Abstract
The objective of this study was to elucidate the cytotoxic mechanism of Compound K, with respect to the involvement of reactive oxygen species (ROS) and the mitochondrial involved apoptosis, in HT-29 human colon cancer cells. Compound K exhibited a concentration of 50% growth inhibition (IC50) at 20 μg/mL and cytotoxicity in a time dependent manner. Compound K produced intracellular ROS in a time dependent fashion; however, N-acetylcysteine (NAC) pretreatment resulted in the inhibition of this effect and the recovery of cell viability. Compound K induced a mitochondria-dependent apoptotic pathway via the modulation of Bax and Bcl-2 expressions, resulting in the disruption of the mitochondrial membrane potential (Δψm). Loss of the Δψm was followed by cytochrome c release from the mitochondria, resulting in the activation of caspase-9, -3, and concomitant poly ADP-ribosyl polymerase (PARP) cleavage, which are the indicators of caspase-dependent apoptosis. The apoptotic effect of Compound K, exerted via the activation of c-Jun NH2-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK), was abrogated by specific MAPK inhibitors. This study demonstrated that Compound K-mediated generation of ROS led to apoptosis through the modulation of a mitochondria-dependent apoptotic pathway and MAPK pathway.
Keywords: Compound K, reactive oxygen species, mitochondrial membrane potential, c-Jun NH2-terminal kinase, p38 mitogen-activated protein kinase
1. Introduction
Reactive oxygen species (ROS) are the by-products of normal cellular oxidative processes, and are mainly generated in the mitochondria. They attack lipid membranes, proteins, and DNA, leading to serious cell damage, and regulate apoptotic signal transduction [1–3]. Indeed, ROS induce the depolarization of the mitochondrial membrane, and lead to increased levels of pro-apoptotic molecules in the cytosol [4–6]. Apoptosis is followed by cell shrinkage, nuclear fragmentation, membrane blebbing, DNA fragmentation, and finally the breakdown of the cell into apoptotic bodies [7–9]. Capases, a family of cysteine-dependent aspartate-directed proteases, play a critical role in the initiation and execution of apoptosis [10–12]. Among this family, caspase-9 and -3 are the most crucial for the initiation and execution of apoptosis in various cell types [13,14]. Cancer is a disease that involves excessive proliferation of cells and insufficient cell suicide via apoptotic process. [20-O-(β-d-glucopyranosyl)-20(S)-protopanaxadiol] (Compound K, Figure 1) is the main metabolite of protopanaxadiol-type ginsenoside formed in the intestine after oral administration [15–18]. We recently reported that Compound K exhibited cytotoxicity through the induction of apoptosis, arrest at the G1 phase of cell cycle, and inhibition of telomerase activity in human leukemia cells [19–21]; that the combined treatment of Compound K and radiation enhanced the cell death in human lung cancer cells [22]; and that Compound K induced apoptosis in MCF-7 breast cancer cells through the modulation of AMP-activated protein kinase [23]. The gastrointestinal tract, especially the colon, is constantly exposed to ROS originating from endogenous and exogenous sources [24]. Colorectal cancer is the fourth most prevalent carcinoma in western society and the second cause of cancer death [25]. And genetic alterations by ROS are the ultimate underlying mechanisms of colorectal carcinogenesis [26,27]. Compound K has been shown to exhibit anti-proliferative effects on colon cancer cells, which was mediated through apoptosis [28–30]. Despite evidence of its anti-proliferative effects in colon cancer, the cytotoxic mechanism of this effect with respect to the involvement of ROS and mitochondrial involved apoptosis, has not been investigated. Our study showed that Compound K significantly induced ROS generation, which in turn led to apoptotic signals including mitochondria-dependent and caspase-dependent processes.
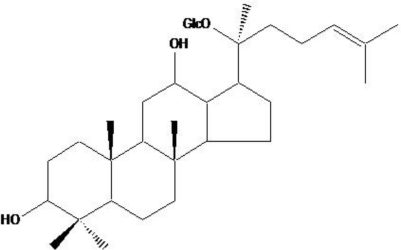
Figure 1.
Chemical structure of Compound K, [20-O-d-glucopyranosyl-20(S)-protopanaxadiol].
