Abstract
Rhamnolipids are known as very efficient biosurfactant molecules. They are used in a wide range of industrial applications including food, cosmetics, pharmaceutical formulations and bioremediation of pollutants. The present review provides an overview of the effect of rhamnolipids in animal and plant defense responses. We describe the current knowledge on the stimulation of plant and animal immunity by these molecules, as well as on their direct antimicrobial properties. Given their ecological acceptance owing to their low toxicity and biodegradability, rhamnolipids have the potential to be useful molecules in medicine and to be part of alternative strategies in order to reduce or replace pesticides in agriculture.
Keywords: rhamnolipids, plant immunity, animal immunity, antimicrobial properties
1. Introduction
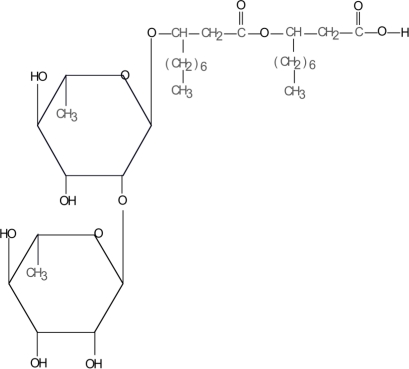
Rhamnolipids (RLs) are glycolipid biosurfactants produced by various bacterial species including some Pseudomonas sp. and Burkholderia sp. [1]. The structure of RLs is highly diverse and those produced by Pseudomonas aeruginosa have been extensively studied. These RLs are amphiphilic molecules typically composed of 3-hydroxyfatty acids linked through a beta-glycosidic bond to mono- or di-rhamnoses (Figure 1) [2]. RLs have several potential functions in bacteria. They are involved in the uptake and biodegradation of poorly soluble substrates and are essential for surface motility and biofilm development [1]. From a biotechnological point of view, RLs are powerful biosurfactants with applications related to environmental concerns, such as bioremediation of hydrocarbon, organic pollutants and heavy-metal-contaminated sites. These topics have been extensively reviewed including some very recent articles [3–6]. RLs have also been used in the production of fine chemicals, surface coatings, as well as additives for food and cosmetics [7]. Finally, a new role for RLs as potential players in the combat of plants and animals against microbes has recently emerged. For years RLs have been extensively studied regarding their direct toxicity to microorganisms but recently they have also been reported to be involved in the stimulation of plant and animal defense responses. The present review provides an update of the current knowledge on the antimicrobial properties of RLs and also highlights the recent discoveries of the involvement of these molecules in the stimulation of immunity in plants and animals. The potential use of these molecules to fight against pathogenic microorganisms in medical and agricultural field will be discussed.
Figure 1.
The major form of rhamnolipid produced by Pseudomonas aeruginosa (Rha-Rha-C10-C10).
2. Rhamnolipids as Antimicrobial Agents
RLs have been shown to display antibacterial activities against plant and human pathogenic bacteria. RLs are known to be active against the Gram-negative bacteria P. aeruginosa, Enterobacter aerogenes, Serratia marcescens and Klebsiella pneumonia, as well as against Gram-positive Micrococcus sp., Streptococcus sp., Staphylococcus sp. and Bacillus sp [8–13] (Table 1). RLs have direct impact on bacterial cell surface structures. Al-Tahhan et al. [14] observed a loss of lipopolysaccharides (LPS) in P. aeruginosa strains treated with RLs at low concentrations and this resulted in increased cell surface hydrophobicity. Recently, Sotirova et al. [15] showed that RLs from Pseudomonas sp. PS-17 interact with P. aeruginosa causing a reduction in LPS content and changes in the outer membrane proteins of the bacteria. These changes had a direct impact on bacterial cell surface morphology. Sotirova et al. [15] concluded that RLs from Pseudomonas sp. PS-17 have a potential application in the field of biomedicine against pathogenic bacteria. Several studies described antifungal activity of RLs mainly against phytopathogens including Botrytis sp., Rhizoctonia sp., Pythium sp., Phytophtora sp. and Plasmopara sp. (Table 1) [16–22]. Additionally, RLs were also shown to be active against Mucor miehei and Neurospora crassa [12]. The main mode of action of RLs against zoospore-producing plant pathogens is the direct lysis of zoospores via the intercalation of RLs within plasma membranes of the zoospore which are not protected by a cell wall [16,21,23]. Recent studies also demonstrated an effect of RLs in the reduction of mycelia growth of Pythium myriotylum [18] and Botrytis cinerea [23]. These data suggest that RLs may also have an adverse effect on cell structures that are protected by a cell wall. Properties of RLs against the algae Heterosigma akashiwo, viruses, amoeba like Dictyostelium discoideum and mycoplasma have also been reported [24–29]. However, RLs’ applications have no significant effects on yeasts [10,12,17,28]. In addition to their in vitro antimicrobial activity, RLs have proven to be also efficient in in vivo plant systems. Treatments with RLs have been shown to protect pepper plants from Phytophthora blight disease and also prevent the development of Colletotrichum orbiculare infection on leaves of cucumber plants [17]. Yoo et al. [22] investigated RLs as alternative antifungal agents against typical plant pathogenic oomycetes, including Phytophthora sp. and Pythium sp. They showed that RLs significantly decrease the incidence of water-borne damping-off disease. Sharma et al. [19] obtained similar results in field trials on chili pepper and tomato. Using bacterial mutants, Perneel et al. [18] clearly showed that phenazine and RLs interact in the biological control of soil-borne diseases caused by Pythium spp. Recent studies also demonstrated that a combination mixture of SRE (Syringomycin E) and RLs is efficient against pathogenic and opportunistic fungi recovered from diseased grape [30,31].
