Abstract
A surprisingly small number of signalling pathways are used reiteratively during neural development, eliciting very different responses depending on the cellular context. Thus, the way a neural cell responds to a given signal is as important as the signal itself and this responsiveness, also called competence, changes with time. Here we describe recent advances in elucidating the signalling pathways that operate in brain development.
Introduction and context
One of the most formidable challenges in biology is to understand the generative program underlying the development of a functional nervous system and, in the case of vertebrates, the astonishingly complex structure of the brain. The neuroepithelium that will make the brain and spinal cord is induced early in development, partly through inhibition of anti-neuralizing signals. In vertebrates, the emerging epithelial sheet - the neural plate - is then patterned coarsely along its anteroposterior (AP, head-to-tail) and dorsoventral (DV, back-to-belly) axes by gradients of secreted factors (morphogens) that specify different regional neural fates in a dose-dependent fashion. Subsequently, regional identities become stabilized through transcriptional feedback and through the establishment of cell-tight compartments. The neural plate rolls up and compacts to form a neural tube that displays increasingly pronounced bulges, constrictions and flexures - the first indication of the morphological complexity of the central nervous system (CNS) at later stages (Figure 1).
Figure 1. Lateral view of embryonic vertebrate (chick) brain.

Principal signalling centres are highlighted in green (floor plate, basal forebrain, zona limitans intrathalamica (ZLI) - Shh expression), red (roof plate - BMP and Wnt expression) and blue (midbrain-hindbrain boundary (MHB), anterior neural ridge/commissural plate (ANR/CP) - FGF expression). Note that the pallial-subpallial boundary (PSB) and the boundaries between rhombomeres in the hindbrain (HB) have also been suggested to exert signalling functions. The notochord (light grey) is a non-neural signalling centre that regulates ventral neural patterning. Di, diencephalon; MB, midbrain; Tel, telencephalon.
Local signalling centres are established within the neuroepithelium, often along the boundaries between compartments, which refine the pattern of neural subdivisions by releasing diffusible signalling factors. A surprisingly small set of signalling factors is employed reiteratively throughout development, and different populations of cells may respond to the same signal very differently, a phenomenon called ‘differential cellular competence’. Eventually, neural identities become determined when neural progenitors exit the cell cycle and differentiate into mature neurons that form dendrites and project axons to establish the complex connectional architecture of the CNS. Understanding the developmental history of cells in specific regions of the emerging brain will provide us with more rational and targeted strategies to produce these cells in a Petri dish from embryonic stem cells.
The initial step in CNS development in vertebrates - the induction of a neural plate from the embryonic ectoderm - occurs early in embryogenesis before the onset of gastrulation. In the 1990s the ‘default model’ for neural induction was proposed: all ectodermal cells will become neural unless they are exposed to epidermis-inducing bone morphogenetic proteins (BMPs) [1-3]. Thus, neural fates are induced either by the mere absence of BMP signals (by default) or by an active inhibition of the BMP signalling pathway. Over the past 15 years, it has been shown that embryos throughout the animal kingdom produce inhibitory factors that sequester BMPs in the extracellular space and relieve cells from their anti-neuralizing effect, thereby inducing neural identity [4,5].
During gastrulation, a crude pattern is established within the neural plate by gradients of signalling factors that determine AP polarity (fibroblast growth factors (FGFs), retinoic acid, secreted signalling proteins of the Wnt family) and mediolateral polarity (BMPs, members of the Hedgehog family) by inducing the expression of region-specific transcription factors in a dose-dependent fashion [6-13]. In many cases, the borders between domains of transcription factor expression are then sharpened by the mutual repression of pairs of factors. For example, the expression domains of the homeobox genes Otx2 in the prospective midbrain and Gbx2 in the anterior hindbrain region initially overlap [14], but mutual repression between the two transcription factors encoded by these genes results in a binary choice, with cells exclusively expressing either Otx2 or Gbx2 [15-18]. Furthermore, cells in adjacent regions may start to express different sets of surface molecules, resulting in an enhanced affinity between cells within a region, decreased affinity and miscibility with cells from neighbouring regions, and the formation of a sharp regional interface - similar to the formation of a phase interface between oil and water [19].
Occasionally, a regional interface becomes a cell-tight boundary that confines cells to lineage-restricted compartments; this is best exemplified in the hindbrain, which consists of a series of compartments called rhombomeres [19,20]. Apart from stabilizing emerging regionalization, boundaries often appear to function as local organizers, specialized cell populations that influence the development of their flanking regions by secreting molecular signals [11,19,21]. For example, the boundary between midbrain and hindbrain (MHB) induces the tectum anteriorly and the cerebellum posteriorly by releasing FGF8 [9,11,16,18]. Thus, the themes of (1) patterning by diffusible signalling factors, (2) mutual repression of transcription factors and (3) boundary/compartment formation are reiterated at multiple stages of brain development, resulting in a progressively refined subdivision of the neuroepithelium.
