Abstract
This study presents the removal of trace level arsenic to meet drinking water standards using an iron oxide-multi-walled carbon nanotube (Fe-MWCNT) hybrid as a sorbent. The synthesis was facilitated by the high degree of nanotube functionalization using a microwave assisted process, and a controlled assembly of iron oxide was possible where the MWCNT served as an effective support for the oxide. In the final product, 11 % of the carbon atoms were attached to Fe. The Fe-MWCNT was effective in arsenic removal to below the drinking water standard levels of 10 µg L−1. The absorption capacity of the composite was 1723 µg g−1 and 189 µg g−1 for As(III) and As(V) respectively. The adsorption of As(V) on Fe-MWCNT was faster than that of As(III). The pseudo-second order rate equation was found to effectively describe the kinetics of arsenic adsorption. The adsorption isotherms for As(III) and As(V) fitted both the Langmuir and Freundlich models.
Keywords: Arsenic, carbon nanotubes, iron oxide, water, adsorption, kinetics
Introduction
Arsenic in natural waters is a problem that affects many parts of the world including those in North America and Asia1–3. Arsenic is also listed as a carcinogenic contaminant which is responsible for other health effects such as spontaneous abortion 4 and diabetes5. As a result of this, more stringent standards are being established by the US-EPA6. Typically, arsenite [As(III)], which is neutral, uncharged and a soluble molecule, is considered more toxic than arsenate or As(V)7,8. Existing technologies for arsenic removal include oxidation/precipitation, coagulation/coprecipitation, nanofiltration, reverse osmosis, electrodialysis, adsorption, ion exchange, foam flotation, solvent extraction and bioremediation9. Most of these techniques are well established, and have their merits and inherent limitations such as the generation of toxic waste, low arsenic removal efficiencies and/or high cost 9.
Adsorption has proven to be an efficient method for arsenic removal and a wide range of materials including lanthanum/iron compounds, mineral oxides, and biological materials10 have been studied. The use of polymeric resins, activated carbon, activated alumina, iron coated sand11, hydrous ferric oxide12, and natural ores have generated much interest and novel metal modified adsorbents have demonstrated superior performance13,14. At present there is a need for the development of higher capacity sorbents for arsenic removal that will be effective at the trace level so that they may be used for drinking water purification.
Carbon nanotubes (CNTs) are made from graphene sheets seamlessly rolled into cylindrical tubes, and are found as single-walled (SWCNT) and multiwall carbon nanotubes (MWCNT), with the latter being relatively inexpensive15,16. Their unique characteristics such as high aspect ratio, superior mechanical, electrical and thermal properties make them well suited for many applications. CNTs also exhibit exceptional sorption properties towards various organic compounds and inorganic ions17. The potential for sidewall functionalization and surface modification make them attractive as support phases for water treatment18–20. Recently we have reported the functionalization of carbon nanotubes with tetragonal zirconyl oxide particles, which have proved effective in water defluoridation21. A recent study has reported the implementation of a fabric supported magnetite multiwalled carbon nanotube composite based supercapacitor for removal of sodium chloride and arsenic at high mg L−1 levels from sea water22. From the standpoint of practical applications, the CNTs can be implemented in water treatment as a replacement for activated carbon with the added advantage that they can be self assembled on supports via chemical vapor deposition23, and can also be immobilized in membranes24 and filters22. However, cost and other factors need to be taken into consideration before such a process could be commercialized.
The goal of this project was to synthesize iron oxide-MWCNT using highly functionalized nanotubes and study the removal of µg L−1 levels of arsenic from water, to meet the US EPA drinking water standard of 10 µg L−1.
Experimental
Materials and Methods
Multiwall carbon nanotubes (OD (20 to 40) nm, Purity 95%) were purchased from Cheap Tubes Inc., and all other chemicals were purchased from Sigma Aldrich with purity higher than 95 %. Ten mg L−1 stock solutions of As(III) and As(V) were prepared by dissolving weighed amounts of NaAsO2 and Na2HAsO4 respectively in measured volumes of MilliQ water. The stock solutions were preserved with 1% trace metal grade HNO3. 1mg L−1 working solutions were then prepared from the stock for analysis. pH values were maintained constant during the analysis using 100 mM acetate buffer (pH 4 and 5) and 0.1 M Tris buffer (pH 6 – 8).
The MWCNT was functionalized in a Microwave Accelerated Reaction System (Mode: CEM Mars) fitted with internal temperature and pressure controls according to an experimental procedure previously published by our laboratory25,26. Pre-weighed amounts of purified MWCNT were treated with a mixture of concentrated H2SO4 and HNO3 solution by subjecting them to microwave radiation at 120 °C for (20 to 40) min. This led to the formation of carboxylic groups on the surface along with some sulphonation and nitration. The resulting solid was filtered through a 10 µm membrane filter, washed with water to a neutral pH and dried under vacuum at 80 °C to a constant weight. This product (f-MWCNT) was used in the subsequent synthesis of the Fe-MWCNT composite.
The iron oxide coated (Fe-MWCNT) hybrid was synthesized using a method reported previously22,27. This was accomplished by dispersing a weighed amount of the f-MCWNT in a 2:1 aqueous solution of FeCl3: FeSO4 under ultrasonication at room temperature. The dispersion was gently stirred at 70 °C for 5 minutes, after which 5 M NaOH solution was added and stirred vigorously at 85 °C for 1 hour. The pH of the reaction was kept between 5 and 11 with the final pH being 10. The composite thus formed was filtered through a 10 µm membrane filter paper, washed and acidified to pH 5 with a few drops of 1 M hydrochloric acid and dried under vacuum at 105 °C for 24 hours.
