Plasmacytoid dendritic cells via their production of IFN-α render B cells more receptive to T cell contact which facilitates B cell proliferation and differentiation.
Keywords: CD27, CD86, type I IFN, IgM
Abstract
The development and quality of a humoral immune response are largely influenced by the environment that supports the activation of naïve B cells. Human PDCs, through their unique capacity to produce high levels of IFN-α, have been shown earlier to enhance B cell responses stimulated by selected TLR ligands. In this study, we investigated whether PDCs also promote B cell activation induced by Th cell interactions and BCR ligation. Sorted human naive CD19+ CD27– B cells were activated in vitro with anti-Ig and irradiated CD4+ T cells. Under these conditions, the presence of supernatants from TLR-stimulated PDCs increased B cell proliferation, the frequency of B cells that differentiated to CD27high CD38high cells, and secretion of IgM. Similar results were observed when the B cells were activated in the presence of purified IFN-α. In contrast, supernatants from stimulated MDCs did not augment these functions. Also, IFN-α treatment of B cells up-regulated the expression of costimulatory molecule CD86 but not CD40, CD80, MHC class II, or CD25. Although direct IFN-α exposure of T cells suppressed their proliferative capacity, IFN-α treatment of B cells led to a small increase in their capacity to induce superantigen-driven activation of autologous CD4+ T cells. In summary, PDCs, via their production of IFN-α, may render B cells more responsive to T cell contact, which in turn, facilitates B cell proliferation and differentiation to antibody-producing cells.
Introduction
DCs are critical in orchestrating immune responses and are well documented for their essential function in priming T cell responses. Recent studies also indicate that they play an important role in the development of B cell responses [1–4]. In addition to the role of DCs in stimulating and regulating the differentiation of CD4+ Th cells that promote T cell-dependent B cell responses, there are several examples of direct interactions between DCs and B cells [5–10]. Through the presentation of unprocessed antigens on their cell surface, DCs can enhance priming of B cell responses [5, 7, 10]. In mice, DCs in LNs were shown to present antigen to BCRs, which led to activation of the B cells shortly after their entry via HEVs and before migration into the follicle [8]. In addition, various DC subsets produce soluble factors, such as IL-6, IL-10, IL-12, and the BAFF, which also support the development of B cell responses [3, 6, 11, 12]. For generation of antiviral immune responses, important early innate cytokines also include the type I IFNs (IFN-α/IFN-β), which can be produced by most cell types through distinct innate signaling pathways in response to virus infection but in particular in large amounts by PDCs [13]. PDCs have been shown to regulate B cell responses by secreting type I IFNs and IL-6 [3, 4, 6, 14, 15] and by direct cell-to-cell contact [6, 9]. As reported by us and others previously [3, 14, 15], IFN-α produced by human PDCs strongly enhances T cell-independent, TLR7/8-stimulated B cell responses in vitro. This enhancement may in part result from IFN-α-induced up-regulation of the expression of TLR7 and the adaptor molecule MyD88, rendering the cells more responsive to TLR7/8 ligand stimulation [14, 16]. It was shown in mice that type I IFN-mediated B cell activation significantly affected the quality and magnitude of the antiviral humoral response [17–19]. Also, IFN-α administered together with soluble protein antigen increased antigen-specific antibody responses [20]. Using two different types of mixed chimeras, where B cells or T cells were devoid of the IFNR-α, direct stimulation of both of these cell types by IFN-α was required for the enhanced antibody responses [20]. Increased survival of B cells and T cells by IFN-α may contribute to the enhanced cellular and humoral responses [21–24]. In contrast, earlier studies showed antiproliferative effects of IFN-α on these cell types [25, 26]; thus, the effects of IFN-α on B cell responses remain somewhat controversial. In this study, we investigated whether human PDCs, via their production of IFN-α, have the capacity to directly enhance T cell-dependent B cell responses in vitro. We chose to study the activation of naïve B cells, as they are the key cells involved in the development of primary humoral responses. Consequently, understanding how they are regulated by central cell and cytokine milieus during the onset of an immune response is of particular relevance. Using purified primary human naïve B cells, T cells, and DCs, we show that the presence of supernatants from TLR-stimulated PDCs or direct addition of purified human IFN-α during B cell stimulation increased their proliferation and differentiation into CD27high CD38high IgM-secreting cells in a T cell help-dependent manner. B cells cultured in the presence of stimulated PDCs or IFN-α up-regulated surface expression of the costimulatory molecule CD86 and showed a small but consistent increased ability to stimulate superantigen-driven T cell activation during coculture with autologous CD4+ T cells. These data suggest that IFN-α produced by PDCs renders B cells more responsive to T cell help, thereby facilitating T cell-dependent B cell activation.
MATERIALS AND METHODS
Isolation of cells
Human PBMCs were obtained from healthy blood donors by collection of buffy coats or automated apheresis as described previously [15, 27]. Following apheresis, enriched populations of lymphocytes and monocytes were obtained by counterflow centrifugal elutriation. Naïve CD19+ CD20+ CD27– IgD+ IgG– B cells were isolated from buffy coats or elutriated lymphocytes by sequential magnetic bead separation on an AutoMacs (Miltenyi Biotec, Bergisch Gladbach, Germany) using a B cell multisort kit (Miltenyi Biotec). The purity of sorted naïve B cells was determined by staining for CD20, CD27, IgD, IgG, CD3, and CD14 (see Fig. 1A). PI staining (Sigma-Aldrich, Schelldorf, Germany) was used to measure cell viability. Total CD4+ T cells were isolated using a CD4+ T cell enrichment cocktail (Stem Cell Technologies, Grenoble, France). The purity of sorted T cells was determined by staining for CD3, CD4, CD14, and CD20. PDCs and MDCs were isolated from elutriated monocytes using magnetic beads labeled with the specific DC markers blood DC antigen-4 and CD1c, respectively (Miltenyi Biotec), as described [15, 27–29]. The purity of the subsets of DCs was determined by staining for CD3, CD14, CD15, CD20, CD56 plus HLA-DR, CD123, and CD11c.
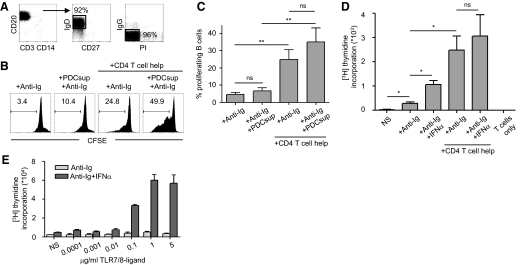
Figure 1. PDCs enhance naïve B cell proliferation induced by T cell help.
(A) Highly enriched CD19+CD27–IgD+IgG– naïve B cells were isolated from PBMCs by magnetic bead isolation. Numbers indicate percentage of gated cells. (B) Anti-Ig and supernatants from TLR7/8-stimulated PDCs (PDCsup) were added to CFSE-labeled, naïve B cells in the presence or absence of T cell help for 6 days. Proliferation was measured by flow cytometry. Numbers indicate percentage of dividing B cells. (C) Compiled data are shown (mean±sd; n≥4 donors). (D) Naïve B cells were stimulated with anti-Ig and human IFN-α in the presence or absence of T cell help, and proliferation was measured by thymidine incorporation (n=3). (E) Naïve B cells were stimulated with increasing concentrations of TLR7/8 ligand in the presence or absence of IFN-α, which is known to increase the sensitivity of B cells to TLR7/8 ligand. Proliferation by thymidine incorporation was measured at Day 5.
