Abstract
Objective
Interleukin-1 beta (IL1β) is a pro-inflammatory cytokine that mediates arthritic pathologies. Our objective was to evaluate pain and limb dysfunction resulting from IL1β over-expression in the rat knee and investigate the ability of local IL1 receptor antagonist (IL1Ra) delivery to reverse associated pathology.
Design
IL1β over-expression was induced in the right knees of 30 Wistar rats via intra-articular injection of rat fibroblasts retrovirally infected with human IL1β cDNA. A subset of animals received a 30 µL intra-articular injection of saline or human IL1Ra on day 1 after cell delivery (0.65 µg/µL hIL1Ra, n=7 per group). Joint swelling, gait, and sensitivity were investigated over 1 week. On day 8, animals were sacrificed and joints were collected for histological evaluation.
Results
Joint inflammation and elevated levels of endogenous IL1β were observed in knees receiving IL1β infected fibroblasts. Asymmetric gaits favoring the affected limb and heightened mechanical sensitivity (allodynia) reflected a unilateral pathology. Histopathology revealed cartilage loss on the femoral groove and condyle of affected joints. Intra-articular IL1Ra injection failed to restore gait and sensitivity to pre-operative levels and did not reduce cartilage degeneration observed in histopathology.
Conclusion
Joint swelling and degeneration subsequent to IL1β over-expression is associated limb hypersensitivity and gait compensation. Intra-articular IL1Ra delivery did not result in marked improvement for this model; this may be driven by rapid clearance of administered IL1Ra from the joint space. These results motivate work to further investigate the behavioral consequences of monoarticular arthritis and sustained release drug delivery strategies for the joint space.
Keywords: Arthritis, Animal Model, Pain, Joint Dysfunction, Interleukin-1
Introduction
Osteoarthritis (OA) is a degenerative joint disease that is the most prevalent joint disorder globally [1–3]. There is substantial evidence implicating a role for inflammatory mediators, such as interleukin-1 beta (IL-1β) in the development of OA [4–12]. Interleukin-1 receptor antagonist (IL1Ra) is a native antagonist of IL-1 that functions by competitively inhibiting IL-1 binding to its cell receptor, thereby preventing downstream activation of proteolytic events that promote cartilage destruction [13, 14]. Several investigators have shown utility of IL1Ra as a therapy for OA when administered in protein form, via the IL1Ra gene, or in conditioned serum [15–19]. Biweekly intra-articular injections of IL1Ra protein in a canine ACL transection model demonstrated an ability for IL1Ra to protect against cartilage lesion formation and collagenase-1 expression, particularly at the highest doses (4 mg) [15]. In rabbit and canine models of either OA or inflammatory arthropathy, transfer of the IL1Ra gene directly to the joint space has led to a marked reduction in synovitis, joint swelling (diameter), cartilage lesion formation, and biochemical markers of arthritis [16, 20, 21]. However, the therapeutic efficacy of IL1Ra in human clinical trials for the treatment of OA has been less impressive with no reported changes in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score from baseline to four weeks following a single intra-articular injection of high or low-dose IL1Ra [22–24]. Here and elsewhere, the translation of anatomical changes in joint structures in the preclinical OA model to the functional and symptomatic consequences of human OA is complex, with frequent reports of discordant findings between the human condition and the animal model.
Patients afflicted with OA may experience pain and joint dysfunctions that have deleterious effects on activity levels and lifestyle. These OA sequelae can be clinically evaluated through patient-reported pain and disability scales, such as the Oswestry disability index [25] and the WOMAC scale [26]. Some correlates of pain and disability scales have been utilized in preclinical OA animal models, such as quantifying features of animal locomotion, measuring sensitivity to mechanical stimuli, or quantifying an animal’s ability to perform a challenge or task [27–29]. Gait changes have been measured in monoarticular models of inflammatory joint disease in rodents [30–32], as well as in large animal models of surgically-induced joint instability in sheep or dogs [33–37]. In addition, mechanical allodynia, a hypersensitivity to a non-noxious mechanical stimulus, is commonly reported as a result of peripheral inflammation and may also be a disease sequela in animal OA models [27].
The objective of this study was to evaluate pain-related behaviors and gait dysfunctions in a rat monoarticular model of OA induced by IL-1β over-expression. Gait characteristics and rodent sensitivity to mechanical stimuli were recorded and compared between pre- and post-operative time points, followed by treatment with a single intra-articular injection of IL1Ra. Anatomic changes in the knee joint were assessed via a gross morphological grade and histological grade of cartilage degeneration. Results suggest that IL1β-induced inflammation causes a moderate limp with preferential weight bearing on the unaffected limb and a hypersensitivity to non-noxious mechanical stimuli in the affected limb that coincide with the anatomic presentation of OA signs. A single intra-articular injection of IL1Ra on day 1 after IL1β-induced inflammation had limited therapeutic efficacy in reversing these behavioral markers of joint dysfunction and pain.
Methods
Experimental Design
The primary goal of this study was to characterize a previously developed model of IL-1β mediated joint inflammation [16] for changes in animal gait and pain-related behaviors. In a first study designed to characterize the arthritis model, rats (n=6) received an intra-articular injection of rat skin fibroblasts genetically altered to over-express human IL-1β as a means to promote knee joint inflammation and arthritis. These rats were assessed for their body weight gain over 7 days to insure that hIL-1β dosing did not result in a systemic pathology. In addition, knee joint diameters in these rats were recorded on days 1, 3, 5 and 7 after intra-articular cell delivery, and compared to pre-operative values (“day -2”). Animals were sacrificed on day 8; knees were collected, opened, and assessed for gross appearance of joint pathology (detailed methods below). Thereafter, joints were placed in explant culture, where media was collected to detect the production of rat and human IL-1β.
A second set of rats was studied longitudinally for measures of gait and pain-related behaviors in this model. Rats (n=10) underwent testing for gait and sensitivity measures preoperatively (see detailed methods), then received an intra-articular injection of hIL-1β-infected fibroblasts on day 0. Rats were tested again for measures of gait and sensitivity post-operatively on days 2 or 3 and 6 or 7. Animals were sacrificed on day 8, and knees were assessed for gross and histological appearance of joint pathology (see detailed methods).
A third set of animals underwent testing to determine if a single injection of human IL1Ra protein could ameliorate the behavioral consequences of hIL-1β-mediated joint pathology in the rat knee. As before, rats (n=14) underwent testing for gait and sensitivity measures preoperatively (see detailed methods), then received an intra-articular injection of hIL-1β-infected fibroblasts on day 0. A subset of these animals received a 30 µL injection of either saline or 0.65 µg/µL hIL1Ra in saline on day 1 after cell delivery (n=7 per group). All animals underwent longitudinal testing for gait measures on days 2 and 6 and mechanical sensitivity measures on days 1, 3, and 5. Animals were sacrificed on day 8, and knees were assessed for gross and histological appearance of joint pathology (see detailed methods).
Intra-articular Over-expression of IL-1β in the Rat Knee (Animal Model)
A rat skin fibroblast cell line was retrovirally infected with a plasmid carrying human IL-1β cDNA (MFG-hIL-1β) and used here, as described previously [16]. Intra-articular injection of these cells modified to over-express IL-1β has been shown to generate an inflammatory arthritis in prior studies. This pathology is characterized by synovial hypertrophy and variable stages of cartilage destruction and subchondral bone remodeling depending on the number of cells injected and beyond that caused by the injection of naïve fibroblasts [38]. To induce knee arthritis, male Wistar rats (187–222 kg) received a 30 µl injection of 12,500 IL-1β infected fibroblasts suspended in sterile PBS to the right knee joint. A total of 30 male Wistar rats were used for this investigation; all procedures were approved by the Duke University’s Institutional Animal Care and Use Committee.