The Fe-MWCNT was characterized using a scanning electron microscope (SEM) fitted with an energy dispersive x-ray spectrometer (EDS), thermogravimetric analysis (TGA), Fourier Transform Infrared spectroscopy (FTIR) and BET surface area. SEM data was collected on a LEO 1530 VP scanning electron microscope equipped with an energy-dispersive X-ray analyzer. Energy dispersive spectroscopic (EDS) data was collected on the EDAX silicon drift detector (SDD) (Apollo XV) mounted on a Hitachi S-3000N electron microscope with specific light element performance. TGA was performed using a Pyris 1 TGA from Perkin-Elmer Inc from 30 °C to 900 °C under a flow of air at 10 mL/min, at a heating rate of 10 °C per min. FTIR measurements were carried out in purified KBr pellets using a Perkin-Elmer (Spectrum One) instrument. Specific surface area, micropore volume, and average pore radius were measured using a Quantachrome NOVA 3000 series (Model N32-11) High Speed Gas Sorption Analyzer at 77.40 K. Before each experiment, the samples were heated at 180 °C and degassed at this temperature until constant vacuum for four hours. The pH of point of zero charge (pHpzc) was determined based on a previously published procedure13.
Kinetics and Adsorption Isotherms
10 mL of 100 µg L−1 arsenic solution [As(III) and As(V)] was contacted with 0.01 g of the adsorbent in a series of conical flasks and samples were collected at (5, 10, 15, 30, and 45) min and (1, 3, 6, 12, 15 and 24) h for kinetic studies. The mass of the adsorbent was varied from (0.01 to 0.1) g in the isothermal adsorption studies keeping all other parameters constant (equilibrium contact time 1 h and 12 h for As(V) and As(III) respectively and pH 4). The arsenic solutions and the adsorbents were mixed thoroughly at a speed of 175 rpm on a platform shaker (Lab systems Wellmix). The mixture was filtered through a 0.45 µm membrane syringe filter.
Residual arsenic was measured using Agilent 7500 ICP-MS. All standards were prepared from multi-element solution 2A, 10 mg L−1 (Spex Certiprep) with addition of an internal standard mix (Li6, Ge, Y, In, Tb, Bi). A Buffer solution was used for all dilutions. Multi-element instrument calibration standard (No. 1, 20 mg/L (Spex Certiprep)) was used for the verification of the calibration.
Results and Discussion
Sorbent Characterization
The different oxide forms expected in the Fe-MWCNT composite are: magnetite (Fe3O4), maghemite (γ-Fe2O3), hematite (α-Fe2O3) and goethite (α-FeO(OH))27. Acid functionalization of the carbon nanotubes produced carboxylic groups on the surface and this enhanced iron oxide loading. The BET surface area of MWCNT and Fe-MWCNT were 110 m2 g−1 and 153 m2 g−1 respectively, which shows that the surface area of the carbon nanotubes was increased by approximately 40 % with the iron oxide coating. The pH at zero point charge (pHZPC) for MWCNT, f-MWCNT and Fe-MWCNT were 6.8, 3.91 and 7.35 respectively. This is the point where the surface charge of the carbon nanotube is independent of the electrolyte concentration. Therefore the carboxylic groups on the f-MWCNT had been replaced in the Fe-MWCNT.
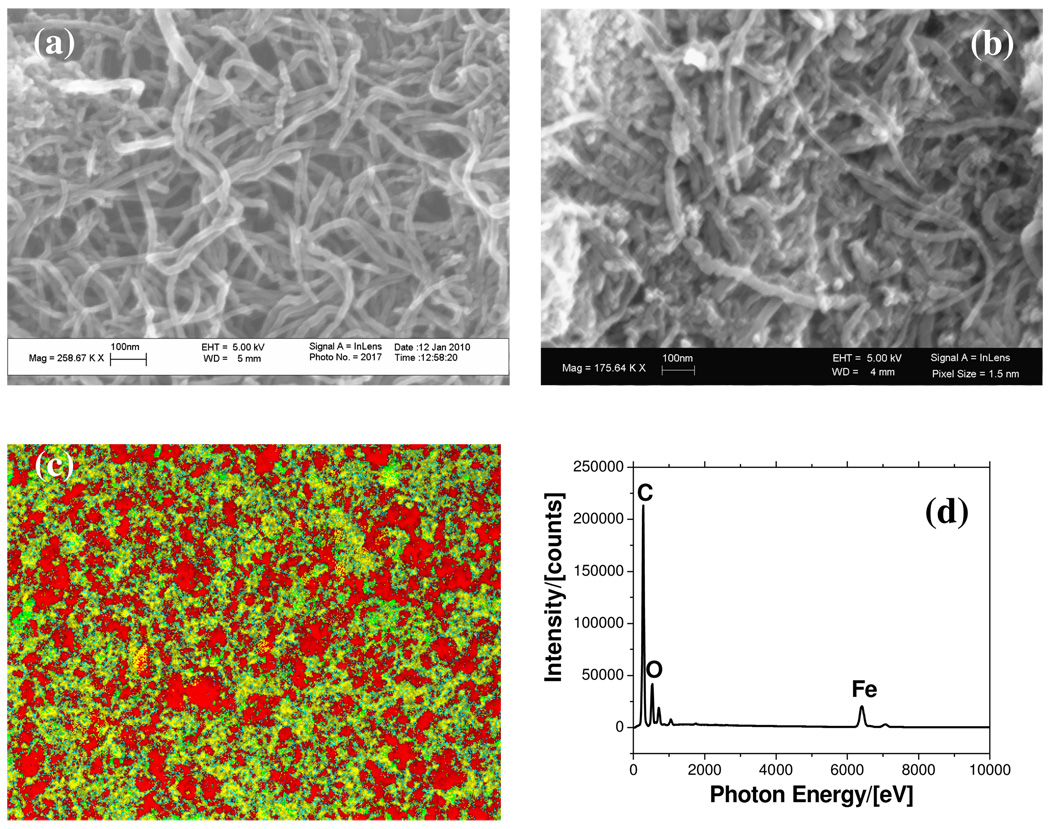
SEM images of f-MWCNT and the Fe-MWCNT hybrid are shown in Figure 1. The original MWCNTs had a diameter in the range of (20 to 40) nm and the length was about (10 to 30) µm. There was no detectable change in tube morphology after acid treatment or iron oxide coating, implying minimal damage to the tube structure. It is quite evident from Figure 1b that in the case of Fe-MWCNT, the surface was heavily coated with iron oxide.