Table 1.
Antimicrobial properties of rhamnolipids.
| Organisms affected | Observed effects | RL application | RL origin | Ref. |
|---|---|---|---|---|
| Fungi | ||||
| Alternaria alternata | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa LBI | [9] |
| Alternaria mali | growth inhibition (MIC) | Rha-Rha-C10-C10 | P. aeruginosa strain B5 | [17] |
| Aspergillus niger | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa LBI | [9] |
| Aureobasidium pullulans | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa LBI | [9] |
| Botrytis cinerea | growth inhibition (MIC) | Rha-Rha-C10-C10 | P. aeruginosa strain B5 | [17] |
| growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] | |
| inhibition of spore germination and mycelium growth | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10 (Jeneil Biosurfactant Company JBR599) | P. aeruginosa | [23] | |
| Candida albicans | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa LBI | [9] |
| Cercospora kikuchii | growth inhibition (MIC) | Rha-Rha-C10-C10 | P. aeruginosa strain B5 | [17] |
| Chaetonium globosum | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] |
| growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa LBI | [9] | |
| Cladosporium cucumerinum | growth inhibition (MIC) | Rha-Rha-C10-C10 | P. aeruginosa strain B5 | [17] |
| Colletotrichum orbiculare | growth inhibition (MIC) | Rha-Rha-C10-C10 | P. aeruginosa strain B5 | [17] |
| Cylindrocarpon destructans | growth inhibition (MIC) | Rha-Rha-C10-C10 | P. aeruginosa strain B5 | [17] |
| Didymella bryoniae | growth inhibition (MIC) | Rha-Rha-C10-C10 | P. aeruginosa strain B5 | [17] |
| Fusarium solani | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] |
| Fusarium sp. | growth inhibition (MIC) | Rha-Rha-C10-C10 | P. aeruginosa strain B5 | [17] |
| growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] | |
| Gliocadium virens | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] |
| growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa LBI | [9] | |
| Magnaporthe grisea | growth inhibition (MIC) | Rha-Rha-C10-C10 | P. aeruginosa strain B5 | [17] |
| Mucor miehei | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10 | P. aeruginosa LBI | [12] |
| Neurospora crassa | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10 | P. aeruginosa LBI | [12] |
| Penicillium funiculosum | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] |
| growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa LBI | [9] | |
| Phytophthora sp. | zoospore lysis by RL intercalation into membrane | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10 | P. aeruginosa | [21] |
| growth inhibition (MIC), lytic effect on zoospores | Rha-Rha-C10-C10 | P. aeruginosa strain B5 | [17] | |
| zoospore motility inhibition, zoospore lysis, hyphae growth inhibition | nd | nd | [22] | |
| reduction of disease incidence and of disease severity | biosurfactant PRO1 (formulation of 25% Rls) Plant support (the Netherlands) | P. aeruginosa | [16] | |
| reduction of damping-off disease | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C10:1, Rha-C10-C10, Rha-Rha-C10-C12:1, Rha-C10-C12:1, Rha-C10-C12, Rha-Rha-C10-C12, Rha-Rha-C10-C8, Rha-C8-C10, Rha-Rha-C8-C10, Rha-Rha-C12-C12, Rha-Rha-C12-C12:1) | Pseudomonas sp. GRP3 | [19] | |
| Pythium sp. | zoospore lysis by RL intercalation into membrane | nd | P. aeruginosa | [21] |
| zoospore motility inhibition, zoospore lysis, hyphae growth inhibition | nd | nd | [22] | |
| reduction of damping-off disease | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C10:1, Rha-C10-C10, Rha-Rha-C10-C12:1, Rha-C10-C12:1, Rha-C10-C12, Rha-Rha-C10-C12, Rha-Rha-C10-C8, Rha-C8-C10, Rha-Rha-C8-C10, Rha-Rha-C12-C12, Rha-Rha-C12-C12:1) | Pseudomonas sp. GRP3 | [19] | |
| mycelial growth inhibition, reduction of disease symptoms, hyphae damages | RL-deficient mutant | P. aeruginosa PA01 | [18] | |
| Rhizoctonia solani | growth inhibition (MIC) | Rha-Rha-C10-C10 | P. aeruginosa strain B5 | [17] |
| growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] | |
| Bacteria | ||||
| Gram-negative | ||||
| Enterobacter aerogenes | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] |
| growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa LBI | [9] | |
| Erwinina carotovora | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] |
| Escherichia coli | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] |
