Abstract
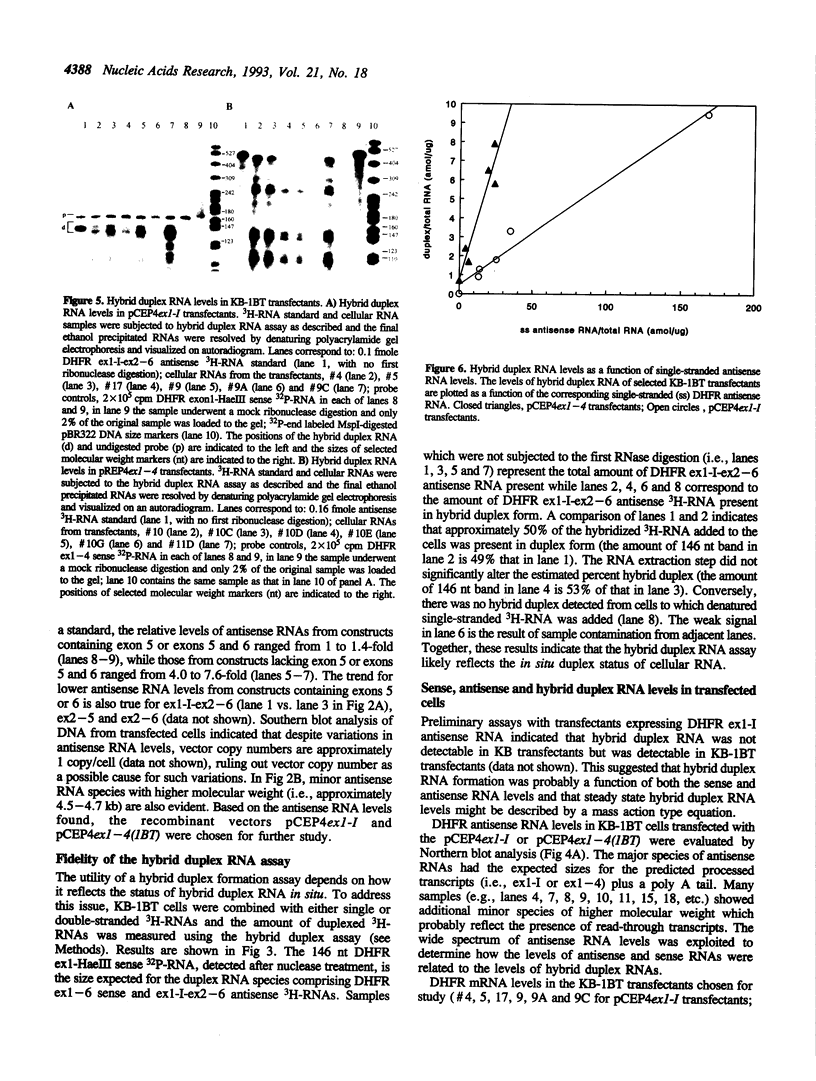
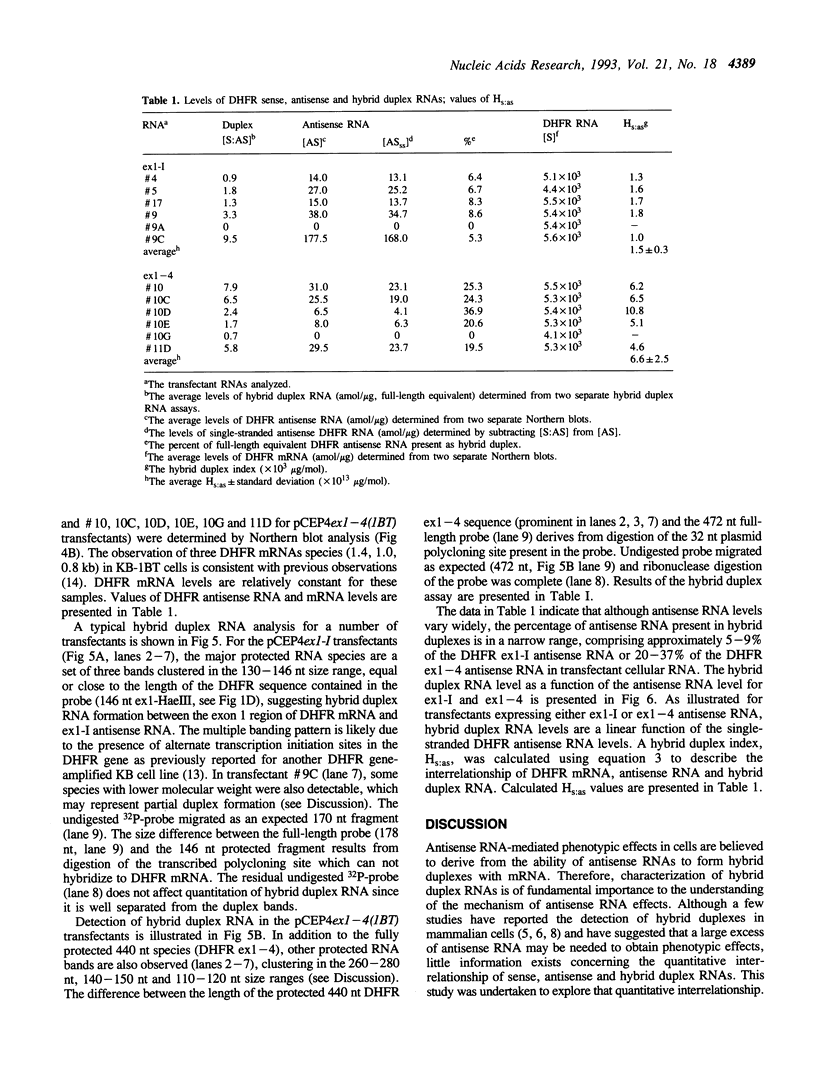
Previous studies have demonstrated that for an antisense RNA to be effective in attenuating gene expression, a large but indeterminate excess of antisense RNA is required. To quantitatively evaluate RNA hybrid duplex formation, expression vectors containing antisense dihydrofolate reductase (DHFR) cDNAs were transfected into KB and KB-1BT (a DHFR overexpressing variant) cells and transfectants expressing antisense transcripts of exon 1 through intron I (ex1-I) or exons 1 through 4 (ex1-4) were analyzed for hybrid duplex formation. Stable duplexes were detectable in KB-1BT but not in KB cells. Approximately 5-9% of antisense ex1-I RNA and 20-37% of antisense ex1-4 RNA were found in duplexes. The amount of each hybrid duplex RNA was found to be a linear function of intracellular single-stranded antisense RNA levels and a hybrid index, Hs:as, was devised to describe this relationship. Based upon the value of Hs:as for each antisense RNA:mRNA duplex, it is calculated that an approximate 2,800- and 600-fold excess of ex1-I and ex1-4 antisense RNA are respectively required for 50% of DHFR mRNA to be present in duplexes. Results support the hypothesis that intracellular sense:antisense RNA hybrid duplex formation is inefficient and dependent upon the levels, lengths and possibly the structures of the RNAs involved.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass B. L., Weintraub H. A developmentally regulated activity that unwinds RNA duplexes. Cell. 1987 Feb 27;48(4):607–613. doi: 10.1016/0092-8674(87)90239-x. [DOI] [PubMed] [Google Scholar]
- Bass B. L., Weintraub H. An unwinding activity that covalently modifies its double-stranded RNA substrate. Cell. 1988 Dec 23;55(6):1089–1098. doi: 10.1016/0092-8674(88)90253-x. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Bunch T. A., Goldstein L. S. The conditional inhibition of gene expression in cultured Drosophila cells by antisense RNA. Nucleic Acids Res. 1989 Dec 11;17(23):9761–9782. doi: 10.1093/nar/17.23.9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. J., Shimada T., Moulton A. D., Cline A., Humphries R. K., Maizel J., Nienhuis A. W. The functional human dihydrofolate reductase gene. J Biol Chem. 1984 Mar 25;259(6):3933–3943. [PubMed] [Google Scholar]
- Colman A. Antisense strategies in cell and developmental biology. J Cell Sci. 1990 Nov;97(Pt 3):399–409. doi: 10.1242/jcs.97.3.399. [DOI] [PubMed] [Google Scholar]
- Dolnick B. J. Antisense agents in cancer research and therapeutics. Cancer Invest. 1991;9(2):185–194. doi: 10.3109/07357909109044229. [DOI] [PubMed] [Google Scholar]
