Inadequate cord blood plasma concentrations of vitamin D adversely impact the innate immune response; exogenous supplementation of 25(OH)D3 in vitro recovers innate immune activity.
Abstract
Objectives:
Our objectives were to 1) assess cord blood vitamin D concentrations from healthy term newborns, 2) ascertain whether cord blood vitamin D insufficiency precludes optimal induction of the Toll-like receptor (TLR) antimicrobial pathway in monocytes, and 3) determine whether in vitro supplementation with 25-hydroxyvitamin D3 [25(OH)D3] and/or 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] restores TLR-induced antimicrobial responses.
Study Design:
Plasma concentrations of 25(OH)D and 1,25(OH)2D were measured from cord blood of 23 newborns. Human monocytes were cultured in cord blood plasma and stimulated with TLR2 and TLR4 ligands, and then antimicrobial gene expression was analyzed using quantitative PCR.
Results:
Cord blood 25(OH)D and 1,25(OH)2D concentrations were positively correlated to each other (r = 0.78; P <0.0001). Compared with those conditioned in vitamin D-sufficient plasma [25(OH)D > 75 nmol/liter], monocytes cultured in severely vitamin D-deficient plasma [25(OH)D < 30 nmol/liter] exhibited decreased TLR-induced cathelicidin expression (P <0.05). Supplementation in vitro of vitamin D-deficient plasma with 25(OH)D3 increased antimicrobial peptide gene expression.
Conclusions:
Cord blood vitamin D deficiency, by its effects on TLR-induced antimicrobial production, altered in vitro monocyte responses. The observation that exogenous 25(OH)D3 in vitro recovered TLR-induced antimicrobial responses suggests the need for additional prospective investigations to further delineate the role of vitamin D in the newborn immune response.
Microbial infections continue to significantly and negatively impact global morbidity and mortality rates in neonates (1). Concurrently, numerous studies have alerted clinicians to the rising incidence of vitamin D deficiency, i.e. 25-hydroxyvitamin D [25(OH)D] below 50 nmol/liter, among neonates and children (2–5), including the reemergence of rickets in the pediatric population. Newborns, particularly those who are exclusively breastfed, are at risk for vitamin D deficiency because their 25(OH)D concentrations are significantly correlated with, and dependent upon, circulating maternal stores (6–8). Recent studies implicate vitamin D insufficiency [25(OH)D < 75 nmol/liter] as an important risk factor for neonates and children acquiring infections such as tuberculosis (9, 10), acute lower respiratory tract infections (11, 12), pneumonia (13), and influenza (14).
The mechanisms contributing to the increased infections observed in vitamin D-insufficient pediatric patients have not been adequately elucidated. It is known that activation of Toll-like receptors (TLR) on adult macrophages leads to an antimicrobial pathway dependent upon 1) induction of CYP27B1, which converts 25(OH)D into 1,25-dihydroxyvitamin D [1,25(OH)2D]; 2) availability of substrate 25(OH)D to the CYP27B1-hydroxylase; 3) vitamin D receptor (VDR) expression; and 4) subsequent 1,25(OH)2D-VDR-directed expression of the genes encoding antimicrobial peptides cathelicidin and β-defensin 2 gene (DEFB4) (15, 16). For this innate immune response, it is the conversion of 25(OH)D to active 1,25(OH)2D within innate immune cells that is key. Adequate circulating concentrations of 25(OH)D are presumably critical to providing the necessary substrate for this conversion to occur. This is best performed by serum or plasma measurements of 25(OH)D. The active form of vitamin D, 1,25(OH)2D, is not a good indicator of vitamin D status, because physiological 1,25(OH)2D concentrations vary based on conditions such as 25(OH)D substrate deficiency, pregnancy (17), and/or PTH status of the host (18).
There is a preponderance of epidemiological evidence indicating circulating 25(OH)D concentrations from cord blood correlate indirectly with increased susceptibility to infection in newborns. Little is known, however, whether cord blood vitamin D status directly affects immune function. The current studies investigate the relationship between cord blood 25(OH)D status and in vitro measures of human monocyte function.
Subjects and Methods
Study cohorts
Cord blood from 23 healthy term newborn infants delivered either vaginally or by cesarean section from the Southern California area was randomly collected for investigation. Cord blood from newborn infants with congenital anomalies, deliveries complicated by antenatal passage of meconium, and/or the prenatal diagnosis of maternal chorioamnionitis was not included in this study. Newborn infants born to mothers with intrapartum fever, HIV/AIDS, diabetes, and/or or preeclampsia were also excluded from providing cord blood samples at the time of their delivery.
Peripheral blood was obtained from healthy adults, to derive monocytes and serum for experiments performed in vitro. Adult subjects were recruited from the Los Angeles County area. Subjects with recent use of antibiotics, international travel and/or illness were excluded.
Institutional review board (IRB) approvals
Acquisition of cord blood was approved by the IRB at the David Geffen School of Medicine at UCLA with the Ronald Reagan Medical Center at UCLA (Los Angeles, CA) and the Olive View Medical Center at UCLA (Sylmar, CA). An exemption for written informed consent before collection of cord blood was permitted by the IRB because the placenta was considered a discarded specimen, and no genetic identifiers were permanently collected. Before collection of peripheral blood from adults, written informed consent was obtained as approved by the IRB at the David Geffen School of Medicine at UCLA.
