Abstract
Deoxyhypusine synthase (DHS) catalyzes the post-translational formation of the amino acid hypusine. Hypusine is unique to the eukaryotic translational initiation factor 5A (eIF5A), and is required for its functions in mRNA shuttling, translational elongation and stress granule formation. In recent studies, we showed that DHS promotes cytokine and ER stress signaling in the islet β cell and thereby contributes to its dysfunction in the setting of diabetes mellitus. Here, we review the evidence supporting a role for DHS (and hypusinated eIF5A) in cellular stress responses, and provide new data on the phenotype of DHS knockout mice. We show that homozygous knockout mice are embryonic lethal, but heterozygous knockout mice appear normal with no evidence of growth or metabolic deficiencies. Mouse embryonic fibroblasts from heterozygous knockout mice attenuate acute cytokine signaling, as evidenced by reduced production of inducible nitric oxide synthase, but show no statistically significant defects in proliferation or cell cycle progression. Our data are discussed with respect to the utility of sub- maximal inhibition of DHS in the setting of inflammatory states, such as diabetes mellitus.
Key words: inflammation, post-translational modification, cytokine, diabetes, mRNA translation, hypusine
β-Cell Dysfunction in Type 2 Diabetes Mellitus
Insulin is a highly conserved peptide hormone that is required for the cellular uptake of glucose into adipose and muscle tissue, and for the suppression of glucose output by the liver. Resistance to the action of insulin or a defect in the secretion of insulin by islet β cells are considered as risk factors for the development of type 2 diabetes (T2DM). Of the two risk factors, evidence is accumulating that β-cell dysfunction, and the consequent inability to maintain appropriately elevated insulin secretion in the face of insulin resistance, is likely the more important factor precipitating the development of T2DM.1 Several physiologic stressors have been proposed to explain the cause of this β-cell impairment, including glucolipotoxicity,2 cytokines released from adipocytes and activated macrophages,3,4 and islet amyloid deposition5,6 among others (reviewed in ref. 7). Pro-inflammatory cytokines (e.g., IL-1β, IFNγ) trigger mitogen-activated protein kinase and NFκB pathways, promoting β-cell necrosis via production of nitric oxide in the short term, and triggering apoptosis via endoplasmic reticulum (ER) and oxidative stress pathways in the longer term.8,9 Similarly, lipotoxicity arising from high fat diets triggers impairments in insulin release and eventual apoptosis.10 Despite its central importance in metabolic physiology, the β cell in general has a poor capacity to withstand stresses, a feature that is compounded by its limited numbers and low inherent replication rate.11
From a teleological perspective, the extent to which an individual's β cells adapt to stress may distinguish those insulin resistant individuals who develop diabetes from those who do not. Studying differences in β-cell stress responses in these two populations of individuals might allow for the identification of differentially expressed genes or proteins that serve as therapeutic targets to sustain β cell functionality. In this respect, our lab has been interested in studying islet β cells in obese mouse models of insulin resistance that phenotypically mimic human populations at risk for T2DM. The db/db mouse contains an inactivating mutation of the leptin receptor, causing excessive food intake that leads to obesity and insulin resistance. Importantly, whether such mice develop frank diabetes or not depends upon the background strain: the C57BL/6J-db/db mouse exhibits robust β-cell compensation with normoglycemia whereas the C57BL/KsJ-db/db mouse exhibits β-cell failure and frank diabetes.12,13 In a recent study, we showed that one feature that distinguished the two mouse strains was the apparent rate at which intact islets perform the crucial post-translation modification (known as “hypusination”) of a protein known as eukaryotic translation initiation factor 5A (eIF5A), with the C57BL/6J-db/db strain attenuating hypusination relative to the C57BL/KsJ-db/db strain.14 These studies identified eIF5A and its hypusine modification as potential contributors to islet β-cell inflammation and ER stress, as seen in T2DM.
