Abstract
The heterologous production of iso-migrastatin (iso-MGS) was successfully demonstrated in an engineered S. lividans SB11002 strain, which was derived from S. lividans K4–114, following introduction of pBS11001, which harbored the entire mgs biosynthetic gene cluster. However, under similar fermentation conditions, the iso-MGS titer in the engineered strain was significantly lower than that in the native producer - Streptomyces platensis NRRL 18993. To circumvent the problem of low iso-MGS titers and to expand the utility of this heterologous system for iso-MGS biosynthesis and engineering, systematic optimization of the fermentation medium was carried out. The effects of major components in the cultivation medium, including carbon, organic and inorganic nitrogen sources, were investigated using a single factor optimization method. As a result, sucrose and yeast extract were determined to be the best carbon and organic nitrogen sources, resulting in optimized iso-MGS production. Conversely, all other inorganic nitrogen sources evaluated produced various levels of inhibition of iso-MGS production. The final optimized R2YE production medium produced iso-MGS with a titer of 86.5 mg/L, about 3.6-fold higher than that in the original R2YE medium, and 1.5 fold higher than that found within the native S. platensis NRRL 18993 producer.
Keywords: iso-Migrastatin, heterologous expression, Streptomyces lividans SB11002, Streptomyces platensis NRRL 18993, fermentation condition
1. Introduction
Natural products are a major resource for the discovery of novel drugs and drug leads, and play an important role in the battle against a myriad of human diseases [1]. However, the very low yield of most available natural products, either from microbial fermentation or total synthesis efforts, has greatly limited their use in mechanistic studies and clinical development. [2]. Recently, model microbial strains, not previously known as producers of specific natural products, have been shown capable of producing natural products following introduction of an appropriate biosynthetic gene cluster [3]. Given the extensive knowledge and the expedient tools available for producing these model organisms, such heterologous hosts represent an important advance in combinatorial biosynthesis, in attempts to make more readily accessible natural products whose titers from the native producers are insufficient to support subsequent development efforts. The feasibility of using heterologous hosts for natural product biosynthesis has been demonstrated previously [4–6], although the unexplored capacity of each heterologous host to produce its targeted natural product has posed a bottleneck for the further application of heterologous hosts.
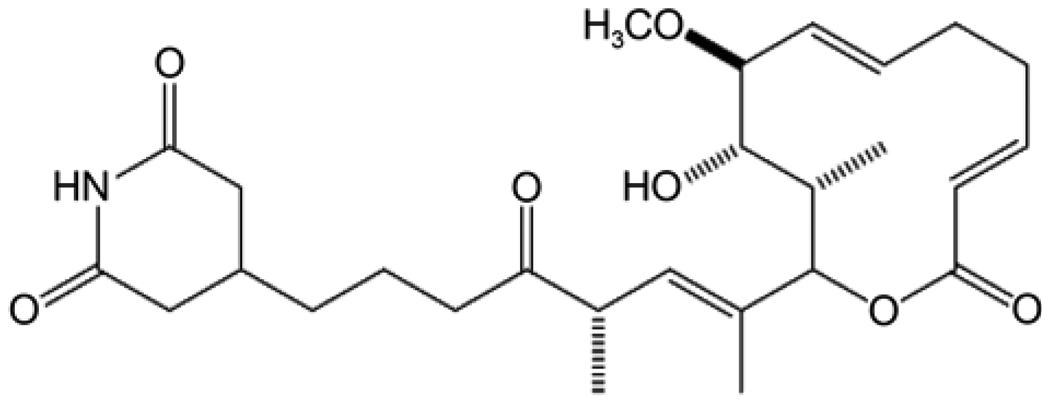
Iso-migrastatin (iso-MGS in Fig. 1) belongs to the class of glutarimide-containing polyketides [7]. Other members of this family include lactimidomycin, migrastatin, and dorrigocins, which have emerged and been actively pursued as outstanding candidates for antimetastasis agents [8–11]. iso-MGS is not only a potent inhibitor of human tumor cell migration, but is also a tool used to study cellular signal transduction, an antagonist of the muscarinic acetylcholine receptor and is associated with the suppression of multidrug resistance [12–14]. In previous work, the MGS gene cluster has been completely characterized, and a model for iso-MGS biosynthesis is proposed, based on functional assignments derived from bioinformatics and supported by the results of in vivo gene inactivation experiments [14]. The bacterial artificial chromosome (BAC) vector pBS11001, which contains the 65 kb inserts, including the entire mgs gene cluster and flanking regions derived from native iso-MGS producer Streptomyces platensis NRRL 18993, was successfully isolated and transformed into Streptomyces lividans K4–114, to generate the engineered SB11002 strain for heterologous production of iso-MGS. However, the titer of iso-MGS from SB11002 was significantly lower than that of the original strain [15]. A thorough examination of SB11002 as a producer of iso-MGS has not yet been undertaken.
Fig. 1.
Structure of iso-Migrastatin.
Our study describes the medium optimization efforts which have been explored to advance the engineered S. lividans SB11002 as the iso-MGS producing strain of choice. We have investigated the relationship between the major components of fermentation medium and the biosynthesis of iso-MGS using the single factor optimization method; the yield of iso-MGS was significantly improved using the fermentation medium resulting from these efforts.
2. Materials and Methods
2.1. Microorganisms
The engineered S. lividans strain SB11002 [i.e., S. lividans K4–114 (pBS11001)] and the native iso-MGS producer S. platensis NRRL 18993 were described previously [15] and maintained as spore suspensions in 20% glycerol at −20°C.