While the assignment of regional identities is under way, the neuroepithelium undergoes a no less complex series of morphological transformations. The neural plate rolls up and its borders fuse to form a neural tube that displays increasingly pronounced constrictions and bulges (some of which correspond to the boundaries and compartments discussed above) [19,22,23]. At present, little is known about the molecular dynamics underlying brain morphogenesis, but differential growth is likely to be one of the driving forces, and some of the signalling factors secreted by local organizers act as growth factors in addition to their role in patterning. Whereas AP patterning of the neural tube continues to be under the influence of several discrete local organizers (such as the MHB), DV patterning is regulated by two continuous signalling centres that stretch along almost the entire neural tube: the floor plate at the ventral midline that controls ventral identity by secreting Sonic hedgehog (Shh) [24-26] and the roof plate at the dorsal midline that emits BMPs and Wnts [6,27,28].
Neural progenitors are kept in a proliferative state until, at the onset of neurogenesis, their cell cycle lengthens and both postmitotic neurons and radial glial cells are produced. Basic helix-loop-helix transcription factors such as NeuroD and the neurogenins, together with the Notch signalling pathway, are key regulators of this process [29,30]. Throughout the brain, neurons become organized to form either nuclei (in the diencephalon, tegmentum and brain stem) or layers (cortex, tectum and cerebellum). In the cerebral cortex, layering is achieved by the sequential radial (outward) migration of newborn neurons that are generated in the ventricular zone.
Once the basic architecture of the brain has been established, some postmitotic neurons become redistributed by tangential migratory processes. For example, GABAergic neurons that originate in the basal forebrain migrate dorsally into the cortex in mammals [31]. Finally, neurons form dendrites and project axons to targets within the brain and in the periphery. Axons are guided by extracellular cues that can act as attractants (netrin and its receptor DCC) or as repellants (ephrins and Eph receptors, netrin and its receptor UNC5, semaphorins and plexin receptors, Slit and its receptor Robo) [32-35]. Sensory input is often represented in an orderly fashion in various brain structures. The best example of such topographic mapping is the projection pattern of retinal axons into the optic tectum, where the two axes of the retina (nasal-temporal, ventral-dorsal) correspond to projection targets along the caudal-rostral and medial-lateral axes of the tectum, respectively (Figure 2). This geometric organization is achieved, at least in part, by the graded expression of ephrins within the tectum and of Eph receptors on retinal axons [36,37]. It is likely that these gradients of expression are set up by the gradients of signalling factors that pattern the neuroepithelium at earlier stages, highlighting a direct link between early patterning and functional brain architecture.
Figure 2. Topographic mapping in the retinotectal pathway.

(a) Axons originating from dorsal (D) aspects of the retina project into the lateral (L) tectum (bright blue) whereas axons from the ventral (V) retina project into the medial (M) tectum (dark blue). Nasal (N) retinal axons target caudal (C) areas of the tectum (pink) whereas temporal (T) ones target rostrally (R, red). (b) Simplified, vectorial depiction of retinotectal projection patterns.
Major recent advances
Neural induction
Support for the default model of neural induction has recently come from experiments in frog embryos in which the simultaneous depletion of three BMP inhibitors resulted in the absence of a neural plate [38] while the simultaneous depletion of three BMPs resulted in massive neural induction [39]. At the same time, however, experiments in chick suggested that prospective neural cells require activation by FGFs before BMP inhibition [9,40,41]. This is consistent with the results of other groups who have demonstrated that BMP inhibition is required, but not sufficient, for neural plate formation in frog and fish embryos [42-45].
Crucial steps in embryogenesis, such as neural induction, are often governed by multiple parallel pathways, providing a safety mechanism that ensures increased fidelity in cellular decision-making. In addition to BMP inhibition and FGF signalling, both activation and inhibition of the Wnt pathway have also been implicated in neural induction [46-49]. The De Robertis lab and others have shown that BMP, FGF/MAP kinase and Wnt pathways are all integrated at the level of Smad1 phosphorylation (Figure 3), providing an elegant explanation for the neural-inducing and neural-inhibiting activities of these pathways [46,50,51]. The integration of three different pathways safeguards the formation of neural cells in a spatially and temporally restricted manner in the embryo. However, even the simultaneous manipulation of BMP, FGF and Wnt signalling may not be sufficient to induce neural tissue in all experimental systems, suggesting the presence of other, as yet unidentified, neural-inducing signals [45].
Figure 3. Integration of BMP, FGF and Wnt signalling at the level of Smad1 during neural induction.