Joint Diameter and Body Weight
Rats were restrained and joint swelling was assessed by measuring the diameter of the affected (right) and contralateral knee joint in the sagittal and coronal planes using digital calipers. These data were transformed into the cross-sectional area of the knee joint, under the assumption that this area could be approximated by an ellipse. Data were analyzed with a two-factor analysis of variance (ANOVA) with a post-hoc Dunnett’s test to assess differences between pre-operative and post-operative time points and a post-hoc Tukey’s HSD test to evaluate differences between the affected and contralateral limb.
IL1β Production In Vitro
At the time of sacrifice, the left and right knees were harvested; skin and muscle were, dissected and discarded. The fibula was removed, the tibia was cut just below the tibial tubercle, and the femur was cut at the transition of the shaft to the lateral epicondyle. The patellar attachments to the femur were transected, opening the joint space. The remaining explants including cartilage, subchondral bone, joint capsule and fat pad was cultured in 1.75 ml of DMEM at 37°C for 24 hours. The amount of human and rat IL-1β secreted into media was quantified by ELISA (human IL-1β, Endogen ELISA kit, Pierce, Rockford, IL; rat IL-1β, Quantikine ELISA kit, R&D Systems Inc). A one-factor ANOVA with a post-hoc Tukey’s HSD was performed to test for differences between affected and contralateral joint values in order to determine if joints receiving an injection of IL-1β infected fibroblasts developed a greater potential to produce IL-1β..
Gait Assessments
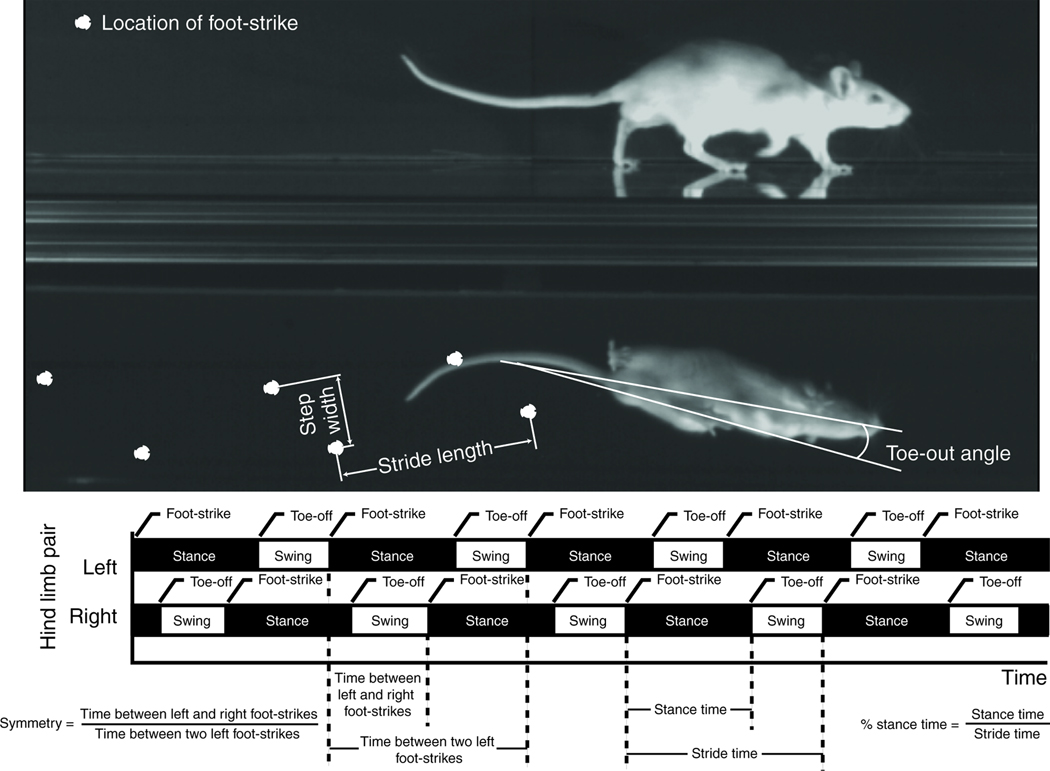
To assess rodent gait, animals were placed in a custom-built acrylic gait arena with transparent floor and sides (5’6” × 1’6”, camera set to record 4’). Underneath the arena, a mirror oriented at 45° allowed for recording of both the sagittal and ventral planes. An animal was allowed to freely explore the arena without an external stimulus for up to 25 minutes or until 5 acceptable trials had been recorded; only trials with a minimum of two complete gait cycles and a consistent velocity (less than 15% about the mean) were saved for processing. All video data were collected at 200 frames per second (1.5–4.0 secs of recorded data, Phantom V4.2; Vision Research, Wayne, NJ). The video frame number (time) and spatial position of foot-strike and toe-off events were determined by visual digitization of the videos in DLTdataviewer2 [39]; the position of the rat’s nose and center of area in the ventral plane was tracked using a custom MATLAB code. From these data, the following gait parameters were calculated: velocity, stride length/stride frequency, step width, toe-out angle, percentage stance time, and gait symmetry (Figure 1).
Figure 1.
Velocity was assessed using analysis of variance (ANOVA) with a post-hoc Dunnett’s test to compare to the pre-operative control only. Percentage stance time and symmetry can indicate the favoring of a limb within a fore- or hind-limb pair. To assess these data, the difference between left and right hind-limb percentage stance time was evaluated for variation from 0.0 (equal stance times on left and right limb) and symmetry was assessed for a variation from 0.5 (symmetric gait) using repeated measures t-test. The remaining gait parameters can have strong correlations to the animal’s selected velocity; therefore, these data were analyzed via a generalized linear model (GLM, Statistica). GLMs include a linear dependence on velocity, followed by a post-hoc Dunnett’s test (pre-op vs. post-op data) or Tukey’s HSD test (saline vs. IL1Ra) when indicated.
Mechanical Hypersensitivity
Rats were acclimated to wire-bottom caging and the testing paradigm over a period of 2 days prior to the 1st measurement. Thereafter, rats were acclimated to the caging for a period of 20 minutes at each time point prior to the application of the von Frey hairs (Stoelting, Wood Dale, IL). Using the up-down protocol for rats [40], the 50% withdrawal frequencies was determined for the hind paws on the affected (right) and contralateral limb. Differences in the withdrawal thresholds between pre- and post-operative values, between affected and contralateral limbs at each timepoint, or between groups receiving saline or hIL1Ra treatments as appropriate, were assessed using multi-factor ANOVAs, with decreases in the withdrawal threshold indicating a heightened sensitivity to non-noxious mechanical stimuli. Post-hoc Dunnett’s test (post-op vs. pre-op control) or Tukey’s HSD tests were used when indicated.