EIF5A: A Protein of Many Functions
EIF5A is a small (17 kDa) protein that is highly conserved throughout evolution and appears to be expressed in a broad range of cell types in mammals. A second isoform not present in islet β cells, eIF5A2 (19 kDa), has a more restricted tissue distribution and has not been studied as completely.15 Despite its name, eIF5A is now thought to function primarily as a translational elongation factor: depletion of eIF5A in yeast and mammalian cells results in the accumulation of polysomes and in prolonged ribosome transit times.16,17 Importantly, loss of eIF5A in yeast results in a ∼30% decrease in protein synthesis rates;18 more strikingly, in unstressed mammalian cells, it has been reported that depletion of eIF5A results in the impaired translational elongation of only about 5% of mRNAs.16 Both of these findings argue against a role for eIF5A as a general translational factor, and instead point to a more restricted role in the translation of a subset of proteins. Another purported function of eIF5A is in the nucleocytoplasmic shuttling of specific proteins and mRNAs. However, it is worth noting that this shuttling function of eIF5A has been shrouded in some controversy. For example, Hauber and colleagues19 have observed that eIF5A binds and transports the HIV Rev protein from the nuclear to cytoplasmic compartment in conjunction with the nuclear protein exportin1/CRM1, yet other studies have disputed this particular function.20,21 Nonetheless, our own studies in the context of inflammation in β cells suggest that a nuclear species of eIF5A binds and shuttles the mRNA encoding inducible nitric oxide synthase (iNOS) across the nuclear membrane in a manner dependent upon exportin1/CRM1.22 We interpret this controversy as reflecting more the disparity of cell types and conditions that have been used to study eIF5A rather than actual functionality of the protein. In this respect, we feel that islet β cells represent a particularly notable cell type for which proteins that govern cellular survival have a profound effect on whole-body metabolic homeostasis.
Deoxyhypusine Synthase: Friend or Foe?
An unusual feature of eIF5A (and eIF5A2) is its posttranslational modification, known as hypusine. Hypusine is required for virtually all of the known functions of eIF5A studied to date, including RNA binding, mRNA shuttling and translational elongation.23 Hypusine synthesis is catalyzed by the sequential actions of deoxyhypusine synthase (DHS) and deoxyhypusine hydroxylase, which together transfer the polyamine moiety of spermidine to the ε-amino group of Lys50 in eIF5A. DHS has been shown to be the rate limiting and reversible enzyme in the biosynthetic pathway, and as such, represents an obvious target for inhibiting the overall rate of hypusine formation. Several inhibitors of DHS have been described, all classified as polyamines with structural homology to spermidine. Perhaps the best-studied and most potent inhibitor is N1-guanyl-1,7-diaminoheptane (GC7), which exhibits a Ki of 10 nM in vitro (about 450-fold lower than the Km for spermidine).24 X-ray crystallographic analysis of DHS at near-optimal pH and ionic conditions shows that GC7 is specifically bound within a deep acidic active site tunnel.25 Inhibition of DHS using GC7 and similar polyamines was shown to have a repressive effect on proliferation in both yeast and mammalian cell cultures as soon as 24 h, with a particularly striking inhibition at the G1/S transition of the cell cycle.22,26–28 The specificity of these inhibitors with respect to the hypusination pathway is at least partially verified in studies showing similar G1/S block in temperature-sensitive mutants of eIF5A in yeast,18,29,30 in DOHH mutants in Drosophila,31 and by using inhibitors of DOHH in mammalian cells.32 Whereas all of these studies have been performed in cultured cells or in lower organisms, to date no formal accounts of eIF5A, DHS or DOHH knockouts in intact mice have been reported (of note, one review states in passing that the homozygous knock-out of alleles encoding DHS is embryonic lethal).23