Figure 1.
SEM Image of (a) acid functionalized MWCNT, (b) the Fe-MWCNT Composite, (c) EDS map (red – carbon, green – oxygen, and yellow – iron), (d) EDS spectra (carbon (C), oxygen (O), and iron(Fe)
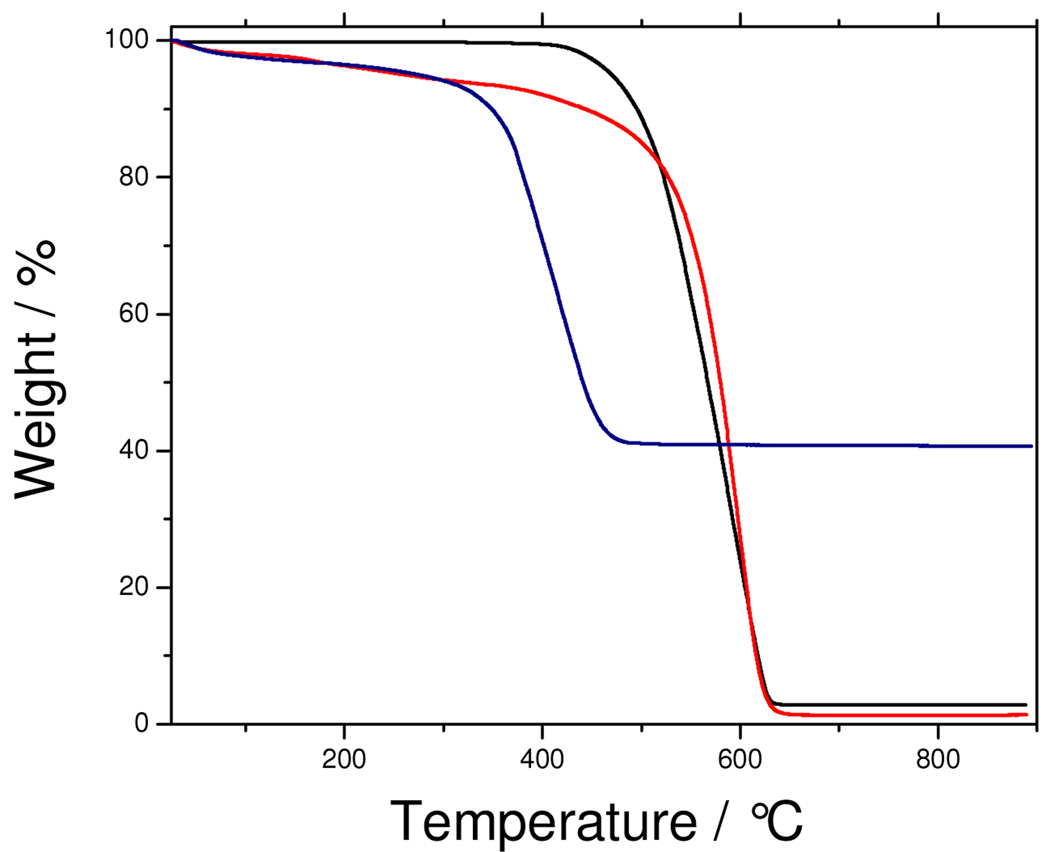
The EDS data shown in Figure 1c and 1d also confirmed the presence of relatively large amounts of iron oxide on the surface of the carbon nanotubes. TGA was used to quantify the iron oxide loading in the MWCNT as shown in Figure 2(a). The resulting weight above 600 °C was attributed to the weight of residual metal or metal oxide. The Fe-MWCNT hybrid was found to contain as much as 40 % (by weight) of iron implying an approximate atomic ratio between Fe and carbon of 11:100. The catalytic activity of the iron is evident from the TGA data, where it altered the thermal stability of the MWCNT. The Fe-MWCNT hybrid degraded at a significantly lower temperature (by nearly 200 °C) compared to the original carbon nanotubes, because the iron catalyzed its oxidation, consistent with previous observation28.
Figure 2.
TGA data for MWCNT (—), f-MWCNT ( ) and Fe-MWCNT (
) and Fe-MWCNT ( ).
).