| growth inhibition (MIC) | nd | P. fluorescens HW-6 | [13] | |
| Klebsiella pneumoniae | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] |
| Proteus mirabilis | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa LBI | [9] |
| Pseudomonas aeruginosa | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa LBI | [9] |
| increase in released proteins | Biosurfactant PS (rhamnolipid+alginate) | Pseudomonas sp. S-17 | [20] | |
| reduction of LPS contents, increase in cell hydrophobicity and in extracellular protein release, changes in outer membrane proteins | Biosurfactant PS (rhamnolipid+alginate) | Pseudomonas sp. S-17 | [15] | |
| growth inhibition, increase in cell permeability and in released proteins | nd | P. fluorescens HW-6 | [13] | |
| Ralstonia solanacearum | growth inhibition (MIC) | Rha-Rha-C10-C10 | P. aeruginosa strain B5 | [17] |
| Salmonella thyphimurium | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa LBI | [9] |
| Serratia marcescens | growth inhibition (MIC) | Rha-Rha-C10-C10 | P. aeruginosa strain B5 | [17] |
| growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] | |
| Xanthomonas campestris | growth inhibition (MIC) | Rha-Rha-C10-C10 | P. aeruginosa strain B5 | [17] |
| Gram-positive | ||||
| Bacillus cereus | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] |
| growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa LBI | [9] | |
| growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10 | P. aeruginosa LBI | [12] | |
| Bacillus sp. | growth inhibition (MIC) | nd | P. fluorescens HW-6 | [13] |
| Bacillus subtilis | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] |
| growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa LBI | [9] | |
| Micrococcus luteus | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] |
| growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa LBI | [9] | |
| growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10 | P. aeruginosa LBI | [12] | |
| Staphylococcus aureus | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] |
| growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10 | P. aeruginosa LBI | [12] | |
| Staphylococcus epidermidis | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-Rha-C10-C12, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa 47T2 | [10] |
| Streptococcus faecalis | growth inhibition (MIC) | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10, Rha-Rha-C10-C12:1 | P. aeruginosa LBI | [9] |
| Amoeba(Dictyostelium discoideum) | growth inhibition, cell lysis | Rhl quorum-sensing mutants | P. aeruginosa PA01 | [24] |
| Algae(Heterosigma akashiwo) | growth inhibition, cell lysis, plasma membrane and organelles damages, condensation of chromatin | RL mixture: Rha-Rha-C10-C10, Rha-C10-C10 | P. aeruginosa | [29] |
| Virus | ||||
| potato virus X, red clover mottle virus | reduction of local lesions, reduction of virus number | nd | nd | [25] |
| herpes simplex virus HSV) | inhibition of cytopathic effects | biosurfactant PS-17 (rhamnolipid+alginate) | Pseudomonas sp. S-17 | [27] |
MIC: minimum inhibitory concentrations ; nd : not done or not communicated
3. Rhamnolipids in Plant and Animal Immunity
During the last decade, pattern recognition emerged as a fundamental process in the immune response of plants and animals. Perception by pattern recognition receptors (PRRs) of molecular signatures that identify whole classes of microbes but are absent from the host allows this nonself recognition [32,33]. Once recognized, these molecular signatures, conventionally named microbe-associated molecular patterns (MAMPs) [34], trigger complex signaling pathways leading to transcriptional activation of defense-related genes and accumulation of antimicrobial metabolites in plant cells [32]. In mammals, MAMP perception leads to the inflammatory response with the production of cytokines including interleukins and the tumor necrosis factor α (TNFα). Years ago, lipopeptides were shown to stimulate human innate immune responses through the PRR Toll-like receptor TLR2 perception, by activating the transcriptional activator of multiple host defense genes NFkB, the production of interleukin (IL)-12 and the respiratory burst [35–39]. Lipopeptides are also involved in the stimulation of innate immunity in plants [40]. It is quite recent that RLs have been shown to be involved in triggering plant and animal defense responses and can be described as a new class of MAMPs.