In studies recently published, we demonstrated the potential utility of DHS inhibition on stress signaling in pancreatic islets.14 Because obese, non-diabetic C57BL/6J-db/db mice show attenuated eIF5A hypusination in their islets, we hypothesized that a downregulation in the activity and/or expression of the rate-limiting enzyme DHS may be a factor that favors islet β-cell function in the setting of obesity. To test this, we employed the C57BL/KSJ-db/db mouse strain, which exhibits obesity with islet dysfunction and frank T2DM. C57BL/KSJ-db/db mice were treated with intraperitoneal injections of GC7 (or control saline) for two weeks, after which metabolic parameters for glycemic control were measured. Our studies revealed that GC7-treated mice exhibited improved fasting glucoses, improved glucose tolerance, elevated insulin levels and enhanced β-cell mass compared to saline-treated mice. Based on follow up studies using cultured β cells, we proposed that this improved metabolic phenotype resulted from a reduced incidence of β-cell death caused by signaling pathways converging on ER stress, particularly the translation of C/EBP homologous protein (CHOP). Whereas this finding provided important insight on the pathogenesis of disease progression, the use of DHS inhibition as a therapeutic approach to T2DM has its caveats. Most notably, and apart from off-target and untoward side effects, there is the worry that long-term treatment with DHS inhibitors has the potential to inhibit proliferation of crucial cell types in an organism, not the least of which is the islet β cell itself. As a first step to address the potential effects of DHS deficiency, we generated mice harboring an allelic knock-out of the gene encoding DHS (known as “Dhps”), and studied glucose homeostasis in these mice and interrogated cells from these mice for proliferative and cell cycle alterations.
Dhps Heterozygosity does not Alter Growth or Metabolic Homeostasis
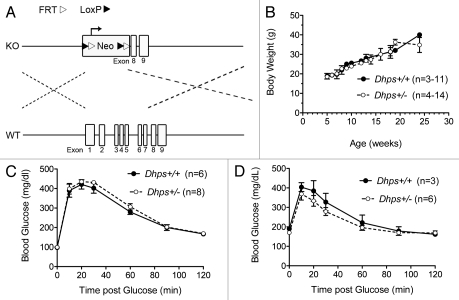
Figure 1A shows the our Dhps gene targeting strategy. Exons 1–7 were replaced by a neomycin selection cassette, preserving exons 8 and 9 (note: exon 9 contains a gene, Wdr83, on the reverse strand). This strategy ensured that no fragments containing the catalytic or binding domain of the DHS protein is produced. Using this targeting strategy, mice harboring a Dhps knockout allele (Dhps+/−) were generated on a mixed C57BL6/129SvEv genetic background. Based on the analysis of offspring from 20 separate matings between Dhps+/− mice (total of 99 offspring), we have obtained 34 Dhps+/+ mice, 65 Dhps+/− mice and no Dhps−/− mice. Given the expected Mendelian ratio of 2:1 for Dhps+/−:Dhps+/+ mice, and the absence of Dhps−/− mice, we conclude that the Dhps−/− mutation is embryonic lethal. We subsequently analyzed embryos from an additional three pregnancies at embryonic day 8.5–9.5, but were unable to identify any Dhps−/− embryos. We therefore believe that DHS may be required for the very early stages of development of the mouse embryo, although it is still unclear at this point whether this requirement stems from the lack of hypusinated eIF5A or an alteration in polyamine homeostasis. Nevertheless, it is possible that DHS is important either in the proliferation of early embryonic cells and/or in the ability of those cells to activate stress signaling pathways during differentiation. We next focused on the phenotype of Dhps+/− mice. As shown in Figure 1B–D, there is no difference between Dhps+/+ and Dhps+/− mice with respect to body weight as mice age from 8–20 weeks, or in glucose homeostasis at 8 and 20 weeks of age (as assessed by intraperitoneal glucose tolerance testing). Examination of the pancreas did not reveal any gross morphologic differences in islet mass or architecture between the two genotypes (data not shown).
Figure 1.
Dhps heterozygosity in mice does not alter growth or metabolic homeostasis. (A) Schematic diagram of the Dhps gene targeting vector (KO) and the wild-type mouse locus (WT). Targeting construct and mice were generated by a contract to InGenious Targeting Labs (Stony Brook, NY). Neo, neomycin selection cassette. Dotted lines indicate homologous recombination regions; (B) serial body weights of Dhps+/+ and Dhps+/− mice between 5–25 weeks of age; (C) results of glucose tolerance tests in mice at 8 weeks of age. Glucose (1 g/kg body weight) was injected intraperitoneally following an overnight fast; (D) results of glucose tolerance tests in mice at 20 weeks of age. Glucose (1 g/kg body weight) was injected intraperitoneally following a 6 h fast.
