obese CRP ≥3 (n = 1128) | 68 | 6.0 | 1.51 | 0.77 | 2.96 | 0.24 |
| 107/1838 = 5.9% | ||||||
Underweight (BMI <20 kg/m2); normal weight (BMI 20–24.9 kg/m2); overweight (BMI 25–29.9 kg/m2); obese (BMI 30–34.9 kg/m2); extreme obese (≥35 kg/m2). CRP concentration is in milligrams per liter.
Adjusted for age, sex, hypertension, diabetes, smoking, alcohol use, and physical activity.
Table 3.
Relationship CRP and mortality within BMI categories in blacks (n = 11,168)
| n = dead | Absolute risk (%) | HRa | 95% CI | P value | ||
|---|---|---|---|---|---|---|
| Underweight CRP <1 (n = 120) | 12 | 10 | 1 | |||
| Underweight CRP 1–3 (n = 63) | 9 | 14 | 1.07 | 0.42 | 2.75 | 0.88 |
| Underweight CRP ≥3 (n = 68) | 14 | 21 | 2.09 | 0.90 | 4.86 | 0.07 |
| 35/251 = 14.0% | ||||||
| Normal CRP <1 (n = 628) | 26 | 4.1 | 1 | |||
| Normal CRP 1–3 (n = 567) | 48 | 8.5 | 2.37 | 1.40 | 4.01 | <0.0001 |
| Normal CRP ≥3 (n = 586) | 75 | 12.8 | 3.17 | 1.91 | 5.26 | 0.001 |
| 149/1781 = 8.4% | ||||||
| Overweight CRP <1 (n = 952) | 43 | 4.5 | 1 | |||
| Overweight CRP 1–3 (n = 1310) | 65 | 5 | 1.05 | 0.70 | 1.57 | 0.82 |
| Overweight CRP ≥3 (n = 1485) | 117 | 7.9 | 1.62 | 1.12 | 2.35 | 0.01 |
| 225/3747 = 6.0% | ||||||
| Obese CRP <1 (n = 412) | 23 | 5.6 | 1 | |||
| Obese CRP 1–3 (n = 916) | 32 | 3.5 | 0.75 | 0.43 | 1.32 | 0.32 |
| Obese CRP ≥3 (n = 1525) | 89 | 5.9 | 1.13 | 0.70 | 1.85 | 0.62 |
| 114/2853 = 4.0% | ||||||
| Severe obese CRP <1 (n = 183) | 5 | 2.7 | 1 | |||
| Severe obese CRP 1–3 (n = 572) | 27 | 4.7 | 2.20 | 0.84 | 5.73 | 0.11 |
| Severe obese CRP ≥3 (n = 1781) | 84 | 4.7 | 2.58 | 1.04 | 6.41 | 0.04 |
| 116/2536 = 4.6% | ||||||
Underweight (BMI <20 kg/m2); normal weight (BMI 20–24.9 kg/m2); overweight (BMI 25–29.9 kg/m2); obese (BMI 30–34.9 kg/m2); severe obese (≥35 kg/m2). CRP concentration is in milligrams per liter.
Adjusted for age, sex, hypertension, diabetes, smoking, alcohol use, and physical activity.
Among whites, CRP 3 mg/L or greater compared with CRP less than 1 mg/liter was associated with increased risk of death across all categories of waist circumference without a significant interaction (P = 0.47 for whites and P = 0.25 for blacks; Tables 4 and 5). For example, whites in the highest sex-race specific waist quartile with a CRP 3 mg/liter or greater had nearly a 2-fold greater risk compared with those with CRP less than 1 mg/liter (HR 1.90, 95% CI 1.30–2.76), with similar results for blacks (HR 1.94, 95% CI 1.13–3.33) (Table 5). Point estimates were similar between men and women within each race group; however, calculating the results stratified by both sex and race subgroups was not feasible due to small sample sizes.
Table 4.