Grading of Gross Appearance and Histology
Immediately after opening of knee joint capsules, the gross appearance of joint structures was evaluated by two blinded reviewers coming to consensus using the following custom three-point scale: 0 = no pathology, 1 = synovial erythema, or 2 = synovial erythema and synovial hypertrophy. Thereafter, knees were fixed in 10% formalin for 48 hours, decalcified for 72–96 hours using Cal-Ex decalcifying agent (Fisher Scientific, Fair Lawn, NJ), and embedded in paraffin wax using standard practices. Serial sagittal histological sections (8 µm) of both right and left knee joints were acquired. One section representing the most severe lesion formation on the femoral condyles, patellofemoral compartment, and tibial plateau were chosen and stained with H&E and toluidine blue. Changes in the articular cartilage were graded by two blinded reviewers coming to consensus using the OARSI Osteoarthritis Histopathology Assessment System [41]. This system assigns one of seven grades to a section based on evidence of progressive cartilage and subchondral bone damage encompassing normal cartilage, chondrocyte cell death, fibrillation, fissures, cartilage erosion and denudation, osteophyte formation, and subchondral bone remodeling (grade 0 = cartilage intact; grade 6 = deformation and evidence of bone remodeling). Changes in the synovial tissue were graded by two blinded reviewers coming to consensus using a histopathological assessment described by Krenn et al. [42]. This system assesses the synovial cell layer (grade 0–3; 0 = synovial lining cell layer is 1–2 cells to 3 = synovial lining cell layer is greater than 10 cells), density of cells in the synovial stroma (grade 0–3; 0 = normal to 3 = cellularity greatly increased with pannus formation or rheumatoid granulomas), and the presence of intra-articular cellular debris (grade 0–2; 0 = no cellular debris to 2 = large quantity of cellular debris). A total grade (0–8) was determined by summing the grades from each category. As gross and histological grades are ordinal data, Kruskal-Wallis median tests were used to detect differences between affected and contralateral limbs or between groups receiving saline or hIL1Ra treatments as appropriate.
Results
Joint Swelling and IL1β Production
The affected joints showed significant increases in cross-sectional area over pre-operative values that persisted out to post-operative day 7 (p < 0.01, Dunnett’s Test, Table 1). Moreover, swelling in the affected joint was significantly greater than the contralateral joint at days 3, 5, and 7 (p < 0.05, Tukey’s HSD). Swelling was not observed in the contralateral joint, as cross-sectional areas remained similar to that of pre-operative control throughout the experiment. Animal body weight s were maintained or increased equally in all experimental animals, indicating that a systemic pathology did not occur from the intra-articular injection.
Table 1.
| Cross-section(mm2) | ||
|---|---|---|
| Days following cell delivery | Affected limb | Contralateral limb |
| Pre-operative | 67.9 ± 5.4 | 67.5 ± 2.3 |
| Day 1 | 84.0 ± 9.4* | 76.6 ± 8.0 |
| Day 3 | 100.5 ± 11.7*T | 69.9 ± 8.0 |
| Day 5 | 101.8 ± 13.6*T | 73.7 ± 5.1 |
| Day 7 | 91.6 ± 10.8*T | 68.1 ± 4.1 |
Rat IL1β concentrations in the media of explant cultures were 120.2 ± 69.3 and 34.3 ± 10.6 µg for the affected and contralateral explant cultures, respectively; human IL-1β concentrations were near the level of detection in both knees, with 11.7 ± 15.0 and 14.8 ± 14.8 µg in the affected and contralateral explant cultures, respectively (mean ± st. dev.). These data confirm that knees injected with hIL-1β infected fibroblasts had potential to produce significantly more rat IL1β, but not human IL1β, relative to contralateral control knees (p < 0.05, Tukey’s HSD).
Gait
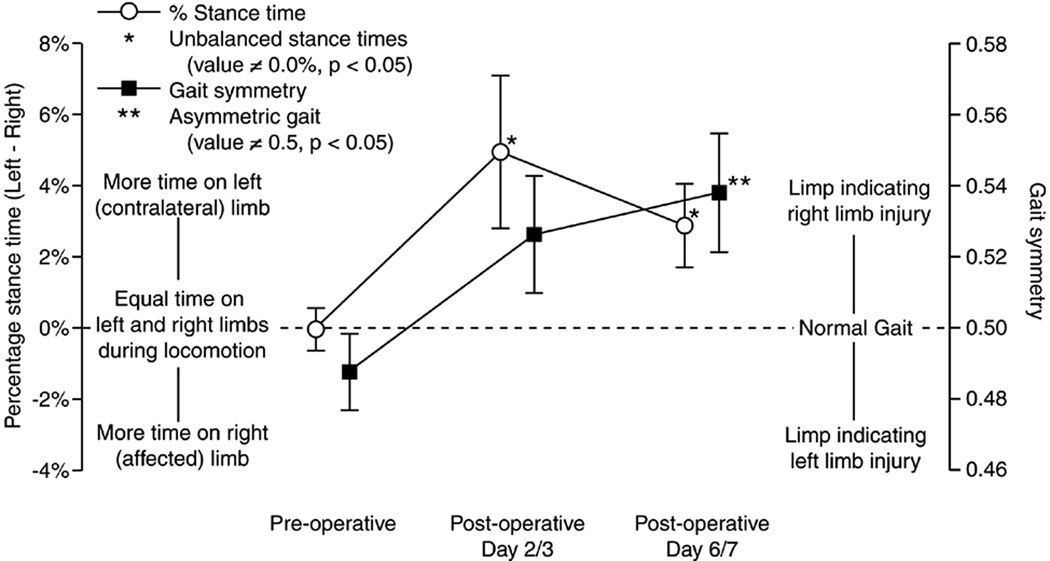
Following the injection of IL-1β infected fibroblasts, rats walked with gaits indicative of unilateral injury to the right hind limb (Figure 2). Pre-operatively, rats walked with symmetric gaits with balanced stance times between the left and right hind limb. By day 2–3, rats spent significantly less time on their affected (right) limb relative to the contralateral limb (p < 0.05, t-test), with gait symmetry tending to be greater than 0.5. The imbalance in stance times continued at day 6–7 (p < 0.05, t-test), with gait symmetry becoming significantly greater than 0.5 (p < 0.05, t-test).
Figure 2.
Animal velocity also increased following the injection of IL-1β infected fibroblasts (Table 2), with velocities at day 2–3 tending to be larger than pre-operative measures (p = 0.09, Dunnett’s) and velocities at day 6–7 being significantly larger than pre-operative measures (p < 0.001, Dunnett’s). Moreover, stride lengths increased above that predicted by the increases in velocity alone following the injection of IL-1β infected fibroblasts (p < 0.001, Dunnett’s), and toe-out angles in both the affected and contralateral limb trended up with time (p < 0.01, Dunnett’s).