Dhps Heterozygosity Attenuates Acute Cytokine Signaling in Mouse Embryonic Fibroblasts
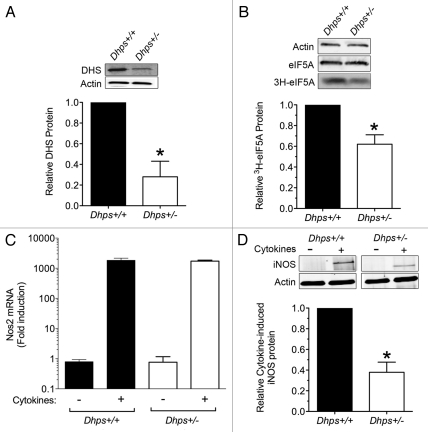
Although the growth and metabolic characteristics of Dhps+/− mice appear unaffected, they do not rule out the possibility that reductions in DHS protein may still lead to phenotypic differences under conditions of stress. As an initial approach to address this possibility, we isolated mouse embryonic fibroblasts (MEFs) from Dhps+/+ and Dhps+/− embryos at embryonic day 13.5. Compared to Dhps+/+ MEFs, Dhps+/− MEFs contain approximately 50% less DHS protein as assessed by immunoblot analysis (Fig. 2A). Although total eIF5A protein is unchanged between Dhps+/+ and Dhps+/− MEFs, the rate of 3H-spermidine incorporation into eIF5A is reduced by approximately 50% in Dhps+/− MEFs (Fig. 2B), a finding consistent at the protein level with haploinsufficiency of Dhps at the genetic level. To assess the response of haploinsufficient cells to the type of stress observed in T2DM, we subjected MEFs to incubation with a cocktail of pro-inflammatory cytokines (IL-1β, TNFα and IFNγ) for 4 h. In prior studies, we showed that pro-inflammatory cytokines cause acute induction of the mRNA and protein for iNOS, and that inhibition of DHS with GC7 blocks activation of iNOS protein, but not its mRNA.22 As shown in Figure 2C and D, activation of the mRNA encoding iNOS (Nos2) is unaffected in Dhps+/− MEFs, but the production of iNOS protein is reduced by 50%. Although these data are not direct evidence of translational control of Nos2 mRNA, the dissociation between the mRNA levels and protein levels are nonetheless suggestive. We feel these findings align with our prior studies, but more importantly, they verify at the level of Dhps haploinsufficiency what we observed in vitro and in vivo with DHS inhibitors.22
Figure 2.
Dhps heterozygosity attenuates acute cytokine signaling in mouse embryonic fibroblasts. (A) Top: immunoblot analysis for DHS and actin from whole-cell extracts of Dhps+/+ and Dhps+/− MEFs. Immunoblots were visualized using a LiCor Odysseyr fluorescence system following electrophoresis on a 4–20% SDS-polyacrylamide gel. Bottom: quantitation of DHS protein levels (normalized to actin levels) from three independent experiments. (B) Top: MEFs were incubated with 1 µCi/ml 3H-spermidine for 4 h, then whole cell extracts were subjected to immunoblot analysis (for actin and eIF5A) or to fluorography (for 3H-eIF5A) following electrophoresis on a 4–20% SDS-polyacrylamide gel. Bottom: quantitation of 3H-eIF5A levels (normalized to actin levels) expressed as the mean ± SEM from 5 independent experiments. (C) MEFs were incubated in the absence or presence of a cytokine mixture (5 ng/ml IL-1β, 10 ng/ml TNFα and 100 ng/ml IFNγ) for 4 h, then total RNA was isolated and subjected to real-time RT-PCR analysis for Nos2 mRNA encoding iNOS. Data were normalized to Actb mRNA levels (encoding actin), and are presented as fold-induction relative to non-cytokine treatment. (D) Top: immunoblot analysis for iNOS and actin from whole cell extracts of MEFs treated without and with a cytokine mixture for 4 h. Bottom: quantitation of iNOS protein levels (normalized to actin levels) following incubation with cytokines. Data represent the mean ± SEM from three independent experiments. In all parts, * indicates that the value for Dhps+/− MEFs is statistically different from corresponding value for Dhps+/+ MEFs (p < 0.05 by a Student's t-test).