Stimulation of DCs and measurement of their cytokine production
Freshly isolated PDCs and MDCs were cultured at 1 × 106 cells/ml in complete medium [RPMI-1640 medium containing 10% FCS, 2 mM L-glutamine, 100 U/ml penicillin, 100 μM streptomycin, 2% HEPES, and 50 μM 2-ME (all from Sigma-Aldrich)], supplemented with rIL-3 (1 ng/ml; R&D Systems, Minneapolis, MN, USA) or GM-CSF (2 ng/ml; PeproTech, Rocky Hill, NJ, USA), respectively, in polystyrene round-bottom tubes. DCs were exposed for 24 h at 37°C to a TLR7/8 ligand (3M-012; 1 μg/ml; 3M Pharmaceuticals, St. Paul, MN, USA), a TLR9 ligand CpG A (2336; 10 μg/ml; Coley Pharmaceutical Group, Ottawa, Canada), or a TLR3 ligand Poly I:C (10 μg/ml; Sigma-Aldrich). Supernatants from the stimulated DCs were collected and frozen at –70°C until used in the B cell cultures or analyzed further. The supernatants were analyzed for levels of IFN-α by ELISA (kit by PBL Biomedical Laboratories, Piscataway, NJ, USA). The assay was performed according to the manufacturer's instructions.
B cell cultures
Purified, naïve B cells were cultured for 6 days at 37°C in polystyrene round-bottom tubes at 5 × 105 cells/ml in complete medium. B cells were cultured alone or together with CD4+ T cells (B cell:T cell ratio, 1:4) that had been γ-irradiated at 50 Gy using an IBL 637 (CIS-BioInternational, Gif sur Yvette, France), then washed, and pulsed with the superantigen TSST-1 (5 ng/ml; Sigma-Aldrich). In addition, rIL-2 (50 ng/ml, PeproTech) was added. All cultures were carried out in the presence of BCR ligation, accomplished by addition of F(ab′)2 anti-human IgM/IgG/IgA (2.5 μg/ml; Jackson ImmunoResearch, West Grove, PA, USA). Where indicated, cultures were exposed to supernatants (diluted 1:4–1:100) from TLR-stimulated DC subsets. For sequential exposure to specific DC supernatants, naïve B cells were first exposed to one supernatant for 3 days, which was then replaced with the second for a further 3 days of culture. Human leukocyte IFN-α (1000 U/ml; PBL Biomedical Laboratories) was added in indicated experiments. The B cell cultures were analyzed for proliferation and differentiation after 5–6 days.
B cell proliferation assays
Sorted, naïve B cells were labeled with 0.25 μM CFSE (Molecular Probes, Eugene, OR, USA) for 7 min at 37°C and washed thoroughly with complete medium as described earlier [15]. The B cells were cultured at the various conditions described above, and proliferation was measured by flow cytometry (FACSCalibur, BD PharMingen, San Jose, CA, USA). Data were analyzed using FlowJo software (Tree Star Inc., San Carlos, CA, USA). Live cells were gated by exclusion of PI staining (Sigma-Aldrich) in initial experiments. B cells were then gated, based on a lack of CD4, CD8, and CD14 expression. Alternatively, proliferation was measured by thymidine incorporation, where the cells were pulsed with [3H]thymidine (1 μCi/well; Amersham Bioscience, GE Healthcare Biosciences AB, Uppsala, Sweden) for 16 h after 4 days of culture. The level of incorporation of [3H]thymidine was measured by a 1450 MicroBeta PLUS counter (Wallac, PerkinElmer Sverige AB, Upplands Väsby, Sweden) and expressed as cpm.
Analysis of B cell maturation, differentiation, and viability
Up-regulation of costimulatory markers MHC class II, CD80, CD86, CD40, and CD25 on B cells was monitored by flow cytometry at various time-points (24 h–4 days) of culture. The cells were stained with the respective mAb (all from BD PharMingen) at room temperature for 20 min, washed in PBS containing 2% FCS, and analyzed immediately. Differentiation of B cells into CD27high CD38high plasmablasts was assessed by flow cytometry using the respective mAb (BD PharMingen) after up to 6 days of culture. Staining for CD4, CD8, and CD14 was used to exclude the T cells and any potential small contaminating monocyte fractions to evaluate the B cells only. Cell viability in the total population plus the divided and undivided populations was monitored by 7-AAD (eBioscience, San Diego, CA, USA), added to the samples 5 min prior to analysis.
Evaluation of antibody production
The presence of IgM in the supernatants from B cells in the indicated culture conditions was detected by ELISA from supernatants that were harvested after 6, 9, or 12 days. Briefly, 96-well Nunc Maxisorp microtiter plates were coated with 1 μg/ml purified goat anti-human IgM (Jackson ImmunoResearch). After washing with PBS containing 0.05% Tween and blocking with PBS supplemented with 2% milk, standards and supernatants of the cultured cells at different dilutions were added to the plates and incubated for 2 h at 37°C. The plates were then washed and incubated with biotin-conjugated, isotype-specific secondary antibodies for IgM (BioSource International, Camarillo, CA, USA), followed by washing and incubation with streptavidin-HRP (Mabtech, Stockholm, Sweden). The reaction was then developed using OPD in hydrogen peroxide/buffer (SIGMAFAST™ OPD, Sigma-Aldrich) as a soluble substrate for the detection of peroxidase activity. Substrate reactions were terminated with 2.5 M H2SO4, and the OD was read at 490 nm.
T cell proliferation assay
Enriched CD4+ T cells were cultured for 5 days at 37°C in 96-well plates at 5 × 104 cells/well in 0.2 ml complete medium. IFN-α and the superantigen SEB from Staphylococcus aureus (Sigma-Aldrich) were added at different concentrations as indicated. Proliferation was measured by thymidine incorporation as for B cells.
B cell stimulation of T cells
After 4 days of culture in the presence of IFN-α (1000 U/ml), CpG C (2395; 5 μg/ml; Coley Pharmaceutical Group), or media only, total CD19+ B cells were exposed to superantigens SEB or TSST-1 at the indicated concentrations for 1 h. After exposure to the superantigens, the B cells were washed thoroughly, and autologous T cells were added at a B cell:T cell ratio of 1:10 and incubated for 1 h at 37°C, followed by an additional 16 h in the presence of the secretion inhibitor Brefeldin A (10 μg/ml; Sigma-Aldrich). Responding T cells were detected by analysis of intracellular cytokine production by flow cytometry as described previously [28, 29]. Briefly, the cells were washed and surface-stained for 20 min with antibodies to CD3, CD4, and CD8 (BD PharMingen) and thereafter, washed, fixed, and permeabilized for 10 min using a Cytofix/Cytoperm kit (BD PharMingen). The cells were washed twice and stained intracellularly with IFN-γ (BD PharMingen)-, TNF-α (BD PharMingen)-, and IL-2 (Caltag Laboratories, Burlingame, CA, USA)-specific antibodies. The cells were analyzed on a FACSCalibur flow cytometer (BD PharMingen). Data were analyzed by FlowJo software (Tree Star Inc.).
Statistical analyses
Statistical analyses were performed using Wilcoxon′s paired t-test or Mann-Whitney′s unpaired t-test with GraphPad Prism software (*P<0.05; **P<0.01).