Table 2.
| Gait parameter | Pre-operative | Day 2/3 | Day 6/7 | |
|---|---|---|---|---|
| Velocity [cm/sec] | 41.0 ± 1.81 | 47.8 ± 2.5 | 54.4 ± 2.5* | |
| IL-1β over-expression | Stride length [cm] | 13.2 ± 0.2 | 14.5 ± 0.3* | 15.2 ± 0.3* |
| No treatment | Step width [cm] | 3.4 ± 0.2 | 3.4 ± 0.1 | 3.8 ± 0.2 |
| (n = 10) | Left foot toe-out angle [deg] | 4.58 ± 0.41 | 5.08 ± 0.54 | 7.37 ± 1.44* |
| Right foot toe-out angle [deg] | 3.99 ± 0.45 | 5.02 ± 0.63 | 6.22 ± 0.91* |
Gait following IL1Ra or Saline Treatment
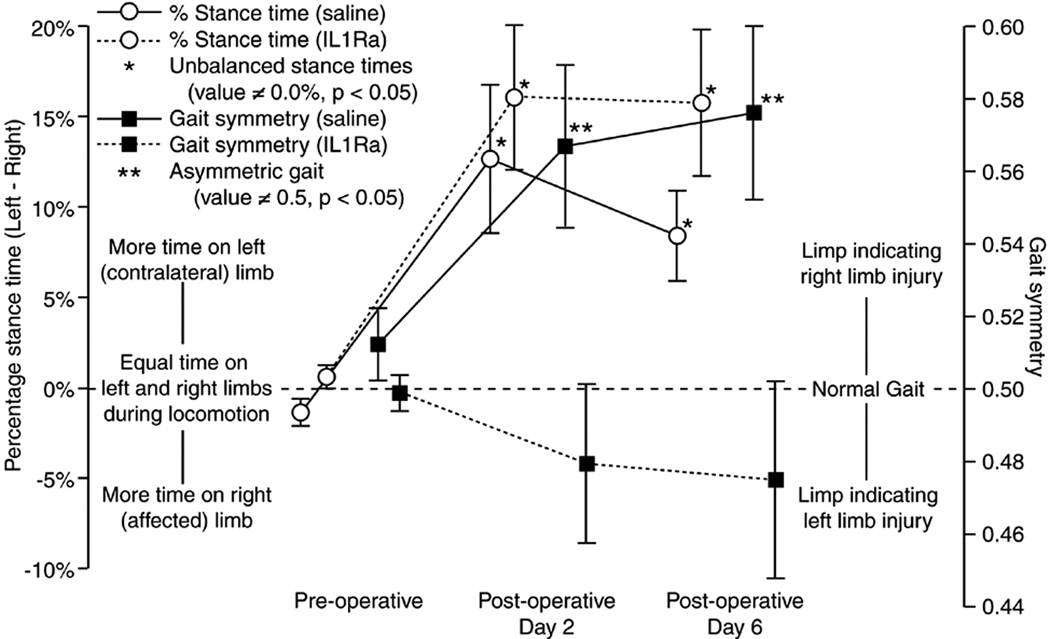
Animals receiving an injection of either saline or IL1Ra at one day after cell delivery had marked increases in the imbalance of stance times between the affected and contralateral limb (Figure 3), with the difference between the affected (right) and contralateral limb stance times being approximately 10% or greater on post-operative day 2 and day 6. This sizable difference of stance time between the two hind limbs resulted in the appearance of an aerial phase where neither hind limb was in stance during the gait cycle. Aerial phases were 2.2 ± 0.9% and 1.4 ± 0.6% of the gait cycle for saline treated animals at day 2 and day 6, respectively; aerial phase were 4.4 ± 1.4% and 6.9 ± 1.9% for IL1Ra treated animals at day 2 and day 6, respectively (mean ± SEM). These gaits are in stark contrast to animals that did not receive an additional injection beyond that of the IL-1β infected fibroblasts on day 0, where percentage stance time differences were generally less than 5% and aerial phases were not observed (compare left-hand y-axis in Figure 2 and 3).
Figure 3.
Similar to that observed in untreated rats, all animals treated with either saline or IL1Ra increased their selected velocities (p < 0.01, Dunnett’s), stride lengths (p < 0.0001, Dunnett’s), and greater toe-out angles (p < 0.05, Dunnett’s) as compared to pre-operative values following the injection of IL-1β infected fibroblasts (Table 3). Step widths also increased relative to preoperative values in animals receiving a saline or IL1Ra injection on day 1 after the injection of IL-1β infected fibroblasts (p < 0.01, Dunnett’s).
Table 3.
| Gait parameter | Pre-operative | Day 2/3 | Day 6/7 | |
|---|---|---|---|---|
| Velocity [cm/sec] | 40.7 ± 2.0 | 47.3 ± 2.5* | 54.0 ± 2.7* | |
| Stride length [cm] | 12.8 ± 0.2 | 14.2 ± 0.4* | 15.1 ± 0.4* | |
| Saline (n = 7) | Step width [cm] | 3.2 + 0.1 | 3.5 ± 0.1* | 3.8 ± 0.1* |
| Left foot toe-out angle [deg] | 3.54 ± 0.30 | 4.76 ± 0.57 | 4.06 ± 0.56* | |
| Right foot toe-out angle [deg] | 3.75 ± 0.51 | 4.66 ± 1.16 | 5.74 ± 2.16* | |
| Velocity [cm/sec] | 34.9 ± 1.8 | 42.7 ± 2.2* | 53.1 ± 2.9* | |
| Stride length [cm] | 12.7 ± 0.3 | 14.4 ± 0.3* | 15.6 ± 0.3* | |
| IL1Ra(n = 7) | Step width [cm] | 3.4 ± 0.1 | 4.0 ± 0.1*T | 4.0 ± 0.2* |
| Left foot toe-out angle [deg] | 4.16 ± 0.35 | 5.33 ± 0.80 | 6.86 ± 1.29*T | |
| Right foot toe-out angle [deg] | 3.61 ± 0.27 | 7.67 ± 1.71 | 7.17 ± 2.03* |
The differences between the gaits of IL1Ra- and saline-treated animals were mild. Most notably, the injection of IL1Ra did tend to keep gaits “near symmetric” while saline-treated animals had highly asymmetric gaits. However, it should be noted that variability in gait symmetry for IL1Ra-treated animals also increased between pre-operative and post-operative time points. Moreover, step widths were wider in IL1Ra-treated animals at day 2 and the left limb toe-out angle was larger at day 6 (p < 0.05, Tukey’s HSD).
Mechanical Hypersensitivity
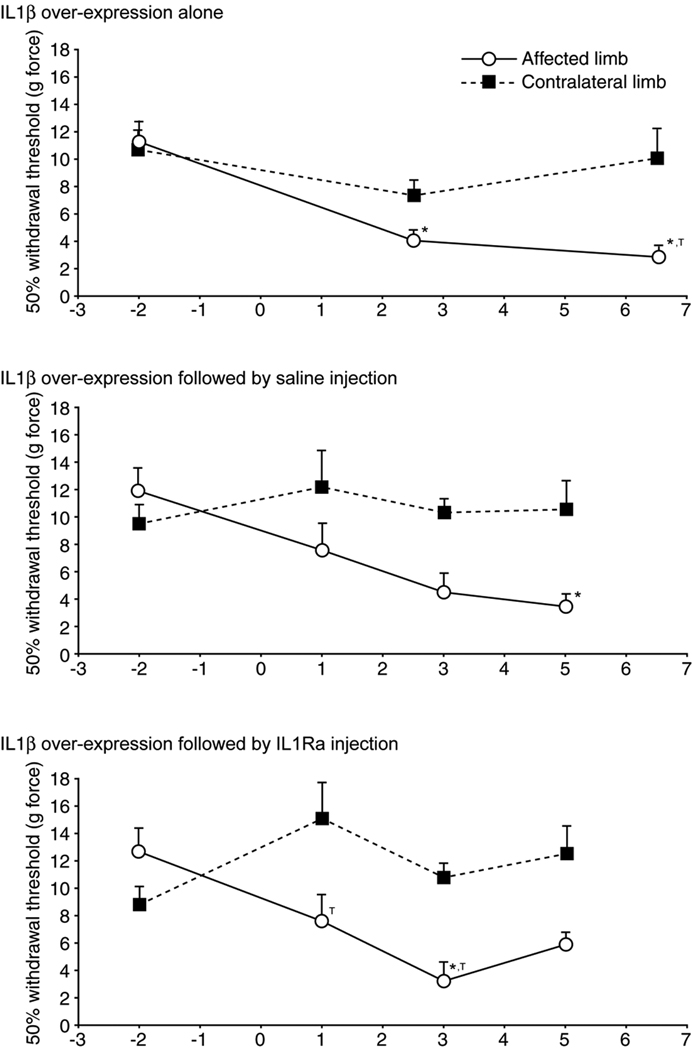
In addition to alterations in gait, 50% paw withdrawal thresholds for the affected limb decreased on post-operative days relative to pre-operative values (p < 0.01, Dunnett’s, Figure 4). Withdrawal threshold for the affected limb also tended to be lower than the contralateral limb (p < 0.01, Tukey’s HSD). A marked improvement as a result of IL1Ra treatment relative to saline control was not found.