3.1. Rhamnolipids as Stimulators of Human and Animal Immunity
RLs have been long known as exotoxins produced by the human pathogen P. aeruginosa [41–44] and several recent papers have highlighted their role in the stimulation of innate immunity in animal cells. The heat-stable Rha-Rha-C14-C14 produced by Burkholderia plantarii and some synthetic derivatives have been particularly studied [45–47]. Rha-Rha-C14-C14 is structurally quite similar to the RL exotoxin from P. aeruginosa and identical to the RL of Burkholderia pseudomallei, the causative agent of melioidosis, an infectious disease of humans and animals leading to skin infection, lung nodules and pneumonia [45]. This RL exhibits strong stimulatory activity on human mononuclear cells to produce TNFα, a pleiotropic inflammatory cytokine. Such a property has not been noted so far for RL exotoxins but only for the lipopolysaccharide (LPS) bacterial endotoxins. Like LPS, the cell stimulating activity of this RL could be inhibited by incubation with polymyxin B. Interestingly, immune cell activation by Rha-Rha-C14-C14 does not occur via receptors that are involved in LPS (TLR4) or lipopeptide signaling (TLR2) [45]. Synthetic RLs derived from B. plantarii Rha-Rha-C14-C14 were also analyzed for their immune cell activation [47]. These synthetic RLs differ by variations in the length, stereochemistry, number of lipid chains, number of rhamnoses and the occurrence of charged or neutral groups. The authors also compared these synthetic RLs to the well-characterized LPS MAMP from Salmonella minnesota. Immunostimulatory properties of RLs were monitored by assaying the secretion of TNFα and the induction of chemiluminescence in monocytes. Howe et al. [47] found that biological test systems showed large variations, depending on particular chemical structures and physicochemical parameters. LPS were, however, more efficient to induce luminescence and TNFα production than the RLs tested. Furthermore, they found that biologically inactive RLs with lamellar aggregate structures antagonize the induced activity in a way similar to lipid A-derived antagonists of LPS [47]. An extended study on structure-activity relationships of synthetic RLs derivatives also indicated a specific, recognition-based mode of action, with small structural variations in the RLs resulting in strong effects on the immunostimulatory activities [46]. RLs also stimulated the release of interleukin (IL)-8, granulocyte-macrophage colony-stimulating factor, and IL-6 from nasal epithelial cells at non-cytotoxic levels [48]. Interestingly, it was recently demonstrated that RLs could also potentiate the recognition of other MAMPs by the human innate immune system. Several MAMPs of P. aeruginosa are known to activate the innate immune system in epithelial cells, particularly the production of antimicrobial peptides such as the human beta-defensin-2 (hBD-2) and proinflammatory cytokines such as interleukin (IL)-8 [49]. In this study, RLs were found to interact with the well-known MAMP flagellin. The authors provide evidence that RLs are responsible for the release of flagellin from the flagella. Their findings indicate that upon adhesion to surfaces, P. aeruginosa may alter the outer membrane composition in an RL-dependent manner, thereby shedding flagellin from the flagella. In turn, epithelial cells recognize flagellin leading to synthesis of anti-microbial peptides as well as recruitment of inflammatory cells by induction of proinflammatory cytokines [49].
3.2. Rhamnolipids as Stimulators of Plant Immunity
RLs have very recently been characterized as new MAMPs involved in non-specific immunity in plants. They have been also shown to induce resistance in plants, which is effective against a broad range of pathogens [23]. It is demonstrated that Rha-C10-C10 and Rha-Rha-C10-C10 from P. aeruginosa and Rha-Rha-C14-C14 from B. plantarii trigger strong defense responses in grapevine including early events of cell signaling like Ca2+ influx, reactive oxygen species (ROS) production and MAP kinase activation. These RLs also induce a large battery of defense genes including some pathogenesis-related protein genes and genes involved in oxylipins and phytoalexins biosynthesis pathways [23]. Interestingly, depending on the concentrations tested, RLs were able to activate a programmed cell death reminiscent of animal apoptosis [23]. It was also demonstrated that RLs potentiate defense responses induced by other elicitors (i.e., chitosan and a culture filtrate of the fungus B. cinerea). Another novel role of RLs consists in protecting grapevine against the necrotropic pathogen B. cinerea. RLs are also active in other plant species. They are able to stimulate defense genes in tobacco, wheat and Arabidopsis thaliana (Sanchez, L. unpublished work, 2010). RLs are also potent protectors in monocotyledonous plants against biotrophic fungi (Couleaud, G. Arvalis. Private communication, 2009). To date, it is not known whether the perception of RLs requires specific receptors in the plant plasma membrane [23]. Interestingly, lipopeptide biosurfactants, which are lipid derivatives with similar properties to RLs, have also been described as potent MAMP elicitors. Surfactin, the most studied cyclic lipopeptide from Bacillus subtilis, has been shown to trigger early signaling events and late defense responses in tobacco cell suspensions [50]. Some cyclic lipopeptides including Massetolide A and fengycin originating, respectively, from Pseudomonas fluorescens SS101 and B. subtilis S499 were identified as elicitors inducing a systemic resistance in tomato and bean [51,52]. As for RLs, it is yet unclear whether the induction of defense responses by lipopeptides requires specific receptors in the plant plasma membrane [40]. An alternative hypothesis is that lipopeptides could induce defense responses by membrane disturbance [50,53] and this could also be the case for RLs.