Dhps Heterozygosity does not Lead to Significant Inhibition of Proliferation and G1/S Cell Cycle Progression
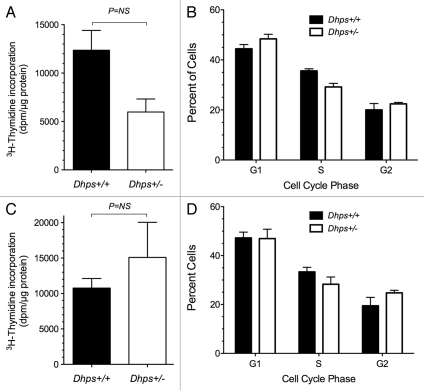
To address the possibility that inhibition of DHS leads to defects in cellular proliferation, we performed 3H-thymidine uptake studies in MEFs. As shown in Figure 3A, Dhps+/− MEFs demonstrated a roughly 40% lower 3H-thymidine uptake over a 4 h period compared to Dhps+/+ cells, although this difference did not strictly approach statistical significance (p = 0.06). This reduction in cellular proliferation in Dhps+/− cells correlated to a small, but again statistically insignificant, increase in the proportion of cells in G1 phase and a decrease in the proportion of cells in the S phase, a finding consistent with trend to G1/S block (Fig. 3B). By contrast, under cytokine stress conditions, no obvious differences in proliferation or cell cycle populations were observed between the two genotypes (Fig. 3C and D). Overall, however, the lack of statistically significant changes in cell cycle populations is consistent with the observation that Dhps+/− mice displayed no obvious differences in growth or weight up to six months of age.
Figure 3.
Dhps heterozygosity does not lead to significant inhibition of proliferation or cell cycle progression. (A) MEFs were incubated with 1 µCi/ml of 3H-methyl thymidine for 4 h, then washed and lysed. 3H-thymidine incorporation was measured and normalized to protein content. (B) MEFs were incubated with Guava cell cycle reagent (Millipore) for 30 min. Intercalation of propidium iodide into cellular DNA was quantitated using a FACS Calibur instrument, and the data were analyzed for cell cycle status using Modfit software. (C) same as in (A), except cells were concurrently treated with a cytokine mixture (5 ng/ml IL-1β, 10 ng/ml TNFα and 100 ng/ml IFNγ) during the 4 h 3H-methyl thymidine incubation period. (D) Same as in (B), except cells were treated with a cytokine mixture for 4 h prior to incubation with Guava reagent.
Concluding Remarks
To date, our studies have pointed to a potential role for DHS and hypusinated eIF5A in the normal responses of islet β cells to pro-inflammatory and ER stressors. Given the totality of the data on the two proteins, as summarized here, it is fair to ask how precisely this pair should be viewed with respect to cellular survival. On the one hand, depletion of eIF5A or inhibition of DHS appears to be beneficial, preventing hyperglycemia in mouse models of inflammation-induced diabetes;14,22 in another particularly striking study, depletion of eIF5A protects mice against death in the setting of sepsis induced by lipopolysaccharide.33 On the other hand, inhibition of DHS or depletion of eIF5A can also have negative effects, leading to defects in both cell cycle progression and the formation of stress granules;16 and in this report, we show that Dhps-null mice are early embryonic lethal. With respect to the islet β cell, we feel it is best to view DHS and eIF5A as representing stress-responsive proteins whose functions are accentuated in periods of acute stress (such as inflammation, viral infection, sepsis, etc.). Under these stress conditions, we propose that DHS and eIF5A are necessary for regulating the ultimate translation of proteins that are necessary for stress remediation or adaptation; however, as the stress continues unabated, these proteins may be necessary to trigger the cellular execution response in an attempt to limit the extent of stress. Thus, DHS and eIF5A may regulate the balance between adaptive and pro-death responses in the cell. As such, it is possible that too much or too little of these proteins may shift the balance in favor of cellular death. The db/db mouse model may represent a particularly dramatic example of this balance in the islet: the C57BL/6J-db/db strain attenuates eIF5A hypusination rates sufficiently to favor islet adaptation and normoglycemia, whereas the C57BKLS/J-db/db strain maintains hypusination rates that favor islet death.