Figure 4.
Histopathology
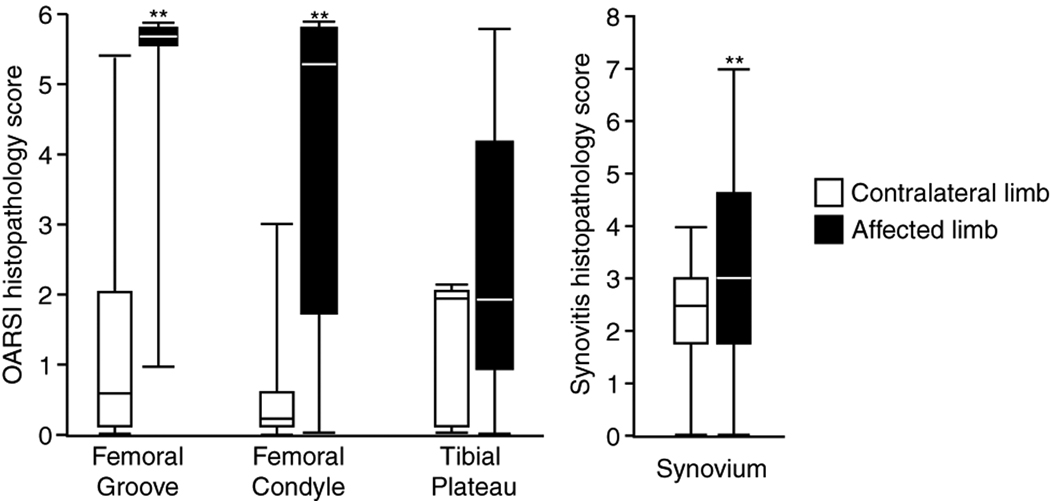
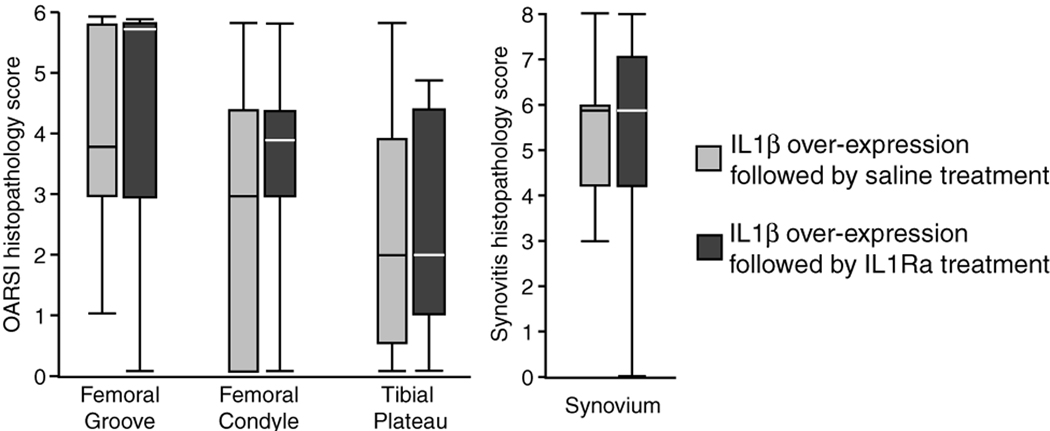
Significant differences were detected in gross pathology scores between affected and contralateral knee joints in all rats studied here. Synovial erythema and hypertrophy were seen in the majority of affected knee joints, while contralateral control joints demonstrated little pathology. Histological grading confirmed the results observed via gross inspection; affected knee joints showed substantial cartilage degeneration in the femoral groove and on the femoral condyle relative to the contralateral joints and significant synovitis in injected knees relative to contralateral control knees (Figure 5, p < 0.05, Kruskall-Wallis). Moreover, the intra-articular injection of IL1Ra protein on day 1 did not improve the histological grades of the knee joint at day 8 relative to saline controls (Figure 6).
Figure 5.
Figure 6.
Discussion
This study provides new data for unilateral joint dysfunction and mechanical hypersensitivity in a monoarticular model of joint arthritis of intra-articular over-expression of IL-1β. Following the delivery of rat fibroblasts infected to express and secrete human IL-1β, knee joints exhibited significant pathology described by joint swelling and potential to produce of endogenous (rat) IL-1β. With signs of swelling and inflammation, behavioral signs of unilateral joint dysfunction and mechanical hypersensitivity occurred. Moreover, joint histology revealed significant pannus formation associated with cartilage loss in the patellofemoral groove and on the femoral condyle that was quantified as a detectable difference in histopathology and synovitis scores at these sites. While not an inflammatory histopathology grading scheme, it is noteworthy that the OARSI score captured some features representative of differences due to the IL-1β induced inflammation in this study. However, this degeneration is in contrast to rat knee instability models of osteoarthritis where degeneration is most severe on the tibial plateau and progresses over the course of weeks [27, 43]. While we did not observe a significant attenuation in behavioral metrics from a single injection of IL1Ra protein, our data does provide motivation to investigate these same parameters in a host of alternative therapeutic strategies including repeated drug administrations, sustained drug release vehicles, and gene therapy approaches.
The data presented herein note the ability to detect unilateral joint dysfunctions using basic descriptors of rodent gait in this model of osteoarthritis. Once pathology was induced, rats selected walking gaits where significantly less time was spent on the affected limb relative to the contralateral limb. Moreover, post-operative gaits demonstrated slight asymmetries where the amount of time between contralateral limb and affected limb foot-strike was greater than the amount of time between affected limb and the next contralateral limb foot-strike. This asymmetry may imply that once weight is on the uninjured contralateral limb, animals are hesitant to shift weight to the affected limb; and if weight is on the affected limb, animals are quick to transfer that weight to the uninjured limb. It is worthy of note that these temporal descriptors of rodent gait can not be detected via traditional inkpad foot-printing methods; instead, the use of high-speed equipment, either video or force-plate, is required.
In addition to changes in temporal gait descriptors, toe-out angles increased following the injection of IL-1β infected fibroblasts. Increased toe-out angles are observed in the OA patient population as well. This gait adaptation reduces the adduction moment, shifts load away from the medial compartment during the early stance phase, and has been associated with changes of the sciatic nerve functionality index [44–46].