2. Results and Discussion
2.1. ROS-Induced Cytotoxic Effect of Compound K on HT-29 Colon Cancer Cells
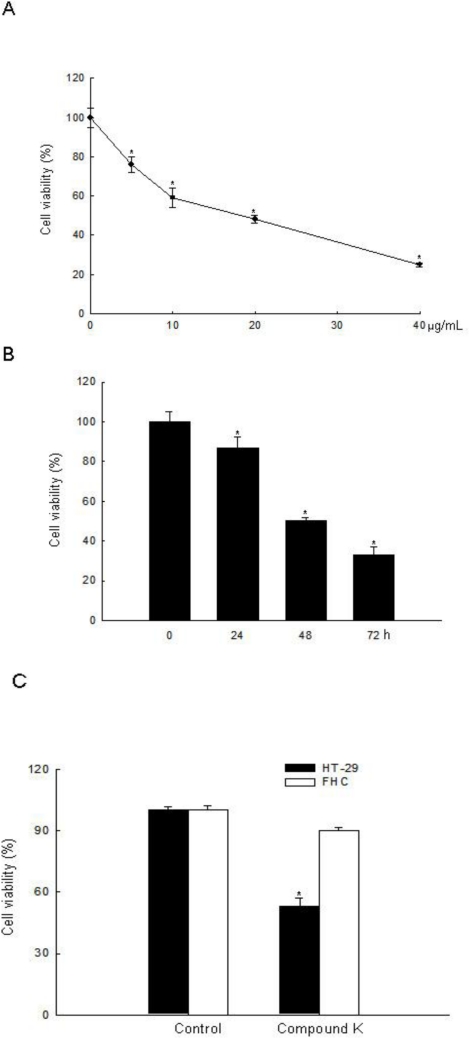
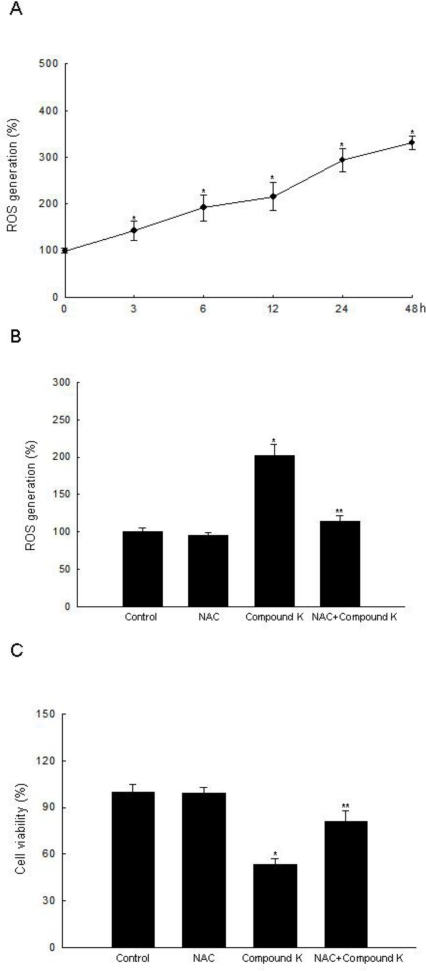
Compound K is an active metabolite of ginsenosides and exhibits anti-tumor effects against various types of cancer cells [16,17,19–23,28–36]. In the present study, we investigated the effects and mechanism of action of Compound K in ROS-mediated apoptosis in HT-29 cancer cells. Although it had been previously shown that Compound K induced apoptosis via a Ca2+/calmodulinactivated protein kinase-IV/AMP-activated protein kinase pathway in HT-29 colon cancer cells [28,29], Compound K-induced ROS-mediated apoptosis in colon cancer cells had not been investigated. Several anticancer agents used in the treatment of cancer have been shown to cause increased cellular ROS generation [37–39]. Compound K inhibited HT-29 cell growth in a dose-dependent manner at 10, 20, 30, and 40 μg/mL at 48 h, and the concentration effecting 50% growth inhibition (IC50) was 20 μg/mL (Figure 2A). Compound K at 20 μg/mL also inhibited HT-29 cell growth in a time-dependent manner (Figure 2B), but did not exhibit cytotoxicity in FHC normal colon cells compared to HT-29 cells at day 2 (Figure 2C). Intracellular ROS, as signaling intermediates, are involved in cell death signal transduction pathways. Compound K induced ROS generation as compared to control in a time-dependent manner (Figure 3A), and NAC, a distinct antioxidant and ROS scavenger, exerted a scavenging effect on the ROS generated by Compound K (Figure 3B). Subsequently, NAC significantly abolished the Compound K-triggered cell death; cell survival was increased to 81% in NAC-pretreated, Compound K-treated cells, compared to 53% in cells treated only with Compound K (Figure 3C). This result suggests that the ROS generated by Compound K induces the cell death.
Figure 2.
Cytotoxic effect of Compound K in human colon cells. Cell viability (A) at the indicated concentrations of Compound K at 48 h in HT-29 cancer cells; (B) at the indicated times with Compound K at 20 μg/mL in HT-29 cells; (C) at 20 μg/mL of Compound K in FHC normal colon cells and HT-29 colon cancer cells was assessed using MTT test. *Significantly different from control (p < 0.05).
Figure 3.
Intracellular ROS generation induced by Compound K treatment. (A) Intracellular ROS generated by Compound K were detected at indicated times by a spectrofluorometer after DCF-DA treatment. (B) After treatment with NAC and/or Compound K, intracellular ROS were detected at 24 h by spectrofluorometer after DCF-DA treatment. (C) After treatment with NAC and/or Compound K, cell viability was assessed by MTT assay. *Significantly different from control (p < 0.05), and **significantly different from Compound K-treated cells (p < 0.05).
