Abstract
Trypanosoma cruzi, the etiological agent of Chagas disease, causes vasculopathy and cardiomyopathy in humans and is associated with elevated levels of several vasoactive molecules such as nitric oxide, endothelin-1 and thromboxane A2 (TXA2). Parasite derived TXA2 modulates vasculopathy and other pathophysiological features of chagasic cardiomyopathy. Previously, we demonstrated that in response to infection with T. cruzi, TXA2 receptor (TP) null mice displayed increased parasitemia; mortality and cardiac pathology compared with wild type (WT) and TXA2 synthase null mice. In order to further study the role of TXA2-TP signaling in the development of Chagas disease, GeneChip microarrays were used to detect transcriptome changes in rat fat pad endothelial cells (RFP-ECs) which is incapable of TXA2 signaling (TP null) to that of control (wild type) and RFP-EC with reconstituted TP expression. Genes that were significantly regulated due to infection were identified using a time course of 2, 18 and 48 hrs post infection. We identified several key genes such as suppressor of cytokine signaling (SOCS-5), several cytokines (CSF-1, CXCF ligands) and MAP kinases (MAPK-1, Janus kinase) that were upregulated in the absence of TP signaling. These data underscore the importance of the interaction of the parasite with mammalian TP and may explain the increased mortality and cardiovascular pathology observed in infected TP null mice.
Key words: Trypanosoma cruzi, Chagel disease, thromboxane signaling, microarray, suppressor of cytokine signaling
Introduction
Eicosanoids are a family of lipid mediators that participate in a wide range of biological activities including vascular tone, inflammation, ischemia and tissue homeostasis.1 In mammals, the biosynthetic pathways for these important biological mediators are dependent upon liberation of arachidonic acid from the inner leaflet of the plasma membrane. Thromboxane A2 (TxA2), an eicosanoid generated during arachidonic acid metabolism, is the most potent vasoconstrictor known and acts via its receptors TPα and its splice variant TPβ, both of which are expressed on human endothelial cells (ECs). Several parasitic organisms are known to produce eicosanoids, many of which are known to modulate host response and the progress of an infection.2–6
Infection with the protozoan parasite Trypanosoma cruzi causes Chagas disease, characterized by acute myocarditis and vasculitis that evolves into a chronic cardiomyopathy in 15 to 30% of infected persons. Chagasic cardiomyopathy is an important cause of morbidity and mortality in endemic areas of Mexico, Central and South America.7,8 Transmission to humans may occur by several means including natural transmission via the insect vector, blood transfusion, laboratory accidents, organ transplantation, congenital transmission9,10 and ingestion of contaminated food and water.11 Chagas disease is also recognized as an opportunistic infection in immune-compromised individuals including those with HIV/AIDS.12
The parasite has a complex life cycle involving a mammalian host and a insect vector.7 The insect forms include epimastigotes, which multiply extracellularly, inside the insect midgut and give rise to infective non-dividing metacyclic trypomastigotes (MT). The insect introduces MTs into the mammalian host while taking a blood meal, through its feces near the punctured skin. The MTs immediately transforms into non-dividing, blood form trypomastigotes (BFT). BFTs can infect a variety of host cell types and multiply intracellularly as amastigotes.13 Amastigotes are released as infected cells rupture and transform back to BFTs, which infect adjacent tissues or are swept into the blood and lymphatics to remote areas of the body. In the cardiovascular system cardiac myocytes, cardiac fibroblasts, ECs and vascular smooth muscle cells are readily infected by this parasite.
Acute T. cruzi infection results in the upregulation of the inflammatory responses in many tissues and has been studied most extensively in the heart. During acute infection there is an increased expression of cytokines,14 chemokines,15 endothelin-1,16,17 vascular adhesion molecules18 and nitric oxide synthases19 which is accompanied by an intense inflammatory infiltrate, myonecrosis, pseudocyst formation, vasculitis and platelet activation and aggregation. Chronic chagasic cardiomyopathy is an example of a dilated cardiomyopathy associated with chronic inflammation and fibrosis, myocytolysis, congestive heart failure and thombo-embolic events. Notably, few parasites are observed in the myocardium during the chronic phase. Many of the features of acute and chronic Chagas disease are also associated with the activation of TXA2 signaling pathway via its receptors.20
The role of TXA2 in the pathogenesis of T. cruzi infection was suggested in 1990,21 and recently examined in more detail in reference 22. Our laboratory demonstrated that all T. cruzi life cycle forms were capable of synthesizing TXA2 thereby modulating vasculopathy and other features of chagasic cardiomyopathy including inflammation and platelet activation.22 Additionally, we demonstrated that majority of circulating TXA2 in T. cruzi-infected thromboxane synthase (TXA2S)-null mice was parasite-derived. T. cruzi infection of TP null mice resulted in increased peripheral parasitemia and mortality. Moreover, infection of ECs obtained from TP null mice displayed higher intracellular parasitism compared with wild-type uninfected cells,22 suggesting that the TXA2-TP signaling plays an important role in Chagas disease. These observations suggested that parasite-derived TXA2 is sufficient to stimulate host TP to ensure normal disease progression via stimulation of host TP to affect parasitemia and host survival. The parasite-derived TXA2 may not directly participate in the inflammatory process of the host, but rather contribute to the balance between the rate of parasite proliferation and continued survival of the host so that the disease can progress to the chronic stage. Previously, we demonstrated that TP stimulation inhibits the proinflammatory effects of TNFα via a Gαq mediated mechanism.22
The nature of the signaling pathways resulting from TP activation that control parasite growth and replication is not entirely known, although activation of Gαq appears to be important.22,23 We sought to determine the potential molecular mechanisms by which the parasite-derived TXA2 modulates Chagas disease progression and limit collateral damage to organs. Thus, we performed GeneChip microarray analysis on rat fat pad ECs with normal TP (WT-EC) and TPα null-EC24 responses to TXA2 signaling and compared to null-EC reconstituted with the human TPα isoform (TPα-EC). The changes in the transcription profile were compared with matched uninfected and infected WT-EC. Rats do not express TPβ, therefore, TPα null ECs are functionally incapable of TXA2 signaling. We monitored the host response to TP receptor null environment over a time course of 2, 18 and 48 h post infection (p.i.).