Relationship between CRP and mortality within waist circumference categories in whites (n = 16,434)
| n = dead | Absolute risk (%) | HRa | 95% CI | P value | ||
|---|---|---|---|---|---|---|
| Q1 WC CRP <1 (n = 2005) | 58 | 2.9 | 1 | |||
| Q1 WC CRP 1–3 (n = 1350) | 70 | 5.2 | 1.59 | 1.10 | 2.29 | 0.01 |
| Q1 WC CRP ≥3 (n = 878) | 84 | 9.6 | 2.60 | 1.81 | 3.71 | <0.0001 |
| 212/4233 = 5.0% | ||||||
| Q2 WC CRP <1 (n = 1189) | 43 | 3.6 | 1 | |||
| Q2 WC CRP 1–3 (n = 1284) | 53 | 4.1 | 1.07 | 0.66 | 1.53 | 0.98 |
| Q2 WC CRP ≥3 (n = 985) | 72 | 7.3 | 1.65 | 1.10 | 2.48 | 0.01 |
| 168/3458 = 4.9% | ||||||
| Q3 WC CRP <1 (n = 1070) | 36 | 3.4 | 1 | |||
| Q3 WC CRP 1–3 (n = 1595) | 78 | 4.9 | 1.53 | 1.02 | 2.31 | 0.042 |
| Q3 WC CRP ≥3 (n = 1570) | 108 | 6.9 | 2.20 | 1.47 | 3.27 | 0.0001 |
| 222/4235 = 5.2% | ||||||
| Q4 WC CRP <1 (n = 679) | 37 | 5.5 | 1 | |||
| Q4 WC CRP 1–3 (n = 1547) | 95 | 6.1 | 1.26 | 0.84 | 1.88 | 0.26 |
| Q4 WC CRP ≥3 (n = 2282) | 192 | 8.4 | 1.90 | 1.31 | 2.76 | 0.0008 |
| 324/4458 = 7.3% | ||||||
CRP concentration is in milligrams per liter. Q, Quartile; WC, waist circumference.
Adjusted for age, sex, hypertension, diabetes, smoking, alcohol use, and physical activity.
Table 5.
Relationship between CRP and mortality within waist circumference categories in blacks (n = 11,124)
| n = dead | Absolute risk (%) | HRa | 95% CI | P value | ||
|---|---|---|---|---|---|---|
| Q1 WC CRP <1 (n = 981) | 41 | 4.2 | 1 | |||
| Q1 WC CRP 1–3 (n = 864) | 53 | 6.1 | 1.62 | 1.04 | 2.50 | 0.03 |
| Q1 WC CRP ≥3 (n = 843) | 71 | 8.4 | 1.95 | 1.28 | 2.97 | 0.002 |
| 165/2688 = 6.1% | ||||||
| Q2 WC CRP <1 (n = 612) | 25 | 4.1 | 1 | |||
| Q2 WC CRP 1–3 (n = 998) | 37 | 3.7 | 1.15 | 0.68 | 1.94 | 0.61 |
| Q2 WC CRP ≥3 (n = 1246) | 77 | 6.2 | 1.85 | 1.15 | 2.99 | 0.01 |
| 139/2856 = 4.9% | ||||||
| Q3 WC CRP <1 (n = 397) | 26 | 6.6 | 1 | |||
| Q3 WC CRP 1–3 (n = 786) | 43 | 5.5 | 0.94 | 0.57 | 1.55 | 0.79 |
| Q3 WC CRP ≥3 (n = 1339) | 83 | 6.2 | 1.11 | 0.69 | 1.77 | 0.68 |
| 152/2522 = 6.0% | ||||||
| Q4 WC CRP <1 (n = 297) | 17 | 5.7 | 1 | |||
| Q4 WC CRP 1–3 (n = 769) | 48 | 6.2 | 1.37 | 0.76 | 2.46 | 0.29 |
| Q4 WC CRP ≥3 (n = 1992) | 145 | 7.3 | 1.94 | 1.13 | 3.33 | 0.02 |
| 210/3058 = 6.9% | ||||||
CRP concentration is in milligrams per liter. Q, Quartile; WC, waist circumference.
Adjusted for age, sex, hypertension, diabetes, smoking, alcohol use, and physical activity.