2.2. Induction of Apoptosis by Compound K via a Mitochondria-Dependent Pathway
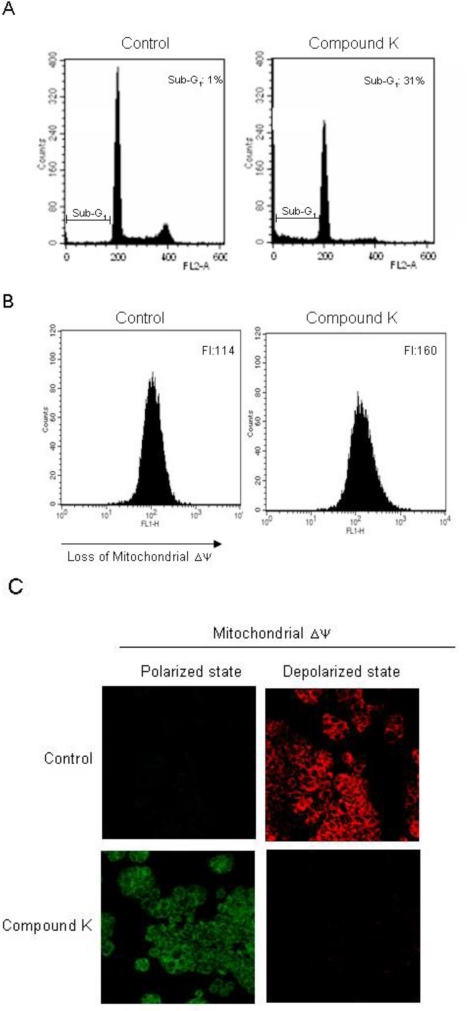
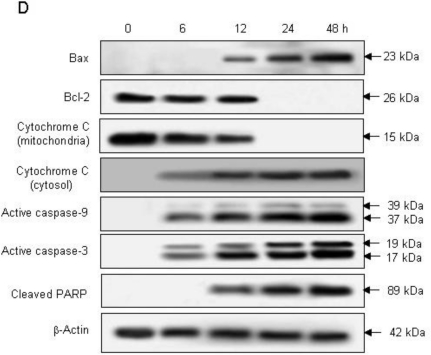
Such increases in intracellular ROS cause loss of mitochondria membrane permeability, resulting in the induction of apoptosis [40,41]. We investigated whether the cytotoxicity of Compound K was associated with the induction of apoptosis. Sub G1-hypodiploid cells, which are an indicator of apoptosis, increased in Compound K-treated cells as compared to control cells (Figure 4A). Apoptotic pathway requires the alteration of the mitochondrial membrane potential (Δψm), which leads to mitochondrial membrane permeabilization and is followed by a release of cytochrome c and caspases activation [4,42]. Compound K-treated cells exhibited a loss of Δψm, as substantiated by an increase of fluorescence intensity in fluorescence (FL-1) using the JC-1 dye (Figure 4B). In non-apoptotic cells, the monomer form of JC-1 accumulates as aggregates in the mitochondria, which then emits red fluorescence; whereas in apoptotic cells, JC-1 does not accumulate and remains a monomer, emitting green fluorescence. The control cells exhibited strong red fluorescence in the mitochondria; however, Compound K-treated cells resulted in a decreased red fluorescence in the mitochondria and increased green fluorescence, suggesting that Compound K treatment disrupted the mitochondrial Δψ (Figure 4C). During the apoptotic process, Bcl-2, an anti-apoptotic regulator, prevents the opening of the mitochondrial membrane pores, whereas Bax, an apoptotic regulator, induces it [43]. Compound K was shown to increase Bax expression while decreasing that of Bcl-2 expression. Therefore, Compound K-induced loss of the Δψm may have been a result of an up-regulation of Bcl-2 and a down-regulation of Bax. The pore opening induces the loss of the Δψm, which in turn induces the release of cytochrome c from the mitochondria [44,45]. Compound K induced the release of cytochrome c from mitochondria into cytosol (Figure 4D). The mitochondrial membrane disruption by Compound K activated caspase-9 (37 and 39 kDa) and caspase-3 (19 and 17 kDa), a target of caspase-9, which was further demonstrated by PARP cleavage (89 kDa) (Figure 4D). These results suggest that Compound K induced apoptosis via a caspase--dependent pathway in the mitochondria. Further, Compound K-induced decreases in the Bcl-2 protein and corresponding increases in the Bax protein, results in the opening of mitochondrial membrane pores, facilitating the release of cytochrome c from mitochondria into cytosol. Cytochrome c is bound to the outer surface of the inner membrane phospholipids. An early process in the release of cytochrome c is its dissociation from the inner membrane. Mitochondrial ROS have been shown to promote cytochrome c release to the cytosol by dissociation from these membrane phospholipids [46,47]. Therefore, Compound K-mediated ROS production may target membrane phospholipids resulting in dissociation of cytochrome c.
Figure 4.