RESULTS
PDCs enhance naïve B cell proliferation induced by CD4+ T cell help and BCR cross-linking
In this study, we investigated whether human PDCs support T cell-dependent B cell responses. Highly enriched human naïve B cells were sorted from blood based on their expression of CD19 and lack of CD27. Isolated cells expressed CD20 and surface IgD but not IgG (Fig. 1A). Freshly sorted B cells were labeled with CFSE and used immediately for the experiments. The cells were incubated with anti-Ig (anti-IgM/G/A) to cross-link the BCR as a substitute for antigen interaction as described [15]. CD4+ T cell help was provided by the addition of rIL-2 and γ-irradiated CD4+ T cells pulsed with the superantigen TSST-1 that links the Vβ region of the TCR to the α-chain of class II MHC molecules and thereby, facilitates the contact between the T cells and B cells [30]. Although TSST-1-pulsed CD4+ T cells induced B cell activation, TSST-1 alone did not stimulate B cells as reported earlier (ref. [31] and data not shown). We first evaluated whether PDCs had an ability to alter B cell proliferation under these culture conditions. PDCs were isolated from blood and exposed to the TLR7/8-binding imidazoquinoline compound 3M-0012 or the TLR9 agonist CpG A, which are known to stimulate PDC efficiently [15, 28, 32]. Supernatants were harvested after 24 h and added to the B cell cultures at a 1:100 dilution. After 6 days of culture, B cell proliferation was assessed by flow cytometry. BCR cross-linking alone led to a slight division of naive B cells (Fig. 1B and C), and the addition of supernatants from stimulated PDCs resulted in a small but noticeable enhancement of proliferation. B cells stimulated by BCR ligation plus T cell help resulted in a markedly higher level of B cell proliferation. This more pronounced B cell proliferation was also often enhanced further when supernatants from stimulated PDCs were added, although when compiling data from several donors, this increase did not reach statistical significance (Fig. 1C). No effects were detected if the B cells were cultured in the presence of supernatants from unstimulated PDCs (data not shown). As IFN-α produced by PDCs has been shown to be an important modulator of B cell responses during certain conditions [3, 14, 15], we also performed these experiments using addition of human IFN-α. IFN-α-mediated enhancement of B cell proliferation in response to anti-Ig alone was larger (mean, 3.9-fold increase) than that observed with the more robust T cell help-driven B cell proliferation (mean, 1.3-fold increase; Fig. 1D). From these initial observations, we concluded that IFN-α produced by PDCs can support proliferation of naïve B cells, although this effect can be diluted by strong T cell help-induced, proliferative responses. CpG A is a potent activator of PDCs but poor at stimulating B cells, and B cell responses to CpG A are not altered drastically by supernatants from stimulated PDCs [15]. In contrast, B cell responses to TLR7/8 ligation are strongly enhanced in the presence of supernatants from stimulated PDC or IFN-α, as this leads to up-regulation of TLR7 in B cells [3, 14, 15]. Nevertheless, it is unlikely that the residual TLR7/8 ligand transferred with the PDC supernatant was responsible for the observed increase in B cell proliferation, as no proliferation was detected in response to the TLR7/8 ligand at the concentration relevant to the PDC supernatant dilution (0.01 μg/ml) or importantly, upon addition of IFN-α (Fig. 1E).
PDCs support differentiation of naive B cells and IgM production
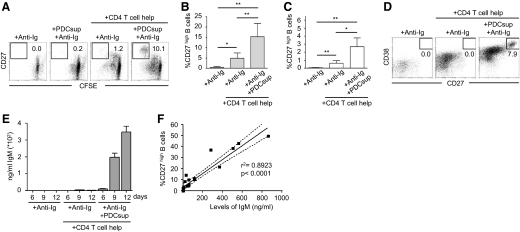
In addition to proliferation, differentiation of naïve B cells into CD27high cells, a marker associated with plasma cell formation [33, 34], was analyzed. No differentiation was observed with BCR ligation alone, and there was only a modest increase in the CD27high population in the presence of BCR ligation/T cell help (Fig. 2A). However, the addition of the PDC supernatant significantly increased the frequency of CD27high B cells under the latter condition. Supernatants from TLR7/8 ligand-stimulated PDCs (Fig. 2B) or CpG A-stimulated PDCs (Fig. 2C) induced an increase in differentiation to CD27high B cells. Although the frequencies of B cells that had differentiated to a CD27high phenotype varied considerably between donors, B cells cultured in the presence of the PDC supernatant consistently showed a higher proportion of differentiated cells compared with the donor-matched control cultures. In addition to CD27 up-regulation, we found that CD38, another marker associated with a plasmablast phenotype [34], was up-regulated when the B cells were stimulated in the presence of supernatants from activated PDCs (Fig. 2D).
Figure 2. PDCs enhance naive B cell differentiation into IgM-secreting plasmablasts.
(A) Naïve B cells were stimulated with anti-Ig and PDC supernatants in the presence or absence of T cell help. Differentiation to CD27high plasmablasts was measured by flow cytometry. Gate numbers indicate percentage of CFSE low B cells that show high expression of CD27 after exclusion of dead cells and T cells. (B and C) Graphs show compiled data on the frequencies of CD27high B cells after culture with supernatants from TLR7/8 ligand-stimulated PDCs (B; n=6) or CpG A-stimulated PDCs (C; n=8). Data indicate mean ± sd. (D) Anti-Ig and PDC supernatants were added to naïve B cells in the presence or absence of T cell help. Differentiation to express high levels of CD27 and CD38 was assessed by flow cytometry. Gate numbers indicate percentage of cells that show high expression of CD27 and CD38. (E) Naïve B cells were cultured in the presence or absence of T cell help for 6, 9, or 12 days in parallel cultures with addition of anti-Ig and supernatants from TLR-stimulated PDCs. IgM production was measured in the culture supernatants using ELISA. Data from one representative donor are shown. (F) IgM levels from different stimulations were compared with the frequencies of CD27high B cells in the respective samples at Day 6. The 95% confidence interval is shown; P < 0.0001.
Next, we examined whether the PDC-mediated enhancement of naïve B cell proliferation and differentiation translated into increased antibody production. Supernatants from B cell cultures stimulated with the various conditions were harvested after 6, 9, and 12 days of culture and analyzed for IgM production by ELISA. BCR ligation/T cell help alone induced low or undetectable levels of IgM secretion from the naïve B cells. In contrast, when supernatant from the CpG A (Fig. 2E)- or TLR7/8 ligand (data not shown)-stimulated PDC was added, the secretion of IgM was markedly higher. As expected, levels of accumulated antibodies were highest at the later time-points. The earliest time-point at which IgM production was detected was at Day 6, and the level of production increased over time. In addition, a strong positive correlation (r2=0.8923) was found between the frequencies of differentiated CD27high B cells at 6 days of culture and the levels of IgM secreted from the same cultures (Fig. 2F; P<0.0001). In summary, TLR-stimulated PDCs promoted naïve B cell differentiation to CD27high cells, which translated into augmented IgM production.
