Administration of kisspeptin to healthy men reveals that a single iv dose of kisspeptin induces prolonged GnRH secretion and also resets the GnRH pulse generator.
Abstract
Context:
Reproduction in all mammals is controlled by a hypothalamic clock that produces periodic secretory pulses of GnRH, but how the timing of these pulses is determined is poorly understood. The neuropeptide kisspeptin potently and selectively stimulates the secretion of GnRH. Although this property of kisspeptin is well described, the effects of kisspeptin on endogenous GnRH pulse generation remain largely unexplored.
Objective:
The objective of the study was to detail the effects of kisspeptin on GnRH secretion, as reflected by LH secretion, in men.
Participants:
Thirteen healthy adult men participated in the study.
Intervention:
The intervention was the administration of a single iv bolus of the C-terminal decapeptide of kisspeptin (amino acids 112–121 of the parent protein).
Results:
Kisspeptin induced an immediate LH pulse, regardless of the timing of the previous endogenous pulse. The kisspeptin-induced pulses were on average larger than endogenous pulses (amplitude 5.0 ± 1.0 vs. 2.1 ± 0.3 mIU/ml, P = 0.02). Comparison of the morphology of kisspeptin-induced LH pulses in healthy men with that of GnRH-induced LH pulses in men with isolated GnRH deficiency suggests that a single iv bolus of kisspeptin triggered sustained GnRH release lasting approximately 17 min. Furthermore, kisspeptin reset the GnRH pulse generator, as it not only induced an immediate LH pulse but also delayed the next endogenous pulse by an interval approximating the normal interpulse interval.
Conclusions:
As the first known agent capable of resetting the hypothalamic GnRH pulse generator, kisspeptin can be used as a physiological tool for studying GnRH pulse generation and opens a door to understanding the mechanisms of biological clocks in general.
Clocks are ubiquitous in living organisms. They drive an extremely wide range of periodic phenomena in individual cells, tissues, organs, and whole organisms (1). These scales have a range on the order of 10 billion, from milliseconds at the neuronal level, to seconds in cardiac rhythms, to a day on the circadian scale, to a year for seasonal changes. Within this milieu lies the hypothalamus, which houses not only the well-known circadian clock but also the clocks that drive the rhythmic secretion of GnRH, GH, and other hormones (1, 2). The pulsatile secretion of GnRH initiates puberty; coordinates ovulation; maintains overall reproductive function; and is influenced by gender, sleep, food intake, photoperiod, and stress (3). Common reproductive disorders, including polycystic ovarian syndrome, hypothalamic amenorrhea, and delayed puberty, are associated with abnormalities in pulsatile GnRH secretion (4–8). Although the importance of pulsatile GnRH secretion has been known for decades (9), the nature of the hypothalamic clock that generates GnRH pulses has remained an enigma.
The hypothalamic neuropeptide kisspeptin is essential for normal reproduction; mutations in the kisspeptin receptor cause abnormal sexual maturation and hypogonadotropic hypogonadism in humans and mice (10–12). Kisspeptin is a potent stimulus for GnRH secretion in all mammalian species tested to date, including humans (13–17). Much has been learned about the roles of kisspeptin in sexual maturation, in mediating sex-steroid feedback, and in conveying the effects of nutritional status and stress to GnRH neurons (13). However, the effects of kisspeptin on the GnRH pulse generator have yet to be explored in detail.
We have administered kisspeptin to healthy adult men and performed frequent blood sampling to chart the morphology and timing of LH pulses before and after kisspeptin administration. This detailed neuroendocrine characterization allowed us to estimate the duration of GnRH secretion in response to kisspeptin and to examine kisspeptin's effects on endogenous GnRH pulse generation.
Subjects and Methods
Subjects
Healthy adult men who received kisspeptin (n = 13) met the following inclusion criteria: 21–40 yr old; self-reported history of normal timing and pace of puberty and normal erectile and ejaculatory function; no use of prescription medications for at least 2 months before the study; body mass index 18.5–30 kg/m2; blood pressure below 140/90 mm Hg; normal physical examination including testicular volume 15 ml or greater by Prader orchidometer; normal white blood cell and platelet counts, normal hemoglobin; no elevation of creatinine or blood urea nitrogen; aspartate aminotransferase and alanine aminotransferase no more than twice the upper limit of the reference range; and normal TSH, prolactin, FSH, LH, and testosterone. Exclusion criteria were the presence of a chronic medical condition, a history of cryptorchidism or microphallus, a history of anaphylaxis, consumption of more than 10 alcoholic drinks per week, and self-reported use of illicit drugs. Men with GnRH deficiency who received GnRH are described elsewhere (18). All protocols were approved by the Institutional Review Board of the Massachusetts General Hospital (MGH) and the Food and Drug Administration, and all subjects gave written informed consent before participation in these studies. The kisspeptin study was registered with www.ClinicalTrials.gov (NCT00914823).