2.2. Medium and fermentation conditions
Inocula were prepared according to the published procedures previously described [7,15,16]. The original production medium - B2 medium (2% glycerol, 2% dextrin, 1% bacto soytone, 0.3% yeast extract, 0.2% (NH4)2SO4, and 0.2% CaCO3, pH 7.0) and R2YE medium (10% sucrose, 1% glucose, 0.5% yeast extract, 0.573% TES buffer, 1.012% MgCl2·6H2O, 0.025% K2SO4, 0.01% Difco Casamino acids, 2 mL/L Trace element solution) [17]; (15 mL/L, 20% L-Proline; 10 mL/L, 0.5% KH2PO4; 7 mL/L, 1 M NaOH; and 4 mL/L, 5 M CaCl2·2H2O; added after autoclaving), which have been previously used for iso-MGS production with the SB11002 strain [15], and commonly used YEME medium (1% glucose, 34% sucrose, 0.3% yeast extract, 0.5% Peptone, 0.3% Malt extract, and 2 mL/L 2.5 M MgCl2·6H2O added after autoclaving) were tested, respectively. The seed medium and production medium were almost identical except for the addition of 5% Amberlite XAD-16 resin (Sigma) in the production medium. Briefly, 50 µL of SB11002 strain spore suspension was added to 50 mL of seed medium in 250 mL flasks. The seed cultures were incubated on a rotary shaker at 250 rpm and 28°C for 72 h until cells reached the log phase. The resultant 2.4 mL of seed cultures were transferred to 30 mL of production medium in 250 mL flasks, and incubation continued on a rotary shaker at 250 rpm and 28°C for 5 to 6 days.
2.3. Isolation and HPLC analysis
Isolation of iso-MGS was carried out using previously described methods [7,15–18]. For HPLC analysis, a 15 µL aliquot of pretreated extracts was injected into an Agilent 1100 series HPLC system equipped with the Agilent UV detector. The mobile phase was comprised of buffer A (15% CH3CN in H2O containing 0.1% HOAc) and buffer B (80% CH3CN in H2O containing 0.1% HOAc). Analytical HPLC was conducted using a Hypersil BDS C18 reversed-phase analytical column (250 × 4.6 mm I.D., 5 µm particle) eluted with a linear gradient of 100% buffer A and 0% buffer B, to 20% buffer A and 80% buffer B over a 20 min period, followed by 10 min at 20% buffer A and 80% buffer B at a flow rate of 1.0 mL/min, with UV detection at 205 nm.
2.4. Fermentation medium optimization for the improvement of iso-MGS production
Based on the comparison of iso-MGS titers using B2, R2YE, and YEME media, respectively, the medium best suited to support high titer production of iso-MGS was used as the starting point for fermentation medium optimization. Different carbon sources, organic nitrogen sources, and inorganic nitrogen sources were studied individually to determine their effects on iso-MGS production. Following the identification of ideal carbon and nitrogen sources, experiments were carried out to identify concentrations of each component which resulted in optimized iso-MGS production. Optimal pH values and inoculum volumes were also determined on the basis of stepwise variations using a single variable manner. All experiments were performed in triplicate and all quantitative data were examined for statistical significance (p < 0.05).
3. Results
3.1. Comparison of iso-MGS production among different media
Three commonly used production media, including B2, R2YE, and YEME were evaluated. The titer of iso-MGS in each original medium was 11.4, 24, and 18.8 mg/L, respectively. Comparably, R2YE medium showed the best ability to support iso-MGS production and was therefore chosen as the starting point for fermentation optimization.
3.2. Effect of medium pH and inocula on the production of iso-MGS
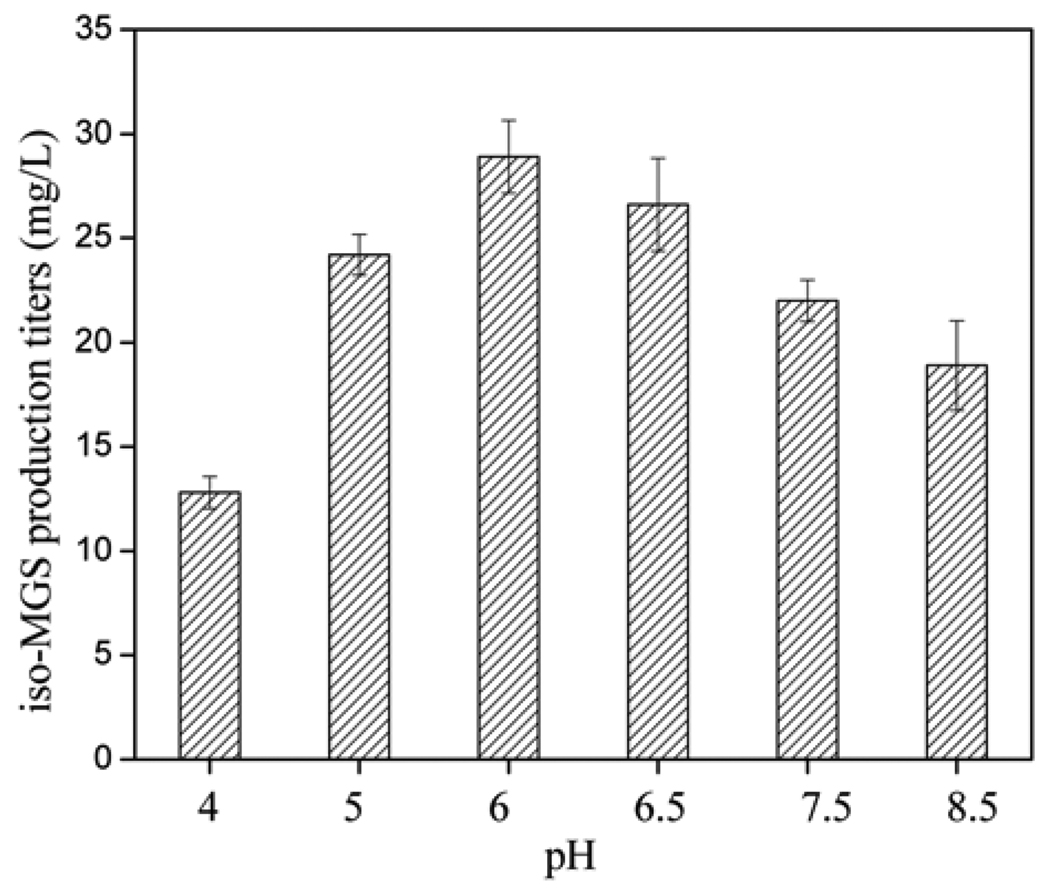
The pH of the fermentation environment is a critical factor found to affect both cell growth and secondary metabolite biosynthesis [19]. Although the exact relationship of pH to cell growth and metabolite production is still not clear, some elementary tests were carried out to find the pH capable of supporting high iso-MGS production. The effect of various initial pH values in the range of 4.0 ~ 8.5 (4.0, 5.0, and 6.0 in the first trial, 6.5, 7.5, and 8.5 in the second trial) was investigated. As shown in Fig. 2, the maximum yield of 28.9 mg/L iso-MGS was produced when the pH of fermentation medium was 6.0. Thus, a pH of 6.0 was selected for all subsequent fermentation media optimization trials.