For the gene expression and antimicrobial studies performed, we determined from previous preliminary data that an estimated 20 donors (five donors per group: 25(OH)D sufficient, insufficient, deficient, and severely deficient) would be needed to reach reasonable statistical power (0.8) for the study of gene induction in monocytes. Cord blood was randomly collected and sampled from subjects until there were five samples per designated group. Effort was made to collect samples within the same season given the known seasonal variability of vitamin D in populations. Normality analysis of data generated in previous work has indicated a normal distribution of antimicrobial activity induction by TLR2/1 ligand (TLR2/1L) and TLR4L in monocytes.
Cord blood acquisition
Approximately 45 ml cord blood was obtained from the umbilical artery after delivery of the placenta. Blood was collected into a 50-ml conical tube containing 1:1000 heparin at no more than 2% of the sample volume. Before processing, samples were stored at 4 C. Within 24 h of birth, however, specimens were processed via centrifugation at 3200 rpm for 20 min at room temperature. No significant hemolysis occurred. The plasma layer was then collected and sterile filtered. A portion of the plasma was subsequently stored at −80 C for future experiments.
Vitamin D assay characteristics
Serum collected from healthy adult donors was assayed for 25(OH)D and 1,25(OH)2D by RIA as previously described by Hollis et al. (19, 20). Cord blood plasma concentrations of 25(OH)D and 1,25(OH)2D were determined in the same manner. In both, antisera were raised against the synthetic vitamin D analog 23,24,25,26,27-pentanor vitamin D-C (22)-carboxylic acid. The synthesis of this analog and its 125I-labeled counterpart have been described in detail (19, 20). Using this antigen, antibodies were generated that were cospecific with regard to detecting total 25(OH)D, 25(OH)D2, and 25(OH)D3. This assay was shown to compare closely with HPLC-UV and HPLC tandem mass spectrometry quantitation of 25(OH)D in human samples, and information regarding cross-reactivity with other vitamin D metabolites has been previously published (19–23). Additionally, this assay does not exhibit cross-reactivity with the 3-epi-25(OH)D or other 25-hydroxylated vitamin D metabolites. In the laboratory of B.W.H., the inter- and intraassay variation is 10% or less. In the RIA for 1,25(OH)2D, antisera were raised against the synthetic vitamin D analog 23,24,25,26,27-pentanor-1-α vitamin D-C (22)-carboxylic acid and was not cross-reactive with other 25-hydroxylated vitamin D metabolites. The synthesis of this analog and its 125I-labled counterpart has likewise been described in detail (20). Using this antigen, antibodies were generated that were cospecific with regard to detecting both 1,25(OH)2D2 and 1,25(OH)2D3 or total 1,25(OH)2D. In the laboratory of B.W.H., the inter- and intraassay variation is less than 12%. The 25(OH)D and 1,25(OH)2D assay sensitivities and specificities are 2.8 μg/liter and 97%, respectively.
No standard classification of 25(OH)D concentrations in cord blood exists. In considering the ongoing discussion by experts as well as the variable use of terms throughout the literature, classification of vitamin D sufficiency, insufficiency, deficiency, and severe deficiency were defined as concentrations of 25(OH)D of 75 nmol/liter or higher, 50–75 nmol/liter, 30–50 nmol/liter, and less than 30 nmol/liter, respectively, for the purpose of the current study (24–30).
In vitro monocyte studies
Whole blood from healthy adult donors was collected as previously indicated. We used adult monocytes, which respond to TLR activation in the presence of vitamin D-sufficient adult serum and plasma (15), allowing us to focus the investigation on the relative contribution of the divergent cord blood vitamin D concentrations to innate immune responses. Peripheral blood mononuclear cells (PBMC) were isolated from buffy coats obtained by Ficoll-Hypaque (GE Healthcare, Piscataway, NJ) density centrifugation. Cells were allowed to adhere to culture wells for 2 h at 37 C with 4% CO2, in RPMI supplemented with 1% fetal calf serum, after which, the nonadherent cells were removed by washing with RPMI.
Adherent cells were then stimulated with either a TLR2/1L (10 μg/ml) or a TLR4L (10 ng/ml) for 24 h with their respective unstimulated adherent cells as appropriate controls. Cord blood plasma was added to provide a 10% final concentration to the cells. Additionally, some cell culture wells were supplemented with 10% pooled adult human serum (generated from the blood of healthy adult volunteers with a 25(OH)D > 75 nmol/liter), to serve as a positive control. We also compared responses using adult serum and heparinized plasma from the same individual, finding no significant difference in TLR-induced responses (data not shown).
For the supplementation studies, aliquots of synthetic 25(OH)D3 and 1,25(OH)2D3 at 10−4 m each were diluted in sterile-filtered ethanol to a concentration of 10−5 m for both. For each appropriate 1-ml cell culture condition well, 1 μl of either synthetic 25(OH)D3 or 1,25(OH)2D3 at 10−5 m was added to bring the final supplemented concentration to 10−8 m for the respective cell culture condition. Diluent-only additions were included as appropriate for control conditions.
Quantitative PCR (qPCR)
RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA), and cDNA was synthesized using the iSCRIPT cDNA synthesis kit (Bio-Rad, Hercules, CA). The quantity of total mRNA was assessed by spectrophotometry at 280 nm using ND-1000 nanospectrometer (NanoDrop Technologies, Wilmington, DE). The concentrations of mRNA were then standardized for each sample before assessment by qPCR. The primer sequences used were as follows: CYP27B1, 5′-ACC CGA CAC GGA GAC CTT C and 3′-ATG GTC AAC AGC GTG GACAC; VDR, 5′-AAG GAC AAC CGA CGC CAC T and 3′-ATC ATG CCG ATG TCC ACA CA; and cathelicidin, 5′-TGG GCC TGG TGA TGC CT and 3′-CGA AGG ACA GCT TCC TTG TAG C. Sequences for DEFB4 and h36B4 have been previously described (31).