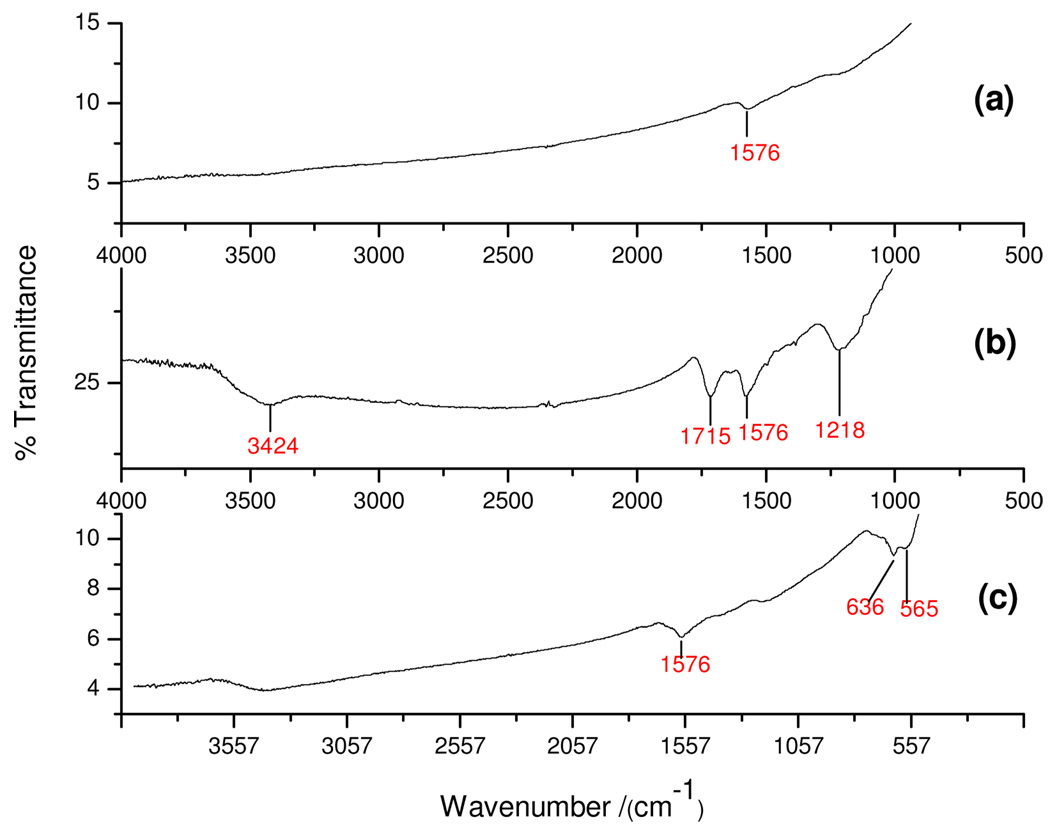
The IR spectrum (Figure 3) confirmed the presence of functional groups in MWCNT, f-MWCNT and the Fe-MWCNT hybrid. The carboxylic stretching frequency in f-MCWNT occurred at 1715 cm−1 (C=O) and 1218 cm−1 (C – O). The stretching (O – H) vibration occurred at 3424 cm−1 in the f-MWCNT spectrum (Fig. 3b) which is clearly absent from the MWCNT spectrum (Fig. 3a). In the Fe-MWCNT spectrum, the characteristic peaks at 1715 cm−1, 1218 cm−1 and 1400 cm−1 belonging to the C=O, C–O and O–H vibrations of carboxylic acid disappear. This may be due to the binding of iron oxide unto the oxidized MWCNTs surface. Based on the disappearance of the C–O and O–H peaks, it is evident that the iron oxide particles are anchored to the MWCNTs by an ester-like bond29. In all the samples, the peak around 1576 cm−1 was assigned to the C=C stretching of the carbon skeleton. The appearance of two new bands at (636 and 565) cm−1 in (c) confirmed the formation of the Fe-O bonds.
Figure 3.
FTIR spectra of (a) MWCNT, (b) f-MWCNT, and (c) Fe-MWCNT.
Arsenic Removal and its kinetics
The arsenic removal capacity of Fe-MWCNT was compared to those of the original multiwall carbon nanotubes (MWCNT) and functionalized multiwall carbon nanotubes (f-MWCNT). The adsorption capacity for arsenic shown by Fe-MWCNT for both As(III) and As(V) (1723 µg g−1 and 189 µg g−1 respectively) was much higher than the values obtained for MWCNT (10 µg g−1 and 23 µg g−1 respectively) and f-MWCNT (3 µg g−1 and 9 µg g−1 respectively). The adsorption capacity for As(III) and As(V) using Fe-MWCNT was also found to be higher than that for iron coated sand,11,30,31 ferrihydrite,31 and hardened paste of Portland cement32. It was higher than the values reported for iron oxide-coated biomass33 but much less than those shown by activated carbon and activated alumina, mostly due to the fact that the initial concentrations of arsenic in these studies were as high as 100 mg L−1.
Oxyanionic arsenic species such as arsenate and arsenite adsorb at the iron oxyhydroxide surface by forming complexes with the surface sites34. This assertion is supported by the poor adsorption capacities obtained from analysis with MWCNT and f-MCWNT. As(V) and As(III) showed different sorption behavior as can be seen from the isothermal and kinetic data. This difference in their sorption characteristics may be due to their ionic forms at a pH of 4. In the pH range studied, As(V) existed in the anionic form H2AsO4−, whereas As(III) is partially ionized below pH 9.22, existed in the molecular form (H3AsO3). This may account for the better adsorption of As(V) compared to As(III). Anionic arsenic species (H2AsO3−, H2AsO4−) are reported to be adsorbed with the positively charged iron oxide-coated adsorbents through electrostatic attraction. There are also reports of the surface potential of iron loaded adsorbents becoming less negative with increasing iron loading35. We therefore postulate that arsenic removal with Fe-MWCNT may occur via multiple mechanisms. Negatively charged arsenic species may adsorb onto positively charged sites on the adsorbent surface resulting in their removal from water.