Fig. 2.
iso-MGS titers as a function of initial pH.
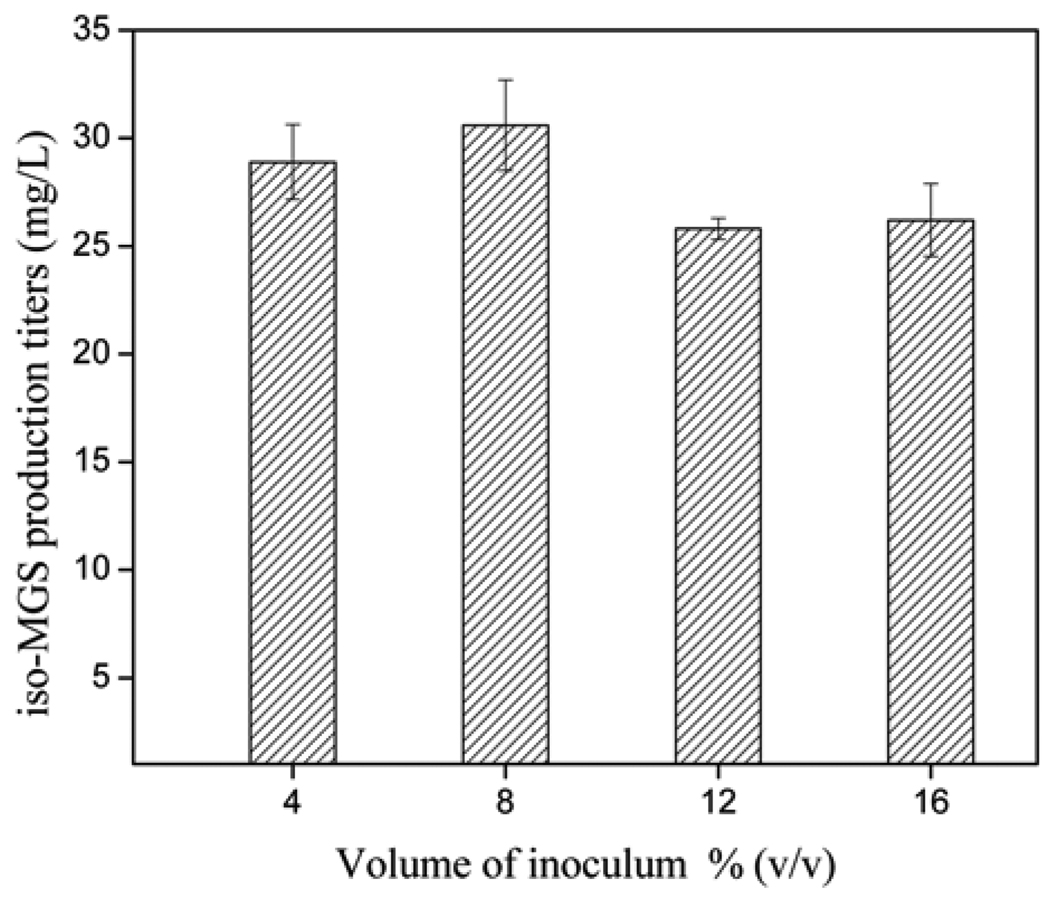
The volume of each inoculum was another important factor found to affect the fermentation period and iso-MGS titers [20]. The ideal inoculum size should effectively balance the cell number and the nutritional material in the broth culture. As shown in Fig. 3, the maximum yield of iso-MGS reached 30.6 mg/L when the volume of inoculum was 8% (v/v) of the total fermentation culture. Thus, inocula used in all subsequent experiments were set to a volume corresponding to 8% (v/v) of the final medium volume, following inoculum addition.
Fig. 3.
iso-MGS titers as a function of inoculum volumes (as v/total v %).
3.3. Effect of carbon sources on the iso-MGS production
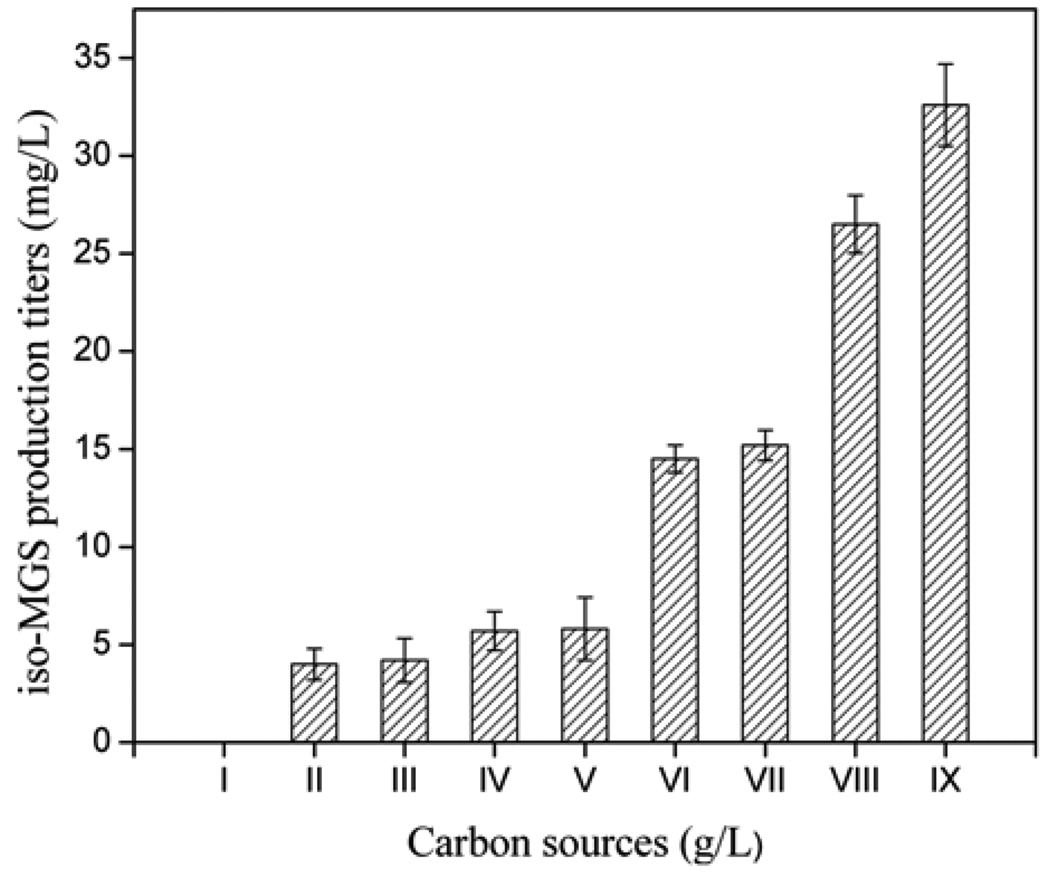
The medium carbon source is essential to support both cell growth and various metabolic pathways [21]. Nine different carbon sources including xylose, fructose, galactose, glycerol, mannitol, dextrin, starch, maltose, and sucrose were tested, each in the concentration ranging from 50 to 200 g/L, together with 20 g/L glucose (Fig. 4). It was found that xylose could not be utilized by SB11002; fructose, galactose, glycerol, and mannitol could only