Reagents
PBMC were cultured in RPMI 1640 (Biochrom, Holliston, MA) supplemented with glutamine (2 mm; Sigma-Aldrich, St. Louis, MO), 13 mm Na-pyruvate, 100 μg/ml streptomycin, and 6 μg/ml penicillin (all from Biochrom). The TLR2/1L is a synthetic 19-kDa Mycobacterium tuberculosis-derived lipopeptide (EMC Microcollections, Tuebingen, Germany). The TLR4L is a synthetic lipopolysaccharide (Invitrogen). Synthetic 25(OH)D3 and 1,25(OH)2D3 were purchased from BioMol (Plymouth Meeting, PA) and resuspended in ethanol at 10−4 m in amber tubes and then stored at −80 C in small aliquots. For experimental purposes, they were each used at a concentration of 10−8 m.
Statistical analysis
The distribution of all quantitative data was evaluated for normality, and the statistical analysis was conducted accordingly. The Pearson product-moment correlation coefficient was determined for 25(OH)D and 1,25(OH)2D concentrations in both the cord blood and adult populations used for the purpose of this study. Comparison of fold induction, as assessed by qPCR, was determined by the Student's t test. These data are expressed as means ± sem. Findings were considered significant if P < 0.05. GraphPad (GraphPad Software, San Diego, CA) was used to conduct the analysis of the data.
Results
Subject demographics
The mean estimated gestational age of newborns in this study (n = 23) was 39.5 ± 1.2 wk. The mean age for the adult comparison group (n = 44) was 63.3 ± 14.9 yr. There was a preponderance of females in the newborn population (60%). The majority of newborns were characterized as Latino. For the adult subjects, 80% were female and were almost exclusively (>90%) White.
Cord blood vitamin D concentrations
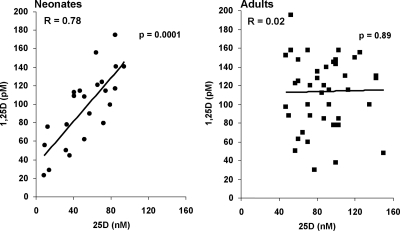
Vitamin D insufficiency, defined as 25(OH)D concentrations below 75 nm (30 ng/ml) was identified in 18 (78%) of the 23 cord blood samples obtained. Of those, 10 (56%) met the criteria for vitamin D deficiency with 25(OH)D concentrations below 50 nmol/liter (20 ng/ml). Evidence of severe vitamin D deficiency [25(OH)D concentrations < 30 nmol/liter (12 ng/ml)] was further identified in four (40%) of those 10 subjects. The mean 25(OH)D concentration of the cord blood specimens was 52.7 ± 5.5 vs. 87.8 ± 4 nmol/liter measured in adults. The average cord blood 1,25(OH)2D concentration was 96.3 ± 8.3 vs. 113.8 ± 6.1 pmol/liter in adults. Cord blood concentrations of 25(OH)D and 1,25(OH)2D were tightly correlated compared with the relationship observed in the adult study population, in which the 1,25(OH)2D remained relatively constant over a range of 25(OH)D concentrations (Fig. 1; for cord blood, r = 0.78, and P = 0.0001 vs. adults, r = 0.02, and P = 0.89).
Fig. 1.
The relationship between 25(OH)D and 1,25(OH)2D is unique in cord blood compared with adults. Blood samples taken from cord blood and healthy adults donors had concentrations of 25(OH)D and 1,25(OH)2D determined by RIA. Cord blood measurements of 25(OH)D and 1,25(OH)2D demonstrated a strong correlation to each other (r = 0.78; P = 0.0001). This was in contrast to the lack of correlation between 25(OH)D and 1,25(OH)2D in adult measurements where conversion of 25(OH)D to 1,25(OH)2D is tightly regulated by PTH and renal 1α-hydroxylase.
TLR4-mediated gene expression
Infection by gram-negative bacteria in the newborn period is a major cause of sepsis. A key mechanism by which the innate immune system responds to gram-negative bacteria is by TLR recognition of lipopolysaccharide. We therefore investigated whether cord blood plasma could support TLR4 induction of antimicrobial peptide expression, including cathelicidin and DEFB4. The data are represented as fold change in gene expression, determined by the comparison of mRNA induction in the stimulated vs. nonstimulated control cells.
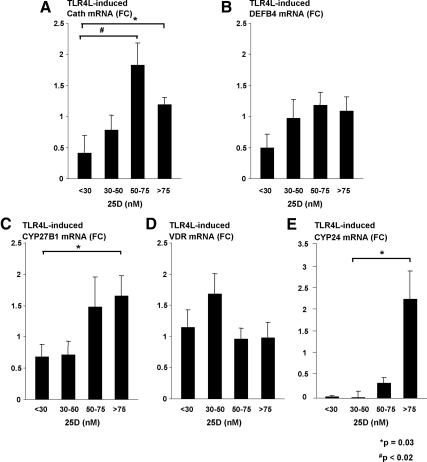
In investigating the effects of cord blood vitamin D concentrations on TLR4L-stimulated monocytes, cathelicidin gene expression was notably diminished in adherent cells cultured with plasma samples taken from severely vitamin D-deficient samples [25(OH)D < 30 nm] compared with vitamin D-sufficient ones (P = 0.03, Fig. 2A). Even when compared with samples considered insufficient [25(OH)D between 50–75 nm], this finding remained statistically significant (P < 0.02). The TLR4-induced expression of DEFB4 in monocytes, when cultured in the vitamin D-deficient vs. -sufficient cord blood plasma, was also diminished (Fig. 2B) but was not statistically significant.