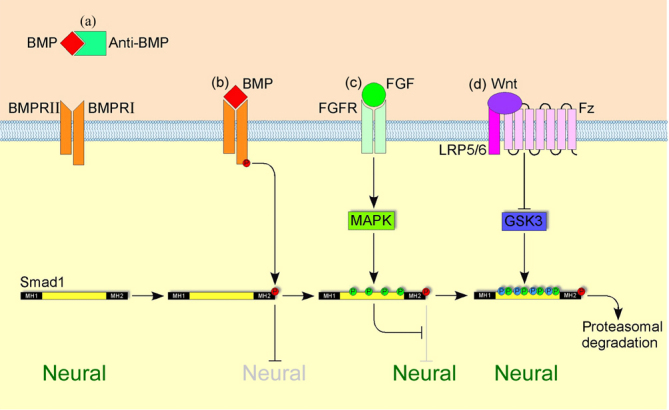
(a) Anti-BMPs (such as Chordin and Noggin) prevent BMPs from binding to their receptor complex; Smad1 remains inactive; neural identity is induced. (b) Binding of a ligand to a BMP receptor complex results in transphosphorylation of the BMP type I receptor by the BMP type II receptor and the subsequent phosphorylation of Smad1 in its carboxy-terminal MH2 domain; this activated Smad1 forms a complex with co-Smads, translocates to the nucleus and blocks neural identity. (c) Activation of the mitogen-activated protein kinase (MAPK) pathway by FGF (or by hepatocyte growth factor (HGF) or insulin-like growth factor (IGF)) signalling results in phosphorylation of Smad1 in its linker region (yellow); this phosphorylation blocks the anti-neuralizing effect of activated Smad1. (d) Phosphorylation in its linker region primes Smad1 for phosphorylation by glycogen synthase kinase 3 (GSK3); doubly phosphorylated Smad1 is then targeted for degradation in the proteasome.
In both chick and frog, the FGF target gene churchill (chch) was proposed to block BMP signalling by encoding a zinc finger transcription factor that induced the expression of the Smad inhibitor Sip1 [52]. However, a recent structural analysis of Chch has indicated that the protein is unlikely to bind to DNA directly, raising the question of how Chch induces Sip1 - possibly by interacting with another DNA-binding cofactor [53].
Patterning
In the 1930s, Otto Mangold proposed that the early neural plate is already subdivided into multiple AP domains [54]. This model was called into question by many studies that found a high level of regional plasticity within the neural plate, indicating that it is a relatively naive sheet of cells. A recent fate-mapping study in zebrafish has, however, revealed a high level of determination within subregions of the neural plate during gastrulation (in particular in the area of the presumptive prethalamus) [55]. Analysis of the promoter of a Xenopus orthologue of the Drosophila genecaudal, which encodes a homeodomain transcription factor expressed in the posterior neural plate, has revealed the presence of multiple regulatory elements that are able to integrate BMP, FGF and Wnt signals, providing evidence for the idea that several pathways interact not only during neural induction but also during the establishment of the posterior CNS [56]. Anteriorly, the homeobox transcription factor Six3 protects forebrain identity by repressing the posteriorizing activity of Wnt1 [57,58].
Local signalling centres are likely to release a secreted signal in a more or less symmetrical fashion. Yet, the response to the same signal on either side of the signalling centre is often strikingly asymmetrical. For example, why do cells on either side of the MHB interpret the same signal, FGF8, differently by forming a tectum anteriorly and a cerebellum posteriorly? A recent study has highlighted a central role for the homeobox transcription factor Irx2 in conferring competence upon cells posterior to the MHB to form cerebellum in response to FGF8 [59]. We and others were able to show that the zona limitans intrathalamica (ZLI) in the posterior forebrain is also a local signalling centre that regulates thalamic development by emitting Shh. Irx3, a close relative of the competence factor Irx2, is expressed posterior to the ZLI and determines the ‘thalamic response’ of the posterior cells to Shh [60]. The expression domains of these competence factors are established early in development, at neural plate stages, linking the coarse pre-pattern that is set up during gastrulation with the later refinement of this pattern. The boundaries between hindbrain rhombomeres have also been shown to exert signalling activity by producing Wnts that regulate the pattern of neurogenesis within the rhombomeres [61,62].
Two studies from the Partanen lab have shown that FGF receptor signalling is essential not only for patterning in the MHB area, but also for maintaining the integrity of the MHB itself [63] and for promoting progenitor proliferation [64]. These studies emphasize the multifunctionality of secreted signalling factors.
Morphogenesis
Wnt proteins are able to activate two alternative intracellular pathways. One is the ‘canonical’ Wnt pathway that results in stabilization of β -catenin and its translocation to the nucleus, where it associates with various cofactors to activate the transcription of target genes [65]. The other is a ‘noncanonical’ Wnt pathway that interacts with the cytoskeleton independently of transcription and regulates epithelial cell polarity [66,67]. It has become increasingly clear that the noncanonical branch of the Wnt pathway (activated by Frizzled3 and Frizzled6) is required for the morphogenetic process of neural tube closure [68,69].