be poorly used by SB11002. The use of dextrin, starch, maltose, and sucrose resulted in iso-MGS titers of 14.5, 15.2, 26.5, and 32.6 mg/L, respectively. Thus, sucrose was chosen as the main carbon source in combination with 20 g/L glucose which was constant in R2YE medium.
Fig. 4.
iso-MGS titers as a function of differing carbon sources. I: Xylose, II: Fructose, III: L-galactose, IV: Glycerol, V: Mannitol, VI: Dextrin, VII: Starch, VIII: Maltose, and IX: Sucrose.
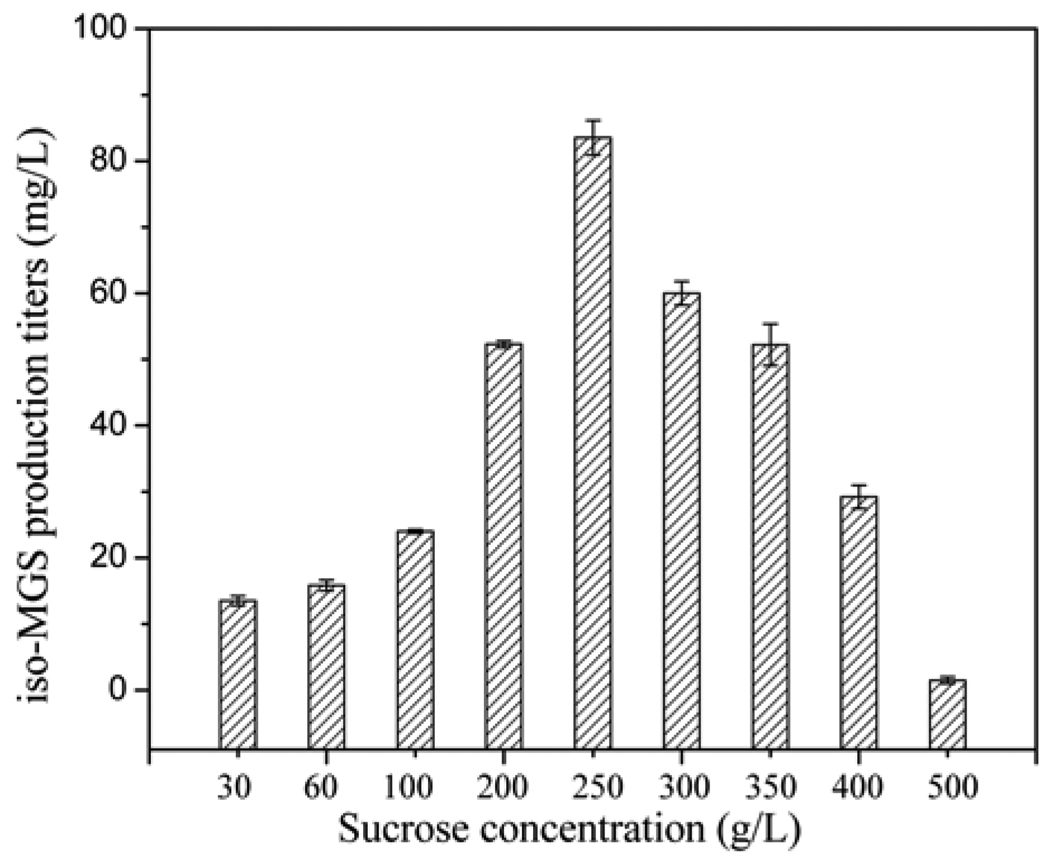
Successive experiments were conducted to determine the optimal sucrose concentration. The concentrations of sucrose in the different media vary significantly; R2YE medium contains 100 g/L sucrose while YEME medium contains up to 340 g/L sucrose. Thus, various initial sucrose concentrations in the range of 30.0 ~ 550 g/L were investigated via two series of experiments (30, 60, and 100 mg/L in the first trial; and 100 ~ 500 mg/L in the second trial). As shown in Fig. 5, the yield of iso-MGS peaked at 83.5 mg/L when sucrose concentration was increased up to 250 g/L. Concentrations of sucrose higher than 250 g/L resulted in diminished iso-MGS titers.
Fig. 5.
Maximum iso-MGS titers as a function of different initial sucrose concentrations.