Fig. 2.
TLR4L induction of the vitamin D-dependent antimicrobial pathway in monocytes is perturbed in the presence of vitamin D insufficiency. Primary human monocytes were stimulated with TLR4L for 24 h in 10% cord blood plasma. These plasma samples were either vitamin D sufficient [25(OH)D > 75 nm], insufficient [25(OH)D 50–75 nm], deficient [25(OH)D 30–50 nm], or severely deficient [25(OH)D < 30 nm]. Cathelicidin, DEFB4, CYP27B1, VDR, and CYP24 gene expression were determined by qPCR (mean fold change ± sem). A, Cathelicidin gene expression was notably diminished in adherent cells cultured with severely vitamin D-deficient cord blood plasma samples vs. vitamin D-sufficient ones (P = 0.03). When compared with insufficient samples, this finding remained statistically significant (P < 0.02). B, DEFB4 exhibited a trend toward diminished gene expression when monocytes were cultured in the markedly deficient plasma, but it did not approach statistical significance. C, CYP27B1 gene expression was notably less in monocytes cultured in deficient plasma when compared with the sufficient plasma group (P = 0.03). D, However, there were no differences in VDR gene expression. E, After TLR4L stimulation, there was greater induction of CYP24 gene expression in the monocytes cultured with vitamin D-sufficient plasma (P = 0.03).
TLR4-induced expression of CYP27B1 mRNA was notably less in the monocytes cultured in deficient plasma vs. sufficient plasma (P = 0.03, Fig. 2C). No other differences in TLR4L-induced CYP27B1 mRNA were observed in monocytes cultured with the cord blood plasma when comparing the other donor groups. When assessing TLR4L-induced up-regulation of VDR mRNA, the amount of 25(OH)D in the plasma samples did not have a significant effect on gene expression (Fig. 2D). The TLR4-induced expression of CYP24 in monocytes, when cultured in the vitamin D-insufficient or -deficient plasma, was diminished vs. gene expression of monocytes cultured in 25(OH)D-sufficient plasma (P = 0.03, Fig. 2E). Although 25(OH)D and 1,25(OH)2D concentrations were previously observed to be significantly correlated to each other, 1,25(OH)2D values were not found to share the same correlations with antimicrobial gene expression of stimulated monocytes as those demonstrated with 25(OH)D. In fact, no statistically significant relationship was evident for 1,25(OH)2D and cathelicidin, CYP27B1, VDR, or CYP24 mRNA expression after TLR4L stimulation.
TLR2/1-mediated gene expression
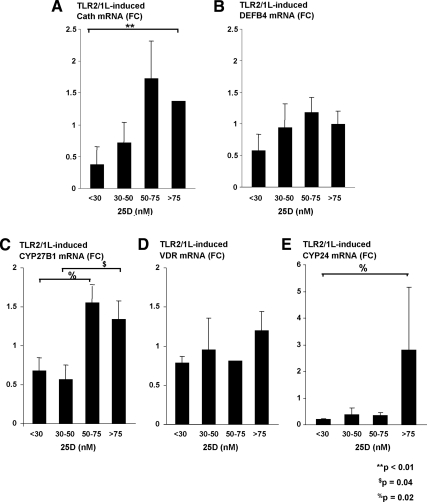
To combat infection by gram-positive bacteria, TLR2/1 responses are considered relevant. Activation of monocytes by the TLR2/1L cultured with vitamin D-deficient plasma demonstrated diminished cathelicidin expression compared with vitamin D-sufficient plasma (P < 0.01, Fig. 3A). There was no difference detected in DEFB4 mRNA expression after stimulation of monocytes with a TLR2/1L when compared with cord blood plasma vitamin D concentrations (Fig. 3B). This finding was similar to the observations from TLR4L-stimulated monocytes.
Fig. 3.
TLR2/1L induction of the vitamin D-dependent antimicrobial pathway in monocytes is also perturbed in the presence of vitamin D insufficiency. Primary human monocytes were then stimulated with a TLR2/1L for 24 h in 10% cord blood plasma of the same varying vitamin D concentrations as described in Fig. 2. Cathelicidin, DEFB4, CYP27B1, VDR, and CYP24 gene expression were again determined by qPCR (mean fold change ± sem). A, Cathelicidin gene expression was notably diminished in adherent cells cultured with severely vitamin D-deficient plasma samples vs. vitamin D-sufficient ones (P < 0.01). B, No difference was detected in DEFB4 expression after stimulation with a TLR2/1L when compared with cord blood 25(OH)D concentrations of the plasma. C, CYP27B1 gene expression was notably less in monocytes cultured in plasma with 25(OH)D below 50 nm. D, There were no differences in VDR gene expression after stimulation with the TLR2L. E, There was a greater induction of CYP24 gene expression in the monocytes cultured with vitamin D-sufficient plasma as previously described with CYP24 gene expression after TLR4L stimulation (P = 0.02).