PDCs but not MDCs support B cell responses induced by CD4+ T cell help
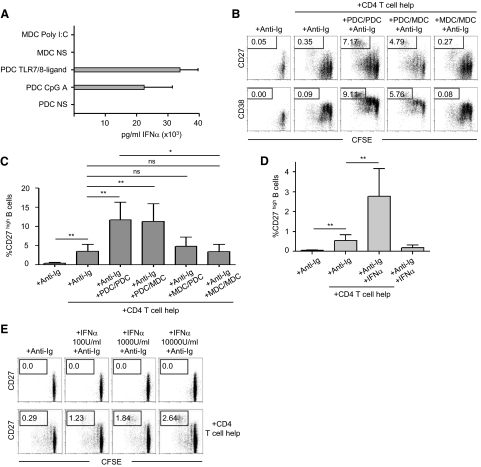
We next investigated whether MDCs, which are phenotypically and functionally distinct from PDCs, also have the capacity to support B cell responses, alone or together with PDCs. In vitro-generated precursor or MDDCs were the first DCs shown to have the capacity to support humoral responses induced by CD4+ T cell help [1, 2, 11]. This effect was shown to be related to the production of IL-6 and IL-12 by the DCs upon CD40L stimulation [11]. More recent studies have shown that IFN-α produced by primary PDCs is a superior modulator of B cell responses [3, 14, 15]. Other DC-mediated factors shown to support B cell responses are IL-10 and BAFF [11, 12]. We have, in earlier studies, found that primary MDCs can produce IL-6, IL-10, IL-12, and BAFF [15, 28]. We first verified the IFN-α production pattern by PDCs and MDCs. As found previously, TLR7/8 ligand- or CpG A-stimulated PDCs produced high levels of IFN-α, whereas low or undetectable levels were measured from donor-matched Poly I:C-stimulated MDCs (Fig. 3A) [15, 28]. The TLR ligands were chosen according to the expression of their respective receptors on the DC subsets [35, 36]. Supernatants from the stimulated DC subsets were harvested after 24 h and added at a 1:100 dilution to B cells stimulated with T cell help and BCR ligation for 3 days. The cells were then washed and cultured for three more days with fresh media and DC supernatants. Proliferation and CD27high and CD38high differentiation induced by these sequential stimulations were measured by flow cytometry. We found that supernatants from activated MDCs had no or very small augmentative effects on B cell function (Fig. 3B and C). Furthermore, sequential addition of PDC supernatants followed by MDC supernatants did not increase the B cell responses above that induced by the PDC supernatants alone. Similarly, MDC supernatants followed by PDC supernatants or simultaneous addition of MDC and PDC supernatants did not enhance the effect induced by the PDC supernatant alone (data not shown) nor did MDC supernatants show any effect when added at a lower dilution (1:4; data not shown). In summary, in large contrast to PDCs, MDCs did not enhance the naïve B cell responses induced by CD4+ T cell help/BCR cross-linking.
Figure 3. PDCs, by their production of IFN-α, have a unique capacity to support B cell differentiation.
(A) TLR ligand stimulation induces IFN-α production, as measured by ELISA, in PDCs but not MDCs (n≥6). (B) Supernatants from PDCs or MDCs were sequentially added to CFSE-stained and anti-Ig-stimulated, naïve B cells. One supernatant was first added for 3 days and then replaced for an additional 3 days in the presence of CD4+ T cell help, and then the cells were analyzed by flow cytometry for proliferation and differentiation to CD27high CD38high plasmablasts. Numbers indicate percentage of cells expressing high levels of CD27 or CD38. (C) Compiled data for CD27 are shown (mean±sd; n=9). (D) Human IFN-α was added to anti-Ig-stimulated, naïve B cells in the presence of T cell help for 6 days. Frequencies of CD27high plasmablasts compiled from nine separate donors are shown (mean±sd). (E) Human IFN-α was added at different concentrations to anti-Ig-stimulated, naïve B cells in the presence or absence of T cell help for 6 days. Numbers indicate percentage of cells expressing high levels of CD27.
Addition of IFN-α (1000 U/ml, a dose equivalent to the levels in the PDC supernatants) to the B cell cultures showed a comparable pattern of enhancement of B cell differentiation to a CD27high phenotype to the PDC supernatants (Fig. 3D). In addition, increasing the dose of IFN-α resulted in a larger portion of the B cells differentiating to a CD27high phenotype (Fig. 3E). Thus, we concluded from these data that PDCs, via their production of IFN-α, have a unique ability to support naïve B cell differentiation in the presence of T cell help and BCR ligation.
IFN-α exposure does not support B cell survival in vitro
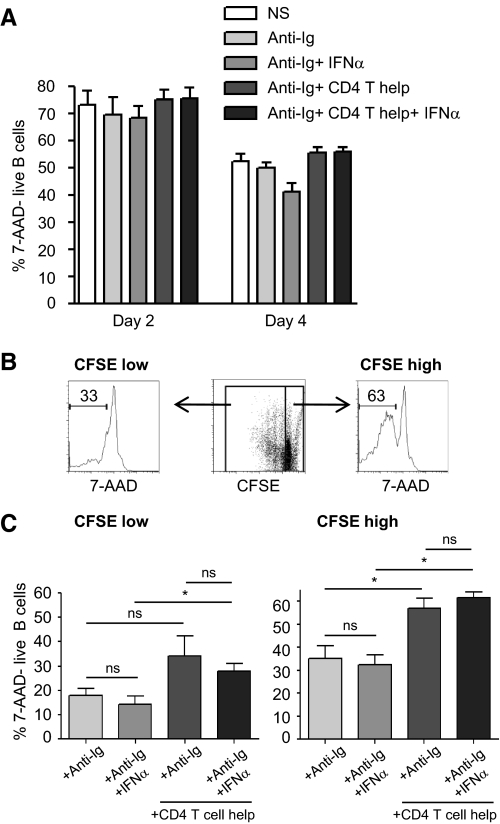
We next addressed whether increased B cell survival by IFN-α exposure contributed to the observed increase in proliferation and differentiation. B cell viability under the various culture conditions was monitored by staining with 7-AAD at Days 2, 4, and 6 of culture. As expected, overall B cell survival was found to decline over time (Fig. 4A). No statistical difference in total B cell viability was found between any of the culture conditions used, although the B cells cultured in the presence of T cell help tended to show a slightly higher viability. However, analyzing the undivided B cells per se, based on their high CFSE stain (Fig. 4B), showed that these cells exhibited increased viability when cocultured with T cell help (Fig. 4C). Notably, no increase in cell survival was found in any of the culture conditions with the addition of IFN-α. There was a proportionally higher number of dead cells among the dividing population than in the undivided population (Fig. 4C). This may result from division leading to exhaustion and/or terminal differentiation of the B cells under these in vitro conditions. Again, no improvement in cell survival was found with IFN-α (Fig. 4C). In conclusion, using 7-AAD to assay cell viability, we did not find any evidence that increased cell survival is a central contributing factor for the IFN-α-mediated enhancement of B cell proliferation or differentiation observed.
Figure 4. IFN-α does not enhance naïve B cell viability.
CFSE-labeled, naïve B cells were stimulated with anti-Ig and IFN-α in the presence or absence of T cell help. (A) Viability was assessed at Days 2 and 4. The graph shows the percentage of 7-AAD-live B cells. Data are compiled from three donors. (B) Gating strategy for the separation of proliferating and nonproliferating B cells based on CFSE expression level. (C) Viability of proliferating and nonproliferating cells was assessed as above at Day 4 or Day 6 (n=6). Data indicate mean ± sd.