3.4. Effect of organic nitrogen sources on the iso-MGS production
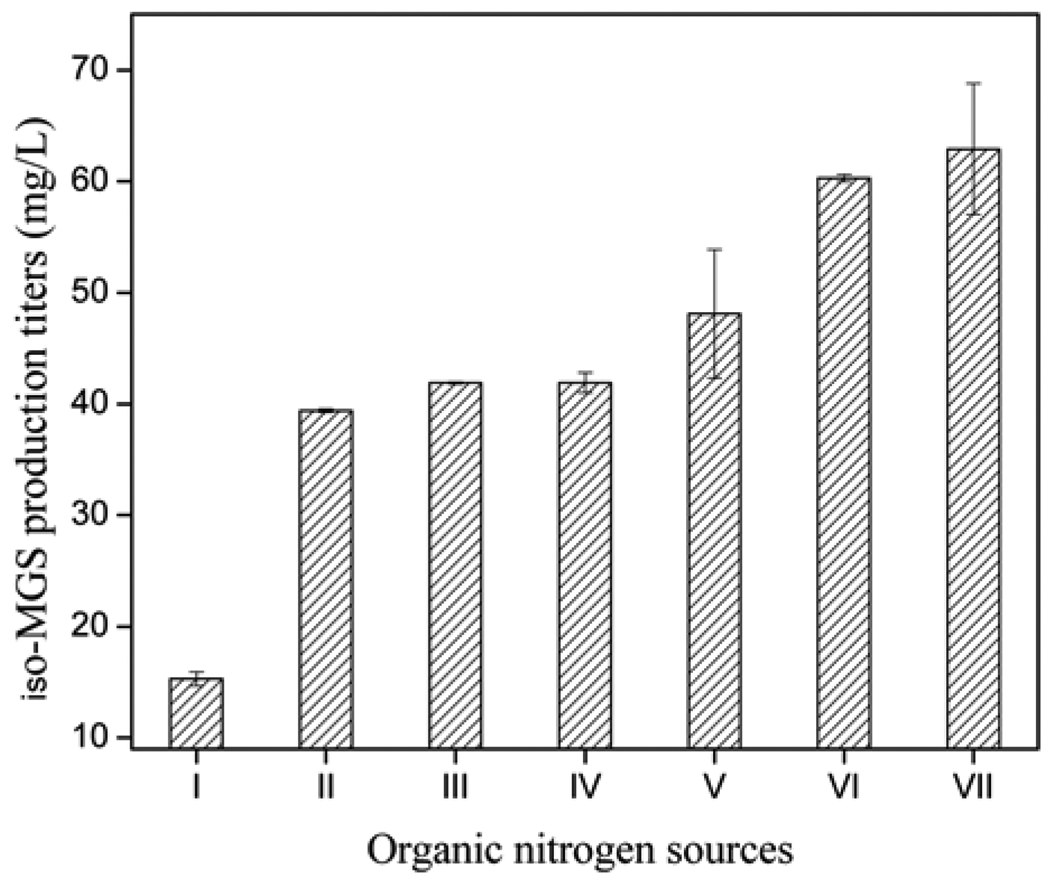
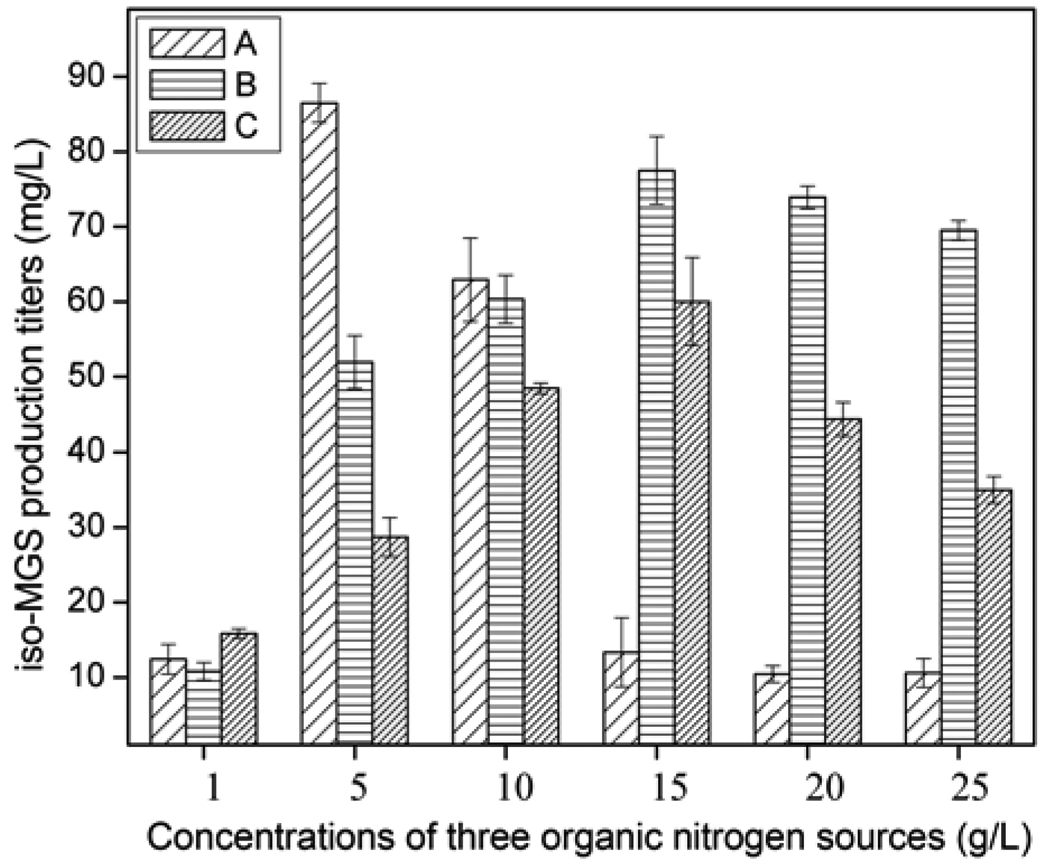
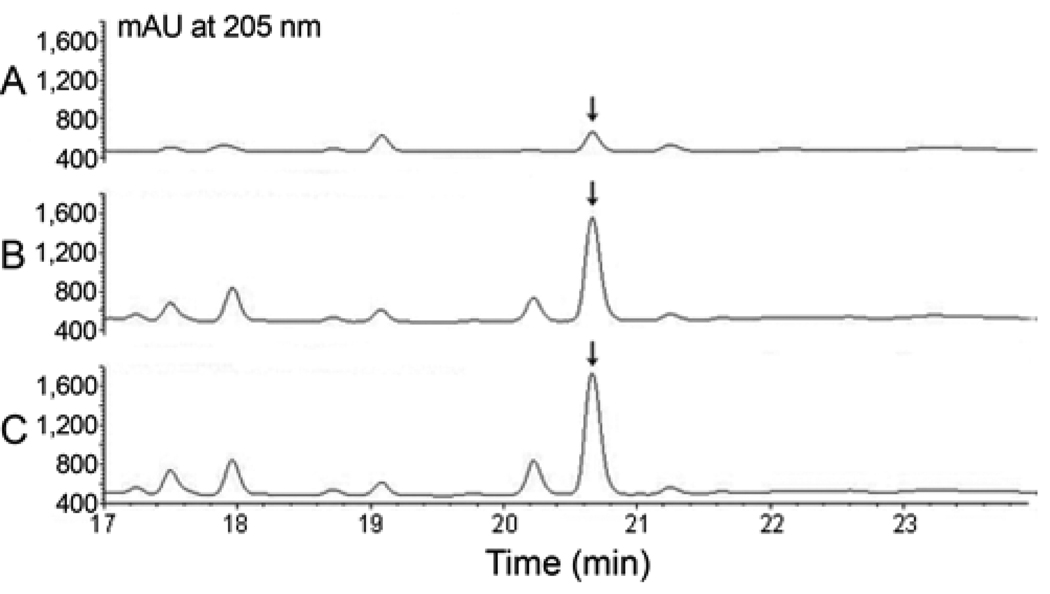
Seven different organic nitrogen sources including polypeptone, malt extract, corn gluten meal, corn flour, fish meal, soybean flour, and yeast extract were tested respectively, at a concentration of 10 g/L. As shown in Fig. 6, polypeptone was poorly utilized by SB11002; malt extract, corn gluten meal, corn flour, fish meal, and soybean flour could only support moderate levels of iso-MGS production; yeast extract, soybean flour, and fish meal could support high levels of iso-MGS production. Among them, relative to soybean flour and fish meal, yeast extract was found to be the best organic nitrogen source, leading to satisfactory cell growth and the highest titers of iso-MGS. However, since the optimal concentration of nitrogen source was not determined, the effects of different initial concentrations of these three nitrogen sources were further investigated in the concentration range of 1.0 ~ 25 g/L to avoid possible neglect (Fig. 7). The comparison results indicated that the maximum iso-MGS production titer, 86.5 mg/L, was achieved when a concentration of 5 g/L yeast extract was used. Higher concentrations of this nitrogen source resulted in a dramatic decrease of iso-MGS titer. Thus, we determined that the original nitrogen source component in the R2YE medium was already the optimal one, after studies to optimize the carbon source. The HPLC profiles of iso-MGS extracts revealed the obvious improvement of iso-MGS concentration after the optimization of carbon and nitrogen sources (Fig. 8).