Discussion
Among middle-aged and older adults, obesity was associated with higher mortality risk among whites but not blacks. For a given level of adiposity, CRP 3 mg/liter or greater was most informative among the underweight, not the severely obese. This negated our hypothesis that elevated levels of CRP would be a strong risk marker for mortality among individuals with excess adiposity.
The results of the present study regarding BMI and mortality risk are concordant with other studies (7–9), especially among older adults (6). Clearly, obesity earlier in life has profound consequences on the life span (24). However, among individuals older than 60 yr, our study suggests that obesity tends to have a weaker association with mortality compared with other known and unknown factors, especially among black men. This phenomenon, known as the obesity paradox, has been previously described, (30, 31) and tends to be most relevant among older individuals and those with shorter follow-up time in population-based studies (32). In addition, BMI may not be a suitable surrogate for obesity. This is supported by one previous study demonstrating a J-shaped association between BMI and death, with opposite associations on mortality risk for body fat and fat-free mass (33). In the current study, we also assessed waist circumference, a measure of central adiposity more closely related to metabolic disease (34). Higher waist circumference was associated with higher mortality risk as in other studies (7, 35), although overall, it was not an informative measure among black men. Taken together, explanations surrounding the obesity paradox regarding age-related differences in risk, follow-up time, and body composition raise important points for future research studies and design to determine the cumulative risks of obesity.
With regard to ethnic differences in mortality risk among the obese, it is known that blacks have a greater degree of adiposity (1) as well as higher concentrations of CRP (36). We hypothesized that a potential obesity-inflammation association with mortality risk would be most relevant among blacks. Although the U-shaped mortality curve for whites (7) has been well described and confirmed in the present study, neither BMI nor waist circumference was associated with mortality risk in black men, and only very high waist circumference was associated with mortality in black women. Elevated CRP was associated with higher risk among severely obese blacks, although in general, absolute mortality rates were highest for underweight blacks with or without high CRP. These results suggest that obesity among middle-aged and older blacks is a less powerful predictor of death compared with other risk factors common among the underweight (37).
Our primary hypothesis that increased circulating levels of CRP among obese individuals would magnify mortality risk was not substantiated in the current study. These results may reflect an inability of BMI or waist circumference to adequately capture metabolically dangerous fat depots or a lack of CRP as a biomarker to act as a surrogate for inflamed fat. Several lines of evidence suggest that ectopic fat deposition, such as hepatic and pericardial fat, are proinflammatory and associated with cardiometabolic dysregulation (38, 39). Evidence also suggests that genetic susceptibility for ectopic fat, specifically hepatic fat, may differ by racial groups (40). With regard to CRP, although a known biomarker of disease risk (23), it may lack specificity for upstream disease pathways. IL-6 and TNF-α, which are highly concentrated within fat tissue, may be more informative for this line of questioning (41). Additionally, adiponectin and leptin, secreted directly from adipose cells and related to cardiovascular health (10, 42), are critical proteins to study among the obese. To this end, future studies focused on the interplay between ectopic fat deposition, adipocytokines, and mortality risk will be an important area for future research. This will be especially informative among women and ethnic groups with different body composition characteristics.
Strengths and limitations of the current study are worth noting. REGARDS represents a well-phenotyped population with BMI and waist circumference measured among a large sample of black and white U.S. adults. The addition of CRP measurement provides the first data concerning the impact of inflammation on the association of adiposity and mortality among blacks and whites. However, cause of death was not available for the current study and generalizations to younger adults cannot be made. In addition, the present study assessed mortality risk over only 6 yr and was not meant to determine the impact of adiposity or CRP measurement on lifetime risk. Lastly, American Heart Association/Centers for Disease Control and Prevention guidelines recommend two separate CRP measurements to account for the within-individual variability. CRP was measured once at the baseline examination in the REGARDS cohort. This report, however, provides new data regarding the efficiency of CRP in predicting death among older blacks and whites across the BMI and waist circumference distribution.
In summary, future studies will be needed to unravel the competing risks among the underweight, which are responsible for premature death, especially among blacks. Second, because CRP did not distinguish the healthy from metabolically dangerous obese in the current study, research is needed to determine the utility of measuring ectopic fat deposition and adipocytokines as alternative strategies to detect individuals at greatest risk due to excess adiposity (43). Ultimately, this line of research will allow for earlier detection and prevention of obesity-related mechanisms related to adverse outcomes.