Materials
Kisspeptin 112–121 and GnRH were synthesized using good manufacturing practices by PolyPeptide Laboratories (San Diego, CA). Resuspended aliquots underwent additional tests for sterility, pyrogenicity, purity, and concentration.
Kisspeptin administration and frequent blood sampling
Subjects were admitted to the Harvard Catalyst Clinical Research Center of MGH for 12 h of blood sampling every 10 min. Kisspeptin was given as an iv bolus at a dose of 0.24 nmol/kg (0.313 μg/kg) at the 6-h time point. There were no adverse events related to kisspeptin immediately after administration or at 2-wk follow-up.
GnRH administration
Men with GnRH deficiency received an “ED50” dose of GnRH as an instantaneous bolus or as a 1-, 5-, or 30-min infusion as described previously (18). Only data from the 10-, 20-, 30-, 40-, 50-, and 60-min time points were analyzed to match the data obtained every 10 min in the kisspeptin administration study.
Laboratory assays
Measurements of LH for each sample and FSH and testosterone on 2-h pools were performed by the MGH Clinical Laboratory Research Core as described elsewhere (19).
Pulse identification and calculated pulse characteristics
We identified LH pulses using a modification of the criteria of Santen and Bardin (20) augmented by the following deconvolution algorithm: the LH decay curves for all pulses for each subject (starting from the first point after each peak to the nadir preceding the next pulse) were used to calculate a single best-fitting decay rate and plateau (assuming single rate exponential decay in the presence of a tonic level of LH secretion). For each LH measurement, these decay constants were used to calculate the LH expected at the next (+10 min) time point, which was then subtracted from the actual measured LH to derive an instantaneous secretory rate (ISR) above baseline. Because ISR during periods of decay are expected to be zero, the sd of ISR during decay periods (as defined above) was used as a measure of variability attributable to sample collection and hormone measurement. ISR greater than 2 sd above zero were flagged as potential secretory events. If the ISR immediately preceding or following this ISR was negative in sign and within 1 sd in magnitude, these data points were attributed to errors in sample collection/measurement and removed from analysis. Remaining secretory rates greater than 2 sd above zero were considered to mark true secretory events (i.e. pulses). To detect gradual pulses, ISR were also calculated based on the LH expected at the +20 and +30 min time points. Decay constants were then recalculated and the analysis repeated until no additional data points were removed and no additional pulses were added or removed.
The amplitude of each pulse was calculated by subtracting the LH concentration at the nadir preceding each pulse from the LH concentration at the peak of the pulse. The results of analysis were unchanged if other methods of calculating pulse amplitude were used, such as totaling the ISR from the nadir to the peak of each pulse. The area under the curve (AUC) for each LH pulse was calculated as reported elsewhere (18). The interpulse interval was defined as the time between pulse nadirs.
Kisspeptin pharmacokinetics
Kisspeptin was added to human plasma to a concentration of 500 ng/ml and then incubated at 37 C for 0, 1, 2, 4, 6, 8, 10, 15, 30, 60, 120, 240, 360, and 1440 min, followed by extraction and quantitation by tandem liquid-chromatography mass-spectroscopy (details in Supplemental Information, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org, and Ref. 21). Kisspeptin was below the limit of quantitation (0.5 ng/ml) in samples incubated for more than 60 min. The assay was linear from 0.5 to 1000 ng/ml (Supplemental Fig. 1). Within-run coefficients of variation were 16.5, 10.4, 8.2, 4.1, and 4.2% at 0.5, 1, 5, 50, and 500 ng/ml, respectively, with six replicates each.
Statistical analysis
Data are presented in text and figures as mean ± sem. One-sample Kolmogorov-Smirnoff tests were used to compare the cumulative number of pulses over time in the separate 6-h periods before and after kisspeptin to an idealized uniform distribution (cumulative probability rising from 0 at the beginning to 1 at the end of each 6-h period). Paired, two-tailed t tests were used to compare pulse amplitude, AUC, time from nadir to peak, and pulse intervals for the kisspeptin-induced pulses to the mean of the values for each individual's endogenous pulses before kisspeptin. If a subject lacked a data point for a particular comparison, that subject's data were excluded from that analysis; conclusions were unchanged if time-to-event analysis was performed using SigmaPlot (SyStat Software, San Jose, CA). Repeated-measures, one-way ANOVA with Bonferroni post hoc analysis was used to compare FSH and testosterone for each 2-h pool after kisspeptin administration (i.e. the 6–8, 8–10, and 10–12 h pools) to the mean of each individual's three pools before kisspeptin (i.e. the 0–2, 2–4, and 4–6 h pools). Correlations were tested using linear regression. Kisspeptin decay in vitro was fitted to a biexponential decay model using WinNonlin version 5 (Pharsight Corp., Sunnyvale, CA). Statistical calculations were performed using Microsoft Excel (Richmond, CA) and GraphPad Prism (La Jolla, CA).