PDCs and IFN-α stimulate up-regulation of CD86 on naïve B cells
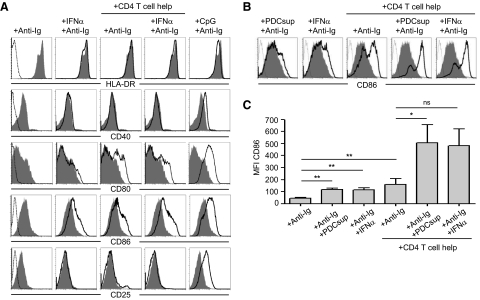
To find an explanation for the increased B cell responsiveness to T cell help in this system, we examined the levels of MHC class II and the costimulatory molecules CD40, CD80, and CD86 on B cells to determine if the presence of PDC supernatants or IFN-α increased their surface expression. The development of a potent and specific humoral immune response to many foreign antigens is largely mediated by CD4+ T cell-dependent B cell responses. Upon antigen encounter, BCR-antigen complexes are internalized, processed into peptide fragments, and presented to CD4+ T cells on MHC class II molecules [37]. In addition, several costimulatory receptors on B cells interact with cognate ligands on T cells [38]. Therefore, naïve B cells were stimulated in the presence or absence of IFN-α in addition to BCR ligation/T cell help and analyzed at various time-points (24 h–4 days). As a positive control for direct activation of B cells, CpG C was used. In addition to MHC class II and the costimulatory molecules, expression of the IL-2Rα chain CD25 was measured. At all time-points, the levels of MHC class II represented by HLA-DR were high and did not further increase following any of the stimulations (Fig. 5A). The expression of CD40 as well as CD25 was up-regulated only upon stimulation with CpG C (Fig. 5A). In contrast, B cells cultured with T cell help showed an up-regulation of CD80 and CD86, and CD86 expression was increased further with the addition of IFN-α. IFN-α itself also led to up-regulation of CD86 but not of CD40, CD80, CD25, or MHC class II. The increased expression of CD86 induced by IFN-α was also confirmed using PDC supernatants, which showed a similar pattern (Fig. 5B and C). From these data, we speculated that increased CD86 surface expression may render B cells more responsive to T cell help, potentiating the enhancement of B cell responses in the presence of IFN-α. As there was no change in CD25 expression upon IFN-α treatment, the observed effects on B cell activation are likely not influenced by increased responsiveness to IL-2.
Figure 5. PDCs and IFN-α stimulate up-regulation of the costimulatory molecule CD86 on B cells.
(A) Naïve B cells were exposed to IFN-α (1000 U/ml) or CpG C in the presence or absence of T cell help for 4 days and analyzed for up-regulation of surface expression of the molecules HLA-DR, CD40, CD80, CD86, and CD25 by flow cytometry. (B) CD86 expression was assessed upon exposure to supernatant from TLR-activated PDCs or IFN-α in the presence or absence of T cell help. An unstained control (dotted line) and B cells cultured in media with BCR ligation only (filled gray) are overlaid with B cells stimulated further (black line) as indicated. (C) Mean fluorescence intensity (MFI) values of CD86 expression on B cells cultured with the various conditions are depicted (n≥3). Data indicate mean ± sd.
IFN-α supports B cell interactions with T cells but decreases T cell proliferation
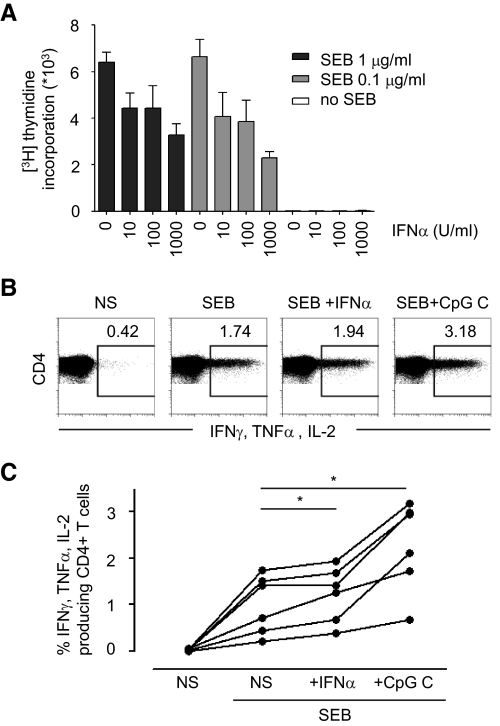
As well as receiving help from T cells to promote antibody responses, B cells also have the ability to present antigen and thereby activate T cells. Their costimulatory molecules CD80/CD86 bind to CD28 on T cells, interactions that are critical not only for the delivery of T cell help to B cells but also vice versa for the activation of CD4+ T cells [37, 39]. To understand whether the antigen-presenting function of B cells was increased in the presence of IFN-α, we examined the effect of IFN-α on B cell-induced stimulation of CD4+ Th cells. We first examined how CD4+ T cells in the absence of B cells were affected by IFN-α treatment. We found that CD4+ T cells, purified as described above but not γ-irradiated, responded with strong proliferation when stimulated by the superantigen SEB, as measured by thymidine incorporation at Day 5. This proliferation was suppressed significantly in the presence of IFN-α (Fig. 6A), consistent with earlier studies [25, 26]. The suppression of T cell proliferation precluded studies aimed at understanding the effect of IFN-α on B–T cell interactions in long-term cocultures, and thus, we assessed short-term cocultures. B cells were therefore first treated with IFN-α for a sufficient time to allow up-regulation of CD86 (4 days) and then pulsed with superantigen. For these studies, the superantigens SEB and TSST-1 were used as a substitute for protein antigens, as antigen uptake functions decrease with phenotypic differentiation, and the B cells could therefore not first be exposed to IFN-α and then provided with antigens for uptake and processing (data not shown). As described above, these superantigens bind directly to MHC class II molecules and do not require conventional loading such as intracellularly processed antigens do. B cells were cultured in media or stimulated with IFN-α or CpG C for 4 days before being pulsed with SEB or TSST-1 for 1 h and then washed and cocultured with autologous CD4+ T cells. Intracellular cytokine production in responding T cells was measured after 16 h. B cells pulsed with suboptimal concentrations of SEB (0.1 μg/ml) or TSST-1 (0.5 ng/ml) stimulated a readily detectable T cell response, resulting in production of one or several of the cytokines IL-2, IFN-γ, and TNF-α (Fig. 6B, and data not shown). Preincubation of B cells with IFN-α resulted in a consistently modest but significant increase in the number of cytokine-producing T cells as compared with B cells pulsed with SEB or TSST-1 alone (Fig. 6B and C, and data not shown). As expected, preincubation with CpG C led to a markedly enhanced capacity of the B cells to activate cytokine production by CD4+ T cells in response to SEB (Fig. 6B and C) and TSST-1 (data not shown). In summary, the modest, albeit notable increase in T cell responses that we observed may reflect an improved capacity of IFN-α-exposed B cells to interact with and induce superantigen-driven activation of CD4+ T cells. This may in return augment the activation and differentiation of B cells.
Figure 6. IFN-α affects T cell activation.
(A) CD4+ T cells were stimulated with SEB and IFN-α as indicated. Proliferation was measured by thymidine incorporation. (B) Naive B cells were cultured in the presence or absence of IFN-α or CpG C for 4 days, pulsed with SEB for 1 h before being added at a ratio of 1:10 to autologous CD4+ T cells. Production of effector cytokines by CD4+ T cells was measured by intracellular flow cytometry. (C) Compiled data from six donors are shown.