4. Potential Use of Rhamnolipids in Agricultural and Biomedical Fields
Major breakthroughs allowing production, separation and purification of RLs in industrial quantities and laboratory purities have allowed the application of these molecules in different fields from cosmetic to industrial and more recently from agriculture to medicine. As previously stated, the major advantage of using RL biosurfactants, which have diverse roles in plant and animal systems, is that they are natural and organic biodegradable compounds, originating from a large number of bacteria [1]. RLs have also been proposed to be used in food industry applications [12]. RLs have a direct biocide action on bacteria and fungi. They also increase the susceptibility of certain Gram-positive bacteria to specific antibiotics. RLs have been demonstrated to control zoosporic pathogens through lysis of their zoospores [21]. Clinical trials using RLs for the treatment of psoriasis, lichen planus, neurodermatitis and human burn wound healing have confirmed excellent ameliorative effects of RLs when compared to conventional therapy using corticosteroids [54,55]. RLs also display differential effects on human keratinocyte and fibroblast cultures [55]. The advantages of these biosurfactants are low irritancy and even anti-irritating effects, as well as compatibility with human skin [55]. Moreover, RLs have permeabilizing effects on Gram-positive and Gram-negative human bacterial strains, reinforcing their potential in biomedicine [20]. An important issue to be taken into account is the study of side effects of biosurfactants on plants and animals. Attention should be paid while using surfactants on plants as the latter could be affected in many different ways. Parameters like negative impact on crop yield or other important agronomical traits should not be neglected and should be studied in parallel to avoid any impact on plant growth or metabolism, while boosting plant immunity. For instance, it is known that high concentrations of RLs cause necrosis in plants [23]. Dose/response experiments in the field are a necessity in order to ensure use of non-toxic concentrations of RLs. In addition, in animal systems, RLs are known as virulence factors especially for immunocompromised patients and individuals suffering from cystic fibrosis (CF) [1]. At some concentrations, RLs also have hemolytic activity [56,57]. Thus, care should be taken in the use of RLs, albeit some applications such as fungicide and bactericide are already considered especially for skin treatments [54,55].
5. Conclusion
RLs are new actors in animal and plant defense and their low toxicity and biodegradability make them promising molecules to be used against pathogens. In this respect, there are some clues now available for the success of RL applications in greenhouses to fight phytopathogens. A better understanding of RL mode of action, especially their perception and the signaling pathways activated, will be very important to potentiate their beneficial effects in plants. RLs have a dual mode of action: they are antimicrobial and also stimulate plant defense responses. This dual property is probably very important for the efficiency of new biopesticides. In animals, the use of RLs is also at an advanced stage. RLs are successfully used as antimicrobial agents, especially for skin disease treatment. Deep insight into the physiochemical effects of RLs and their biological importance would reveal new dimensions in the fields of research like agriculture and medicine, precisely in plant defense, disease control and pathogenesis. An understanding of bacterial genera producing RLs that are not yet well studied would provide light on these fascinating aspects.
Acknowledgments
This work is supported by Europôl’Agro.
References
- 1.Abdel-Mawgoud AM, Lepine F, Deziel E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010;86:1323–1336. doi: 10.1007/s00253-010-2498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soberon-Chavez G, Lépine F, Déziel E. Production of rhamnolipids by Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2005;68:718–725. doi: 10.1007/s00253-005-0150-3. [DOI] [PubMed] [Google Scholar]
- 3.Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti MG, Fracchia L, Smyth TJ, Marchant R. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010;87:427–444. doi: 10.1007/s00253-010-2589-0. [DOI] [PubMed] [Google Scholar]