Neuronal phenotype
Postmitotic neurons acquire characteristic neurotransmitter phenotypes depending on their gene-expression profile. For example, Otx2, which acts as a pre-patterning factor at earlier stages, promotes glutamatergic differentiation and represses GABAergic differentiation in the thalamus [70]. Similarly, somatosensory neurons in the hindbrain are replaced by viscerosensory relay neurons in mice lacking the transcription factor Lbx1 [71]. A recent paper from the Briscoe lab [72] has shown that the mutual repression of transcription factors is not only important for the establishment of neuroepithelial subregions in the hindbrain, but also determines the decision between a visceral motor neuron and a serotonergic neuron fate. Thus, early brain patterning and later neuronal function are inherently linked by the characteristic gene-expression profile of a given brain area.
Axon guidance and map formation
A host of novel molecular players and interactions in axon guidance have been identified [73,74], with mutagenesis screens in zebrafish proving a particularly useful tool for such gene-mining projects [75]. As with patterning and the establishment of neuronal phenotypes, the expression or absence of single transcription factors can have a profound influence on axon projections and the formation of functional synapses [76]. Recently, the formation of the facial somatosensory map was shown to depend on the Hox-gene-regulated rhombomeric organization of the hindbrain, providing yet another example of a direct link between early brain regionalization and the establishment of functional architecture [77].
Hodge et al. [78] have shown that the regionalized expression of BMP4 in facial structures of the mouse embryo regulates gene expression in trigeminal sensory neurons and, as a consequence, influences the spatial projection pattern of these neurons. This study indicates that target-derived signals play a crucial role in shaping neuronal architecture.
Future directions
Neural induction is still a hotly debated topic, but it seems likely that FGFs and other signals mediate the earliest steps of this process and that the inhibition of BMPs (probably in combination with Wnt inhibitors and ongoing FGF signalling) serves to stabilize the early-induced neural fate [79]. It has become increasingly clear that secreted signalling factors perform different functions at different developmental stages, not only regulating patterning [56,60,63,64,80], but also proliferation [64], the acquisition of neuronal phenotypes [64,78] and even axon guidance [6,8,9,81-83].
Our deepening knowledge of the molecular processes that establish specific subregions of the brain allows us to mimic these steps in a Petri dish in order to drive embryonic stem cells along certain predictable developmental routes. So far, motor neurons [84], telencephalic precursors [85], dopaminergic midbrain neurons [86] and cerebellar granule cells [87] have been generated in vitro and in some cases have been shown to integrate successfully into the corresponding structures of a developing brain. Thus, basic research in developmental neurobiology has opened up new avenues and can offer more specific, target-oriented approaches in producing stem cells for therapeutic purposes.
Acknowledgments
We would like to apologize to all researchers whose work we were not able to mention due to space constraints.
Abbreviations
- AP
anteroposterior
- BMPs
bone morphogenetic proteins
- chch
the FGF target gene churchill
- CNS
central nervous system
- DV
dorsoventral
- FGFs
fibroblast growth factors
- MHB
boundary between midbrain and hindbrain
- Shh
Sonic hedgehog
Competing interests
The authors declare that they have no competing interests.
The electronic version of this article is the complete one and can be found at: http://F1000.com/Reports/Biology/content/1/1
References
- 1.De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munoz-Sanjuan I, Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci. 2002;3:271–80. doi: 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- 3.Stern CD. Neural induction: 10 years on since the ‘default model’. Curr Opin Cell Biol. 2006;18:692–7. doi: 10.1016/j.ceb.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 4.De Robertis EM. Evo-devo: variations on ancestral themes. Cell. 2008;132:185–95. doi: 10.1016/j.cell.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine AJ, Brivanlou AH. Proposal of a model of mammalian neural induction. Dev Biol. 2007;308:247–56. doi: 10.1016/j.ydbio.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6:351–62. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 7.De Robertis EM, Larraín J, Oelgeschläger M, Wessely O. The establishment of Spemann's organizer and patterning of the vertebrate embryo. Nat Rev Genet. 2000;1:171–81. doi: 10.1038/35042039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8:755–65. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- 9.Mason I. Initiation to end point: the multiple roles of fibroblast growth factors in neural development. Nat Rev Neurosci. 2007;8:583–96. doi: 10.1038/nrn2189. [DOI] [PubMed] [Google Scholar]