When the iron oxide was exposed to water, metal ions on the oxide surface completed their coordination shells with OH groups36, which either bound to or released H+ ions depending on the pH, and in the process developed a surface charge. The adsorption properties of oxides are due to the existence of these OH2+, OH and O− functional groups37. Arsenic may be removed by ligand exchange with OH and OH2+ functional groups on the surface forming an inner-sphere complex. An incompletely dissociated acid H2AsO4− is required to provide a proton for complexation with the OH group to form H2O and providing space for anion adsorption36. Therefore arsenic species may be removed through complexation with oxyhydroxide sites on the adsorbent surface according to equations 1 and 233.
| (1) |
| (2) |
The kinetics of arsenic uptake was investigated by the Lagergren38 and Ho and McKay39 kinetic models. Lagergren models the rate of adsorption of pollutants on an adsorbent after a pseudo-first order equation:
| (3) |
where qe and qt are the sorption capacity (µg g−1) of the adsorbent at equilibrium and at time t (h), respectively and k1 is the pseudo-first order sorption rate constant (h−1). Ho and McKay proposed a pseudo-second order equation of the form:
| (4) |
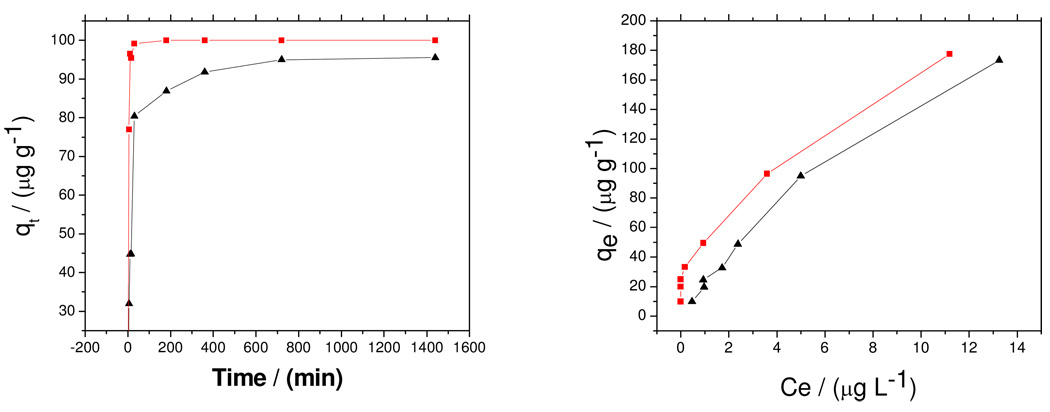
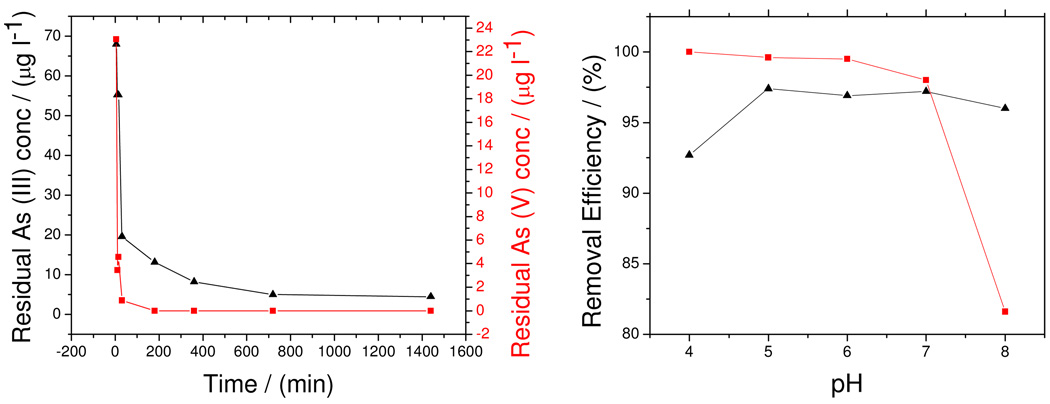
where k2 is the pseudo-second order sorption rate constant (g h µg−1) and t is time (h). Figure 5(a) shows the residual As(III) and As(V) concentrations versus time at pH 4. After 10 minutes of contacting the adsorbent with 100 µg L−1 Arsenic solution, 96 % of As(V) was removed, whereas only 45 % of As(III) was removed. 99 % of As(V) and 80 % of As(III) were removed after 30 minutes of contact. Ho and McKay’s pseudo-second kinetic equation was a better fit for adsorption of both As(III) and As(V) than the Lagergren’s pseudo-first order equation. The pseudo-second order kinetic parameters for As(III) and As(V) removal are presented in Table 1. The R2 values for both As (III) and As (V) were close to unity implying that their adsorption can best be described by the pseudo-second order kinetic model with chemisorption being the rate limiting step. This means that the adsorption rate is proportional to the amount of adsorbent and the square of the number of free sites. The latter corresponds to the term (qe −qt)2 in the pseudo second order model.
Figure 5.
Kinetic and equilibrium data at pH 4 for As(III) and As(V) adsorption by Fe-MWCNT. Symbols ▲ As(III);  As(V).
As(V).
Table 1.
Pseudo-second order kinetic parameters for As(III) and As(V) adsorption
| qe/(µg g−1) | k2/(g (min µg)−1) | h/(µg (g min)−1) | R2 | |
|---|---|---|---|---|
| As(III) | 97.09 | 7.8 × 10−4 | 7.353 | 0.9999 |
| As(V) | 103.84 | 7.4 × 10−3 | 79.79 | 0.9992 |
The rate of As(V) removal was faster than that of As(III) as shown by the pseudo-second order kinetic parameters in Table 1. The higher removal rate of As(V) relative to As(III) may be due to the easy formation of ferric arsenate40. Faster adsorption of arsenate than arsenite has also been attributed to the smaller radius of the arsenate ion [(4.0 to 4.2) Å] compared with that of the arsenite ion (4.8 Å)41.