Results
Kisspeptin immediately induces a single, large LH pulse in men
Thirteen healthy adult men underwent blood sampling every 10 min, and LH was measured at each time point to chart LH pulses (22), which serve as a well-validated surrogate measure of GnRH secretory pulses (23, 24). After 6 h of blood sampling to establish baseline GnRH secretory patterns, subjects received a single iv bolus of 0.24 nmol/kg of the human kisspeptin decapeptide (amino acids 112–121 of the precursor molecule), followed by an additional 6 h of blood sampling. LH pulses were identified using the method of Santen and Bardin (20) augmented by deconvolution analysis.
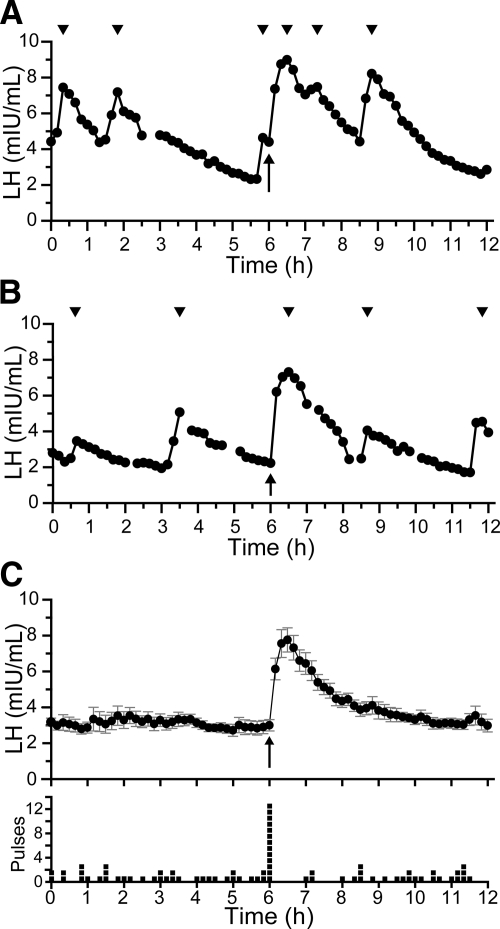
In all subjects, kisspeptin induced an immediate LH pulse, regardless of the timing of the previous endogenous pulse (Fig. 1, A and B, and Supplemental Fig. 2). To isolate the effects of kisspeptin from those of endogenous GnRH secretion, data from all subjects were averaged at each time point. This caused endogenous pulses, which occurred asynchronously across subjects, to even out to an apulsatile baseline and revealed that kisspeptin induces a single pulse, with LH returning to baseline by 4 h (Fig. 1C).
Fig. 1.
Effects of kisspeptin on LH secretion. A and B, Representative patterns of LH secretion from two men. C, Average serum LH (upper panel) and distribution of pulses (lower panel) across all 13 subjects. Each box in the lower panel indicates that a subject had a pulse nadir at that time point. Error bars show sem in this and all figures. Arrows, Time of kisspeptin administration; arrowheads, peaks of pulses.
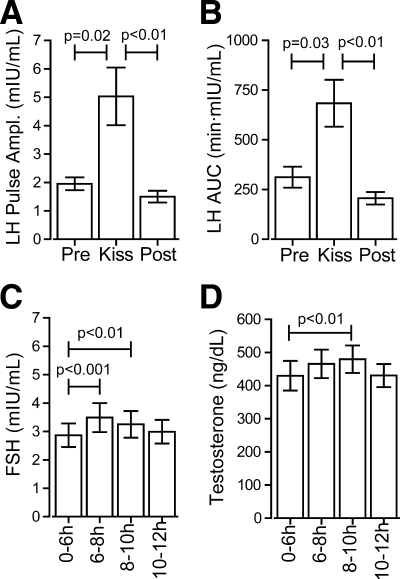
The mean amplitude and AUC of the kisspeptin-induced LH pulses were larger than those of endogenous pulses (amplitude 5.0 ± 1.0 vs. 2.1 ± 0.3 mIU/ml, P = 0.02; AUC 684 ± 118 vs. 312 ± 52 min·mIU/ml, P = 0.03; Fig. 2, A and B). Notably, however, the ranges of amplitudes of kisspeptin-induced and endogenous pulses demonstrated considerable overlap (1.6–10.9 and 0.5–7.8 mIU/ml, respectively). Serum FSH and testosterone also rose after kisspeptin administration (pooled values 2–4 h after kisspeptin vs. 6 h before kisspeptin: FSH 3.3 ± 0.5 vs. 2.9 ± 0.4 mIU/ml, P < 0.01; testosterone 480 ± 41 vs. 430 ± 45 ng/dl, P < 0.01; Fig. 2, C and D). Of several linear regression analyses performed (Supplemental Fig. 3), a positive correlation was observed between the interval between the kisspeptin-induced pulse and the succeeding endogenous pulse and the amplitude of the succeeding endogenous pulse (R2 = 0.6, P < 0.005).