Increases in gait velocity and stride length were unexpected; it was anticipated that an injured animal will walk at slower self-selected speeds with shorter stride lengths. However, the data for all animals receiving a single injection of IL-1β infected fibroblasts show increased speeds. Moreover, stride lengths at these increased velocities were longer than that predicted by pre-operative measures. Certainly, emotional stress associated with a limb injury may cause the animals to be more nervous in the gait arena, and rather than exploring, animals may be rapidly searching for a safe, protected area. In addition, the linear dependence of gait parameters on velocity formed from pre-operative data represented normal, symmetric walking gaits. With unilateral injury, gaits became progressively asymmetric and may begin to approach that of a half-bound. With this altered gait pattern, an animal may use longer strides at a given velocity.
A single IL1Ra injection did not markedly improve animal gait characteristics or reduce mechanical hypersensitivity. In fact, a second injection to the affected joint, whether it be saline or IL1Ra, decreased the affected limb stance time and was associated with the appearance of an aerial phase during locomotion at normal walking velocities. Animals receiving IL1Ra did walk with gaits that were near-symmetric; however, a return of the gait symmetry variable to near-symmetric should not be misinterpreted as an improvement in gait and joint function. Although symmetric gaits are most common for walking velocities, both asymmetric and symmetric gaits (as defined by the symmetry variable) can occur naturally in rodents. However, significant differences in stance times for the limbs in a limb pair are rare, and since stance times remained markedly shifted to the contralateral limb, animal gait following treatment of IL1Ra still likely indicates a unilateral pathology and dysfunction. ,A more sophisticated analysis of ground reaction forces in a future study could improve our knowledge of how the limb is used to bear weight and generate push-off forces during locomotion. However, these data still point to a significant concern that a second therapeutic intra-articular injection, timed so closely after the first pathology-inducing injection, was itself responsible for initiating or promoting further pain and hypersensitivity in the rat model. While the rats were particularly sensitive to the repeated injections, there is no evidence that this phenomena extends to the human or even to larger animal models such as the canine [15]. This observation may also provide additional support for the use of gene therapy and sustained-release drug delivery strategies in the intra-articular space, that have the goal of reducing overall drug administration over time [47, 48].
Prior work has demonstrated the potential of IL1Ra to attenuate cartilage degeneration; however, multiple injections are often necessary [15]. In our model, the inability of a single injection of IL1Ra to reduce or reverse the effects of intra-articular over-expression of IL-1β may be related to artifacts associated with repeated intra-articular injections, the rapid and efficient clearance of IL1Ra from the joint space, and/or an inability to maintain an effective concentration of IL1Ra in the joint space. Intra-articular synovial fluid is cleared through lymphatics and vasculature in the synovium with evidence that 10–50 kDa molecules have half-lives in the joint space on the order of minutes to hours [45]. Moreover, the IL-1β infected fibroblasts remain in the joint and may re-incite pathology once the IL1Ra protein is cleared from the joint space. This study again motivates the need to increase the residence time via either gene delivery [16, 21] or local drug depot formation [49] in order to increase IL1Ra’s efficacy in reducing the progression of cartilage degeneration. However, the behavioral metrics described herein may provide for an improved evaluation of the therapeutic efficacy of these approaches in future studies.
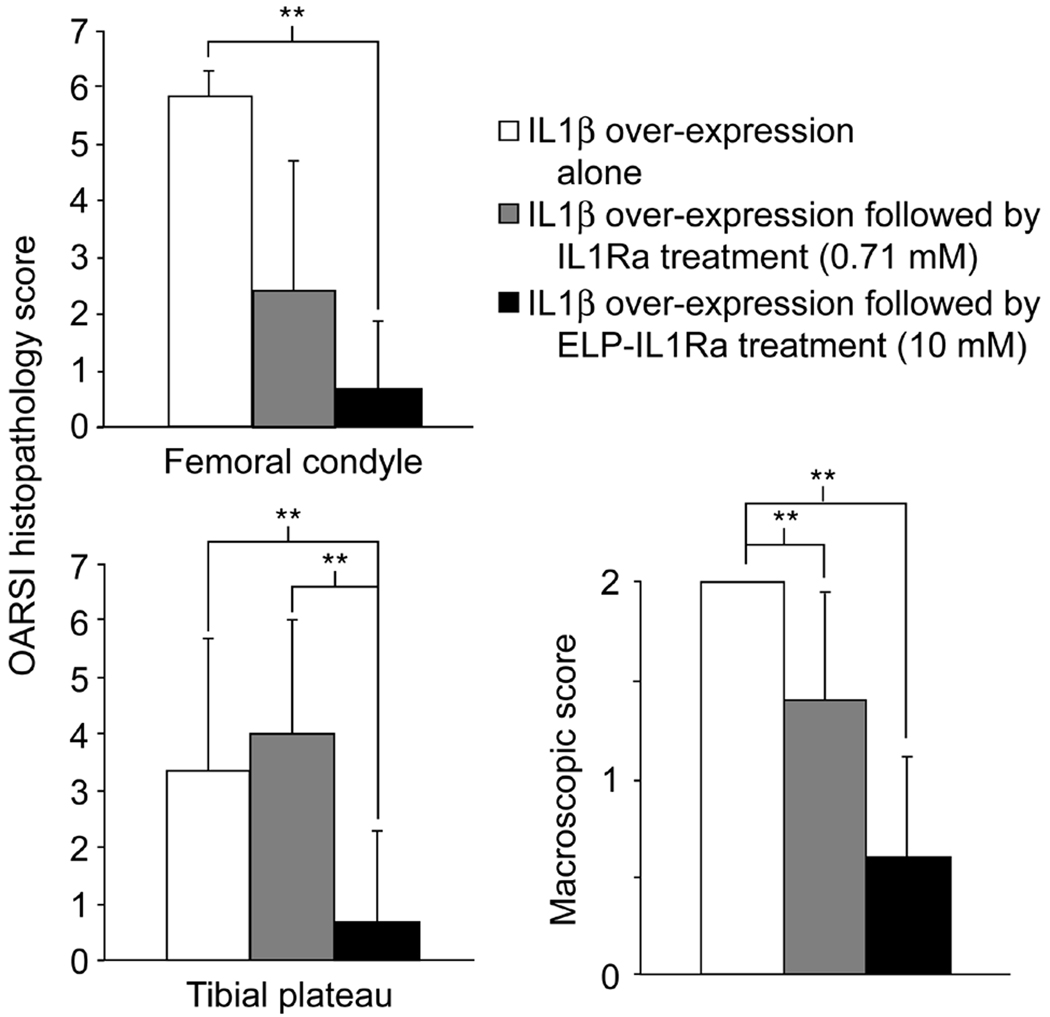
In exploratory work, we have incited the same pathology described above in 18 additional animals: six each receiving either no treatment, a 20 µL intra-articular injection of IL1Ra (0.71 mM), or a 20 µL intra-articular injection of IL1Ra fused to an elastin-like polypeptide tag (ELP-IL1Ra, 10 mM) [48]. Since the ELP tag may interfere with accessibility of the IL1Ra domain to its receptor and is associated with lower bioactivity than that of IL1Ra (IC50 = 1.4nM vs. 310 nM, IL1Ra vs. ELP-IL1Ra) [48], the doses were chosen to insure that both IL1Ra and ELP-IL1Ra protein groups received an equivalent amount of bioactive drug. ELP-IL1Ra treated animals showed a trend towards less macroscopic and microscopic joint and cartilage degeneration relative to no treatment groups at the medial femoral condyle, patellofemoral groove and tibial plateau sites (Figure 7). These preliminary observations suggest the potential for modifications and conjugations of IL1Ra that increase the joint residence time as a method to provide for IL-1 antagonism while minimizing total drug administrations and drug use as a means to protect cartilaginous tissues from degradation.