The TLR2/1L-induced expression of CYP27B1 expression was also positively correlated with the cord blood plasma 25(OH)D concentrations added to the monocyte cultures. CYP27B1 mRNA measured from cells cultured in 25(OH)D-deficient plasma samples also consistently exemplified diminished expression compared with cells cultured in plasma with 25(OH)D concentrations higher than 50 nmol/liter. Specifically, TLR2/1L activation of monocytes cultured in 25(OH)D-deficient plasma (concentrations from 30–50 nm) had less CYP27B1 mRNA measured than those monocytes cultured in plasma with sufficient vitamin D concentrations (P = 0.04). Even in the vitamin D-insufficient group, there was a greater induction of CYP27B1 mRNA compared with the severely deficient group (1.5- vs. 0.7-fold change, P = 0.02). Although it did not reach statistical significance (P = 0.07), the fold change in the sufficient group was also more than twice that of the severely deficient group [25(OH)D <30 nm]. With VDR gene expression, however, there were no differences in gene expression after stimulation with a TLR2/1L using plasma from the various vitamin D-deficient, -insufficient, and -sufficient groups (Fig. 3D). This finding was identical to the observation after TLR4L stimulation. When assessing TLR2L-induced up-regulation of CYP24 mRNA, the monocytes cultured in the vitamin D-insufficient or -deficient cord blood plasma exhibited diminished CYP24 mRNA expression vs. monocytes cultured in vitamin D-sufficient plasma (P = 0.02, Fig. 3E).
When cord blood plasma 1,25(OH)2D values were compared with the TLR2/1L-induced antimicrobial gene expression of stimulated monocytes, no statistically significant relationship was evident between the active vitamin D metabolite and cathelicidin, CYP27B1, VDR, or CYP24 mRNA after TLR2L stimulation (data not shown). The lack of relationship between measured plasma 1,25(OH)2D values and antimicrobial gene expression of TLR2/1L-stimulated primary monocytes was also consistent with the data collected after TLR4L stimulation. In summary, the results indicate that cord blood plasma with 25(OH)D concentrations below 50 nm do not support TLR2/1L or TLR4L induction of the vitamin D-dependent antimicrobial pathway in the same manner observed in cord blood plasma 25(OH)D concentrations greater than 50 nm or even in adult vitamin D-sufficient sera and plasma.
In vitro vitamin D supplementation
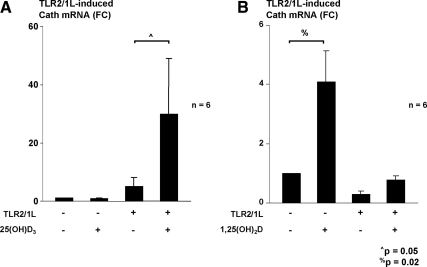
We next wanted to determine whether augmentation of 25(OH)D or 1,25(OH)2D concentrations could achieve a TLR-induced antimicrobial response in samples where it was previously absent. Monocytes were cultured in vitamin D-deficient cord blood plasma with and without supplementation of either exogenous 25(OH)D3 or exogenous 1,25(OH)2D3 at the time of stimulation with the TLR2/1L. Again, TLR2/1L activation of monocytes did not induce robust amounts of cathelicidin mRNA. However, the addition of 10−8 m 25(OH)D3 resulted in a greater than 5-fold induction of cathelicidin mRNA compared with the unsupplemented samples (n = 6, Fig. 4A). The addition of 10−8 m 1,25(OH)2D3 induced cathelicidin production independently of TLR2/1 stimulation as previously reported (32). With TLR2/1 stimulation, there was more induction of cathelicidin mRNA in monocytes supplemented with 1,25(OH)2D3 compared with the unsupplemented samples (n = 6, Fig. 4B) but not as robustly as 25(OH)D3 supplementation. Given that the 25(OH)D and 1,25(OH)2D concentrations in the cord blood samples were 52.7 ± 5.5 nm and 96.3 ± 8.3 pm compared with the mean in adult samples of 87.8 ± 4 nm and 113.8 ± 6.1 pm, the data indicate that supplementation of 25(OH)D-deficient cord blood plasma with exogenous 25(OH)D3 was capable of augmenting TLR2/1L-induced antimicrobial responses.
Fig. 4.
Restoration of the TLR-stimulated antimicrobial pathway is achieved with exogenous 25(OH)D3 supplementation in vitro. A, Primary monocytes were cultured in vitamin D-deficient cord blood plasma with and without supplementation of exogenous 25(OH)D3 at the time of stimulation with the TLR2/1L. In the unsupplemented samples, TLR2/1L activation of monocytes did not induce robust amounts of cathelicidin mRNA. However, the addition of 10−8 m 25(OH)D3 resulted in a greater than 5-fold induction of cathelicidin mRNA compared with the unsupplemented samples. B, Primary monocytes were cultured in vitamin D-deficient cord blood plasma with and without the addition of exogenous 1,25(OH)2D3 at the time of stimulation with the TLR2/1L. Independent induction of cathelicidin gene expression occurred in monocytes that were supplemented with 1,25(OH)2D3 in the absence of TLR2/1L stimulation. In monocytes stimulated with a TLR2/1L, the addition of 1, 25(OH)2D3 led to only a slightly greater expression of cathelicidin mRNA compared with those without supplementation.