Fig. 2.
Effects of kisspeptin on hormone concentrations. A, Amplitude of LH pulses. B, AUC of LH pulses. C, FSH in 2-h study pools. D, Testosterone in 2-h study pools.
The morphology of kisspeptin-induced LH pulses reveals that kisspeptin induces sustained GnRH release
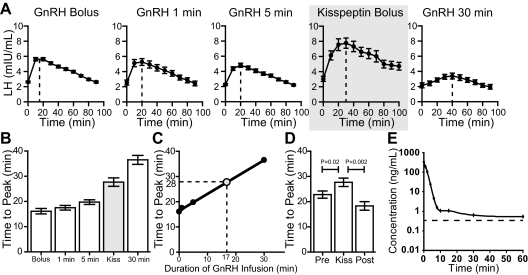
The LH pulses induced by kisspeptin were more rounded and more prolonged than endogenous pulses (Fig. 1 and Supplemental Fig. 1), with a longer time from nadir to peak (kisspeptin-induced, 27.7 ± 1.7 min; endogenous, 22.8 ± 1.4 min, P < 0.02; Fig. 3D). A similar pulse morphology was seen in a previous study from our group, in which 10 men with isolated GnRH deficiency were given a fixed dose of GnRH as an instantaneous bolus or as 1-, 5-, or 30-min infusions (18). Because of these men's isolated GnRH deficiency, their pituitary responses to exogenous GnRH could be charted without interference from endogenous GnRH secretion. Compared with the LH pulses after an instantaneous bolus of GnRH, those stimulated by the 1-, 5-, and 30-min infusions of GnRH became progressively more blunted and prolonged (Fig. 3, A–C). By comparing the shape of the kisspeptin-induced LH pulses in normal men to the shape of the GnRH-induced LH pulses in GnRH deficient men, we determined that the LH response produced by the dose of kisspeptin used in this study could be mimicked by a 17-min infusion of GnRH (Fig. 3, A–C). To assess the possibility that this could be due to prolonged pharmokinetics of kisspeptin, the half-life of kisspeptin 112–121 in human plasma in vitro was determined using a tandem liquid-chromatography mass-spectroscopy assay. This revealed that kisspeptin 112–121 has a half-life of 55 sec at 37 C (Fig. 3E).
Fig. 3.
Contour of LH pulses induced by administration of GnRH to men with GnRH deficiency and by administration of kisspeptin to healthy men. A, Average LH after an instantaneous bolus, after a 1-, 5-, or 30-min infusion of GnRH or after an instantaneous bolus of kisspeptin. [Adapted with permission with modifications from F. P. Pralong, et al.: Neuroendocrinology 64:247–256, 1996 (18) with permission from S. Karger AG, Basel.] Vertical dashed line, Time of peak LH. B, Average time from nadir to peak of LH pulses. C, Linear regression of time from nadir to peak. D, Time from nadir to peak of endogenous pulses and kisspeptin-induced pulses. Pre, Prekisspeptin pulses; Post, postkisspeptin pulses, excluding the immediate kisspeptin-induced pulse. E, Pharmacokinetics of kisspeptin 112–121 in human plasma in vitro. Dashed line, Lower limit of quantitation.