Figure 7.
In this study, we demonstrate that intra-articular injection of fibroblasts genetically modified to express and secrete human IL-1β can result in behavioral changes associated with unilateral joint dysfunction and mechanical hypersensitivity. Findings for increased production of endogenous (rat) IL-1β, increased joint swelling, and increased cartilage degeneration in this model corroborates prior work and suggests that IL-1β is a significant mediator of the resulting behavioral changes. The intra-articular delivery of IL1Ra on day 1 after inciting pathology did not result in marked improvement of the macroscopic and microscopic signs of cartilage development, nor attenuate the behavioral signs of unilateral joint dysfunction and mechanical hypersensitivity. This may be due to challenges associated with repeated intra-articular injections in this animal mode, the rapid clearance of IL1Ra from the intra-articular joint space, or an inability of IL1Ra to interfere with a broad spectrum of inflammatory agents. Drug delivery strategies that can provide for IL-1 antagonism while reducing therapeutic dosing amounts or frequency may provide a working solution to protect joint tissues from degenerative remodeling.
Acknowledgments
The contributions of Mr. Steve Johnson and Dr. Mostafa Gabr are gratefully acknowledged. This work was supported with funds from the NIH (R01EB002263, R21AR052745, P01AR050245, K99AR057426 (KDA), F32AR056190 (KDA)), the North Carolina Biotechnology Center (CFG-8013), and an OREF/DePuy Orthopaedic Resident Educational Grant (SBA, Jr.).
Footnotes
Author Contributions
All authors have contributed to the conception and design of the study, acquisition, analysis, and interpretation of data, or the drafting the manuscript for important intellectual content. All authors approved the final version of the submitted manuscript. Dr. Adams and Dr. Allen share the primary researcher responsibilities of this report: Dr. Adams was the primary researcher for establishing the animal model and pathological evaluations; Dr. Allen was the primary researcher for establishing tests of animal behavior and joint dysfunction in all experiments.
References
- 1.Birchfield PC. Osteoarthritis overview. Geriatr Nurs. 2001;22:124–130. doi: 10.1067/mgn.2001.116375. quiz 130-121. [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter JA, Stanish WD, Rosier RN, Schenck RC, Jr., Dennis DA, Coutts RD. The increasing need for nonoperative treatment of patients with osteoarthritis. Clin Orthop Relat Res. 2001:36–45. doi: 10.1097/00003086-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 4.Attur MG, Patel IR, Patel RN, Abramson SB, Amin AR. Autocrine production of IL-1 beta by human osteoarthritis-affected cartilage and differential regulation of endogenous nitric oxide, IL-6, prostaglandin E2, and IL-8. Proc Assoc Am Physicians. 1998;110:65–72. [PubMed] [Google Scholar]
- 5.Buckwalter JA, Lotz M, Stoltz JF. Osteoarthritis, inflammation, and degradation : a continuum. Amsterdam ; Washington, DC: IOS Press; 2007. [Google Scholar]
- 6.Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 7.Joosten LA, Helsen MM, Saxne T, van De Loo FA, Heinegard D, van Den Berg WB. IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol. 1999;163:5049–5055. [PubMed] [Google Scholar]
- 8.Myers SL, Brandt KD, Ehlich JW, Braunstein EM, Shelbourne KD, Heck DA, et al. Synovial inflammation in patients with early osteoarthritis of the knee. J Rheumatol. 1990;17:1662–1669. [PubMed] [Google Scholar]
- 9.Pelletier JP, McCollum R, DiBattista J, Loose LD, Cloutier JM, Martel-Pelletier J. Regulation of human normal and osteoarthritic chondrocyte interleukin-1 receptor by antirheumatic drugs. Arthritis Rheum. 1993;36:1517–1527. doi: 10.1002/art.1780361106. [DOI] [PubMed] [Google Scholar]
- 10.Pelletier JP, Mineau F, Fernandes JC, Duval N, Martel-Pelletier J. Diacerhein and rhein reduce the interleukin 1beta stimulated inducible nitric oxide synthesis level and activity while stimulating cyclooxygenase-2 synthesis in human osteoarthritic chondrocytes. J Rheumatol. 1998;25:2417–2424. [PubMed] [Google Scholar]
- 11.Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24:365–371. [PubMed] [Google Scholar]
- 12.van de Loo FA, Joosten LA, van Lent PL, Arntz OJ, van den Berg WB. Role of interleukin-1, tumor necrosis factor alpha, and interleukin-6 in cartilage proteoglycan metabolism and destruction. Effect of in situ blocking in murine antigen- and zymosan-induced arthritis. Arthritis Rheum. 1995;38:164–172. doi: 10.1002/art.1780380204. [DOI] [PubMed] [Google Scholar]
- 13.Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–1652. [PubMed] [Google Scholar]
- 14.Granowitz EV, Porat R, Mier JW, Pribble JP, Stiles DM, Bloedow DC, et al. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine. 1992;4:353–360. doi: 10.1016/1043-4666(92)90078-6. [DOI] [PubMed] [Google Scholar]
- 15.Caron JP, Fernandes JC, Martel-Pelletier J, Tardif G, Mineau F, Geng C, et al. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collagenase-1 expression. Arthritis Rheum. 1996;39:1535–1544. doi: 10.1002/art.1780390914. [DOI] [PubMed] [Google Scholar]
- 16.Gouze E, Pawliuk R, Gouze JN, Pilapil C, Fleet C, Palmer GD, et al. Lentiviral-mediated gene delivery to synovium: potent intra-articular expression with amplification by inflammation. Mol Ther. 2003;7:460–466. doi: 10.1016/s1525-0016(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 17.Frisbie DD, Kawcak CE, Werpy NM, Park RD, McIlwraith CW. Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am J Vet Res. 2007;68:290–296. doi: 10.2460/ajvr.68.3.290. [DOI] [PubMed] [Google Scholar]
- 18.Nixon AJ, Haupt JL, Frisbie DD, Morisset SS, McIlwraith CW, Robbins PD, et al. Gene-mediated restoration of cartilage matrix by combination insulin-like growth factor-I/interleukin-1 receptor antagonist therapy. Gene Ther. 2005;12:177–186. doi: 10.1038/sj.gt.3302396. [DOI] [PubMed] [Google Scholar]
- 19.Wehling P, Moser C, Frisbie D, McIlwraith CW, Kawcak CE, Krauspe R, et al. Autologous conditioned serum in the treatment of orthopedic diseases: the orthokine therapy. BioDrugs. 2007;21:323–332. doi: 10.2165/00063030-200721050-00004. [DOI] [PubMed] [Google Scholar]
- 20.Oligino T, Ghivizzani S, Wolfe D, Lechman E, Krisky D, Mi Z, et al. Intra-articular delivery of a herpes simplex virus IL-1Ra gene vector reduces inflammation in a rabbit model of arthritis. Gene Ther. 1999;6:1713–1720. doi: 10.1038/sj.gt.3301014. [DOI] [PubMed] [Google Scholar]
- 21.Pelletier JP, Caron JP, Evans C, Robbins PD, Georgescu HI, Jovanovic D, et al. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997;40:1012–1019. doi: 10.1002/art.1780400604. [DOI] [PubMed] [Google Scholar]