Acknowledgments
We acknowledge the participants and the staff for making the REGARDS study possible.
Financial Disclosures: No financial disclosures are reported.
This work was supported by Cooperative Agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, U.S. Department of Health and Human Services. The funding organizations played no role in the design of the study, choice of enrolled patients, review, and interpretation of data, or preparation or approval of the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- Body mass index
- CI
- confidence interval
- CRP
- C-reactive protein
- HDL
- high-density lipoprotein
- HR
- hazard ratio
- LDL
- low-density lipoprotein
- REGARDS
- Reasons for Geographic and Racial Differences in Stroke.
References
- 1. Flegal KM, Carroll MD, Ogden CL, Curtin LR. 2010. Prevalence and trends in obesity among U.S. adults, 1999–2008. JAMA 303:235–241 [DOI] [PubMed] [Google Scholar]
- 2. Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH. 2004. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation 110:2952–2967 [DOI] [PubMed] [Google Scholar]
- 3. Eckel RH, York DA, Rössner S, Hubbard V, Caterson I, St. Jeor ST, Hayman LL, Mullis RM, Blair SN. 2004. Prevention Conference VII: obesity, a worldwide epidemic related to heart disease and stroke: executive summary. Circulation 110:2968–2975 [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Differences in prevalence of obesity among black, white, and Hispanic adults—United States, 2006–2008 [Google Scholar]
- 5. Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF. 2006. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med 355:763–778 [DOI] [PubMed] [Google Scholar]
- 6. Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. 1998. The effect of age on the association between body-mass index and mortality. N Engl J Med 338:1–7 [DOI] [PubMed] [Google Scholar]
- 7. Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, van der Schouw YT, Spencer E, Moons KG, Tjonneland A, Halkjaer J, Jensen MK, Stegger J, Clavel-Chapelon F, Boutron-Ruault MC, Chajes V, Linseisen J, Kaaks R, Trichopoulou A, Trichopoulos D, Bamia C, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PH, May AM, Bueno-de-Mesquita HB, van Duijnhoven FJ, Hallmans G, Weinehall L, Manjer J, Hedblad B, Lund E, Agudo A, Arriola L, Barricarte A, Navarro C, Martinez C, Quirós JR, Key T, Bingham S, Khaw KT, Boffetta P, Jenab M, Ferrari P, Riboli E. 2008. General and abdominal adiposity and risk of death in Europe. N Engl J Med 359:2105–2120 [DOI] [PubMed] [Google Scholar]
- 8. Durazo-Arvizu R, Cooper RS, Luke A, Prewitt TE, Liao Y, McGee DL. 1997. Relative weight and mortality in U.S. blacks and whites: findings from representative national population samples. Ann Epidemiol 7:383–395 [DOI] [PubMed] [Google Scholar]
- 9. Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr 1999. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med 341:1097–1105 [DOI] [PubMed] [Google Scholar]
- 10. Tilg H, Moschen AR. 2006. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6:772–783 [DOI] [PubMed] [Google Scholar]
- 11. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr 2003. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wellen KE, Hotamisligil GS. 2005. Inflammation, stress, and diabetes. J Clin Invest 115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. 1995. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest 95:2111–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. 2006. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116:1494–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hotamisligil GS. 2006. Inflammation and metabolic disorders. Nature 444:860–867 [DOI] [PubMed] [Google Scholar]
- 17. Steinberg GR, Michell BJ, van Denderen BJ, Watt MJ, Carey AL, Fam BC, Andrikopoulos S, Proietto J, Görgün CZ, Carling D, Hotamisligil GS, Febbraio MA, Kay TW, Kemp BE. 2006. Tumor necrosis factor α-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab 4:465–474 [DOI] [PubMed] [Google Scholar]
- 18. Harman D. 1956. Aging: a theory based on free radical and radiation chemistry. J Gerontol 11:298–300 [DOI] [PubMed] [Google Scholar]
- 19. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, 3rd, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. 2003. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107:499–511 [DOI] [PubMed] [Google Scholar]