With regard to the islet β cell, hypusination may be an especially attractive target for several reasons: (1) hypusinated eIF5A has a very short half-life in islets (∼6 h),22 compared to other cell types (>20 h),22,34–36 a finding supporting an acute regulatory role for the protein in islets; (2) eIF5A and DHS exhibit rapid and reciprocal nucleo-cytoplasmic shuttling in β cells in response to cytokines or ER stress,14,22 whereas in other cell types specific compartmentation is less clear or controversial;20,37 and (3) the islet β cell has a very slow replicative rate and therefore is less susceptible acutely to agents (such as DHS inhibitors) that affect cellular proliferation. Because our data suggest that Dhps+/− mice can maintain normal growth and glucose homeostasis, with evidence that their cells maintain more robust responses to inflammation, we feel that pharmacologic approaches to inhibiting DHS could be successful in mitigating diabetes progression in an intact animal. Nonetheless, further research using islet and β cell-specific knockout mouse models of DHS and eIF5A would be especially helpful in elucidating the roles of these proteins in islet cell types and diabetes models.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health (R01 DK60581 to R.G.M.) and the American Diabetes Association (Takeda-ADA mentor-based award to R.G.M.).
Abbreviations
- DHS
deoxyhypusine synthase
- DOHH
deoxyhypusine hydroxylase
- eIF5A
eukaryotic translation initiation factor 1
- ER
endoplasmic reticulum
- iNOS
inducible nitric oxide synthase
- MEF
mouse embryonic fibroblast
- T2DM
type 2 diabetes mellitus
References
- 1.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontés G, Zarrouki B, Hagman DK, Latour MG, Semache M, Roskens V, et al. Glucolipotoxicity age-dependently impairs beta cell function in rats despite a marked increase in beta cell mass. Diabetologia. 2010;53:2369–2379. doi: 10.1007/s00125-010-1850-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. Islet-Associated macrophages in type 2 diabetes. Diabetologia. 2009;52:1686–1688. doi: 10.1007/s00125-009-1410-z. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 5.Huang CJ, Lin CY, Haataja L, Gurlo T, Butler AE, Rizza RA, et al. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016–2027. doi: 10.2337/db07-0197. [DOI] [PubMed] [Google Scholar]
- 6.Zraika S, Hull RL, Udayasankar J, Aston-Mourney K, Subramanian SL, Kisilevsky R, et al. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia. 2009;52:626–635. doi: 10.1007/s00125-008-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogihara T, Mirmira RG. An islet in distress: B cell failure in type 2 diabetes. J Diab Invest. 2010;1:123–133. doi: 10.1111/j.2040-1124.2010.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers KT, Unverferth JA, Weber SM, Wek RC, Urano F, Corbett JA. The role of nitric oxide and the unfolded protein response in cytokine-induced beta-cell death. Diabetes. 2008;57:124–132. doi: 10.2337/db07-0944. [DOI] [PubMed] [Google Scholar]
- 9.Hughes KJ, Chambers KT, Meares GP, Corbett JA. Nitric oxides mediates a shift from early necrosis to late apoptosis in cytokine-treated {beta}-cells that is associated with irreversible DNA damage. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00214.2009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontés G. Glucolipotoxicity of the pancreatic beta cell. Biochim Biophys Acta. 2010;1801:289–298. doi: 10.1016/j.bbalip.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perl S, Kushner JA, Buchholz BA, Meeker AK, Stein GM, Hsieh M, et al. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J Clin Endocrinol Metab. 2010;95:234–239. doi: 10.1210/jc.2010-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leiter EH, Coleman DL, Hummel KP. The influence of genetic background on the expression of mutations at the diabetes locus in the mouse. III. Effect of H-2 haplotype and sex. Diabetes. 1981;30:1029–1034. doi: 10.2337/diab.30.12.1029. [DOI] [PubMed] [Google Scholar]