Induction of mitochondria-dependent and caspase-dependent apoptosis by Compound K treatment. (A) Apoptotic sub-G1 cells were detected by flow cytometry after PI staining. (B) Δψm was analyzed by flow cytometry and (C) confocal microscopy after staining cells with JC-1 dye. (D) Cell lysates were electrophoresed and Bax, Bcl-2, cytochrome c, active caspase-9, active caspase-3, and cleaved PARP proteins were detected using their corresponding antibodies.
2.3. Induction of Apoptosis by Compound K via JNK and p38 MAPK Activation
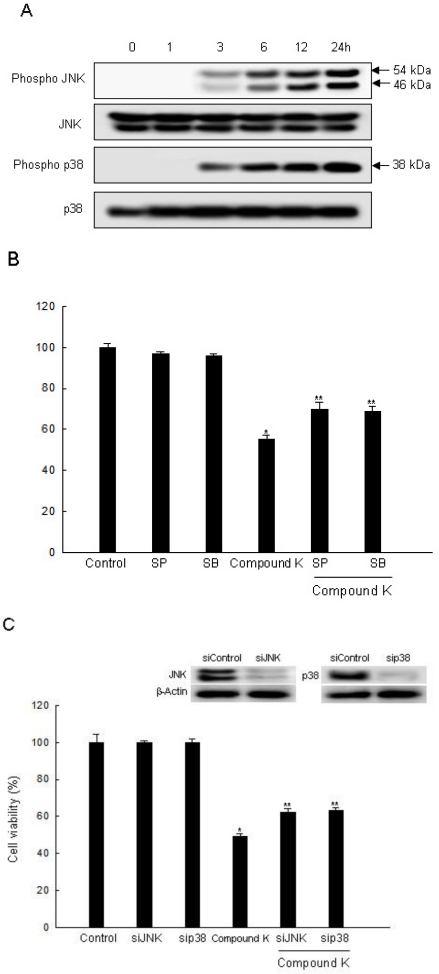
Various studies have suggested a possible mechanism for the JNK and p38 MAPK pathway, as related to mitochondrial depolarization and apoptosis induction [48]. The activated MAPK family members including the phosphorylated form of p38, JNK, and ERK are common components of the apoptotic process [49–51]. Some reports have shown that ROS acted as upstream regulators, resulting in the activation of p38 MAPK and JNK [52,53]. Intracellular ROS are also upstream of AMP-activated protein kinase (AMPK) activation [54]. AMPK is highly sensitive to oxidative stress because increased cellular ROS change the AMP level, which leads to rapid AMPK activation. The involvement of AMPK in the inhibition of carcinogenesis can modulate the regulation of COX-2 [55]. Our previous study demonstrated that Compound K exhibited apoptosis by ROS-mediated AMPK activation in MCF-7 breast cancer cells [23]. In the present study, Compound K induced the activation of JNK and p38 MAPK in a time-dependent manner (Figure 5A). We then examined whether a specific inhibitor of JNK and p38 MAPK could attenuate cell death through activation of JNK and p38 MAPK signaling by Compound K. Results revealed that SP600125 (an inhibitor of JNK) and SB203580 (an inhibitor of p38 MAPK) attenuated the cytotoxic effect of Compound K (Figure 5B). Likewise, siJNK and sip38-transfected cells abolished the cytotoxic effect of Compound K (Figure 5C). Our results revealed that phospho p38 MAPK and phospho JNK were notably increased after Compound K treatment. In contrast, Compound K treatment reduced the level of phospho ERK expression (data not shown). This suggested that activation of p38 MAPK and JNK was related to Compound K-induced apoptotic cell death. These results indicate that JNK and p38 MAPK may play a role in Compound K-induced cytotoxicity in HT-29 cells.
Figure 5.
Effect of Compound K on the MAPK signaling pathway. (A) Cell lysates were electrophoresed and were immunoblotted using anti-JNK, -phospho JNK, -p38, and -phospho p38 antibodies. (B) After treatment with MAPK inhibitors or/and Compound K, cell viability was assessed by MTT assay. (C) After transfection of siRNA against MAPK, and/or Compound K, cell viability was assessed by MTT assay. *Significantly different from control (p < 0.05), and **significantly different from Compound K-treated cells (p < 0.05).
3. Experimental Section
3.1. Chemicals
Compound K was provided by professor Dong Hyun Kim (Kyung Hee University, Seoul, Korea). [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium] bromide (MTT), 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA), N-acetylcysteine (NAC), propidium iodide, SP600125, and SB203580 were purchased from Sigma Chemical Co. (St. Louis, MO, USA). 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine chloride (JC-1) was purchased from Molecular Probes (Eugene, OR, USA). The primary anti-Bcl-2, -Bax, -cytochrome c, -caspase-9, -caspase-3, -poly ADP-ribosyl polymerase (PARP), -c-Jun NH2-terminal kinase (JNK), -phospho JNK, -p38, and -phospho p38 antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA).
3.2. Cells and Cell Culture
Human colon adenocarcinoma (HT-29) and normal colon cells (FHC), from the American type culture collection (Rockville, MD, USA), were maintained at 37 °C in an incubator with a humidified atmosphere of 5% CO2 and cultured in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum, streptomycin (100 μg/mL), and penicillin (100 units/mL).