DISCUSSION
In the current study, we have shown that supernatants from TLR-stimulated PDCs, but not MDCs, promote T cell help-dependent activation of naïve B cells to proliferate and differentiate into IgM-secreting cells in vitro. The observed effect could be reproduced using purified IFN-α in place of supernatants from stimulated PDCs, indicating that IFN-α is likely central for inducing these effects. We further investigated whether the interactions between B cells and CD4+ Th cells were enhanced by IFN-α exposure and found that the costimulatory molecule CD86 was up-regulated on B cells after IFN-α treatment and that the B cells had a modest but consistently increased ability to activate T cells in response to superantigens. In conclusion, these data suggest that PDCs, through their production of IFN-α, enhance interactions between B cells and T cells that facilitate B cell proliferation and differentiation to antibody-secreting cells.
In the generation of an antibody response, B cells need to undergo affinity maturation processes that generally, in addition to BCR engagement, include CD4+ Th cell interactions. These interactions take place in the outer T cell zone of secondary lymphoid organs, and B cells subsequently migrate into the follicles where they form germinal centers or alternatively, to extrafollicular sites where they differentiate rapidly into short-lived, Ig-secreting cells [40, 41]. In this study, PDCs but not MDCs were found to enhance the activation and differentiation of naïve B cells in vitro in response to BCR ligation and Th cell interaction. Interestingly, although PDCs are much more sparse than MDCs in peripheral blood, they are more common in the LNs and spleen [42] and located at sites in proximity to HEVs and T cell-rich areas through which B cells migrate [43]. Thus, there is a high probability that PDCs and B cells meet during the development of humoral responses. In fact, B cell–DC encounters were shown recently to take place in proximity to HEVs in LNs and to lead to activation of the B cells responding to antigens presented by DCs [8].
Our data indicated that IFN-α, produced at high levels by PDCs, mediated the enhancement of B cell functions observed in this study. IFN-α is a highly pleiotropic innate cytokine and central in inducing immune responses to microbial infections. IFN-α is also documented to be critical in mediating the effects of certain vaccine adjuvants and gene-delivery vectors [19, 44]. An expanding body of literature indicates that IFN-α contributes to the shaping of adaptive immune responses [45–47] and that direct type I IFN-mediated B cell activation significantly affects the quality and magnitude of the antiviral humoral responses in vivo [17–20, 48]. As reported previously by us and others [3, 14, 15], human PDCs, via their production of IFN-α, significantly enhance B cell responses to TLR7/8 ligands. This effect may, at least in part, be explained by the up-regulation of the cognate TLR and/or the signaling adaptor molecule MyD88 in B cells by IFN-α [14, 16]. The increased responsiveness of naïve B cells to Th cell signals stimulated by PDCs and IFN-α reported here likely involves a distinctly different mechanism. In previous studies, we found that whereas PDCs produced high levels of IFN-α, MDCs produced IL-6, IL-10, and BAFF [15], cytokines that are all associated with conditioning B cells. Therefore, although MDCs did not enhance B cell responses in the in vitro system described here, they may still play a role in the activation and/or survival of B cells in vivo [49]. The considerably higher levels of cytokines, which in vitro-generated MDDCs produce in comparison with primary MDCs [50], may also explain why the latter cells did not show any effect here.
We found that naïve B cells exposed to PDC supernatant or IFN-α did not exhibit an increased expression of the classical receptors involved in the interaction with T cells such as MHC class II or CD40 but had elevated expression of the costimulatory molecule CD86. These findings do not exclude a possible enhancement in the signaling capacity of all of these molecules by IFN-α exposure or that any differences in expression levels are too subtle to detect using the current conditions and methods. Nevertheless, these data are consistent with previous reports of a type I IFN-dependent increase in CD86 expression on B cells in vitro [15, 21] and in vivo [17, 18]. In addition, ligation of CD86 on B cells to CD28 expressed on T cells has been reported to give a stimulatory signal to B cells leading to enhanced proliferation and IgG secretion [51–54]. Thus, CD86 triggering affects B cell function, which is supported by the fact that CD86 has signaling-associated structural features, such as a cytoplasmic tail containing three potential sites for PKC phosphorylation [39]. Consequently, regulation of CD86 expression and in turn, increased responsiveness of B cells to Th cell interactions may be one mechanism accounting for the observed PDC/IFN-α-mediated enhancement of B cell responses.
Although CD40-mediated T cell help via surface CD40L (CD154) is the most commonly described means of Th cell-induced activation of B cells, we did not observe modulation of CD40 expression on naïve B cells with the PDC supernatant or IFN-α exposure. However, signaling through CD40 could be affected and may be potentiated by IFN-α at some other level. MHC class II expression, on the other hand, was reported to only be up-regulated upon exposure to IFN-γ but not to type I IFNs [55]. Also, we did not see consistent up-regulation of the CD80 molecule upon IFN-α exposure, which might reflect the different requirements of CD80 expression as compared with CD86 [52]. It is unlikely that secreted cytokines from the Th cells play a role in our findings, as the T cells were irradiated.
The central observation in this study was that PDC supernatants or IFN-α augmented B cell differentiation and IgM secretion in response to T cell help. Although IFN-α has been reported to improve cell survival [21–24], the effects reported here are unlikely to relate to better B cell survival, as no significantly increased viability was detected with IFN-α exposure. Further, proliferation of B cells has been shown earlier to be required for the generation of Ig-producing cells [56] and cell division itself to play an important role in the regulation of differentiation of naïve and memory B cells [57]. Here, although IFN-α tended to enhance B cell proliferation in response to anti-Ig alone or anti-Ig plus T cell help, the strongest and significant effects by IFN-α exposure in this culture system were the enhanced differentiation of naïve B cells to express high levels of CD27 and CD38, which translated into higher secretion of IgM. Specifically, this may involve mechanisms where IFN-α exposure renders B cells more responsive to T cell help, which could involve increased costimulation via CD86. This prompted our subsequent interest in whether B cells exposed to IFN-α also had an increased ability to activate Th cells. Indeed, B cells preincubated with IFN-α prior to superantigens showed a small but consistently increased capacity to stimulate IL-2, IFN-γ, and/or TNF-α production in T cells. As the CpG C-stimulated B cells with higher expression of costimulatory molecules, including CD86, proved that this could enhance their ability to activate T cells responding to superantigen, it is possible that the effect by IFN-α exposure could translate into a relevant difference under physiological conditions in vivo. Although there are several prior in vivo studies in mice that have shown that IFN-α can significantly enhance B cell responses where Th cell processes are involved [17–20, 48], our findings remain to be confirmed in humans in vivo.
Taken together, the data from this study support the conclusion that PDCs have the ability to enhance B cell activation under T cell help-dependent conditions and that IFN-α directly renders the B cells more receptive to T cell help. The mechanisms responsible for the observed effects may include the up-regulation of the costimulatory molecule CD86, which could also account for the improved ability of the B cells to in turn activate CD4+ T cells. Both of these effects could shape the magnitude and quality of ensuing B cell responses. Studies of the stimulation requirements of B cells for the induction of potent antibody responses and how such responses can be regulated by DC and IFN-α will increase our understanding of the complexity of immune responses to various infections and provide important information for the design of new vaccine and immunomodulatory treatment formulations.
ACKNOWLEDGMENTS
This study was supported by the Swedish Council for Research (Vetenskapsrådet), the Swedish International Development Agency (Sida) (G.B.K.H and K.L. funding recipients), the Jeansson's Foundation, the Swedish Society for Medicine (K.L. funding recipient), and the Sven Gard Foundation (C.G., K.J.S., and W.C.A. funding recipients). W.C.A. and C.S. are recipients of graduate student scholarships from Karolinska Institutet. The authors are grateful to technical help by Andrea Hildebrandt and the Department of Transfusion Medicine, Karolinska University Hospital, for assistance with leukapheresis and elutriations.