- Dolnick B. J., Pink J. J. 5-fluorouracil modulation of dihydrofolate reductase RNA levels in methotrexate-resistant KB cells. J Biol Chem. 1983 Nov 10;258(21):13299–13306. [PubMed] [Google Scholar]
- Dolnick B. J., Pink J. J. Effects of 5-fluorouracil on dihydrofolate reductase and dihydrofolate reductase mRNA from methotrexate-resistant KB cells. J Biol Chem. 1985 Mar 10;260(5):3006–3014. [PubMed] [Google Scholar]
- Dolnick B. J., Zhang Z. G., Hines J. D., Rustum Y. M. Quantitation of dihydrofolate reductase and thymidylate synthase mRNAs in vivo and in vitro by polymerase chain reaction. Oncol Res. 1992;4(2):65–72. [PubMed] [Google Scholar]
- Ecker J. R., Davis R. W. Inhibition of gene expression in plant cells by expression of antisense RNA. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5372–5376. doi: 10.1073/pnas.83.15.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groger R. K., Morrow D. M., Tykocinski M. L. Directional antisense and sense cDNA cloning using Epstein-Barr virus episomal expression vectors. Gene. 1989 Sep 30;81(2):285–294. doi: 10.1016/0378-1119(89)90189-3. [DOI] [PubMed] [Google Scholar]
- Hambor J. E., Hauer C. A., Shu H. K., Groger R. K., Kaplan D. R., Tykocinski M. L. Use of an Epstein-Barr virus episomal replicon for anti-sense RNA-mediated gene inhibition in a human cytotoxic T-cell clone. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4010–4014. doi: 10.1073/pnas.85.11.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J. L., Ma C. The mammalian dihydrofolate reductase locus. Biochim Biophys Acta. 1990 Oct 23;1087(2):107–125. doi: 10.1016/0167-4781(90)90195-8. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Redner R. L., Nienhuis A. W. An oligomer complementary to c-myc mRNA inhibits proliferation of HL-60 promyelocytic cells and induces differentiation. Mol Cell Biol. 1988 Feb;8(2):963–973. doi: 10.1128/mcb.8.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G. Antisense "pseudogenetics". Cell Motil Cytoskeleton. 1989;14(1):81–91. doi: 10.1002/cm.970140117. [DOI] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Constitutive and conditional suppression of exogenous and endogenous genes by anti-sense RNA. Science. 1985 Jul 26;229(4711):345–352. doi: 10.1126/science.2990048. [DOI] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Inhibition of thymidine kinase gene expression by anti-sense RNA: a molecular approach to genetic analysis. Cell. 1984 Apr;36(4):1007–1015. doi: 10.1016/0092-8674(84)90050-3. [DOI] [PubMed] [Google Scholar]
- Kim S. K., Wold B. J. Stable reduction of thymidine kinase activity in cells expressing high levels of anti-sense RNA. Cell. 1985 Aug;42(1):129–138. doi: 10.1016/s0092-8674(85)80108-2. [DOI] [PubMed] [Google Scholar]