Discussion
Although vitamin D insufficiency in the pediatric population has been linked to a wide variety of adverse health issues (33–38), including increased susceptibility to infectious diseases (10, 14, 39, 40), the mechanisms that contribute to these associations remain unclear. Neonates, in particular, continue to have high rates of morbidity and mortality around the world (1). Infections remain a leading cause for this troubling statistic. Because the neonatal acquired T and B cell responses are not fully developed and, therefore, not as robust, neonates are relatively more dependent on their innate immune response to infections than adults. In the current study, we investigated whether cord blood vitamin D concentrations affect the innate immune response to microbial pathogens, using the TLR-induced, vitamin D-dependent antimicrobial pathway in monocytes as a model system.
Specifically, we studied the TLR2/1- and TLR4-induced responses, given that these represent key host responses to gram-positive and gram-negative bacteria, respectively. Both types of bacteria are also well recognized for the life-threatening infections they cause in neonates. Our data indicate that the ability of cord blood plasma to support the TLR-induced antimicrobial pathway was dependent on circulating 25(OH)D concentrations. Importantly, in vitro supplementation of the vitamin D-deficient cord blood plasma with exogenous 25(OH)D3 recovered TLR-induced antimicrobial gene expression in mature primary monocytes. These data suggest that vitamin D deficiency can perturb innate immune function by altering the ability of immune cells to respond to a challenge from a pathogen.
A key finding of the present study was the differential effect of cord blood plasma vitamin D concentrations on both TLR2/1- and TLR4-induced antimicrobial pathways. When the 25(OH)D concentration was less than 50 nm, there was diminished TLR-mediated induction of CYP27B1 in human monocytes, the key enzyme responsible for monocyte/macrophage conversion of 25(OH)D to the bioactive 1,25(OH)2D. This active form, 1,25(OH)2D, is known to induce expression of downstream genes encoding for antimicrobial peptides. Cord blood plasma concentrations less than 50 nm were also found to correlate with diminished TLR2/1 and TLR4 induction of cathelicidin, which encodes for an antimicrobial peptide. This peptide possesses microbicidal activity against gram-positive (e.g. Staphylococcus aureus) and gram-negative (e.g. Escherichia coli) pathogens (41), common organisms responsible for invasive infections and overwhelming sepsis in neonates. In addition to its antimicrobial activity, cathelicidin possesses proinflammatory properties that assist in amplifying the innate immune response.
In contrast, cord blood plasma vitamin D concentrations did not impact gene expression of another vitamin D-dependent antimicrobial peptide, DEFB4. Our lab recently reported, however, that TLR2/1L induction of IL-1β was also required for up-regulation of DEFB4 (42). Sadeghi et al. (43) previously found that IL-1β expression is diminished in newborns compared with adults after TLR2/1 stimulation. We speculate that our data may support their previous findings with the distinction that plasma from cord blood influences the response of innate immune cells to a more significant degree than plasma or sera from adults. It is also possible that other, but as yet undefined, vitamin D-dependent immune responses contribute to optimal innate defense against microbial pathogens.
The correlation between TLR induction of antimicrobial responses and cord blood plasma vitamin D concentrations was significant to the available concentrations of 25(OH)D measured. No correlation, however, was evident for TLR-induced antimicrobial responses and 1,25(OH)2D concentrations (data not shown). Despite the disparate results of TLR-induced antimicrobial responses and 25(OH)D vs. 1,25(OH)2D concentrations, a very strong correlation between measured plasma concentrations of 25(OH)D and 1,25(OH)2D existed. This was a unique finding that distinctly differed from the pattern in adults where 1,25(OH)2D concentrations remained relatively constant despite differences in 25(OH)D. Markestad (44 previously published similar results while noting that the tight correlation between 25(OH)D and 1,25(OH)2D concentrations was specific to the newborn period even compared with other pediatric subjects. The regulation of normal 1,25(OH)2D concentrations in the context of vitamin D insufficiency is classically achieved by PTH feedback, which induces renal CYP27B1, to maintain calcium homeostasis. Neonates, however, are not born with significant amounts of circulating PTH, providing an explanation for the unique correlation demonstrated between the cord blood 25(OH)D and 1,25(OH)2D values. The lack of correlation between TLR-induced antimicrobial responses and plasma 1,25(OH)2D is difficult to reconcile but is consistent with results previously published by Adams et al. (45). One cannot exclude, however, that the sample size may have limited the ability to determine a correlation.
Likewise, other limitations to the study exist. A classification system for vitamin D status based on general consensus guidelines from experts and published literature is presented, but it has not been validated for cord blood measurements. In terms of subject selection, the majority of cord blood samples were from Latino patients, and differences in vitamin D status are known to vary between ethnicities. Additionally, cord blood samples from newborns born to mothers with chorioamnionitis were excluded. Maternal chorioamnionitis is the most significant risk factor for the development of infections that lead to early-onset sepsis in newborns, so this exclusion may have limited the ability to determine the effects of vitamin D status on the subsequent development of infections in newborns. Furthermore, the study lacks direct clinical correlation because no information was obtained regarding which healthy neonates at birth subsequently developed infections within the first few months of life to determine whether vitamin D deficiency continued to alter innate immune responses vs. those infants who remained healthy.
In summary, the present data indicate a significant decrease in TLR induction of key antimicrobial pathway genes in human monocytes when cultured in cord blood plasma with 25(OH)D values below 50 nmol/liter. These data suggest that newborn 25(OH)D deficiency alters the in vitro monocyte response to TLR stimulation. Subsequent in vitro supplementation of the 25(OH)D-deficient cord blood plasma with exogenous 25(OH)D3 was able to recover TLR-induced antimicrobial gene expression. However, the use of vitamin D supplementation in vivo to increase antimicrobial peptide production by the neonatal immune system will require more investigation. Well-designed prospective correlative studies of vitamin D status, in vitro monocyte function, and host susceptibility to infections should ideally occur in a larger cohort of newborns and infants during their first year of life.