- 10.Niehrs C. Regionally specific induction by the Spemann-Mangold organizer. Nat Rev Genet. 2004;5:425–34. doi: 10.1038/nrg1347. [DOI] [PubMed] [Google Scholar]
- 11.Rhinn M, Picker A, Brand M. Global and local mechanisms of forebrain and midbrain patterning. Curr Opin Neurobiol. 2006;16:5–12. doi: 10.1016/j.conb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Stern CD, Charite J, Deschamps J, Duboule D, Durston AJ, Kmita M, Nicolas JF, Palmeirim I, Smith JC, Wolpert L. Head-tail patterning of the vertebrate embryo: one, two or many unresolved problems? Int J Dev Biol. 2006;50:3–15. doi: 10.1387/ijdb.052095cs. [DOI] [PubMed] [Google Scholar]
- 13.Wilson SW, Houart C. Early steps in the development of the forebrain. Dev Cell. 2004;6:167–81. doi: 10.1016/S1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garda AL, Echevarria D, Martinez S. Neuroepithelial co-expression of Gbx2 and Otx2 precedes Fgf8 expression in the isthmic organizer. Mech Dev. 2001;101:111–8. doi: 10.1016/S0925-4773(00)00567-0. [DOI] [PubMed] [Google Scholar]
- 15.Katahira T, Sato T, Sugiyama S, Okafuji T, Araki I, Funahashi J, Nakamura H. Interaction between Otx2 and Gbx2 defines the organizing center for the optic tectum. Mech Dev. 2000;91:43–52. doi: 10.1016/S0925-4773(99)00262-2. [DOI] [PubMed] [Google Scholar]
- 16.Liu A, Joyner AL. Early anterior/posterior patterning of the midbrain and cerebellum. Annu Rev Neurosci. 2001;24:869–96. doi: 10.1146/annurev.neuro.24.1.869. [DOI] [PubMed] [Google Scholar]
- 17.Raible F, Brand M. Divide et Impera - the midbrain-hindbrain boundary and its organizer. Trends Neurosci. 2004;27:727–34. doi: 10.1016/j.tins.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Wurst W, Bally-Cuif L. Neural plate patterning: upstream and downstream of the isthmic organizer. Nat Rev Neurosci. 2001;2:99–108. doi: 10.1038/35053516. [DOI] [PubMed] [Google Scholar]
- 19.Kiecker C, Lumsden A. Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci. 2005;6:553–64. doi: 10.1038/nrn1702. [DOI] [PubMed] [Google Scholar]
- 20.Pasini A, Wilkinson DG. Stabilizing the regionalisation of the developing vertebrate central nervous system. BioEssays. 2002;24:427–38. doi: 10.1002/bies.10085.abs. [DOI] [PubMed] [Google Scholar]
- 21.Echevarria D, Vieira C, Gimeno L, Martinez S. Neuroepithelial secondary organizers and cell fate specification in the developing brain. Brain Res Brain Res Rev. 2001;43:179–91. doi: 10.1016/j.brainresrev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Colas JF, Schoenwolf GC. Towards a cellular and molecular understanding of neurulation. Dev Dyn. 2001;221:117–45. doi: 10.1002/dvdy.1144. [DOI] [PubMed] [Google Scholar]
- 23.Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–93. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- 24.Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:772–83. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- 25.Placzek M, Briscoe J. The floor plate: multiple cells, multiple signals. Nat Rev Neurosci. 2005;6:230–40. doi: 10.1038/nrn1628. [DOI] [PubMed] [Google Scholar]
- 26.Strähle U, Lam CS, Ertzer R, Rastegar S. Vertebrate floor-plate specification: variations on common themes. Trends Genet. 2004;20:155–62. doi: 10.1016/j.tig.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Chesnutt C, Burrus LW, Brown AM, Niswander L. Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev Biol. 2004;274:334–47. doi: 10.1016/j.ydbio.2004.07.019. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Andrew Lumsden 11 Oct 2004
- 28.Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Annu Rev Neurosci. 1999;22:261–94. doi: 10.1146/annurev.neuro.22.1.261. [DOI] [PubMed] [Google Scholar]
- 29.Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306:343–8. doi: 10.1016/j.yexcr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 31.Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–83. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- 32.Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17:580–9. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Guthrie S. Patterning and axon guidance of cranial motor neurons. Nat Rev Neurosci. 2007;8:859–71. doi: 10.1038/nrn2254. [DOI] [PubMed] [Google Scholar]