Kisspeptin resets the GnRH pulse generator
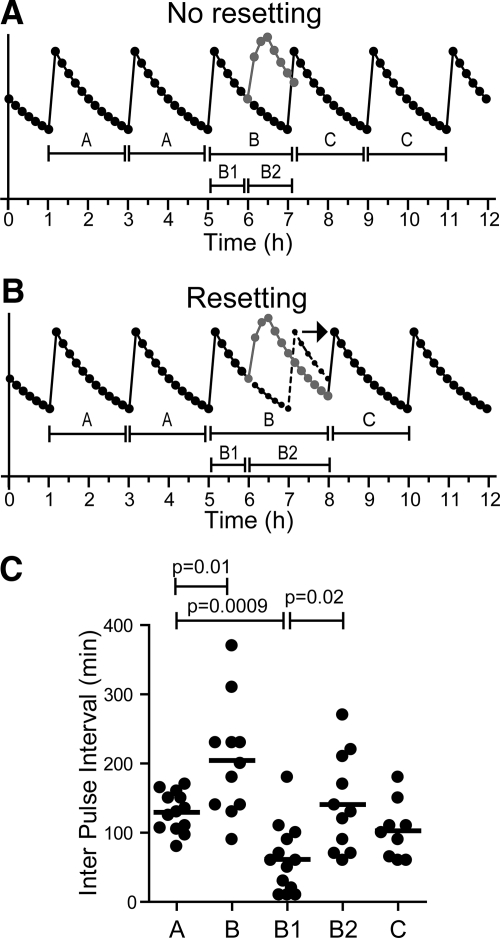
To examine the effects of kisspeptin on endogenous GnRH pulse generation, we tested two competing hypotheses: the null hypothesis, that exogenous kisspeptin, although inducing its own GnRH pulse, has no effect on the timing of endogenous pulses (Fig. 4A), and an alternative hypothesis, that exogenous kisspeptin resets the GnRH pulse generator (Fig. 4B). We first examined the temporal distribution of LH pulses. The null hypothesis predicts that endogenous pulses, which are asynchronous across the subject population and thus evenly distributed before kisspeptin, would also be evenly distributed after kisspeptin. As expected, we observed a uniform distribution of pulses in the 6 h before kisspeptin. However, the distribution of pulses in the 6 h after kisspeptin was significantly nonuniform (P < 0.03), with a paucity of pulses immediately after the kisspeptin-induced pulse (Fig. 1C). Thus, exogenous kisspeptin delayed the appearance of the next endogenous pulse, as would be expected if kisspeptin resets the pulse generator.
Fig. 4.
Effects of kisspeptin on pulse intervals. A, Schematic of the predicted result if kisspeptin were to have no effect on the timing of endogenous pulses. Note that this is an idealized schematic and that actual pulse profiles show more variability in pulse intervals. B, The observed result, that kisspeptin delayed the appearance of the next endogenous pulse and prolongs intervals B and B2. C, Observed pulse intervals. Bars, means.
To characterize this delay in more detail, we next examined the intervals between endogenous pulses. The null hypothesis predicts that the intervals between endogenous pulses would be unaffected by exogenous kisspeptin administration. Specifically, the pulse intervals before kisspeptin (interval A in Fig. 4) would be equal to the interval between the two pulses straddling the kisspeptin-induced pulse (interval B in Fig. 4). We found that interval A (before kisspeptin) averaged 130 ± 8 min (Fig. 4C), consistent with the well-described mean pulse frequency of approximately 2 h in men (20). In sharp contrast, we found that interval B was significantly longer than interval A, averaging 205 ± 25 min (P < 0.02; Fig. 4C).
We then quantified the timing of the kisspeptin-induced pulse relative to the preceding and succeeding endogenous pulses. The null hypothesis predicts that the kisspeptin-induced pulse would be as likely to fall closer to the preceding pulse as it would be to fall closer to the succeeding pulse. Thus, the means of these pulse intervals (B1 and B2 in Fig. 3) would be equal to each other and would be half of interval A. However, we found that interval B2 was longer than predicted by the null hypothesis. Specifically, although interval B1 averaged 62 ± 14 min, approximately half of interval A as expected, interval B2 was significantly longer (141 ± 21 min, P < 0.02, Fig. 3C). Moreover, interval B2 was statistically indistinguishable from interval A (P = 0.6; Fig. 3C). Therefore, the interval from the kisspeptin-induced pulse to the next endogenous pulse approximated the endogenous interpulse interval, exactly what would be expected with resetting. A post hoc power calculation reveals that our study had 90% power to detect a small difference (28 min) between these intervals. Thus, our data support the possibility that these pulse intervals are in fact identical and, by extension, that kisspeptin resets the hypothalamic clock. We did not observe any abiding effects of kisspeptin; the intervals between endogenous pulses after kisspeptin (103 ± 14 min; interval C in Fig. 3) were not different from those before kisspeptin (P = 0.14; Fig. 3C).
Discussion
By detailing the neuroendocrine responses of healthy men to exogenous kisspeptin, we have discovered previously undescribed properties of kisspeptin. A single, short-lived dose of kisspeptin induces sustained GnRH release in vivo, as demonstrated by the distinctive morphology of the kisspeptin-induced LH pulse. Furthermore, kisspeptin has the striking property of resetting the hypothalamic clock that drives pulses of GnRH secretion. Although estrogen, progesterone, testosterone, opioids, and their agonists and antagonists have been shown to modulate GnRH pulse frequency and amplitude (25), kisspeptin is the first known agent that can acutely reset the hypothalamic GnRH clock.