Acknowledgments
This work was supported by NIH/NIAID A147868, NIH/NIAID A173539, and RWJF 053510.
Disclosure Summary: V.P.W., X.Z., I.R., P.T.L., J.S.A., and R.L.M. have nothing to disclose. B.W.H. is a board member and advisory council member of DiaSorin Corp. where he also receives royalties.
Footnotes
- 25(OH)D
- 25-Hydroxyvitamin D
- 1,25(OH)2D
- 1,25-dihydroxyvitamin D
- DEFB4
- β-defensin 2 gene
- IRB
- institutional review board
- PBMC
- peripheral blood monocyte
- qPCR
- quantitative PCR
- TLR
- Toll-like receptor
- TLR2/1L
- TLR2/1 ligand
- VDR
- vitamin D receptor.
References
- 1. Bryce J, Boschi-Pinto C, Shibuya K, Black RE. 2005. WHO estimates of the causes of death in children. Lancet 365:1147–1152 [DOI] [PubMed] [Google Scholar]
- 2. Lubani MM, al-Shab TS, al-Saleh QA, Sharda DC, Quattawi SA, Ahmed SA, Moussa MA, Reavey PC. 1989. Vitamin-D-deficiency rickets in Kuwait: the prevalence of a preventable disease. Ann Trop Paediatr 9:134–139 [DOI] [PubMed] [Google Scholar]
- 3. Ziegler EE, Hollis BW, Nelson SE, Jeter JM. 2006. Vitamin D deficiency in breastfed infants in Iowa. Pediatrics 118:603–610 [DOI] [PubMed] [Google Scholar]
- 4. Kazemi A, Sharifi F, Jafari N, Mousavinasab N. 2009. High prevalence of vitamin D deficiency among pregnant women and their newborns in an Iranian population. J Womens Health (Larchmt) 18:835–839 [DOI] [PubMed] [Google Scholar]
- 5. Rabbani A, Alavian SM, Motlagh ME, Ashtiani MT, Ardalan G, Salavati A, Rabbani B, Rabbani A, Shams S, Parvaneh N. 2009. Vitamin D insufficiency among children and adolescents living in Tehran, Iran. J Trop Pediatr 55:189–191 [DOI] [PubMed] [Google Scholar]
- 6. Hollis BW, Pittard WB., 3rd 1984. Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. J Clin Endocrinol Metab 59:652–657 [DOI] [PubMed] [Google Scholar]
- 7. Nicolaidou P, Hatzistamatiou Z, Papadopoulou A, Kaleyias J, Floropoulou E, Lagona E, Tsagris V, Costalos C, Antsaklis A. 2006. Low vitamin D status in mother-newborn pairs in Greece. Calcif Tissue Int 78:337–342 [DOI] [PubMed] [Google Scholar]
- 8. Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. 2007. Vitamin D deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 46:42–44 [DOI] [PubMed] [Google Scholar]
- 9. Morcos MM, Gabr AA, Samuel S, Kamel M, el Baz M, el Beshry M, Michail RR. 1998. Vitamin D administration to tuberculous children and its value. Boll Chim Farm 137:157–164 [PubMed] [Google Scholar]
- 10. Williams B, Williams AJ, Anderson ST. 2008. Vitamin D deficiency and insufficiency in children with tuberculosis. Pediatr Infect Dis J 27:941–942 [DOI] [PubMed] [Google Scholar]
- 11. Beser E, Cakmakci T. 1994. Factors affecting the morbidity of vitamin D deficiency rickets and primary protection. East Afr Med J 71:358–362 [PubMed] [Google Scholar]
- 12. Wayse V, Yousafzai A, Mogale K, Filteau S. 2004. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr 58:563–567 [DOI] [PubMed] [Google Scholar]
- 13. Muhe L, Lulseged S, Mason KE, Simoes EA. 1997. Case-control study of the role of nutritional rickets in the risk of developing pneumonia in Ethiopian children. Lancet 349:1801–1804 [DOI] [PubMed] [Google Scholar]
- 14. Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. 2006. Epidemic influenza and vitamin D. Epidemiol Infect 134:1129–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. 2006. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770–1773 [DOI] [PubMed] [Google Scholar]
- 16. Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. 2001. Extrarenal expression of 25-hydroxyvitamin D3-1α-hydroxylase. J Clin Endocrinol Metab 86:888–894 [DOI] [PubMed] [Google Scholar]
- 17. Zehnder D, Evans KN, Kilby MD, Bulmer JN, Innes BA, Stewart PM, Hewison M. 2002. The ontogeny of 25-hydroxyvitamin D3 1α-hydroxylase expression in human placenta and decidua. Am J Pathol 161:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Markestad T. 1983. Plasma concentrations of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25,26-dihydroxyvitamin D in the first year of life. J Clin Endocrinol Metab 57:755–759 [DOI] [PubMed] [Google Scholar]
- 19. Hollis BW, Napoli JL. 1985. Improved radioimmunoassay for vitamin D and its use in assessing vitamin D status. Clin Chem 31:1815–1819 [PubMed] [Google Scholar]
- 20. Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. 1993. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem 39:529–533 [PubMed] [Google Scholar]
- 21. Horst RL. 2010. Exogenous versus endogenous recovery of 25-hydroxyvitamins D2 and D3 in human samples using high-performance liquid chromatography and the DiaSorin LIAISON Total-D assay. J Steroid Biochem Mol Biol 121:180–182 [DOI] [PubMed] [Google Scholar]