The optimum pH for As(V) removal was determined to be 4, the equilibrium adsorption qe (µg g−1) of As(V) was found to decrease slightly from pH 4 through 8 as presented in figure 4(b). On the contrary the equilibrium adsorption of As(III) was found to increase slightly from pH 4 to pH 6 and remained constant from pH 6 to pH 8 (Figure 4(b)). As(III) is dominant in the form of neutral species (H3AsO3) below pH 9.22, accounting for the relatively constant As(III) adsorption. A reduced removal of As(V) at pH 8 may be because OH− ion becomes dominant at an alkaline pH and this ion competes with arsenic species [H2AsO4−]. The pH range studied here was within what is normally encountered in water resources.
Figure 4.
(a)Residual arsenic concentration as a function of time, (b) Effect of pH on As(III) and As(V) adsorption. Symbols ▲ As(III);  As(V).
As(V).
Adsorption Isotherms for As(III) and As(V) Removal
The capacity of arsenic removal by the adsorbent was evaluated with the Langmuir42 and Freundlich43 isotherms. The linear form of the Langmuir adsorption isotherm is presented below;
| (5) |
where qm (µg/g) is the maximum sorption capacity for monolayer coverage of the adsorbent, Ce (µg/L) is the equilibrium concentration of arsenic and Langmuir constant b (L/µg) is indirectly related to the enthalpy of adsorption. The linearized form of the Freundlich isotherm involves a plot of log qe and log Ce with n and log kf being the slope and y-intercept respectively.
| (6) |
The Freundlich constants kf and 1/n measure the adsorption capacity and intensity respectively. The bond energy increases proportionally with surface density for n<1 and vice a versa for n>1.
The Langmuir isotherm parameters presented in Table 2 indicate a high maximum sorption capacity for monolayer adsorption (qm) for the adsorbent in the removal of As(III). The adsorption of As(III) and As(V) fit both the Langmuir and Freundlich equations; however the coefficient of determination (R2) value for the Freundlich model in both instances were higher than that of the Langmuir equation. Hence the Freundlich isotherm model effectively explained the removal of As(III) and As(V) by the adsorbent with coefficient of determination (R2) values of 0.9907 and 0.9997 respectively.
Table 2.
Adsorption isotherm parameters for removal of As(III) and As(V) by Fe-MWCNT
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| qm/(µg g−1) | b/(L µg−1) | R2 | Kf/(L µg−1) | n | R2 | |
| As(III) | 1723 | 0.013 | 0.9899 | 21.89 | 1.181 | 0.9907 |
| As(V) | 189 | 0.373 | 0.9899 | 50.83 | 1.946 | 0.9997 |
The Freundlich constants log kf and n were obtained from the y-intercept and slope respectively. The constants kf (mg g−1) and 1/n provide a measure of adsorption capacity and intensity respectively are presented in table 2. The bond energy increases proportionally with surface density for n<1 and vice a versa for n>143. The adsorption capacity for the adsorbent in As(V) removal was much higher than As(III) as was the intensity. The Freundlich isotherm model effectively explained the removal of As(V) by the adsorbent with a coefficient of determination of 0.9995. The value of the constant 1/n (0 – 1) is also indicative of the heterogeneity of the adsorbent surface, with 1/n closer to 0 implying a heterogeneous surface. The kinetic and equilibrium data at pH 4 for As(III) and As(V) adsorption are shown in Figure 5.
Effect of temperature on arsenic removal
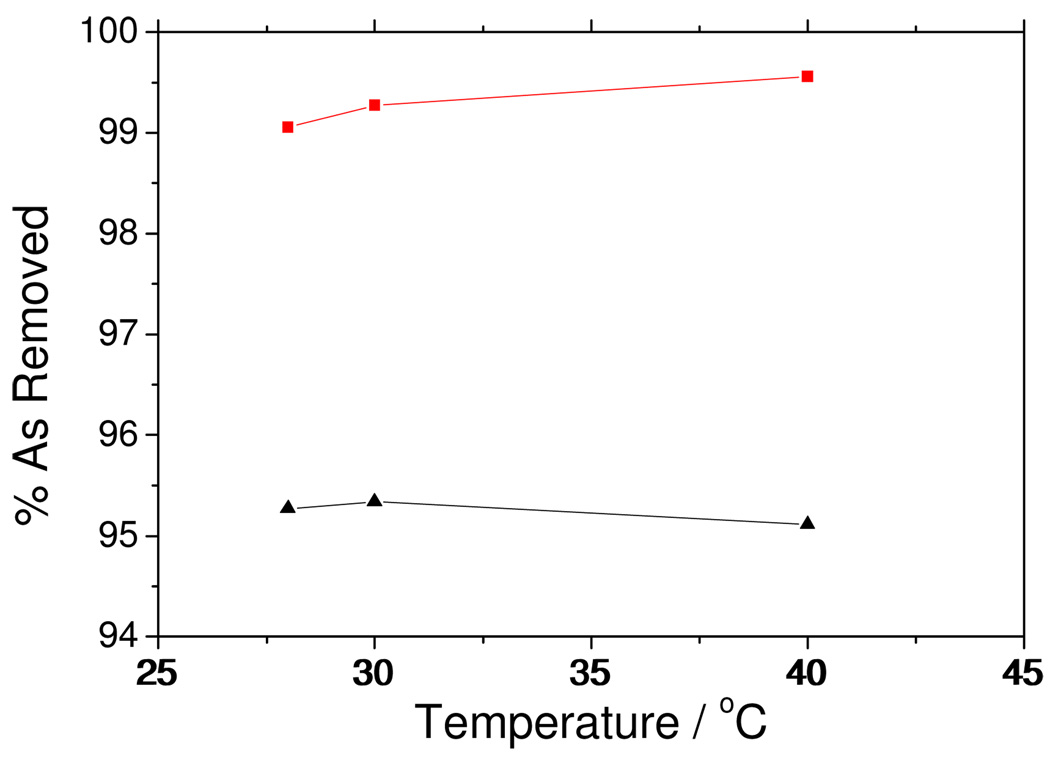
The effect of temperature on arsenic removal efficiency was investigated at pH 4, 1 g L−1 adsorbent dose, 180 rpm agitation, and 1 h and 12 h equilibration time for As(V) and As(III) respectively. The temperature was varied from 28°C to 40°C. It was found that Arsenic removal efficiency was fairly constant over the temperature range. A plot of arsenic removal versus temperature is presented in Figure 6. A study using Fe3+- impregnated granular activated carbon (GAC) reported a decrease in arsenic As (III) and As (V) removal efficiency with increasing temperature from (30 to 60) °C44 but the decrease did not seem substantial.