- Krystal G. W., Armstrong B. C., Battey J. F. N-myc mRNA forms an RNA-RNA duplex with endogenous antisense transcripts. Mol Cell Biol. 1990 Aug;10(8):4180–4191. doi: 10.1128/mcb.10.8.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebhaber S. A., Cash F. E., Shakin S. H. Translationally associated helix-destabilizing activity in rabbit reticulocyte lysate. J Biol Chem. 1984 Dec 25;259(24):15597–15602. [PubMed] [Google Scholar]
- Maher L. J., 3rd, Dolnick B. J. Specific hybridization arrest of dihydrofolate reductase mRNA in vitro using anti-sense RNA or anti-sense oligonucleotides. Arch Biochem Biophys. 1987 Feb 15;253(1):214–220. doi: 10.1016/0003-9861(87)90654-0. [DOI] [PubMed] [Google Scholar]
- McGarry T. J., Lindquist S. Inhibition of heat shock protein synthesis by heat-inducible antisense RNA. Proc Natl Acad Sci U S A. 1986 Jan;83(2):399–403. doi: 10.1073/pnas.83.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A. Injected anti-sense RNAs specifically block messenger RNA translation in vivo. Proc Natl Acad Sci U S A. 1985 Jan;82(1):144–148. doi: 10.1073/pnas.82.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebagliati M. R., Melton D. A. Antisense RNA injections in fertilized frog eggs reveal an RNA duplex unwinding activity. Cell. 1987 Feb 27;48(4):599–605. doi: 10.1016/0092-8674(87)90238-8. [DOI] [PubMed] [Google Scholar]
- Shakin S. H., Liebhaber S. A. Destabilization of messenger RNA/complementary DNA duplexes by the elongating 80 S ribosome. J Biol Chem. 1986 Dec 5;261(34):16018–16025. [PubMed] [Google Scholar]
- Wagner R. W., Nishikura K. Cell cycle expression of RNA duplex unwindase activity in mammalian cells. Mol Cell Biol. 1988 Feb;8(2):770–777. doi: 10.1128/mcb.8.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Will C. L., Dolnick B. J. 5-Fluorouracil augmentation of dihydrofolate reductase RNA containing contiguous exon and intron sequences in KB7B cells. J Biol Chem. 1987 Apr 25;262(12):5433–5436. [PubMed] [Google Scholar]
- Will C. L., Dolnick B. J. 5-Fluorouracil augmentation of dihydrofolate reductase gene transcripts containing intervening sequences in methotrexate-resistant KB cells. Mol Pharmacol. 1986 Jun;29(6):643–648. [PubMed] [Google Scholar]
- Will C. L., Dolnick B. J. 5-Fluorouracil inhibits dihydrofolate reductase precursor mRNA processing and/or nuclear mRNA stability in methotrexate-resistant KB cells. J Biol Chem. 1989 Dec 15;264(35):21413–21421. [PubMed] [Google Scholar]
- Yokoyama K., Imamoto F. Transcriptional control of the endogenous MYC protooncogene by antisense RNA. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7363–7367. doi: 10.1073/pnas.84.21.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon G. Innovations in the use of antisense oligonucleotides. Ann N Y Acad Sci. 1990;616:161–172. doi: 10.1111/j.1749-6632.1990.tb17837.x. [DOI] [PubMed] [Google Scholar]