3.3. Cell Viability Assay
The effect of Compound K on the viability of the cells was determined by the MTT assay, which is based on the reduction of a tetrazolium salt by mitochondrial succinatedehydrogenase in viable cells [56]. The cells were seeded in a 96 well plate at a density of 1 × 105 cells/mL, treated with Compound K, and after incubating for 48 h, 50 μL of the MTT stock solution (2 mg/mL) was added to each well to attain a total reaction volume of 250 μL. After an incubation of 4 h, the supernatants were aspirated. The formazan crystals in each well were then dissolved in 150 μL dimethylsulfoxide, and the absorbance at 540 nm was read on a scanning multi-well spectrophotometer.
3.4. Measurement of Intracellular Reactive Oxygen Species (ROS)
The DCF-DA method was used to detect the levels of intracellular ROS [42]. Cells were seeded onto a 96 well plate at 2 × 104 cells/well. The day after plating, the cells were treated with NAC (2 mM) for 30 min and then treated with Compound K for 24 h. After the addition of 25 mM of the DCF-DA solution for 20 min, the fluorescence of 2′,7′-dichlorofluorescein was measured using a Perkin Elmer LS-5B spectrofluorometer.
3.5. Detection of Sub-G1 Hypodiploid Cells
The amount of apoptotic sub-G1 hypodiploid cells was determined by flow cytometry [57]. Cells were treated with Compound K for 48 h. Harvested cells were then washed twice with phosphate buffered saline (PBS) and fixed in 70% ethanol for 30 min at 4 °C. Subsequently, the cells were incubated in 50 mg/mL propidium iodide solution and 50 μg/mL RNase A in the dark for 30 min at 37 °C. A flow cytometric analysis was performed using a FACS Calibur flow cytometer (Becton Dickinson, Mountain View, CA, USA). The sub-G1 hypodiploid cells were assessed based on histograms generated by Cell Quest and Mod-Fit computer programs.
3.6. Analysis of Mitochondrial Membrane Potential (Δψm)
Cells were stained with JC-1 (10 μg/mL), and were then analyzed using flow cytometry [58]. In addition, for image analysis, the JC-1-stained cells were mounted with mounting medium (DAKO, Carpinteria, CA, USA). Microscopic images were collected using the Laser Scanning Microscope 5 PASCAL program (Carl Zeiss, Jena, Germany) on a confocal microscope.
3.7. Western Blot Analysis
Harvested cells were washed in PBS, lysed in a lysis buffer [120 mM NaCl, 40 mM Tris (pH 8), 0.1% NP 40] and then centrifuged at 13,000 × g for 15 min. Aliquots of the lysates (50 μg of protein) were boiled at 95 °C for 5 min and electrophoresed on SDS–polyacrylamide gels. Gels were transferred onto nitrocellulose membranes for blotting (Bio-Rad, CA, USA), and the membranes were then incubated with primary antibodies. The membranes were further incubated with secondary immunoglobulin G-horseradish peroxidase conjugates, and then underwent enhanced chemiluminescence using a Western blotting detection kit (Amersham, Buckinghamshire, UK). The protein bands were visualized using luminescent image analyzer.
3.8. Transient Transfection of Small RNA Interference (siRNA)
Cells were seeded at 1.5 × 105 cells/well in 24 well plate and allowed to reach approximately 50% confluence on the day of transfection. The siRNA construct used were obtained as mismatched siRNA control (siControl, Santa Cruz Biotechnology, Santa Cruz, CA, USA), siRNA against JNK, and p38 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Cells were transfected with 10–50 nM siRNA using lipofectamineTM 2000 (Invitrogen, Carlsbad, CA, USA) based on the manufacturer’s instruction. At 24 h after transfection, the cells were treated with Compound K for 48 h and examined by either Western blot analysis or MTT assay.
3.9. Statistical Analysis
All the measurements were performed in triplicate and all values were represented as the mean standard error (SE). Results were subjected to an analysis of the variance (ANOVA) using the Tukey test for analysis of the differences. Statistical significance was set at p > 0.05.
4. Conclusions
This study demonstrated that Compound K-mediated generation of ROS led to apoptosis through activation of p38 MAPK and JNK, which modulate the expression of Bcl-2 and Bax, and then trigger loss of mitochondrial membrane potential, cytochrome c release, and caspase activation.
Acknowledgments
This work was supported by a research grant from Jeju National University Hospital.