Fig. 6.
Iso-MGS titers as a function of different 10 g/L organic nitrogen sources. I: Polypeptone, II: Malt extract, III: Corn Gluten meal, IV: corn flour, V: Fish meal, VI: Soybean flour, and VII: Yeast extract.
Fig. 7.
Maximum iso-MGS titers as a function of different initial organic nitrogen source concentrations. A: Yeast extract, B: Soybean flour, and C: Fish meal.
Fig. 8.
HPLC of iso-MGS XAD-16 extracts derived from different medium compositions. A: Initial medium compositions, B: Optimal carbon source, and C: Optimal organic nitrogen source. Arrow indicates iso-MGS peak.
3.5. Effect of inorganic nitrogen sources on the iso-MGS production
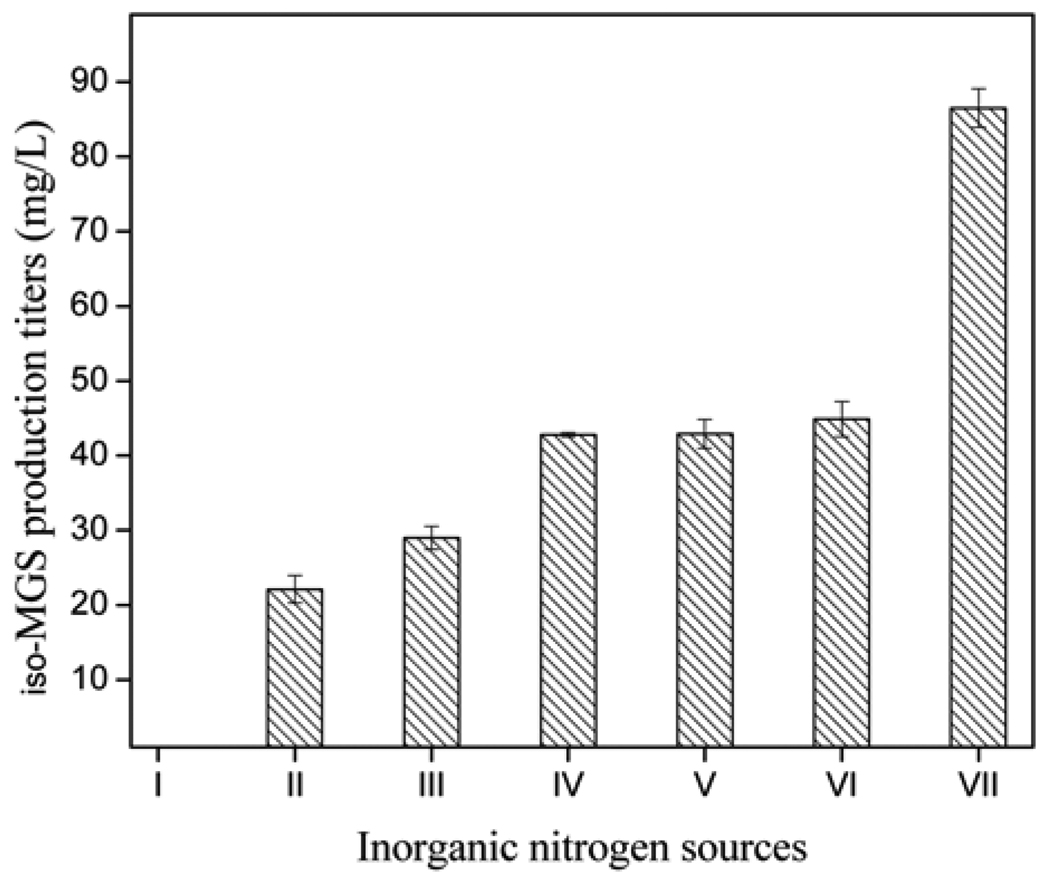
After the major components of the fermentation medium were determined, six different inorganic nitrogen sources including NH4NO3, NaNO3, (NH4)2SO4, NH4Cl, Ca(NO3)2 ·4H2O, and KNO3 each at a concentration of 0.02 g/L were investigated. A medium devoid of any inorganic nitrogen source served as the control. The results revealed that addition of NH4NO3 totally abolished iso-MGS production and the addition of NaNO3, (NH4)2SO4, NH4Cl, Ca(NO3)2 ·4H2O or KNO3 all negatively impaired the iso-MGS production compared with the control medium (Fig. 9). Thus, efficient iso-MGS production was found to require the absence of all the evaluated inorganic nitrogen sources.