- 4.Kosaric N. Biosurfactants and their application for soil bioremediation. Food Technol. Biotechnol. 2001;39:295–304. [Google Scholar]
- 5.Nitschke M, Costa SG, Contiero J. Rhamnolipid surfactants: An update on the general aspects of these remarkable biomolecules. Biotechnol. Prog. 2005;21:1593–1600. doi: 10.1021/bp050239p. [DOI] [PubMed] [Google Scholar]
- 6.Pornsunthorntawee O, Wongpanit P, Rujiravanit R. Rhamnolipid biosurfactants: Production and their potential in environmental biotechnology. Adv. Exp. Med. Biol. 2010;672:211–221. doi: 10.1007/978-1-4419-5979-9_16. [DOI] [PubMed] [Google Scholar]
- 7.Maier RM, Soberon-Chavez G. Pseudomonas aeruginosa rhamnolipids: Biosynthesis and potential applications. Appl. Microbiol. Biotechnol. 2000;54:625–633. doi: 10.1007/s002530000443. [DOI] [PubMed] [Google Scholar]
- 8.Arino S, Marchal R, Vandecasteele JP. Involvement of a rhamnolipid-producing strain of Pseudomonas aeruginosa in the degradation of polycyclic aromatic hydrocarbons by a bacterial community. J. Appl. Microbiol. 1998;84:769–776. doi: 10.1046/j.1365-2672.1998.00412.x. [DOI] [PubMed] [Google Scholar]
- 9.Benincasa M, Abalos A, Oliveira I, Manresa A. Chemical structure, surface properties and biological activities of the biosurfactant produced by Pseudomonas aeruginosa LBI from soapstock. Antonie Van Leeuwenhoek. 2004;85:1–8. doi: 10.1023/B:ANTO.0000020148.45523.41. [DOI] [PubMed] [Google Scholar]
- 10.Haba E, Pinazo A, Jauregui O, Espuny MJ, Infante MR, Manresa A. Physiochemical characterization and antimicrobial properties of rhamnolipids produced by Pseudomonas aeruginosa 47T2 NCBIM 40044. Biotechnol. Bioeng. 2003;81:316–322. doi: 10.1002/bit.10474. [DOI] [PubMed] [Google Scholar]
- 11.Lang S, Katsiwela E, Wagner F. Antimicrobial effects of biosurfactants. Fat Sci Technol. 1989;91:363–366. [Google Scholar]
- 12.Nitschke M, Costa SG, Contiero J. Structure and applications of a rhamnolipid surfactant produced in soybean oil waste. Appl. Biochem. Biotechnol. 2010;160:2066–2074. doi: 10.1007/s12010-009-8707-8. [DOI] [PubMed] [Google Scholar]
- 13.Vasileva-Tonkova E, Sotirova A, Galabova D. The effect of rhamnolipid biosurfactant produced by Pseudomonas fluorescens on model bacterial strains and isolates from industrial wastewater. Curr Microbiol. 2010 doi: 10.1007/s00284-010-9725-z. [DOI] [PubMed] [Google Scholar]
- 14.Al-Tahhan RA, Sandrin TR, Bodour AA, Maier RM. Rhamnolipid-induced removal of lipopolysaccharide from Pseudomonas aeruginosa: Effect on cell surface properties and interaction with hydrophobic substrates. Appl. Environ. Microbiol. 2000;66:3262–3268. doi: 10.1128/aem.66.8.3262-3268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sotirova A, Spasova D, Vasileva-Tonkova E, Galabova D. Effects of rhamnolipid-biosurfactant on cell surface of Pseudomonas aeruginosa. Microbiol. Res. 2009;164:297–303. doi: 10.1016/j.micres.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 16.De Jonghe K, De Dobbelaere I, Sarrazyn R, Höfte M. Control of Phytophthora cryptogea in the hydroponic forcing of witloof chicory with the rhamnolipid-based biosurfactant formulation PRO1. Plant Pathol. 2005;54:219–226. [Google Scholar]
- 17.Kim BS, Lee JY, Hwang BK. In vivo control and in vitro antifungal activity of rhamnolipid B, a glycolipid antibiotic, against Phytophthora capsici and Colletotrichum orbiculare. Pest Manage. Sci. 2000;56:1029–1035. [Google Scholar]
- 18.Perneel M, D’Hondt L, De Maeyer K, Adiobo A, Rabaey K, Hofte M. Phenazines and biosurfactants interact in the biological control of soil-borne diseases caused by Pythium spp. Environ. Microbiol. 2008;10:778–788. doi: 10.1111/j.1462-2920.2007.01501.x. [DOI] [PubMed] [Google Scholar]
- 19.Sharma A, Jansen R, Nimtz M, Johri BN, Wray V. Rhamnolipids from the rhizosphere bacterium Pseudomonas sp. GRP(3) that reduces damping-off disease in Chilli and tomato nurseries. J. Nat. Prod. 2007;70:941–947. doi: 10.1021/np0700016. [DOI] [PubMed] [Google Scholar]
- 20.Sotirova AV, Spasova DI, Galabova DN, Karpenko E, Shulga A. Rhamnolipid-biosurfactant permeabilizing effects on gram-positive and gram-negative bacterial strains. Curr. Microbiol. 2008;56:639–644. doi: 10.1007/s00284-008-9139-3. [DOI] [PubMed] [Google Scholar]
- 21.Stanghellini ME, Miller RM. Biosurfactants: Their identity and potential efficacy in the biological control of zoosporic plant pathogen. Plant Dis. 1997;81:4–12. doi: 10.1094/PDIS.1997.81.1.4. [DOI] [PubMed] [Google Scholar]