- 34.Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–63. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- 35.Polleux F, Ince-Dunn G, Ghosh A. Transcriptional regulation of vertebrate axon guidance and synapse formation. Nat Rev Neurosci. 2007;8:331–40. doi: 10.1038/nrn2118. [DOI] [PubMed] [Google Scholar]
- 36.Lemke G, Reber M. Retinotectal mapping: new insights from molecular genetics. Annu Rev Cell Dev Biol. 2005;21:551–80. doi: 10.1146/annurev.cellbio.20.022403.093702. [DOI] [PubMed] [Google Scholar]
- 37.McLaughlin T, O'Leary DD. Molecular gradients and development of retinotopic maps. Annu Rev Neurosci. 2005;28:327–55. doi: 10.1146/annurev.neuro.28.061604.135714. [DOI] [PubMed] [Google Scholar]
- 38.Khokha MK, Yeh J, Grammer TC, Harland RM. Depletion of three BMP antagonists from Spemann's organizer leads to a catastrophic loss of dorsal structures. Dev Cell. 2005;8:401–11. doi: 10.1016/j.devcel.2005.01.013. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Claudio Stern 8 Mar 2005
- 39.Reversade B, Kuroda H, Lee H, Mays A, De Robertis EM. Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embryos. Development. 2005;132:3381–92. doi: 10.1242/dev.01901. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Claudio Stern 28 Jul 2005
- 40.Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–21. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- 41.Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406:74–8. doi: 10.1038/35017617. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Ali H Brivanlou 30 Nov 2001
- 42.Chang C, Harland RM. Neural induction requires continued suppression of both Smad1 and Smad2 signals during gastrulation. Development. 2007;134:3861–72. doi: 10.1242/dev.007179. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.2 RecommendedEvaluated by David Kimelman 22 Oct 2007, James Briscoe 24 Oct 2007
- 43.Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development. 2005;132:299–310. doi: 10.1242/dev.01582. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by David Kimelman 4 Jan 2005
- 44.Kudoh T, Concha ML, Houart C, Dawid IB, Wilson SW. Combinatorial Fgf and Bmp signalling patterns the gastrula ectoderm into prospective neural and epidermal domains. Development. 2004;131:3581–92. doi: 10.1242/dev.01227. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by David Kimelman 22 Jul 2004
- 45.Linker C, Stern CD. Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development. 2004;131:5671–81. doi: 10.1242/dev.01445. [DOI] [PubMed] [Google Scholar]
- 46.Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–93. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6.5 Must ReadEvaluated by David Kimelman 13 Dec 2007, Manfred Frasch 17 Dec 2007, Andrew Lumsden 18 Dec 2007
- 47.Gomez-Skarmeta J, de La Calle-Mustienes E, Modolell J. The Wnt-activated Xiro1 gene encodes a repressor that is essential for neural development and downregulates Bmp4. Development. 2001;128:551–60. doi: 10.1242/dev.128.4.551. [DOI] [PubMed] [Google Scholar]; F1000 Factor 4.8 Must ReadEvaluated by Harukazu Nakamura 4 Dec 2001, Siew-Lan Ang 20 Dec 2001
- 48.Takemoto T, Uchikawa M, Kamachi Y, Kondoh H. Convergence of Wnt and FGF signals in the genesis of posterior neural plate through activation of the Sox2 enhancer N-1. Development. 2006;133:297–306. doi: 10.1242/dev.02196. [DOI] [PubMed] [Google Scholar]; F1000 Factor 4.8 Must ReadEvaluated by James Briscoe 31 Jan 2006, Claudio Stern 20 Feb 2006
- 49.Wilson SI, Rydstrom A, Trimborn T, Willert K, Nusse R, Jessell TM, Edlund T. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature. 2001;411:325–30. doi: 10.1038/35077115. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by John Rubenstein 9 Oct 2001
- 50.Aubin J, Davy A, Soriano P. In vivo convergence of BMP and MAPK signaling pathways: impact of differential Smad1 phosphorylation on development and homeostasis. Genes Dev. 2004;18:1482–94. doi: 10.1101/gad.1202604. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 4.9 Must ReadEvaluated by Daniel Constam 24 Jun 2004, David Wotton 24 Jun 2004, Claudio Stern 7 Jul 2004
- 51.Pera EM, Ikeda A, Eivers E, De Robertis EM. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev. 2003;17:3023–8. doi: 10.1101/gad.1153603. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.2 RecommendedEvaluated by Andrew Lumsden 13 Jan 2004, David Wotton 21 Jan 2004
- 52.Sheng G, dos Reis M, Stern CD. Churchill, a zinc finger transcriptional activator, regulates the transition between gastrulation and neurulation. Cell. 2003;115:603–13. doi: 10.1016/S0092-8674(03)00927-9. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.4 Must ReadEvaluated by James Briscoe 9 Dec 2003, David Kimelman 11 Dec 2003
- 53.Lee BM, Buck-Koehntop BA, Martinez-Yamout MA, Dyson HJ, Wright PE. Embryonic neural inducing factor churchill is not a DNA-binding zinc finger protein: solution structure reveals a solvent-exposed beta-sheet and zinc binuclear cluster. J Mol Biol. 2007;371:1274–89. doi: 10.1016/j.jmb.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Thomas Friedman 29 Jan 2008