The ability of kisspeptin to reset the GnRH pulse generator was unexpected. We had presumed that kisspeptin acts at a late step in GnRH secretion (exocytosis) because kisspeptin can trigger GnRH secretion in explants of the mediobasal hypothalamus (MBH), which is rich in GnRH neuronal fibers but contains few GnRH neuronal cell bodies (26). Thus, we had predicted that endogenous GnRH pulse generation would be unaffected by exogenous kisspeptin administration. Contrary to our expectation, kisspeptin not only triggered an exocytic event but also had an acute effect on the hypothalamic GnRH pulse generator, such that the interval from the kisspeptin-induced pulse to the next endogenous pulse was about 2 h, approximating the normal endogenous pulse interval in men. Therefore, kisspeptin did not merely delay the next GnRH-induced LH pulse; it reset the hypothalamic GnRH pulse generator to a new zero time point.
The current study adds new information to the ongoing debate on the anatomic location of the GnRH pulse generator. One hypothesis is that GnRH neurons themselves contain autonomous mechanisms to allow coordinated secretion at periodic intervals. Indeed, pulsatile GnRH release has been observed both in vitro in a GnRH-secreting cell line and ex vivo in cultured GnRH neurons from fetal monkey and rat olfactory placodes (27–29). An alternative hypothesis is that the pulse generator lies outside GnRH neuronal cell bodies. This hypothesis was suggested by deafferentation experiments that demonstrated that the MBH can support reproductive endocrine activity and pulsatile LH secretion, even in the absence of connections with the rest of the brain (30). Also, explants of the MBH can produce pulsatile GnRH secretion (31, 32). Furthermore, volleys of multiunit activity in the arcuate nucleus, part of the MBH, are temporally associated with LH pulses (30). Because few GnRH neurons are present in the MBH (30), these results argue against GnRH neuronal cell bodies being the primary site of the pulse generator. Kisspeptin appears to act directly on GnRH neurons because GnRH neurons express the kisspeptin receptor (13) and kisspeptin stimulates GnRH neurons even when inputs from other neurons are blocked (33–36). Thus, our finding that kisspeptin resets the hypothalamic GnRH clock provides evidence that GnRH neurons are an essential component of the pulse generator.
It is important to recognize, however, that intrinsic pulsatility of the GnRH neurons does not preclude the existence of other pulse generators in the reproductive endocrine system. Indeed, kisspeptin secretion itself is also pulsatile (37, 38), and it has been suggested the multiunit activity that correlates with LH secretion originates from kisspeptin neurons in the arcuate nucleus (30). Thus, there appear to be multiple clocks in the reproductive endocrine system operating at different levels within the hypothalamus, with kisspeptin serving as a means of communication between these clocks. This redundancy could protect against disruption of GnRH pulse generation and could also provide multiple sites of regulation of reproductive endocrine function.
The second major finding of this study is that an instantaneous iv kisspeptin bolus results in sustained activation of GnRH neurons lasting approximately 17 min in vivo, a finding that is remarkably congruent with ex vivo studies of GnRH neurons. After transient exposure to kisspeptin, GnRH neurons in cultured brain slices exhibit sustained depolarization and calcium mobilization (33–36, 39), with a mean duration of depolarization of 16 min in one study (36). This sustained effect of kisspeptin could be due to prolonged receptor occupancy or prolonged activation of downstream signaling pathways but does not appear to be due to prolonged pharmacokinetics of kisspeptin because the in vitro half-life of kisspeptin in human plasma is very short. However, it remains possible that the pharmocokinetics of kisspeptin differ in vivo due to tissue distribution or protein binding. It is also unlikely that this is due to a direct effect of kisspeptin on the pituitary gland. Administration of kisspeptin intracerebroventricularly to mice, rats, and rhesus macaques stimulates LH secretion, and this stimulation is blocked by pretreatment with a GnRH receptor antagonist (13). Thus, the results in this study most likely reflect a hypothalamic action of kisspeptin.
The dose of kisspeptin used in this study was chosen based on those reported elsewhere (14), in which an equimolar dose of kisspeptin 68–121 was the smallest dose to produce a maximal rise in LH. In our study, this dose induced responses that ranged from physiological to supraphysiological. Future studies will determine whether kisspeptin can cause sustained GnRH release and resetting of the GnRH pulse generator at other doses.
Kisspeptin's role in reproduction was revealed by the discovery that loss-of-function mutations in the kisspeptin receptor cause GnRH deficiency in humans and mice (10–12), and we have now brought those initial findings through the translational research cycle. By administering kisspeptin to human subjects, we have discovered a previously unknown property of this critical neuropeptide: the ability to reset the GnRH pulse generator. Furthermore, by performing peptide administration studies in both normal and GnRH-deficient men, we have determined that a single iv bolus of kisspeptin induces a prolonged GnRH secretory event in humans. We anticipate that kisspeptin will be a valuable physiological tool for unraveling the mystery of how pulses of GnRH secretion are generated, how they are altered in disease states, and how they might be subject to biopharmaceutical modification to treat reproductive disorders.