Figure 6.
Arsenic removal efficiency as a function of temperature. Symbols ▲ As(III);  As(V).
As(V).
Conclusion
An iron oxide-multi-walled carbon nanotube (Fe-MWCNT) hybrid was effective as a sorbent material for arsenic removal from water. Controlled assembly of iron oxide was possible and the MWCNT served as an effective support for the oxide. The kinetics of As(III) and As(V) removal was explained by the pseudo-second order rate equation and their adsorption by the Langmuir and Freundlich models. It is conceivable that MWCNT with appropriate surface modification can provide a platform for developing potentially useful environmental remediation tools.
Acknowledgments
The authors would like to thank Dr Yuhong Chen for helping with the EDS analysis, and Dr. Larisa Krishtopa for helping with the ICP-MS analysis. Partial financial support for this work was provided by the National Institute of Environmental Health Sciences (NIEHS) under Grant Number RC2 ES018810 and the Schlumberger Foundation through the faculty for the future fellowship.
References
- 1.Mondal P, Majumder CB, Mohanty B. Laboratory based approaches for arsenic remediation from contaminated water: Recent developments. J. Hazard. Mater. 2006;137(1):464–479. doi: 10.1016/j.jhazmat.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee A, Das D, Mandal BK, Chowdhury TR, Samanta G, Chakraborty D. Arsenic in ground water in six districts of West Bengal, India: the biggest arsenic calamity in the world. Part I. Arsenic species in drinking water and urine of the affected people. Analyst. 1995;120:643–656. doi: 10.1039/an9952000917. [DOI] [PubMed] [Google Scholar]
- 3.Dhar RK, Biswas BK, Samanta G, Mandal BK, Chakraborti D, Roy S, et al. Groundwater arsenic calamity in Bangladesh. Curr. Sci. 1997;73(1):48–59. [Google Scholar]
- 4.Richardson SD. Environmental mass spectrometry: Emerging contaminants and current issues. Anal. Chem. 2006;78(12):4021–4045. doi: 10.1021/ac060682u. [DOI] [PubMed] [Google Scholar]
- 5.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Arsenic Exposure and Prevalence of Type 2 Diabetes in US Adults. J. Am. Med. Assoc. 2008;300(7):814–822. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- 6.USEPA. Federal Register. 2001;66(14):6976–7066. [Google Scholar]
- 7.Knowles FC, Benson AA. The biochemistry of arsenic. Trends Biochem. Sci. 1983;8(5):178–180. [Google Scholar]
- 8.Pattanayak J, Mondal K, Mathew S, Lalvani SB. A parametric evaluation of the removal of As (V) and As (III) by carbon based adsorbents. Carbon. 2000;38:589–596. [Google Scholar]
- 9.Mohan D, Pittman CU., Jr Arsenic removal from water/waste water using adsorbents - a critical review. J. Hazard. Mater. 2007;142:1–53. doi: 10.1016/j.jhazmat.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Elizalde-González MP, Mattusch J, Einicke W-D, Wennrich R. Sorption on natural solids for arsenic removal. Chem.Eng. J. 2001;81:187–195. [Google Scholar]
- 11.Thirunavukkarasu OS, Viraraghavan T, Subramanian KS. Arsenic removal from drinking water using iron oxide-coated sand. Water, Air, and Soil Pollut. 2003;142:95–111. [Google Scholar]
- 12.Wilkie JA, Hering JG. Adsorption of arsenic onto hydrous ferric oxide: effects of adsorbate/adsorbent ratios and co-occurring solutes. Colloids Surf., A. 1996;107:97–110. [Google Scholar]
- 13.Chen WF, Parette R, Zou JY, Cannon FS, Dempsey BA. Arsenic removal by iron-modified activated carbon. Water Res. 2007;41:1851–1858. doi: 10.1016/j.watres.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt GT, Vlasova N, Zuzaan D, Kersten M, Daus B. Adsorption mechanism of arsenate by zirconyl-functionalized activated carbon. J. Colloid Interface Sci. 2008;317:228–234. doi: 10.1016/j.jcis.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. [Google Scholar]
- 16.Iijima S. Carbon nanotubes: past, present, and future. Phys. B. 2002;323:1–5. [Google Scholar]
- 17.Tojanowicz M. Analytical applications of carbon nanotubes: a review. Trends Anal. Chem. 2006;25(5):480–489. [Google Scholar]
- 18.Wang Y, Iqbal Z, Mitra S. Microwave-induced rapid chemical functionalization of single-walled carbon nanotubes. Carbon. 2005;43:1015–1020. [Google Scholar]
- 19.Wang Y, Iqbal Z, Mitra S. Rapid, low temperature microwave synthesis of novel carbon nanotube-silicon carbide composite. Carbon. 2006;44(13):2804–2808. [Google Scholar]
- 20.Wang Y, Iqbal Z, Mitra S. Rapidly functionalized, water-dispersed carbon nanotubes at high concentration. J. Am. Chem. Soc. 2006;128(1):95–99. doi: 10.1021/ja053003q. [DOI] [PubMed] [Google Scholar]