Fig. 9.
iso-MGS titers as a function of different 0.02 g/L inorganic nitrogen sources. I: NH4NO3, II: NaNO3, III: (NH4)2SO4, IV: NH4Cl, V: Ca(NO3)2.4H2O, VI: KNO3, and VII: Control.
4. Discussion
Natural product production in heterologous model hosts represents a new and powerful means by which to obtain low titer natural products. However, natural product yields in the engineered heterologous hosts are commonly lower at the outset than in the native producers. Low initial titers in heterologous hosts are likely attributable to the lack of a native environment necessary for the full expression of the target gene cluster [22]. Thus, successful expression of the target gene cluster in the heterologous host may not initially satisfy the requirements for high natural product titers. The engineered heterologous producers must be complemented with the proper choice of fermentation conditions necessary to support cell growth and efficient functional expression of the target biosynthetic machinery.
iso-MGS, isolated originally from S. platensis NRRL 18993, has been previously produced heterologously in the engineered strain S. lividans SB11002. However, the potential of this heterologous system for iso-MGS production was not fully realized as reflected by the heterologous iso-MGS titer of 25 mg/L in SB11002 relative to the titer of 58 mg/L produced by the native S. platensis strain [15]. To explore the capacity of SB11002 to produce iso-MGS, single factor optimization was applied to improve and optimize the SB11002 fermentation medium. Comparative analysis of carbon and nitrogen sources revealed that sucrose and yeast extract are ideally suited to support iso-MGS production in the recombinant host SB11002 strain. The ideal concentrations of these two main components were also identified and found to be 250 g/L sucrose and 5 g/L yeast extract. Additionally, the effects of different inorganic nitrogen sources were evaluated; all inorganic nitrogen sources evaluated negatively impacted iso-MGS production, thus suggesting that such components are best avoided in the SB11002 fermentation medium. Finally, the acquired data allowed the formulation of an optimized production medium containing: 250 g/L sucrose, 20 g/L glucose, 5 g/L yeast extract, 5.73 g/L TES buffer, 10.12 g/L MgCl2·6H2O, 0.25 g/L K2SO4, 0.1 g/L Difco casamino acids, and 2 mL/L trace element solution [17].
The use of genetic engineering and subsequent combinatory biosynthesis are potential approaches to generate novel active natural products or related analogs. However, compromised metabolite production, resulting in low amounts of metabolites produced by engineered strains, is a common problem for combinatorial biosynthesis. Successful and effective genetic manipulation of biosynthetic gene clusters is only one prerequisite for the practical application of combinatorial biosynthesis. Titer improvement is also essential to the fulfillment of the combinatorial biosynthesis premise for practical drug discovery and development. The iso-MGS titer of the recombinant strain SB11002 in the optimized production medium was 86.5 mg/L (Fig. 9), 3.6-fold higher than that in the original R2YE medium, and ~1.5-fold higher than that in the native producer. This exciting result is a good paradigm to exhibit the potential of combining contemporary combinatorial biosynthesis strategies with proven methods of traditional strain improvement and fermentation optimization. This strategy not only reveals the power of heterologous hosts in natural product production, but enables further extensions of this novel approach to other natural products, and encourages further investigation of iso-MGS and its related congeners in mechanistic and clinical development studies.
Acknowledgement
This work was financially supported by NIH grant CA 94426 (B.S.), and Nature Science Foundation of China grant No. 20736008, 20806068 and 20676115 (Z.X).
Contributor Information
Xueyun Wu, Institute of Biochemistry, Zhejiang Sci-Tech University, Hangzhou 310-018, China; Department of Chemical and Biological Engineering, Institute of Bioengineering, Zhejiang University, Hangzhou 310-027, China.
Dong Yang, Department of Chemical and Biological Engineering, Institute of Bioengineering, Zhejiang University, Hangzhou 310-027, China.
Xiangcheng Zhu, Department of Chemical and Biological Engineering, Institute of Bioengineering, Zhejiang University, Hangzhou 310-027, China.
Zhiyang Feng, Division of Pharmaceutical Science, School of Pharmacy, University of Wisconsin-Madison, Madison, WI 53705, USA.
Zhengbin Lv, Institute of Biochemistry, Zhejiang Sci-Tech University, Hangzhou 310-018, China.
Yaozhou Zhang, Institute of Biochemistry, Zhejiang Sci-Tech University, Hangzhou 310-018, China.
Ben Shen, Division of Pharmaceutical Science, School of Pharmacy, University of Wisconsin-Madison, Madison, WI 53705, USA.
Zhinan Xu, Department of Chemical and Biological Engineering, Institute of Bioengineering, Zhejiang University, Hangzhou 310-027, China.