- 54.Mangold O. Über die Induktionsfähigkeit der verschiedenen Bezirke der Neurula von Urodelen. Naturwissenschaften. 1933;21:761–66. doi: 10.1007/BF01503740. [DOI] [Google Scholar]
- 55.Staudt N, Houart C. The prethalamus is established during gastrulation and influences diencephalic regionalization. PLoS Biol. 2007;5:e69. doi: 10.1371/journal.pbio.0050069. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Lilianna Solnica-Krezel 20 Apr 2007
- 56.Haremaki T, Tanaka Y, Hongo I, Yuge M, Okamoto H. Integration of multiple signal transducing pathways on Fgf response elements of the Xenopus caudal homologue Xcad3. Development. 2003;130:4907–17. doi: 10.1242/dev.00718. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by David Kimelman 3 Sep 2003
- 57.Lagutin OV, Zhu CC, Kobayashi D, Topczewski J, Shimamura K, Puelles L, Russell HR, McKinnon PJ, Solnica-Krezel L, Oliver G. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 2003;17:368–79. doi: 10.1101/gad.1059403. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Ali H Brivanlou 19 Mar 2003
- 58.Lavado A, Lagutin OV, Oliver G. Six3 inactivation causes progressive caudalization and aberrant patterning of the mammalian diencephalon. Development. 2008;135:441–50. doi: 10.1242/dev.010082. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Andrew Lumsden 5 Mar 2008
- 59.Matsumoto K, Nishihara S, Kamimura M, Shiraishi T, Otoguro T, Uehara M, Maeda Y, Ogura K, Lumsden A, Ogura T. The prepattern transcription factor Irx2, a target of the FGF8/MAP kinase cascade, is involved in cerebellum formation. Nat Neurosci. 2004;7:605–12. doi: 10.1038/nn1249. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Gordon Fishell 22 Jul 2004
- 60.Kiecker C, Lumsden A. Hedgehog signaling from the ZLI regulates diencephalic regional identity. Nat Neurosci. 2004;7:1242–9. doi: 10.1038/nn1338. [DOI] [PubMed] [Google Scholar]; F1000 Factor 4.8 Must ReadEvaluated by Claudio Stern 5 Nov 2004, Alexandra Joyner 10 Dec 2004
- 61.Amoyel M, Cheng YC, Jiang YJ, Wilkinson DG. Wnt1 regulates neurogenesis and mediates lateral inhibition of boundary cell specification in the zebrafish hindbrain. Development. 2005;132:775–85. doi: 10.1242/dev.01616. [DOI] [PubMed] [Google Scholar]
- 62.Riley BB, Chiang MY, Storch EM, Heck R, Buckles GR, Lekven AC. Rhombomere boundaries are Wnt signaling centers that regulate metameric patterning in the zebrafish hindbrain. Dev Dyn. 2004;231:278–91. doi: 10.1002/dvdy.20133. [DOI] [PubMed] [Google Scholar]
- 63.Trokovic R, Jukkola T, Saarimaki J, Peltopuro P, Naserke T, Weisenhorn DM, Trokovic M, Wurst W, Partanen J. Fgfr1-dependent boundary cells between developing mid- and hindbrain. Dev Biol. 2005;278:428–39. doi: 10.1016/j.ydbio.2004.11.024. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Harukazu Nakamura 11 Feb 2005
- 64.Saarimaki-Vire J, Peltopuro P, Lahti L, Naserke T, Blak AA, Vogt Weisenhorn DM, Yu K, Ornitz DM, Wurst W, Partanen J. Fibroblast growth factor receptors cooperate to regulate neural progenitor properties in the developing midbrain and hindbrain. J Neurosci. 2007;27:8581–92. doi: 10.1523/JNEUROSCI.0192-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Harukazu Nakamura 12 Sep 2007
- 65.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 66.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–77. doi: 10.1016/S1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 67.Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/S1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 68.Kibardin A, Ossipova O, Sokol SY. Metastasis-associated kinase modulates Wnt signaling to regulate brain patterning and morphogenesis. Development. 2006;133:2845–54. doi: 10.1242/dev.02445. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Herbert Steinbeisser 12 Jul 2006
- 69.Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–56. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Jeffrey Axelrod 3 Aug 2006
- 70.Puelles E, Acampora D, Gogoi R, Tuorto F, Papalia A, Guillemot F, Ang SL, Simeone A. Otx2 controls identity and fate of glutamatergic progenitors of the thalamus by repressing GABAergic differentiation. J Neurosci. 2006;26:5955–64. doi: 10.1523/JNEUROSCI.1097-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sieber MA, Storm R, Martinez-de-la-Torre M, Müller T, Wende H, Reuter K, Vasyutina E, Birchmeier C. Lbx1 acts as a selector gene in the fate determination of somatosensory and viscerosensory relay neurons in the hindbrain. J Neurosci. 2007;27:4902–9. doi: 10.1523/JNEUROSCI.0717-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Jean-François Brunet 25 Jun 2007