- 13.Baetens D, Stefan Y, Ravazzola M, Malaisse-Lagae F, Coleman DL, Orci L. Alteration of islet cell populations in spontaneously diabetic mice. Diabetes. 1978;27:1–7. doi: 10.2337/diab.27.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Robbins RD, Tersey SA, Ogihara T, Gupta D, Farb TB, Ficorilli J, et al. Inhibition of deoxyhypusine synthase enhances islet {beta} cell function and survival in the setting of endoplasmic reticulum stress and type 2 diabetes. J Biol Chem. 2010;285:39943–39952. doi: 10.1074/jbc.M110.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clement PM, Henderson CA, Jenkins ZA, Smit-McBride Z, Wolff EC, Hershey JW, et al. Identification and characterization of eukaryotic initiation factor 5A-2. Eur J Biochem. 2003;270:4254–4263. doi: 10.1046/j.1432-1033.2003.03806.x. [DOI] [PubMed] [Google Scholar]
- 16.Li CH, Ohn T, Ivanov P, Tisdale S, Anderson P. Eif5A promotes translation elongation, polysome disassembly and stress granule assembly. PLoS One. 2010;5:9942. doi: 10.1371/journal.pone.0009942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saini P, Eyler DE, Green R, Dever TE. Hypusine-Containing protein eif5a promotes translation elongation. Nature. 2009;459:118–121. doi: 10.1038/nature08034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang HA, Hershey JW. Effect of initiation factor eif-5a depletion on protein synthesis and proliferation of saccharomyces cerevisiae. J Biol Chem. 1994;269:3934–3940. [PubMed] [Google Scholar]
- 19.Rosorius O, Reichart B, Kratzer F, Heger P, Dabauvalle MC, Hauber J. Nuclear pore localization and nucleocytoplasmic transport of eif-5a: Evidence for direct interaction with the export receptor CRM1. J Cell Sci. 1999;112:2369–2380. doi: 10.1242/jcs.112.14.2369. [DOI] [PubMed] [Google Scholar]
- 20.Jao DL, Yu Chen K. Subcellular localization of the hypusine-containing eukaryotic initiation factor 5A by immunofluorescent staining and green fluorescent protein tagging. J Cell Biochem. 2002;86:590–600. doi: 10.1002/jcb.10235. [DOI] [PubMed] [Google Scholar]
- 21.Lipowsky G, Bischoff FR, Schwarzmaier P, Kraft R, Kostka S, Hartmann E, et al. Exportin 4: A mediator of a novel nuclear export pathway in higher eukaryotes. EMBO J. 2000;19:4362–4371. doi: 10.1093/emboj/19.16.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maier B, Ogihara T, Trace AP, Tersey SA, Robbins RD, Chakrabarti SK, et al. The unique hypusine modification of eif5a promotes islet beta cell inflammation and dysfunction in mice. J Clin Invest. 2010;120:2156–2170. doi: 10.1172/JCI38924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park MH, Nishimura K, Zanelli CF, Valentini SR. Functional significance of eif5a and its hypusine modification in eukaryotes. Amino Acids. 2010;38:491–500. doi: 10.1007/s00726-009-0408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakus J, Wolff EC, Park MH, Folk JE. Features of the spermidine-binding site of deoxyhypusine synthase as derived from inhibition studies. Effective inhibition by bis- and mono-guanylated diamines and polyamines. J Biol Chem. 1993;268:13151–13159. [PubMed] [Google Scholar]