- 21.Sai Sathish R, Chen Y, Kalyan MK, Nageswara Rao G, Janardhana C, Mitra S. Carbon nanotube-zirconium dioxide hybrid for defluoridation of water. Nano Sci. Nano Technol. J. Nano Sci. Nano Technol. doi: 10.1166/jnn.2011.3806. In press. [DOI] [PubMed] [Google Scholar]
- 22.Mishra AK, Ramaprabhu S. Magnetite Decorated Multiwalled Carbon Nanotube Based Supercapacitor for Arsenic Removal and Desalination of Seawater. J. Phys. Chem. C. 2010;114:2583–2590. [Google Scholar]
- 23.Karwa M, Iqbal Z, Mitra S. Scaled-up self-assembly of carbon nanotubes inside long stainless steel tubing. Carbon. 2006;44(7):1235–1242. [Google Scholar]
- 24.Hylton K, Chen Y, Mitra S. Carbon nanotube mediated microscale membrane extraction. J. Chromatogr. A. 2008;1211(1–2):43–48. doi: 10.1016/j.chroma.2008.09.092. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Iqbal Z, Mitra S. Microwave-Induced Controlled Purification of Single-Walled Carbon Nanotubes without Sidewall Functionalization. Adv. Funct. Mater. 2007;17:3946–3951. [Google Scholar]
- 26.Chen Y, Mitra S. Fast microwave-assisted purification, functionalization and dispersion of multi-walled carbon nanotubes. J. Nanosci. Nanotechnol. 2008;8(11):5770–5775. doi: 10.1166/jnn.2008.215. [DOI] [PubMed] [Google Scholar]
- 27.Zhang QL, Lin YC, Chenc X, Gao NY. A method for preparing ferric activated carbon composites adsorbents to remove arsenic from drinking water. J. Hazard. Mater. 2007;14:671–678. doi: 10.1016/j.jhazmat.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 28.Brukh R, Mitra S. Kinetics of carbon nanotube oxidation. J. Mater. Chem. 2007;17:619–623. [Google Scholar]
- 29.Yan S, Lian G. Synthesis and characterization of phase controllable ZrO2–carbon nanotube nanocomposites. Nanotechnology. 2005;16:625–630. [Google Scholar]
- 30.Gupta VK, Saini VK, Jain N. Adsorption of As (III) from aqueous solutions by iron oxide-coated sand. J. Colloid Interface Sci. 2005;228(1):55–60. doi: 10.1016/j.jcis.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 31.Thirunavukkarasu OS, Viraraghavan T, Subramanian KS. Removal of arsenic in drinking water by iron oxide-coated sand and ferrihydrite-batch studies. Water Qual. Res. J. Can. 2001;36(1):55–70. [Google Scholar]
- 32.Kundu S, Kavalakatt SS, Pal A, Ghosh SK, Mandal M, Pal T. Removal of arsenic using hardened paste of Portland cement: batch adsorption and column study. Water Res. 2004;38:3780–3790. doi: 10.1016/j.watres.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Pokhrel D, Viraraghavan T. Arsenic removal from an aqueous solution by modified A. niger biomass: batch kinetic and isotherm studies. J. Hazard. Mater. 2008;150:818–825. doi: 10.1016/j.jhazmat.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 34.Edwards M. Chemistry of arsenic removal during coagulation and. Fe-Mn oxidation. J.-Am.Water Works Assoc. 1994;86(9):64–78. [Google Scholar]
- 35.Huang CP, Vane LM. Enhancing As5+ removal by a Fe2+-treated activated carbon. J. Water Pollution Control Federation. 1989;61(9):1596–1603. [Google Scholar]
- 36.Hingston FJ, Posner AM, Quirk JP. Anion adsorption by goethite and gibbsite. J. Soil Sci. 1972;23(2):177–192. [Google Scholar]
- 37.Sposito G. The Surface Chemistry of Soils. New York: Oxford University Press; 1984. The Surface Chemistry of Soils. [Google Scholar]
- 38.Lagergren S. About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar. 1898;24(4):1–39. [Google Scholar]
- 39.Ho YS, McKay G. Kinetic model for lead (II) sorption on to peat. Adsorpt. Sci. Technol. 1998;16(4):243–255. [Google Scholar]
- 40.Joshi A, Chaudhuri M. Removal of Arsenic from Ground Water by Iron Oxide-Coated Sand. J. Environ. Eng. 1996;122(8):769–771. [Google Scholar]
- 41.Kim Y, Kim C, Choi I, Rengaraj S, Yi J. Arsenic removal using mesoporous alumina prepared via a templating method. Environ. Sci. Technol. 2004;38(3):924–931. doi: 10.1021/es0346431. [DOI] [PubMed] [Google Scholar]
- 42.Langmuir I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916;38(11):2221–2295. [Google Scholar]
- 43.Freundlich HMF. Over the adsorption in solution. J. Phys. Chem. 1906;57A:385. [Google Scholar]
- 44.Mondal P, Balomajumder C, Mohanty B. A laboratory study for the treatment of arsenic, iron, and manganese bearing ground water using Fe3+ impregnated activated carbon: Effects of shaking time, pH and temperature. J. Hazard. Mater. 2007;144(1–2):420–426. doi: 10.1016/j.jhazmat.2006.10.078. [DOI] [PubMed] [Google Scholar]