- 20. Wee CC, Mukamal KJ, Huang A, Davis RB, McCarthy EP, Mittleman MA. 2008. Obesity and C-reactive protein levels among white, black, and Hispanic U.S. adults. Obesity (Silver Spring) 16:875–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. 1997. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 336:973–979 [DOI] [PubMed] [Google Scholar]
- 22. Ridker PM, Hennekens CH, Buring JE, Rifai N. 2000. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 342:836–843 [DOI] [PubMed] [Google Scholar]
- 23. Cushman M, Arnold AM, Psaty BM, Manolio TA, Kuller LH, Burke GL, Polak JF, Tracy RP. 2005. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation 112:25–31 [DOI] [PubMed] [Google Scholar]
- 24. Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. 2003. Years of life lost due to obesity. JAMA 289:187–193 [DOI] [PubMed] [Google Scholar]
- 25. Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. 2005. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 25:135–143 [DOI] [PubMed] [Google Scholar]
- 26. Cushman M, McClure LA, Howard VJ, Jenny NS, Lakoski SG, Howard G. 2009. Implications of increased C-reactive protein for cardiovascular risk stratification in black and white men and women in the US. Clin Chem 55:1627–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alberti KG, Zimmet P, Shaw J. 2006. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 23:469–480 [DOI] [PubMed] [Google Scholar]
- 28. Beydoun MA, Wang Y. 2009. Gender-ethnic disparity in BMI and waist circumference distribution shifts in US adults. Obesity (Silver Spring) 17:169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) 2001. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 30. Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. 2009. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373:1083–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cepeda-Valery B, Pressman GS, Figueredo VM, Romero-Corral A. Impact of obesity on total and cardiovascular mortality-fat or fiction? Nat Rev Cardiol. 2011 Jan 25; doi: 10.1038/nrcardio.2010.209. 10.1038/nrcardio.2010.209. [DOI] [PubMed] [Google Scholar]
- 32. Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee IM, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. 2010. Body-mass index and mortality among 1.46 million white adults. N Engl J Med 363:2211–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bigaard J, Frederiksen K, Tjonneland A, Thomsen BL, Overvad K, Heitmann BL, Sorensen TI. 2004. Body fat and fat-free mass and all-cause mortality. Obes Res 12:1042–1049 [DOI] [PubMed] [Google Scholar]
- 34. Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodés-Cabau J, Bertrand OF, Poirier P. 2008. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol 28:1039–1049 [DOI] [PubMed] [Google Scholar]
- 35. Reis JP, Araneta MR, Wingard DL, Macera CA, Lindsay SP, Marshall SJ. 2009. Overall obesity and abdominal adiposity as predictors of mortality in U.S. White and black adults. Ann Epidemiol 19:134–142 [DOI] [PubMed] [Google Scholar]
- 36. Lakoski SG, Cushman M, Criqui M, Rundek T, Blumenthal RS, D'Agostino RB, Jr, Herrington DM. 2006. Gender and C-reactive protein: data from the Multiethnic Study of Atherosclerosis (MESA) cohort. Am Heart J 152:593–598 [DOI] [PubMed] [Google Scholar]
- 37. Kuller LH. 2003. Obesity and years of life lost. JAMA 289:1777; author reply 1777–1778 [DOI] [PubMed] [Google Scholar]
- 38. Browning JD, Horton JD. 2004. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 114:147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rabkin SW. 2007. Epicardial fat: properties, function and relationship to obesity. Obes Rev 8:253–261 [DOI] [PubMed] [Google Scholar]
- 40. Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. 2008. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 40:1461–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berg AH, Scherer PE. 2005. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96:939–949 [DOI] [PubMed] [Google Scholar]
- 42. Han SH, Quon MJ, Kim JA, Koh KK. 2007. Adiponectin and cardiovascular disease: response to therapeutic interventions. J Am Coll Cardiol 49:531–538 [DOI] [PubMed] [Google Scholar]
- 43. Unger RH, Scherer PE. 2010. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends Endocrinol Metab 21:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]