SEE CORRESPONDING EDITORIAL ON PAGE 805
- 7-AAD
- 7-amino-actinomycin D
- BAFF
- B cell-activating factor
- CD40L
- CD40 ligand
- CpG A/C
- CpG oligodeoxynucleotide class A/C
- HEV
- high-endothelial venule
- MDC
- myeloid DC
- MDDC
- monocyte-derived DC
- OPD
- o-phenylenediamine dihydrochloride
- PDC
- plasmacytoid DC
- Poly I:C
- polyinosinic:polycytidiylic acid
- SEB
- Staphylococcal enterotoxin B
- TSST-1
- toxic shock syndrome toxin 1
AUTHORSHIP
C.G., K.J.S., and K.L. designed and performed research, interpreted data, and wrote the manuscript. I.D. designed research and interpreted data. W.C.A. and C.S. performed research and interpreted data. A.S-S., R.A.S., and G.B.K.H. designed research, interpreted data, and wrote the manuscript.
REFERENCES
- 1. Dubois B., Bridon J. M., Fayette J., Barthelemy C., Banchereau J., Caux C., Briere F. (1999) Dendritic cells directly modulate B cell growth and differentiation. J. Leukoc. Biol. 66, 224–230 [DOI] [PubMed] [Google Scholar]
- 2. Dubois B., Vanbervliet B., Fayette J., Massacrier C., Van Kooten C., Briere F., Banchereau J., Caux C. (1997) Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J. Exp. Med. 185, 941–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jego G., Palucka A. K., Blanck J. P., Chalouni C., Pascual V., Banchereau J. (2003) Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 19, 225–234 [DOI] [PubMed] [Google Scholar]
- 4. Poeck H., Wagner M., Battiany J., Rothenfusser S., Wellisch D., Hornung V., Jahrsdorfer B., Giese T., Endres S., Hartmann G. (2004) Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood 103, 3058–3064 [DOI] [PubMed] [Google Scholar]
- 5. Bergtold A., Desai D. D., Gavhane A., Clynes R. (2005) Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity 23, 503–514 [DOI] [PubMed] [Google Scholar]
- 6. Ding C., Cai Y., Marroquin J., Ildstad S. T., Yan J. (2009) Plasmacytoid dendritic cells regulate autoreactive B cell activation via soluble factors and in a cell-to-cell contact manner. J. Immunol. 183, 7140–7149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. MacPherson G., Kushnir N., Wykes M. (1999) Dendritic cells, B cells and the regulation of antibody synthesis. Immunol. Rev. 172, 325–334 [DOI] [PubMed] [Google Scholar]
- 8. Qi H., Egen J. G., Huang A. Y., Germain R. N. (2006) Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science 312, 1672–1676 [DOI] [PubMed] [Google Scholar]
- 9. Shaw J., Wang Y. H., Ito T., Arima K., Liu Y. J. (2010) Plasmacytoid dendritic cells regulate B-cell growth and differentiation via CD70. Blood 115, 3051–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wykes M., Pombo A., Jenkins C., MacPherson G. G. (1998) Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J. Immunol. 161, 1313–1319 [PubMed] [Google Scholar]
- 11. Dubois B., Massacrier C., Vanbervliet B., Fayette J., Briere F., Banchereau J., Caux C. (1998) Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J. Immunol. 161, 2223–2231 [PubMed] [Google Scholar]
- 12. Litinskiy M. B., Nardelli B., Hilbert D. M., He B., Schaffer A., Casali P., Cerutti A. (2002) DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3, 822–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siegal F. P., Kadowaki N., Shodell M., Fitzgerald-Bocarsly P. A., Shah K., Ho S., Antonenko S., Liu Y. J. (1999) The nature of the principal type 1 interferon-producing cells in human blood. Science 284, 1835–1837 [DOI] [PubMed] [Google Scholar]
- 14. Bekeredjian-Ding I. B., Wagner M., Hornung V., Giese T., Schnurr M., Endres S., Hartmann G. (2005) Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J. Immunol. 174, 4043–4050 [DOI] [PubMed] [Google Scholar]
- 15. Douagi I., Gujer C., Sundling C., Adams W. C., Smed-Sorensen A., Seder R. A., Karlsson Hedestam G. B., Lore K. (2009) Human B cell responses to TLR ligands are differentially modulated by myeloid and plasmacytoid dendritic cells. J. Immunol. 182, 1991–2001 [DOI] [PubMed] [Google Scholar]
- 16. Hemmi H., Kaisho T., Takeuchi O., Sato S., Sanjo H., Hoshino K., Horiuchi T., Tomizawa H., Takeda K., Akira S. (2002) Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3, 196–200 [DOI] [PubMed] [Google Scholar]
- 17. Coro E. S., Chang W. L., Baumgarth N. (2006) Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J. Immunol. 176, 4343–4351 [DOI] [PubMed] [Google Scholar]
- 18. Fink K., Lang K. S., Manjarrez-Orduno N., Junt T., Senn B. M., Holdener M., Akira S., Zinkernagel R. M., Hengartner H. (2006) Early type I interferon-mediated signals on B cells specifically enhance antiviral humoral responses. Eur. J. Immunol. 36, 2094–2105 [DOI] [PubMed] [Google Scholar]
- 19. Hidmark A. S., Nordstrom E. K., Dosenovic P., Forsell M. N., Liljestrom P., Karlsson Hedestam G. B. (2006) Humoral responses against coimmunized protein antigen but not against αvirus-encoded antigens require α/β interferon signaling. J. Virol. 80, 7100–7110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Le Bon A., Thompson C., Kamphuis E., Durand V., Rossmann C., Kalinke U., Tough D. F. (2006) Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J. Immunol. 176, 2074–2078 [DOI] [PubMed] [Google Scholar]
- 21. Braun D., Caramalho I., Demengeot J. (2002) IFN-α/β enhances BCR-dependent B cell responses. Int. Immunol. 14, 411–419 [DOI] [PubMed] [Google Scholar]
- 22. Marrack P., Kappler J., Mitchell T. (1999) Type I interferons keep activated T cells alive. J. Exp. Med. 189, 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruuth K., Carlsson L., Hallberg B., Lundgren E. (2001) Interferon-α promotes survival of human primary B-lymphocytes via phosphatidylinositol 3-kinase. Biochem. Biophys. Res. Commun. 284, 583–586 [DOI] [PubMed] [Google Scholar]
- 24. Yang C. H., Murti A., Pfeffer S. R., Basu L., Kim J. G., Pfeffer L. M. (2000) IFNα/β promotes cell survival by activating NF-κ B. Proc. Natl. Acad. Sci. USA 97, 13631–13636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin Q., Dong C., Cooper M. D. (1998) Impairment of T and B cell development by treatment with a type I interferon. J. Exp. Med. 187, 79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petricoin E. F., III, Ito S., Williams B. L., Audet S., Stancato L. F., Gamero A., Clouse K., Grimley P., Weiss A., Beeler J., Finbloom D. S., Shores E. W., Abraham R., Larner A. C. (1997) Antiproliferative action of interferon-α requires components of T-cell-receptor signaling. Nature 390, 629–632 [DOI] [PubMed] [Google Scholar]
- 27. Adams W. C., Bond E., Havenga M. J., Holterman L., Goudsmit J., Karlsson Hedestam G. B., Koup R. A., Lore K. (2009) Adenovirus serotype 5 infects human dendritic cells via a coxsackievirus-adenovirus receptor-independent receptor pathway mediated by lactoferrin and DC-SIGN. J. Gen. Virol. 90, 1600–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loré K., Betts M. R., Brenchley J. M., Kuruppu J., Khojasteh S., Perfetto S., Roederer M., Seder R. A., Koup R. A. (2003) Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J. Immunol. 171, 4320–4328 [DOI] [PubMed] [Google Scholar]