- 22. Maunsell Z, Wright DJ, Rainbow SJ. 2005. Routine isotope-dilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamins D2 and D3. Clin Chem 51:1683–1690 [DOI] [PubMed] [Google Scholar]
- 23. Saenger AK, Laha TJ, Bremner DE, Sadrzadeh SM. 2006. Quantification of serum 25-hydroxyvitamin D2 and D3 using HPLC-tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D deficiency. Am J Clin Pathol 125:914–920 [DOI] [PubMed] [Google Scholar]
- 24. Greer FR. 2009. Defining vitamin D deficiency in children: beyond 25-OH vitamin D serum concentrations. Pediatrics 124:1471–1473 [DOI] [PubMed] [Google Scholar]
- 25. Henry HL, Bouillon R, Norman AW, Gallagher JC, Lips P, Heaney RP, Vieth R, Pettifor JM, Dawson-Hughes B, Lamberg-Allardt CJ, Ebeling PR. 2010. 14th Vitamin D Workshop consensus on vitamin D nutritional guidelines. J Steroid Biochem Mol Biol 121:4–6 [DOI] [PubMed] [Google Scholar]
- 26. Holick MF. 2007. Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- 27. Mansbach JM, Ginde AA, Camargo CA., Jr 2009. Serum 25-hydroxyvitamin D levels among US children aged 1 to 11 years: do children need more vitamin D? Pediatrics 124:1404–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Munns C, Zacharin MR, Rodda CP, Batch JA, Morley R, Cranswick NE, Craig ME, Cutfield WS, Hofman PL, Taylor BJ, Grover SR, Pasco JA, Burgner D, Cowell CT. 2006. Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: a consensus statement. Med J Aust 185:268–272 [DOI] [PubMed] [Google Scholar]
- 29. Roux C, Bischoff-Ferrari HA, Papapoulos SE, de Papp AE, West JA, Bouillon R. 2008. New insights into the role of vitamin D and calcium in osteoporosis management: an expert roundtable discussion. Curr Med Res Opin 24:1363–1370 [DOI] [PubMed] [Google Scholar]
- 30. Wagner CL, Greer FR. 2008. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 122:1142–1152 [DOI] [PubMed] [Google Scholar]
- 31. Miller LS, Sørensen OE, Liu PT, Jalian HR, Eshtiaghpour D, Behmanesh BE, Chung W, Starner TD, Kim J, Sieling PA, Ganz T, Modlin RL. 2005. TGF-α regulates TLR expression and function on epidermal keratinocytes. J Immunol 174:6137–6143 [DOI] [PubMed] [Google Scholar]
- 32. Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH, Hanrahan JH. 2004. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173:2909–2912 [DOI] [PubMed] [Google Scholar]
- 33. Carvalho NF, Kenney RD, Carrington PH, Hall DE. 2001. Severe nutritional deficiencies in toddlers resulting from health food milk alternatives. Pediatrics 107:E46. [DOI] [PubMed] [Google Scholar]
- 34. Zipitis CS, Akobeng AK. 2008. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child 93:512–517 [DOI] [PubMed] [Google Scholar]
- 35. Svoren BM, Volkening LK, Wood JR, Laffel LM. 2009. Significant vitamin D deficiency in youth with type 1 diabetes mellitus. J Pediatr 154:132–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holick MF. 2006. Resurrection of vitamin D deficiency and rickets. J Clin Invest 116:2062–2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wright TB, Shults J, Leonard MB, Zemel BS, Burnham JM. 2009. Hypovitaminosis D is associated with greater body mass index and disease activity in pediatric systemic lupus erythematosus. J Pediatr 155:260–265 [DOI] [PubMed] [Google Scholar]
- 38. Brown J, Nunez S, Russell M, Spurney C. 2009. Hypocalcemic rickets and dilated cardiomyopathy: case reports and review of literature. Pediatr Cardiol 30:818–823 [DOI] [PubMed] [Google Scholar]
- 39. McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM. 2009. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol 44:981–988 [DOI] [PubMed] [Google Scholar]
- 40. Banajeh SM. 2009. Nutritional rickets and vitamin D deficiency. Association with the outcomes of childhood very severe pneumonia: a prospective cohort study. Pediatr Pulmonol 44:1207–1215 [DOI] [PubMed] [Google Scholar]
- 41. Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI. 1998. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother 42:2206–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, Vazirnia A, Zhang X, Steinmeyer A, Zügel U, Hollis BW, Cheng G, Modlin RL. 2009. Convergence of IL-1β and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One 4:e5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sadeghi K, Berger A, Langgartner M, Prusa AR, Hayde M, Herkner K, Pollak A, Spittler A, Forster-Waldl E. 2007. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J Infect Dis 195:296–302 [DOI] [PubMed] [Google Scholar]
- 44. Markestad T. 1983. Plasma concentrations of vitamin D metabolites in unsupplemented breast-fed infants. Eur J Pediatr 141:77–80 [DOI] [PubMed] [Google Scholar]
- 45. Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. 2009. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol 182:4289–4295 [DOI] [PMC free article] [PubMed] [Google Scholar]