- 22.Yoo DS, Lee BS, Kim EK. Characteristics of microbial biosurfactant as an antifungal agent against plant pathogenic fungus. J. Microbiol. Biotechnol. 2005;15:1164–1169. [Google Scholar]
- 23.Varnier AL, Sanchez L, Vatsa P, Boudesocque L, Garcia-Brugger A, Rabenoelina F, Sorokin A, Renault JH, Kauffmann S, Pugin A, Clément C, Baillieul F, Dorey S. Bacterial rhamnolipids are novel MAMPs conferring resistance to Botrytis cinerea in grapevine. Plant Cell Environ. 2009;32:178–193. doi: 10.1111/j.1365-3040.2008.01911.x. [DOI] [PubMed] [Google Scholar]
- 24.Cosson P, Zulianello L, Join-Lambert O, Faurisson F, Gebbie L, Benghezal M, Van Delden C, Curty LK, Kohler T. Pseudomonas aeruginosa virulence analyzed in a Dictyostelium discoideum host system. J. Bacteriol. 2002;184:3027–3033. doi: 10.1128/JB.184.11.3027-3033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haferburg D, Hommel R, Kleber H, Kluge S, Schuster G, Zschiegner H. Antiphytovirale Aktivität von Rhamnolipid aus Pseudomonas aeruginosa. Acta Biotechnol. 1987;7:353–356. [Google Scholar]
- 26.Itoh S, Honda H, Tomita F, Suzuki T. Rhamnolipids produced by Pseudomonas aeruginosa grown on n-paraffin (mixture of C12, C13 and C14 fractions) J. Antibiot. 1971;24:855–859. doi: 10.7164/antibiotics.24.855. [DOI] [PubMed] [Google Scholar]
- 27.Remichkova M, Galabova D, Roeva I, Karpenko E, Shulga A, Galabov AS. Anti-herpesvirus activities of Pseudomonas sp. S-17 rhamnolipid and its complex with alginate. Z. Naturforsch. Sect. C. 2008;63:75–81. doi: 10.1515/znc-2008-1-214. [DOI] [PubMed] [Google Scholar]
- 28.Vasileva-Tonkova E, Galabova D, Karpenko E, Shulga A. Biosurfactant-rhamnolipid effects on yeast cells. Lett. Appl. Microbiol. 2001;33:280–284. doi: 10.1046/j.1472-765x.2001.00996.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Gong L, Liang S, Han X, Zhu C, Li Y. Algicidal activity of rhamnolipid biosurfactants produced by Pseudomonas aeruginosa. Harmful Algae. 2005;4:433–443. [Google Scholar]
- 30.De Lucca A, Klich M, Boue S, Cleveland T, Sien T, Walsh T. Fungicidal activity of plant saponin CAY-1 for fungi isolated from diseased Vitis fruit and stems. Am. J. Enol. Vitic. 2008;59:67–72. [Google Scholar]
- 31.Takemoto JY, Bensaci M, De Lucca AJ, Cleveland TE, Gandhi NR, Skebba VP. Inhibition of fungi from diseased grapeby syringomycin E-rhamnolipid mixture. Am. J. Enol. Vitic. 2010;61:120–124. [Google Scholar]
- 32.Boller T, Felix G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- 33.Boller T, He SY. Innate immunity in plants: An arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science. 2009;324:742–744. doi: 10.1126/science.1171647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackey D, McFall AJ. MAMPs and MIMPs: Proposed classifications for inducers of innate immunity. Mol. Microbiol. 2006;61:1365–1371. doi: 10.1111/j.1365-2958.2006.05311.x. [DOI] [PubMed] [Google Scholar]
- 35.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 36.Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR, Maitland M, Norgard MV, Plevy SE, Smale ST, Brennan PJ, Bloom BR, Godowski PJ, Modlin RL. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 37.Gerold G, Ajaj KA, Bienert M, Laws HJ, Zychlinsky A, de Diego JL. A Toll-like receptor 2-integrin beta3 complex senses bacterial lipopeptides via vitronectin. Nat. Immunol. 2008;9:761–768. doi: 10.1038/ni.1618. [DOI] [PubMed] [Google Scholar]
- 38.Hauschildt S, Hoffmann P, Beuscher HU, Dufhues G, Heinrich P, Wiesmüller K-H, Jung G, Bessler WG. Activation of bone marrow-derived mouse macrophages by bacterial lipopeptide: Cytokine production, phagocytosis and Ia expression. Eur. J. Immunol. 1990;20:63–68. doi: 10.1002/eji.1830200110. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi O, Kaufmann A, Grote K, Kawai T, Hoshino K, Morr M, Muhlradt PF, Akira S. Cutting edge: Preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 2000;164:554–557. doi: 10.4049/jimmunol.164.2.554. [DOI] [PubMed] [Google Scholar]
- 40.Raaijmakers JM, de Bruijn I, Nybroe O, Ongena M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010;34:1037–1062. doi: 10.1111/j.1574-6976.2010.00221.x. [DOI] [PubMed] [Google Scholar]