References
- 1.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic. Biol. Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 2.Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysilogy. 2000;7:153–163. doi: 10.1016/s0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 3.Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 4.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Huang CY, Zheng RL, Cui KR, Li JF. Hydrogen peroxide induces apoptosis in human hepatoma cells and alters cell redox status. Cell Biol. Int. 2000;24:9–23. doi: 10.1006/cbir.1999.0438. [DOI] [PubMed] [Google Scholar]
- 6.Shih CM, Ko WC, Wu JS, Wei YH, Wang LF, Chang EE, Lo TY, Cheng HH, Chen CT. Mediating of caspase-independent apoptosis by cadmium through the mitochondria-ROS pathway in MRC-5 fibroblasts. J. Cell. Biochem. 2004;91:384–397. doi: 10.1002/jcb.10761. [DOI] [PubMed] [Google Scholar]
- 7.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 8.Cobb JP, Hotchkiss RS, Karl IE, Buchman TG. Mechanisms of cell injury and death. Br. J. Anaesth. 1996;77:3–10. doi: 10.1093/bja/77.1.3. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler U, Groscurth P. Morphological features of cell death. News Physiol. Sci. 2004;19:124–128. doi: 10.1152/nips.01519.2004. [DOI] [PubMed] [Google Scholar]
- 10.Thornberry NA, Lazebnik Y. Caspases: Enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 11.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 12.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- 13.Izban KF, Wrone-Smith T, Hsi ED, Schnitzer B, Quevedo ME, Alkan S. Characterization of the interleukin-1beta-converting enzyme/ced-3-family protease, caspase-3/CPP32, in Hodgkin’s disease: Lack of caspase-3 expression in nodular lymphocyte predominance Hodgkin’s disease. Am. J. Pathol. 1999;154:1439–1447. doi: 10.1016/s0002-9440(10)65398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M, Wang J. Initiator caspases in apoptosis signaling pathways. Apoptosis. 2002;7:313–319. doi: 10.1023/a:1016167228059. [DOI] [PubMed] [Google Scholar]
- 15.Akao T, Kida H, Kanaoka M, Hattori M, Kobashi K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J. Pharm. Pharmacol. 1998;50:1155–1160. doi: 10.1111/j.2042-7158.1998.tb03327.x. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa H, Sung JH, Huh JH. Ginseng intestinal bacterial metabolite IH901 as a new anti-metastatic agent. Arch. Pharm. Res. 1997;20:539–544. doi: 10.1007/BF02975208. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa H, Sung JH, Benno Y. Role of human intestinal Prevotella oris in hydrolyzing ginseng saponins. Planta Med. 1997;63:436–440. doi: 10.1055/s-2006-957729. [DOI] [PubMed] [Google Scholar]
- 18.Akao T, Kanaoka M, Kobashi K. Appearance of compound K, a major metabolite of ginsenoside Rb1 by intestinal bacteria, in rat plasma after oral administration--measurement of compound K by enzyme immunoassay. Biol. Pharm. Bull. 1998;21:245–249. doi: 10.1248/bpb.21.245. [DOI] [PubMed] [Google Scholar]
- 19.Kang KA, Kim YW, Kim SU, Chae S, Koh YS, Kim HS, Choo MK, Kim DH, Hyun JW. G1 phase arrest of the cell cycle by a ginseng metabolite, compound K, in U937 human monocytic leukamia cells. Arch. Pharm. Res. 2005;28:685–690. doi: 10.1007/BF02969359. [DOI] [PubMed] [Google Scholar]
- 20.Kang KA, Lim HK, Kim SU, Kim YW, Kim WT, Chung HS, Choo MK, Kim DH, Kim HS, Shim MJ, Chung MH, Hyun JW. Induction of apoptosis by ginseng saponin metabolite in U937 human monocytic leukemia cells. J. Food Biochem. 2005;29:27–40. [Google Scholar]
- 21.Kang KA, Lim HK, Chae S, Kim JK, Seo JY, Ham YH, Lee KH, Kim BJ, Kim HS, Kim DH, Hyun JW. Inhibition of telomerase activity in U937 human monocytic leukemia cells by compound K, a ginseng saponin metabolite. Biotechnol. Bioprocess Eng. 2005;11:7–12. [Google Scholar]
- 22.Chae S, Kang KA, Chang WY, Kim MJ, Lee SJ, Lee YS, Kim HS, Kim DH, Hyun JW. Effect of compound K, a metabolite of ginseng saponin, combined with gamma-ray radiation in human lung cancer cells in vitro and in vivo. J. Agric. Food Chem. 2009;57:5777–5782. doi: 10.1021/jf900331g. [DOI] [PubMed] [Google Scholar]