- 22.Goupille P, Mulleman D, Chevalier X. Is interleukin-1 a good target for therapeutic intervention in intervertebral disc degeneration: lessons from the osteoarthritic experience. Arthritis Res Ther. 2007;9:110. doi: 10.1186/ar2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chevalier X, Giraudeau B, Conrozier T, Marliere J, Kiefer P, Goupille P. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J Rheumatol. 2005;32:1317–1323. [PubMed] [Google Scholar]
- 24.Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009;61:344–352. doi: 10.1002/art.24096. [DOI] [PubMed] [Google Scholar]
- 25.Fairbank JC, Couper J, Davies JB, O'Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 26.Bellamy N, Gilbert JR, Brooks PM, Emmerson BT, Campbell J. A survey of current prescribing practices of antiinflammatory and urate lowering drugs in gouty arthritis in the province of Ontario. J Rheumatol. 1988;15:1841–1847. [PubMed] [Google Scholar]
- 27.Bove SE, Laemont KD, Brooker RM, Osborn MN, Sanchez BM, Guzman RE, et al. Surgically induced osteoarthritis in the rat results in the development of both osteoarthritis-like joint pain and secondary hyperalgesia. Osteoarthritis Cartilage. 2006;14:1041–1048. doi: 10.1016/j.joca.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Kohara A, Nagakura Y, Kiso T, Toya T, Watabiki T, Tamura S, et al. Antinociceptive profile of a selective metabotropic glutamate receptor 1 antagonist YM-230888 in chronic pain rodent models. Eur J Pharmacol. 2007;571:8–16. doi: 10.1016/j.ejphar.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 29.McDougall JJ, Watkins L, Li Z. Vasoactive intestinal peptide (VIP) is a modulator of joint pain in a rat model of osteoarthritis. Pain. 2006;123:98–105. doi: 10.1016/j.pain.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira-Gomes J, Adaes S, Castro-Lopes JM. Assessment of movement-evoked pain in osteoarthritis by the knee-bend and CatWalk tests: a clinically relevant study. J Pain. 2008;9:945–954. doi: 10.1016/j.jpain.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Clarke KA, Heitmeyer SA, Smith AG, Taiwo YO. Gait analysis in a rat model of osteoarthrosis. Physiol Behav. 1997;62:951–954. doi: 10.1016/s0031-9384(97)00022-x. [DOI] [PubMed] [Google Scholar]
- 32.Berryman ER, Harris RL, Moalli M, Bagi CM. Digigait quantitation of gait dynamics in rat rheumatoid arthritis model. J Musculoskelet Neuronal Interact. 2009;9:89–98. [PubMed] [Google Scholar]
- 33.Yu LP, Jr, Smith GN, Jr, Brandt KD, Myers SL, O'Connor BL, Brandt DA. Reduction of the severity of canine osteoarthritis by prophylactic treatment with oral doxycycline. Arthritis Rheum. 1992;35:1150–1159. doi: 10.1002/art.1780351007. [DOI] [PubMed] [Google Scholar]
- 34.Madore E, Huneault L, Moreau M, Dupuis J. Comparison of trot kinetics between dogs with stifle or hip arthrosis. Vet Comp Orthop Traumatol. 2007;20:102–107. doi: 10.1160/vcot-06-06-0052. [DOI] [PubMed] [Google Scholar]
- 35.Brandt KD, Myers SL, Burr D, Albrecht M. Osteoarthritic changes in canine articular cartilage, subchondral bone, and synovium fifty-four months after transection of the anterior cruciate ligament. Arthritis Rheum. 1991;34:1560–1570. doi: 10.1002/art.1780341214. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh P, Read R, Armstrong S, Wilson D, Marshall R, McNair P. The effects of intraarticular administration of hyaluronan in a model of early osteoarthritis in sheep. I. Gait analysis and radiological and morphological studies. Semin Arthritis Rheum. 1993;22:18–30. doi: 10.1016/s0049-0172(10)80016-2. [DOI] [PubMed] [Google Scholar]
- 37.Cake M, Read R, Edwards S, Smith MM, Burkhardt D, Little C, et al. Changes in gait after bilateral meniscectomy in sheep: effect of two hyaluronan preparations. J Orthop Sci. 2008;13:514–523. doi: 10.1007/s00776-008-1279-6. [DOI] [PubMed] [Google Scholar]
- 38.Ghivizzani SC, Kang R, Georgescu HI, Lechman ER, Jaffurs D, Engle JM, et al. Constitutive intra-articular expression of human IL-1 beta following gene transfer to rabbit synovium produces all major pathologies of human rheumatoid arthritis. J Immunol. 1997;159:3604–3612. [PubMed] [Google Scholar]
- 39.Hedrick T. DLT dataviewer. Chapel Hill, NC: 2009. Self-published at http://www.unc.edu/~thedrick/software1.html. [Google Scholar]
- 40.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 41.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B, et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49:358–364. doi: 10.1111/j.1365-2559.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- 43.Janusz MJ, Bendele AM, Brown KK, Taiwo YO, Hsieh L, Heitmeyer SA. Induction of osteoarthritis in the rat by surgical tear of the meniscus: Inhibition of joint damage by a matrix metalloproteinase inhibitor. Osteoarthritis Cartilage. 2002;10:785–791. doi: 10.1053/joca.2002.0823. [DOI] [PubMed] [Google Scholar]
- 44.Chang A, Hurwitz D, Dunlop D, Song J, Cahue S, Hayes K, et al. The relationship between toe-out angle during gait and progression of medial tibiofemoral osteoarthritis. Ann Rheum Dis. 2007;66:1271–1275. doi: 10.1136/ard.2006.062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jenkyn TR, Hunt MA, Jones IC, Giffin JR, Birmingham TB. Toe-out gait in patients with knee osteoarthritis partially transforms external knee adduction moment into flexion moment during early stance phase of gait: a tri-planar kinetic mechanism. J Biomech. 2008;41:276–283. doi: 10.1016/j.jbiomech.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Varejao AS, Cabrita AM, Geuna S, Melo-Pinto P, Filipe VM, Gramsbergen A, et al. Toe out angle: a functional index for the evaluation of sciatic nerve recovery in the rat model. Exp Neurol. 2003;183:695–699. doi: 10.1016/s0014-4886(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 47.Betre H, Liu W, Zalutsky MR, Chilkoti A, Kraus VB, Setton LA. A thermally responsive biopolymer for intra-articular drug delivery. J Control Release. 2006;115:175–182. doi: 10.1016/j.jconrel.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 48.Shamji MF, Betre H, Kraus VB, Chen J, Chilkoti A, Pichika R, et al. Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist: sustained release of a local anti-inflammatory therapeutic. Arthritis Rheum. 2007;56:3650–3661. doi: 10.1002/art.22952. [DOI] [PubMed] [Google Scholar]
- 49.Allen KD, Adams SB, Setton LA. Evaluating intra-articular drug delivery for the treatment of osteoarthritis in a rat model. Tissue Eng Part B Rev. 16:81–92. doi: 10.1089/ten.teb.2009.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]