Supplementary Material
Acknowledgments
We thank the members of the Massachusetts General Hospital Reproductive Endocrine Unit and T.-s. Chan for discussions and reading of the manuscript; the staff of the Harvard Catalyst Clinical Research Center for assistance with the studies; and H. Feldman for statistical advice.
This work was supported by Grant R01 HD43341 from the Eunice Kennedy Shriver National Institute for Child Health and Human Development and Grant UL1 RR025758 (Harvard Clinical and Translational Science Center) from the National Center for Research Resources. Y.-M.C. was supported by a Charles A. King Trust postdoctoral fellowship and a Career Development Award from Children's Hospital Boston.
Present address for F.P.P.: University of Lausanne Medical School, CH-1011 Lausanne, Switzerland.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- Area under the curve
- ISR
- instantaneous secretory rate
- MBH
- mediobasal hypothalamus
- MGH
- Massachusetts General Hospital.
References
- 1. Winfree A. 2001. The geometry of biological time. 2nd ed New York: Springer-Verlag [Google Scholar]
- 2. Veldhuis JD, Keenan DM, Pincus SM. 2008. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev 29:823–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Low M. 2008. Neuroendocrinology. In: Kronenberg H, Melmed S, Polonsky KS, Larsen PR. eds. Williams textbook of endocrinology. Philadelphia: Saunders Elsevier; 85–154 [Google Scholar]
- 4. Kelch RP, Hopwood NJ, Sauder S, Marshall JC. 1985. Evidence for decreased secretion of gonadotropin-releasing hormone in pubertal boys during short-term testosterone treatment. Pediatr Res 19:112–117 [DOI] [PubMed] [Google Scholar]
- 5. Santoro N, Filicori M, Crowley WF., Jr 1986. Hypogonadotropic disorders in men and women: diagnosis and therapy with pulsatile gonadotropin-releasing hormone. Endocr Rev 7:11–23 [DOI] [PubMed] [Google Scholar]
- 6. Waldstreicher J, Santoro NF, Hall JE, Filicori M, Crowley WF., Jr 1988. Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab 66:165–172 [DOI] [PubMed] [Google Scholar]
- 7. Cemeroglu AP, Foster CM, Warner R, Kletter GB, Marshall JC, Kelch RP. 1996. Comparison of the neuroendocrine control of pubertal maturation in girls and boys with spontaneous puberty and in hypogonadal girls. J Clin Endocrinol Metab 81:4352–4357 [DOI] [PubMed] [Google Scholar]
- 8. Perkins RB, Hall JE, Martin KA. 1999. Neuroendocrine abnormalities in hypothalamic amenorrhea: spectrum, stability, and response to neurotransmitter modulation. J Clin Endocrinol Metab 84:1905–1911 [DOI] [PubMed] [Google Scholar]
- 9. Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. 1978. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science 202:631–633 [DOI] [PubMed] [Google Scholar]
- 10. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. 2003. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- 12. Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, Yang S, Monsma FJ, Gustafson EL. 2003. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun 312:1357–1363 [DOI] [PubMed] [Google Scholar]
- 13. Pineda R, Aguilar E, Pinilla L, Tena-Sempere M. 2010. Physiological roles of the kisspeptin/GPR54 system in the neuroendocrine control of reproduction. Prog Brain Res 181:55–77 [DOI] [PubMed] [Google Scholar]
- 14. Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. 2005. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab 90:6609–6615 [DOI] [PubMed] [Google Scholar]
- 15. Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M, Ghatei MA, Bloom SR. 2007. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab 92:3958–3966 [DOI] [PubMed] [Google Scholar]
- 16. Jayasena CN, Nijher GM, Chaudhri OB, Murphy KG, Ranger A, Lim A, Patel D, Mehta A, Todd C, Ramachandran R, Salem V, Stamp GW, Donaldson M, Ghatei MA, Bloom SR, Dhillo WS. 2009. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes tachyphylaxis. J Clin Endocrinol Metab 94:4315–4323 [DOI] [PubMed] [Google Scholar]
- 17. Jayasena CN, Nijher GM, Abbara A, Murphy KG, Lim A, Patel D, Mehta A, Todd C, Donaldson M, Trew GH, Ghatei MA, Bloom SR, Dhillo WS. 2010. Twice-weekly administration of kisspeptin-54 for 8 weeks stimulates release of reproductive hormones in women with hypothalamic amenorrhea. Clin Pharmacol Ther 88:840–847 [DOI] [PubMed] [Google Scholar]
- 18. Pralong FP, Boepple PA, Conn PM, Whitcomb RW, Butler JP, Schoenfeld D, Crowley WF., Jr 1996. Contour of the GnRH pulse independently modulates gonadotropin secretion in the human male. Neuroendocrinology 64:247–256 [DOI] [PubMed] [Google Scholar]