- 29. Loré K., Smed-Sorensen A., Vasudevan J., Mascola J. R., Koup R. A. (2005) Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J. Exp. Med. 201, 2023–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCormick J. K., Yarwood J. M., Schlievert P. M. (2001) Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55, 77–104 [DOI] [PubMed] [Google Scholar]
- 31. Mourad W., Scholl P., Diaz A., Geha R., Chatila T. (1989) The staphylococcal toxic shock syndrome toxin 1 triggers B cell proliferation and differentiation via major histocompatibility complex-unrestricted cognate T/B cell interaction. J. Exp. Med. 170, 2011–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ito T., Amakawa R., Kaisho T., Hemmi H., Tajima K., Uehira K., Ozaki Y., Tomizawa H., Akira S., Fukuhara S. (2002) Interferon-α and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J. Exp. Med. 195, 1507–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Avery D. T., Ellyard J. I., Mackay F., Corcoran L. M., Hodgkin P. D., Tangye S. G. (2005) Increased expression of CD27 on activated human memory B cells correlates with their commitment to the plasma cell lineage. J. Immunol. 174, 4034–4042 [DOI] [PubMed] [Google Scholar]
- 34. Huggins J., Pellegrin T., Felgar R. E., Wei C., Brown M., Zheng B., Milner E. C., Bernstein S. H., Sanz I., Zand M. S. (2007) CpG DNA activation and plasma-cell differentiation of CD27– naive human B cells. Blood 109, 1611–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hornung V., Rothenfusser S., Britsch S., Krug A., Jahrsdorfer B., Giese T., Endres S., Hartmann G. (2002) Quantitative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168, 4531–4537 [DOI] [PubMed] [Google Scholar]
- 36. Krug A., Towarowski A., Britsch S., Rothenfusser S., Hornung V., Bals R., Giese T., Engelmann H., Endres S., Krieg A. M., Hartmann G. (2001) Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol. 31, 3026–3037 [DOI] [PubMed] [Google Scholar]
- 37. Lanzavecchia A. (1985) Antigen-specific interaction between T and B cells. Nature 314, 537–539 [DOI] [PubMed] [Google Scholar]
- 38. Mills D. M., Cambier J. C. (2003) B lymphocyte activation during cognate interactions with CD4+ T lymphocytes: molecular dynamics and immunologic consequences. Semin. Immunol. 15, 325–329 [DOI] [PubMed] [Google Scholar]
- 39. Lenschow D. J., Walunas T. L., Bluestone J. A. (1996) CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14, 233–258 [DOI] [PubMed] [Google Scholar]
- 40. MacLennan I. C., Gulbranson-Judge A., Toellner K. M., Casamayor-Palleja M., Chan E., Sze D. M., Luther S. A., Orbea H. A. (1997) The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol. Rev. 156, 53–66 [DOI] [PubMed] [Google Scholar]
- 41. McHeyzer-Williams L. J., Malherbe L. P., McHeyzer-Williams M. G. (2006) Checkpoints in memory B-cell evolution. Immunol. Rev. 211, 255–268 [DOI] [PubMed] [Google Scholar]
- 42. Loré K. (2004) Isolation and immunophenotyping of human and rhesus macaque dendritic cells. Methods Cell Biol. 75, 623–642 [DOI] [PubMed] [Google Scholar]
- 43. Colonna M., Trinchieri G., Liu Y. J. (2004) Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5, 1219–1226 [DOI] [PubMed] [Google Scholar]
- 44. Longhi M. P., Trumpfheller C., Idoyaga J., Caskey M., Matos I., Kluger C., Salazar A. M., Colonna M., Steinman R. M. (2009) Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J. Exp. Med. 206, 1589–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Biron C. A. (1998) Role of early cytokines, including α and β interferons (IFN-α/β), in innate and adaptive immune responses to viral infections. Semin. Immunol. 10, 383–390 [DOI] [PubMed] [Google Scholar]
- 46. Kadowaki N., Antonenko S., Lau J. Y., Liu Y. J. (2000) Natural interferon α/β-producing cells link innate and adaptive immunity. J. Exp. Med. 192, 219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tough D. F. (2004) Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leuk. Lymphoma 45, 257–264 [DOI] [PubMed] [Google Scholar]
- 48. Uccellini M. B., Busconi L., Green N. M., Busto P., Christensen S. R., Shlomchik M. J., Marshak-Rothstein A., Viglianti G. A. (2008) Autoreactive B cells discriminate CpG-rich and CpG-poor DNA and this response is modulated by IFN-α. J. Immunol. 181, 5875–5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Do R. K., Hatada E., Lee H., Tourigny M. R., Hilbert D., Chen-Kiang S. (2000) Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J. Exp. Med. 192, 953–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jefford M., Schnurr M., Toy T., Masterman K. A., Shin A., Beecroft T., Tai T. Y., Shortman K., Shackleton M., Davis I. D., Parente P., Luft T., Chen W., Cebon J., Maraskovsky E. (2003) Functional comparison of DCs generated in vivo with Flt3 ligand or in vitro from blood monocytes: differential regulation of function by specific classes of physiologic stimuli. Blood 102, 1753–1763 [DOI] [PubMed] [Google Scholar]
- 51. Jeannin P., Delneste Y., Lecoanet-Henchoz S., Gauchat J. F., Ellis J., Bonnefoy J. Y. (1997) CD86 (B7–2) on human B cells. A functional role in proliferation and selective differentiation into IgE- and IgG4-producing cells. J. Biol. Chem. 272, 15613–15619 [DOI] [PubMed] [Google Scholar]
- 52. Kasprowicz D. J., Kohm A. P., Berton M. T., Chruscinski A. J., Sharpe A., Sanders V. M. (2000) Stimulation of the B cell receptor, CD86 (B7–2), and the β 2-adrenergic receptor intrinsically modulates the level of IgG1 and IgE produced per B cell. J. Immunol. 165, 680–690 [DOI] [PubMed] [Google Scholar]
- 53. Rau F. C., Dieter J., Luo Z., Priest S. O., Baumgarth N. (2009) B7–1/2 (CD80/CD86) direct signaling to B cells enhances IgG secretion. J. Immunol. 183, 7661–7671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Suvas S., Singh V., Sahdev S., Vohra H., Agrewala J. N. (2002) Distinct role of CD80 and CD86 in the regulation of the activation of B cell and B cell lymphoma. J. Biol. Chem. 277, 7766–7775 [DOI] [PubMed] [Google Scholar]
- 55. Zhao W., Cha E. N., Lee C., Park C. Y., Schindler C. (2007) Stat2-dependent regulation of MHC class II expression. J. Immunol. 179, 463–471 [DOI] [PubMed] [Google Scholar]
- 56. Jelinek D. F., Lipsky P. E. (1983) The role of B cell proliferation in the generation of immunoglobulin-secreting cells in man. J. Immunol. 130, 2597–2604 [PubMed] [Google Scholar]
- 57. Tangye S. G., Hodgkin P. D. (2004) Divide and conquer: the importance of cell division in regulating B-cell responses. Immunology 112, 509–520 [DOI] [PMC free article] [PubMed] [Google Scholar]