- 72.Jacob J, Ferri AL, Milton C, Prin F, Pla P, Lin W, Gavalas A, Ang SL, Briscoe J. Transcriptional repression coordinates the temporal switch from motor to serotonergic neurogenesis. Nat Neurosci. 2007;10:1433–9. doi: 10.1038/nn1985. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Jean-François Brunet 20 Dec 2007
- 73.Osterfield M, Egelund R, Young LM, Flanagan JG. Interaction of amyloid precursor protein with contactins and NgCAM in the retinotectal system. Development. 2008;135:1189–99. doi: 10.1242/dev.007401. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Harukazu Nakamura 26 Feb 2008
- 74.Uemura M, Nakao S, Suzuki ST, Takeichi M, Hirano S. OL-Protocadherin is essential for growth of striatal axons and thalamocortical projections. Nat Neurosci. 2007;10:1151–9. doi: 10.1038/nn1960. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Frans Van Roy 29 Oct 2007
- 75.Tanaka H, Maeda R, Shoji W, Wada H, Masai I, Shiraki T, Kobayashi M, Nakayama R, Okamoto H. Novel mutations affecting axon guidance in zebrafish and a role for plexin signalling in the guidance of trigeminal and facial nerve axons. Development. 2007;134:3259–69. doi: 10.1242/dev.004267. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Judith S Eisen 18 Dec 2007
- 76.Ince-Dunn G, Hall BJ, Hu SC, Ripley B, Huganir RL, Olson JM, Tapscott SJ, Ghosh A. Regulation of thalamocortical patterning and synaptic maturation by NeuroD2. Neuron. 2006;49:683–95. doi: 10.1016/j.neuron.2006.01.031. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Michael Greenberg 24 Apr 2006
- 77.Oury F, Murakami Y, Renaud JS, Pasqualetti M, Charnay P, Ren SY, Rijli FM. Hoxa2- and rhombomere-dependent development of the mouse facial somatosensory map. Science. 2006;313:1408–13. doi: 10.1126/science.1130042. [DOI] [PubMed] [Google Scholar]; F1000 Factor 4.8 Must ReadEvaluated by Anthony Graham 13 Sep 2006, Andrew Lumsden 14 Sep 2006
- 78.Hodge LK, Klassen MP, Han BX, Yiu G, Hurrell J, Howell A, Rousseau G, Lemaigre F, Tessier-Lavigne M, Wang F. Retrograde BMP signaling regulates trigeminal sensory neuron identities and the formation of precise face maps. Neuron. 2007;55:572–86. doi: 10.1016/j.neuron.2007.07.010. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Filippo Rijli 3 Oct 2007
- 79.Albazerchi A, Stern CD. A role for the hypoblast (AVE) in the initiation of neural induction, independent of its ability to position the primitive streak. Dev Biol. 2007;301:489–503. doi: 10.1016/j.ydbio.2006.08.057. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Patrick Tam 26 Sep 2006
- 80.Houart C, Caneparo L, Heisenberg C, Barth K, Take-Uchi M, Wilson S. Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron. 2002;35:255–65. doi: 10.1016/S0896-6273(02)00751-1. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.5 Must ReadEvaluated by Lilianna Solnica-Krezel 29 Aug 2002, Gordon Fishell 18 Sep 2002, Ali H Brivanlou 3 Oct 2002
- 81.Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/S0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]; F1000 Factor 4.9 Must ReadEvaluated by Andrew Lumsden 2 Apr 2003, Gordon Fishell 16 Apr 2003, Judith S Eisen 14 May 2003
- 82.Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–8. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Yi Rao 8 Jan 2004
- 83.Okada A, Charron F, Morin S, Shin DS, Wong K, Fabre PJ, Tessier-Lavigne M, McConnell SK. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature. 2006;444:369–73. doi: 10.1038/nature05246. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.5 Must ReadEvaluated by M Angela Nieto 27 Nov 2006, Harukazu Nakamura 27 Nov 2006, Simon Hughes 7 Dec 2006
- 84.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–97. doi: 10.1016/S0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]; F1000 Factor 9.9 ExceptionalEvaluated by Constance Scharff 19 Aug 2002, Sally Temple 6 Sep 2002, Andrew Lumsden 24 Sep 2002
- 85.Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, Watanabe Y, Mizuseki K, Sasai Y. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8:288–96. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]; F1000 Factor 4.8 Must ReadEvaluated by Arturo Alvarez-Buylla 9 Mar 2005, Patrick Tam 11 Mar 2005
- 86.Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B, Perlmann T, Ericson J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124:393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.5 Must ReadEvaluated by Andrew S McCallion 11 Apr 2006, Gordon Fishell 20 Apr 2006, Jeffrey Macklis 25 Apr 2006
- 87.Salero E, Hatten ME. Differentiation of ES cells into cerebellar neurons. Proc Natl Acad Sci USA. 2007;104:2997–3002. doi: 10.1073/pnas.0610879104. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Jeffrey Macklis 12 Feb 2008