References
- 1.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 2.Bode HB, Muller R. The impact of bacterial genomics on natural product research. Angew. Chem. Int. Ed. 2005;44:6828–6846. doi: 10.1002/anie.200501080. [DOI] [PubMed] [Google Scholar]
- 3.Wendt-Pienkowski E, Huang Y, Zhang J, Li B, Jiang H, Kwon H, Hutchinson CR, Shen B. Cloning, sequencing, analysis, and heterologous expression of the fredericamycin biosynthetic gene cluster from Streptomyces griseus. J. Am. Chem. Soc. 2005;127:16442–16452. doi: 10.1021/ja054376u. [DOI] [PubMed] [Google Scholar]
- 4.Sosio M, Giusino F, Cappellano C, Bossi E, Puglia AM, Donadio S. Artificial chromosomes for antibiotic-producing actinomycetes. Nat. Biotechnol. 2000;18:343–345. doi: 10.1038/73810. [DOI] [PubMed] [Google Scholar]
- 5.Martinez A, Kolvek SJ, Yip CLT, Hopke J, Brown KA, MacNeil IA, Osburne MS. Genetically modified bacterial strains and novel bacterial artificial chromosome shuttle vectors for constructing environmental libraries and detecting heterologous natural products in multiple expression hosts. Appl. Env. Microb. 2004;70:2452–2463. doi: 10.1128/AEM.70.4.2452-2463.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang HR, Wang Y, Pfeifer BA. Bacterial hosts for natural product production. Mol. Pharmacol. 2008;5:212–225. doi: 10.1021/mp7001329. [DOI] [PubMed] [Google Scholar]
- 7.Ju J, Lim SK, Jiang H, Shen B. Migrastatin and dorrigocins are shunt metabolites of iso-migrastain. J. Am. Chem. Soc. 2005;127:1622–1623. doi: 10.1021/ja043808i. [DOI] [PubMed] [Google Scholar]
- 8.Shan D, Chen L, Njardarson JT, Gaul C, Ma X, Danishefsky SJ, Huang XY. Synthetic analogues of migrastatin that inhibit mammary tumor metastasis in mice. Proc. Natl. Acad. Sci. 2005;102:3772–3776. doi: 10.1073/pnas.0500658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metaferia BB, Chen L, Baker HL, Huang XY, Bewley CA. Synthetic macrolides that inhibit breast cancer cell migration in vitro. J. Am. Chem. Soc. 2007;129:2434–2435. doi: 10.1021/ja068538d. [DOI] [PubMed] [Google Scholar]
- 10.Reymond S, Cossy J. Migrastatin and analogues: New anti-metastatic agents. Comptes Rendus Chim. 2008;11:1447–1462. [Google Scholar]
- 11.Ju J, Rajski SR, Lim SK, Seo JW, Peters NR, Hoffmann F, Shen B. Lactimidomycin, iso-migrastatin and related glutarimide-containing 12-membered macrolides are extremely potent inhibitors of cell migration. J. Am. Chem. Soc. 2009;131:1370–1371. doi: 10.1021/ja808462p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakae K, Nishimura Y, Ohba S, Akamatsu Y. Migrastatin acts as a muscarinic acetylcholine receptor antagonist. J. Antibiot. 2006;59:685–692. doi: 10.1038/ja.2006.91. [DOI] [PubMed] [Google Scholar]
- 13.Takemoto Y, Tashiro E, Imoto M. Suppression of multidrug resistance by migrastatin. J. Antibiot. 2006;59:435–438. doi: 10.1038/ja.2006.62. [DOI] [PubMed] [Google Scholar]
- 14.Lim SK, Ju J, Zazopoulos E, Jiang H, Seo JW, Chen Y, Feng Z, Rajski SR, Farnet CM, Shen B. iso-Migrastatin, migrastatin, and dorrigocin production in Streptomyces platensis NRRL 18993 is governed by single biosynthetic machinery featuring an acyltransferase-less Type I polyketide synthase. J. Biol. Chem. 2009;284:29746–29756. doi: 10.1074/jbc.M109.046805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Z, Wang L, Rajski SR, Xu Z, Coeffet-LeGal MF, Shen B. Engineered production of iso-migrastatin in heterologous hosts. Bioorg. Med. Chem. 2009;17:2147–2153. doi: 10.1016/j.bmc.2008.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ju J, Lim SK, Jiang H, Seo JW, Shen B. Isomigrastatin congeners from Streptomyces platensis and generation of a glutarimide polyketide library featuring the dorrigocin, lactimidomycin, migrastatin, and NK 30424 scaffolds. J. Am. Chem. Soc. 2005;127:11930–11931. doi: 10.1021/ja053118u. [DOI] [PubMed] [Google Scholar]
- 17.Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. Practical Streptomyces genetics: A Laboratory Manual. 2nd Ed. Norwich, U.K: The John Innes Foundation; 2000. [Google Scholar]
- 18.Ju J, Lim SK, Jiang H, Seo JW, Her Y, Shen B. Thermolysis of Iso-migrastatin and its congeners via [3,3]-sigmatropic rearrangment: A new route to the synthesis of migrastatin and its analogues. Org. Lett. 2006;8:5865–5868. doi: 10.1021/ol062470p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buese M, Kopmann A, Diekmann H, Thoma M. Oxygen, pH value, and carbon sources induced changes of the mode of oscillation in synchronous continuous culture of Saccharomyces cerevisiae. Biotechnol. Bioeng. 1999;63:410–417. doi: 10.1002/(sici)1097-0290(19990520)63:4<410::aid-bit4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura M, Pitsch BL. Effect of size of inocula on growth of Shigella sonnei in a chemically defined medium. Can. J. Microbiol. 1961;7:848–849. doi: 10.1139/m61-106. [DOI] [PubMed] [Google Scholar]
- 21.Olano C, Lombó F, Méndez C, José AS. Improving production of bioactive secondary metabolites in actinomycetes by metabolic engineering. Metab. Eng. 2008;10:281–292. doi: 10.1016/j.ymben.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Galm U, Shen B. Expression of biosynthetic gene cluster in heterologous hosts for natural product production and combinatorial biosynthesis. Expert Opin. Drug Discov. 2006;1:409–437. doi: 10.1517/17460441.1.5.409. [DOI] [PubMed] [Google Scholar]