- 23.Kim AD, Kang KA, Zhang R, Lim CM, Kim HS, Kim DH, Jeon YJ, Lee CH, Park J, Chang WY, Hyun JW. Ginseng saponin metabolite induces apoptosis in MCF-7 breast cancer cells through the modulation of AMP-activated protein kinase. Environ. Toxicol. Pharmacol. 2010;30:134–140. doi: 10.1016/j.etap.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Blau S, Rubinstein A, Bass P, Singaram C, Kohen R. Differences in the reducing power along the rat GI tract: Lower antioxidant capacity of the colon. Mol. Cell. Biochem. 1999;194:185–191. doi: 10.1023/a:1006994800272. [DOI] [PubMed] [Google Scholar]
- 25.Rainis T, Keren D, Goldstein O, Stermer E, Lavy A. Diagnostic yield and safety of colonoscopy in Israeli patients in an open access referral system. J. Clin. Gastroenterol. 2007;41:394–399. doi: 10.1097/01.mcg.0000225573.27643.3d. [DOI] [PubMed] [Google Scholar]
- 26.Gushima M, Hirahashi M, Matsumoto T, Fujita K, Fujisawa R, Mizumoto K, Nakabeppu Y, Iida M, Yao T, Tsuneyoshi M. Altered expression of MUTYH and an increase in 8-hydroxydeoxyguanosine are early events in ulcerative colitis-associated carcinogenesis. J. Pathol. 2009;219:77–86. doi: 10.1002/path.2570. [DOI] [PubMed] [Google Scholar]
- 27.Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL, Schauer DB, Dedon PC, Fox JG, Samson LD. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Invest. 2008;118:2516–2525. doi: 10.1172/JCI35073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choo MK, Sakurai H, Kim DH, Saiki I. A ginseng saponin metabolite suppresses tumor necrosis factor-alpha-promoted metastasis by suppressing nuclear factor-kappaB signaling in murine colon cancer cells. Oncol. Rep. 2008;19:595–600. [PubMed] [Google Scholar]
- 29.Kim do Y, Yuan HD, Chung IK, Chung SH. Compound K, intestinal metabolite of ginsenoside, attenuates hepatic lipid accumulation via AMPK activation in human hepatoma cells. J. Agric. Food Chem. 2009;57:1532–1537. doi: 10.1021/jf802867b. [DOI] [PubMed] [Google Scholar]
- 30.Kim do Y, Park MW, Yuan HD, Lee HJ, Kim SH, Chung SH. Compound K induces apoptosis via CAMK-IV/AMPK pathways in HT-29 colon cancer cells. J. Agric. Food Chem. 2009;57:10573–10578. doi: 10.1021/jf902700h. [DOI] [PubMed] [Google Scholar]
- 31.Hasegawa H, Matsumiya S, Uchiyama M, Kurokawa T, Inouye Y, Kasai R, Ishibashi S, Yamasaki K. Inhibitory effect of some triterpenoid saponins on glucose transport in tumor cells and its application to in vitro cytotoxic and antiviral activities. Planta Med. 1994;60:240–243. doi: 10.1055/s-2006-959467. [DOI] [PubMed] [Google Scholar]
- 32.Lee BH, Lee SJ, Hur JH, Lee S, Sung JH, Huh JD, Moon CK. In vitro antigenotoxic activity of novel ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1998;64:500–503. doi: 10.1055/s-2006-957501. [DOI] [PubMed] [Google Scholar]
- 33.Lee JY, Shin JW, Chun KS, Park KK, Chung WY, Bang YJ, Sung JH, Surh YJ. Antitumor promotional effects of a novel intestinal bacterial metabolite (IH-901) derived from the protopanaxadiol-type ginsenosides in mouse skin. Carcinogenesis. 2005;26:359–367. doi: 10.1093/carcin/bgh313. [DOI] [PubMed] [Google Scholar]
- 34.Yim HW, Jong HS, Kim TY, Choi HH, Kim SG, Song SH, Kim J, Ko SG, Lee JW, Kim TY, Bang YJ. Cyclooxygenase-2 inhibits novel ginseng metabolite-mediated apoptosis. Cancer Res. 2005;65:1952–1960. doi: 10.1158/0008-5472.CAN-04-1740. [DOI] [PubMed] [Google Scholar]
- 35.Kim YS, Kim JJ, Cho KH, Jung WS, Moon SK, Park EK, Kim DH. Biotransformation of ginsenoside Rb1, crocin, amygdalin, geniposide, puerarin, ginsenoside Re, hesperidin, poncirin, glycyrrhizin, and baicalin by human fecal microflora and its relation to cytotoxicity against tumor cells. J. Microbiol. Biotechnol. 2008;18:1109–1114. [PubMed] [Google Scholar]
- 36.Choi K, Choi C. Proapoptotic ginsenosides compound K and Rh enhance Fas-induced cell death of human astrocytoma cells through distinct apoptotic signaling pathways. Cancer Res. Treat. 2009;41:36–44. doi: 10.4143/crt.2009.41.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Hwang PM, Bunz F, Yu J, Rago C, Chan TA, Murphy MP, Kelso GF, Smith RA, Kinzler KW. Vogelstein, B. Ferredoxin reductase affects p53-dependent, 5-fluorouracil-induced apoptosis in colorectal cancer cells. Nat. Med. 2001;7:1111–1117. doi: 10.1038/nm1001-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexandre J, Hu Y, Lu W, Pelicano H, Huang P. Novel action of paclitaxel against cancer cells: Bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67:3512–3517. doi: 10.1158/0008-5472.CAN-06-3914. [DOI] [PubMed] [Google Scholar]
- 40.Qanungo S, Das M, Haldar S, Basu A. Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis. 2005;26:958–967. doi: 10.1093/carcin/bgi040. [DOI] [PubMed] [Google Scholar]