- 19. Dwyer AA, Hayes FJ, Plummer L, Pitteloud N, Crowley WF., Jr 2010. The long-term clinical follow-up and natural history of men with adult-onset idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab 95:4235–4243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Santen RJ, Bardin CW. 1973. Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest 52:2617–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ren C, Liu Z, Jones W, Chen P, Seminara S, Smith N, Covey J, Chan K. 2007. An LC-MS/MS method for the quantitation of metastin 45–54 (NSC 741805) and its preclinical pharmacokinetics in rats. AAPS J 9(Suppl 2):2199 (Abstract) [Google Scholar]
- 22. Crowley WF, Jr, Filicori M, Spratt DI, Santoro NF. 1985. The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res 41:473–531 [DOI] [PubMed] [Google Scholar]
- 23. Levine JE, Pau KY, Ramirez VD, Jackson GL. 1982. Simultaneous measurement of luteinizing hormone-releasing hormone and luteinizing hormone release in unanesthetized, ovariectomized sheep. Endocrinology 111:1449–1455 [DOI] [PubMed] [Google Scholar]
- 24. Clarke IJ, Cummins JT. 1982. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology 111:1737–1739 [DOI] [PubMed] [Google Scholar]
- 25. Marshall JC, Dalkin AC, Haisenleder DJ, Griffin ML, Kelch RP. 1993. GnRH pulses—the regulators of human reproduction. Trans Am Clin Climatol Assoc 104:31–46 [PMC free article] [PubMed] [Google Scholar]
- 26. d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. 2008. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology 149:3926–3932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martínez de la Escalera G, Choi AL, Weiner RI. 1992. Generation and synchronization of gonadotropin-releasing hormone (GnRH) pulses: intrinsic properties of the GT1–1 GnRH neuronal cell line. Proc Natl Acad Sci USA 89:1852–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Terasawa E, Quanbeck CD, Schulz CA, Burich AJ, Luchansky LL, Claude P. 1993. A primary cell culture system of luteinizing hormone releasing hormone neurons derived from embryonic olfactory placode in the rhesus monkey. Endocrinology 133:2379–2390 [DOI] [PubMed] [Google Scholar]
- 29. Funabashi T, Daikoku S, Shinohara K, Kimura F. 2000. Pulsatile gonadotropin-releasing hormone (GnRH) secretion is an inherent function of GnRH neurons, as revealed by the culture of medial olfactory placode obtained from embryonic rats. Neuroendocrinology 71:138–144 [DOI] [PubMed] [Google Scholar]
- 30. Maeda K, Ohkura S, Uenoyama Y, Wakabayashi Y, Oka Y, Tsukamura H, Okamura H. 2010. Neurobiological mechanism underlying GnRH pulse generation by the hypothalamus. Brain Res 1364:103–115 [DOI] [PubMed] [Google Scholar]
- 31. Bourguignon JP, Franchimont P. 1984. Puberty-related increase in episodic LHRH release from rat hypothalamus in vitro. Endocrinology 114:1941–1943 [DOI] [PubMed] [Google Scholar]
- 32. Rasmussen DD, Gambacciani M, Swartz W, Tueros VS, Yen SS. 1989. Pulsatile gonadotropin-releasing hormone release from the human mediobasal hypothalamus in vitro: opiate receptor-mediated suppression. Neuroendocrinology 49:150–156 [DOI] [PubMed] [Google Scholar]
- 33. Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. 2005. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25:11349–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pielecka-Fortuna J, Chu Z, Moenter SM. 2008. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang C, Roepke TA, Kelly MJ, Ronnekleiv OK. 2008. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 28:4423–4434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dumalska I, Wu M, Morozova E, Liu R, van den Pol A, Alreja M. 2008. Excitatory effects of the puberty-initiating peptide kisspeptin and group I metabotropic glutamate receptor agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J Neurosci 28:8003–8013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith JT, Rao A, Pereira A, Caraty A, Millar RP, Clarke IJ. 2008. Kisspeptin is present in ovine hypophysial portal blood but does not increase during the preovulatory luteinizing hormone surge: evidence that gonadotropes are not direct targets of kisspeptin in vivo. Endocrinology 149:1951–1959 [DOI] [PubMed] [Google Scholar]
- 38. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. 2008. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149:4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Constantin S, Caligioni CS, Stojilkovic S, Wray S. 2009. Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in gonadotropin-releasing hormone-1 neurons. Endocrinology 150:1400–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.