- 41.Haussler S, Rohde M, von Neuhoff N, Nimtz M, Steinmetz I. Structural and functional cellular changes induced by Burkholderia pseudomallei rhamnolipid. Infect. Immun. 2003;71:2970–2975. doi: 10.1128/IAI.71.5.2970-2975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McClure CD, Schiller NL. Effects of Pseudomonas aeruginosa rhamnolipids on human monocyte-derived macrophages. J. Leukocyte Biol. 1992;51:97–102. doi: 10.1002/jlb.51.2.97. [DOI] [PubMed] [Google Scholar]
- 43.McClure CD, Schiller NL. Inhibition of macrophage phagocytosis by Pseudomonas aeruginosa rhamnolipids in vitro and in vivo. Curr. Microbiol. 1996;33:109–117. doi: 10.1007/s002849900084. [DOI] [PubMed] [Google Scholar]
- 44.Zulianello L, Canard C, Kohler T, Caille D, Lacroix JS, Meda P. Rhamnolipids are virulence factors that promote early infiltration of primary human airway epithelia by Pseudomonas aeruginosa. Infect. Immun. 2006;74:3134–3147. doi: 10.1128/IAI.01772-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andrä J, Rademann J, Howe J, Koch MH, Heine H, Zähringer U, Brandenburg K. Endotoxin-like properties of a rhamnolipid exotoxin from Burkholderia (Pseudomonas) plantarii: Immune cell stimulation and biophysical characterization. Biol. Chem. 2006;387:301–310. doi: 10.1515/BC.2006.040. [DOI] [PubMed] [Google Scholar]
- 46.Bauer J, Brandenburg K, Zähringer U, Rademann J. Chemical synthesis of a glycolipid library by a solid-phase strategy allows elucidation of the structural specificity of immunostimulation by rhamnolipids. Chemistry. 2006;12:7116–7124. doi: 10.1002/chem.200600482. [DOI] [PubMed] [Google Scholar]
- 47.Howe J, Bauer J, Andrä J, Schromm AB, Ernst M, Rössle M, Zähringer U, Rademann J, Brandenburg K. Biophysical characterization of synthetic rhamnolipids. FEBS J. 2006;273:5101–5112. doi: 10.1111/j.1742-4658.2006.05507.x. [DOI] [PubMed] [Google Scholar]
- 48.Bédard M, McClure C, Schiller N, Francoeur C, Cantin A, Denis M. Release of interleukin-8, interleukin-6, and colony- stimulating factors by upper airway epithelial cells: Implication for cystic fibrosis. Am. J. Resir. Cell Mol. Biol. 1993;9:455–462. doi: 10.1165/ajrcmb/9.4.455. [DOI] [PubMed] [Google Scholar]
- 49.Gerstel U, Czapp M, Bartels J, Schroder JM. Rhamnolipid-induced shedding of flagellin from Pseudomonas aeruginosa provokes hBD-2 and IL-8 response in human keratinocytes. Cell. Microbiol. 2009;11:842–853. doi: 10.1111/j.1462-5822.2009.01299.x. [DOI] [PubMed] [Google Scholar]
- 50.Jourdan E, Henry G, Duby F, Dommes J, Barthelemy JP, Thonart P, Ongena M. Insights into the defense-related events occurring in plant cells following perception of surfactin-type lipopeptide from Bacillus subtilis. Mol. Plant-Microbe Interact. 2009;22:456–468. doi: 10.1094/MPMI-22-4-0456. [DOI] [PubMed] [Google Scholar]
- 51.Ongena M, Jourdan E, Adam A, Paquot M, Brans A, Joris B, Arpigny JL, Thonart P. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol. 2007;9:1084–1090. doi: 10.1111/j.1462-2920.2006.01202.x. [DOI] [PubMed] [Google Scholar]
- 52.Tran H, Ficke A, Asiimwe T, Hofte M, Raaijmakers JM. Role of the cyclic lipopeptide massetolide A in biological control of Phytophthora infestans and in colonization of tomato plants by Pseudomonas fluorescens. New Phytol. 2007;175:731–742. doi: 10.1111/j.1469-8137.2007.02138.x. [DOI] [PubMed] [Google Scholar]
- 53.D’Aes J, De Maeyer K, Pauwelyn E, Höfte M. Biosurfactants in plant–Pseudomonas interactions and their importance to biocontrol. Env. Microbiol. Rep. 2010;2:359–372. doi: 10.1111/j.1758-2229.2009.00104.x. [DOI] [PubMed] [Google Scholar]
- 54.Stipcevic T, Piljac A, Piljac G. Enhanced healing of full-thickness burn wounds using di-rhamnolipid. Burns. 2006;32:24–34. doi: 10.1016/j.burns.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stipcevic T, Piljac T, Isseroff RR. Di-rhamnolipid from Pseudomonas aeruginosa displays differential effects on human keratinocyte and fibroblast cultures. J. Dermatol Sci. 2005;40:141–143. doi: 10.1016/j.jdermsci.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujita K, Akino T, Yoshioka H. Characteristics of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect. Immun. 1988;56:1385–1387. doi: 10.1128/iai.56.5.1385-1387.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haussler S, Nimtz M, Domke T, Wray V, Steinmetz I. Purification and characterization of a cytotoxic exolipid of Burkholderia pseudomallei. Infect. Immun. 1998;66:1588–1593. doi: 10.1128/iai.66.4.1588-1593.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]