- 41.Cao XH, Wang AH, Wang CL, Mao DZ, Lu MF, Cui YQ, Jiao RZ. Surfactin induces apoptosis in human breast cancer MCF-7 cells through a ROS/JNK-mediated mitochondrial/caspase pathway. Chem. Biol. Interact. 2010;183:357–362. doi: 10.1016/j.cbi.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 42.Rosenkranz AR, Schmaldienst S, Stuhlmeier KM, Chen W, Knapp W, Zlabinger GJ. A microplate assay for the detection of oxidative products using 2′,7′-dichlorofluorescin-diacetate. J. Immunol. Methods. 1992;156:39–45. doi: 10.1016/0022-1759(92)90008-h. [DOI] [PubMed] [Google Scholar]
- 43.Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssière JL, Petit PX, Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J. Exp. Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zamzami N, Susin SA, Marchetti P, Hirsch T, Gómez-Monterrey I, Castedo M, Kroemer G. Mitochondrial control of nuclear apoptosis. J. Exp. Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai J, Yang J, Jones DP. Mitochondrial control of apoptosis: The role of cytochrome c. Biochim. Biophys. Acta. 1998;1366:139–149. doi: 10.1016/s0005-2728(98)00109-1. [DOI] [PubMed] [Google Scholar]
- 46.Petrosillo G, Ruggiero FM, Pistolese M, Paradies G. Reactive oxygen species generated from the mitochondrial electron transport chain induce cytochrome c dissociation from beef-heart submitochondrial particles via cardiolipin peroxidation. Possible role in the apoptosis. FEBS Lett. 2001;509:435–438. doi: 10.1016/s0014-5793(01)03206-9. [DOI] [PubMed] [Google Scholar]
- 47.Petrosillo G, Ruggiero FM, Paradies G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J. 2003;17:2202–2208. doi: 10.1096/fj.03-0012com. [DOI] [PubMed] [Google Scholar]
- 48.Chen KC, Chang LS. Arachidonic acid-induced apoptosis of human neuroblastoma SK-N-SH cells is mediated through mitochondrial alteration elicited by ROS and Ca(2+)-evoked activation of p38alpha MAPK and JNK1. Toxicology. 2009;262:199–206. doi: 10.1016/j.tox.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 49.Chen HL, Chang CY, Lee HT, Lin HH, Lu PJ, Yang CN, Shiau CW, Shaw AY. Synthesis and pharmacological exploitation of clioquinol-derived copper-binding apoptosis inducers triggering reactive oxygen species generation and MAPK pathway activation. Bioorg. Med. Chem. 2009;17:7239–7247. doi: 10.1016/j.bmc.2009.08.054. [DOI] [PubMed] [Google Scholar]
- 50.Sahu RP, Zhang R, Batra S, Shi Y, Srivastava SK. Benzyl isothiocyanate-mediated generation of reactive oxygen species causes cell cycle arrest and induces apoptosis via activation of MAPK in human pancreatic cancer cells. Carcinogenesis. 2009;30:1744–1753. doi: 10.1093/carcin/bgp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Najjar N, Chatila M, Moukadem H, Vuorela H, Ocker M, Gandesiri M, Schneider-Stock R, Gali-Muhtasib H. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis. 2010;15:183–195. doi: 10.1007/s10495-009-0421-z. [DOI] [PubMed] [Google Scholar]
- 52.Chen YJ, Liu WH, Kao PH, Wang JJ, Chang LS. Involvement of p38 MAPK- and JNK-modulated expression of Bcl-2 and Bax in Naja nigricollis CMS-9-induced apoptosis of human leukemia K562 cells. Toxicon. 2010;55:1306–1316. doi: 10.1016/j.toxicon.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 53.Deng YT, Huang HC, Lin JK. Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol. Carcinog. 2010;49:141–151. doi: 10.1002/mc.20583. [DOI] [PubMed] [Google Scholar]
- 54.Choi SL, Kim SJ, Lee KT, Kim J, Mu J, Birnbaum MJ, Kim SS, Ha J. The regulation of AMP-activated protein kinase by H2O2. Biochem. Biophys. Res. Commun. 2001;287:92–97. doi: 10.1006/bbrc.2001.5544. [DOI] [PubMed] [Google Scholar]
- 55.Yun H, Lee M, Kim SS. Glucose deprivation increases mRNA stability of vascular endothelial growth factor through activation of AMP-activated protein kinase in DU145 prostate carcinoma. J. Biol. Chem. 2005;280:9963–9972. doi: 10.1074/jbc.M412994200. [DOI] [PubMed] [Google Scholar]
- 56.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 57.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 58.Troiano L, Ferraresi R, Lugli E, Nemes E, Roat E, Nasi M, Pinti M, Cossarizza A. Multiparametric analysis of cells with different mitochondrial membrane potential during apoptosis by polychromatic flow cytometry. Nat. Protoc. 2007;2:2719–2727. doi: 10.1038/nprot.2007.405. [DOI] [PubMed] [Google Scholar]