- 25.Umland TC, Wolff EC, Park MH, Davies DR. A new crystal structure of deoxyhypusine synthase reveals the configuration of the active enzyme and of an enzyme. NAD. Inhibitor ternary complex. J Biol Chem. 2004;279:28697–28705. doi: 10.1074/jbc.M404095200. [DOI] [PubMed] [Google Scholar]
- 26.Lee Y, Kim HK, Park HE, Park MH, Joe YA. Effect of n1-guanyl-1,7-diaminoheptane, an inhibitor of deoxyhypusine synthase, on endothelial cell growth, differentiation and apoptosis. Mol Cell Biochem. 2002;237:69–76. doi: 10.1023/a:1016535217038. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura K, Murozumi K, Shirahata A, Park MH, Kashiwagi K, Igarashi K. Independent roles of eif5a and polyamines in cell proliferation. Biochem J. 2005;385:779–785. doi: 10.1042/BJ20041477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park MH, Wolff EC, Lee YB, Folk JE. Antiproliferative effects of inhibitors of deoxyhypusine synthase. Inhibition of growth of chinese hamster ovary cells by guanyl diamines. J Biol Chem. 1994;269:27827–27832. [PubMed] [Google Scholar]
- 29.Chatterjee I, Gross SR, Kinzy TG, Chen KY. Rapid depletion of mutant eukaryotic initiation factor 5A at restrictive temperature reveals connections to actin cytoskeleton and cell cycle progression. Mol Genet Genomics. 2006;275:264–276. doi: 10.1007/s00438-005-0086-4. [DOI] [PubMed] [Google Scholar]
- 30.Zanelli CF, Valentini SR. Pkc1 acts through zds1 and gic1 to suppress growth and cell polarity defects of a yeast eif5a mutant. Genetics. 2005;171:1571–1581. doi: 10.1534/genetics.105.048082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel PH, Costa-Mattioli M, Schulze KL, Bellen HJ. The Drosophila deoxyhypusine hydroxylase homologue nero and its target eif5a are required for cell growth and the regulation of autophagy. J Cell Biol. 2009;185:1181–1194. doi: 10.1083/jcb.200904161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanauske-Abel HM, Park MH, Hanauske AR, Popowicz AM, Lalande M, Folk JE. Inhibition of the G1-S transition of the cell cycle by inhibitors of deoxyhypusine hydroxylation. Biochim Biophys Acta. 1994;1221:115–124. doi: 10.1016/0167-4889(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 33.Moore CC, Martin EN, Lee G, Taylor C, Dondero R, Reznikov LL, et al. Eukaryotic translation initiation factor 5A small interference rna-liposome complexes reduce inflammation and increase survival in murine models of severe sepsis and acute lung injury. J Infect Dis. 2008;198:1407–1414. doi: 10.1086/592222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klier H, Csonga R, Joäo HC, Eckerskorn C, Auer M, Lottspeich F, et al. Isolation and structural characterization of different isoforms of the hypusine-containing protein eif-5a from hela cells. Biochemistry. 1995;34:14693–14702. doi: 10.1021/bi00045a010. [DOI] [PubMed] [Google Scholar]
- 35.Gerner EW, Mamont PS, Bernhardt A, Siat M. Post-Translational modification of the protein-synthesis initiation factor eif-4d by spermidine in rat hepatoma cells. Biochem J. 1986;239:379–386. doi: 10.1042/bj2390379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gosslau A, Jao DL, Butler R, Liu AY, Chen KY. Thermal killing of human colon cancer cells is associated with the loss of eukaryotic initiation factor 5A. J Cell Physiol. 2009;219:485–493. doi: 10.1002/jcp.21696. [DOI] [PubMed] [Google Scholar]
- 37.Lee SB, Park JH, Kaevel J, Sramkova M, Weigert R, Park MH. The effect of hypusine modification on the intracellular localization of eif5a. Biochem Biophys Res Commun. 2009;383:497–502. doi: 10.1016/j.bbrc.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]