Results
TP null endothelial cells (ECs) are functionally deficient of TP activation.
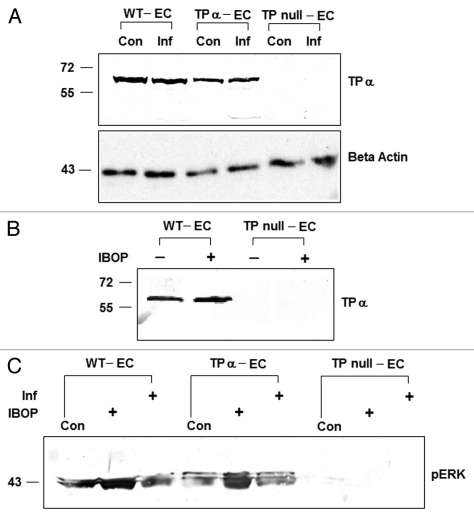
We employed immunoflourescence to detect the expression and abundance of TP in RFPECs. Since, TP null ECs were a functional mutant and not a genetic knockout,24 we could detect a small amount of TP expression in these cells with anti-human TP antibody that also recognizes rat TPs. However, the abundance of TP in TP null ECs was approximately 42% of that of WT-EC and 29% of that of the TP reconstituted (TPα-EC) ECs (Fig. 1). We also analyzed the expression of TP by immunoblotting using the same antibody and found that TP null ECs contain significantly less TP as compared to either the WT-ECGrTPα-EC. Conversely, re-expression of the human TPα isoform in null-EC resulted in levels of expression similar to those observed in WT-EC indicating physiological levels of expression were achieved in TPα-EC (Fig. 2A). TP expression in these cells was not regulated as a result of either infection with T. cruzi treatment with 50 nM IBOP, a TP receptor against (Fig. 2B). We evaluated the functional status of TP in these cells by stimulating with 50 nM IBOP, a thromboxane-mimetic agent and measuring the activation of ERK pathway by immunoblotting using anti phospho ERK antibody.25 Both WT-EC and TPα-EC expressed high levels of phospho ERK when stimulated with IBOP indicating an intact TP signaling pathways in these cells. However, we could not detect ERK activation in TP null ECs stimulated with IBOP (Fig. 2C). These results indicate that reconstitution of TP null ECs with ectopic expression of human TPα isoforms are a reliable system to analyze TP signaling as functional receptors with appropriate coupling. Thus they were employed to determine the role of prostanoid signaling associated with T. cruzi infection.
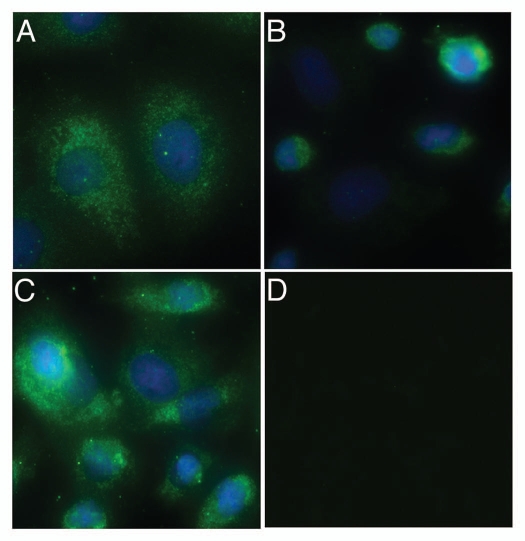
Figure 1.
Detection of TP in WT-EC (A), TP null (B) and TPα-EC (C) by immunoflourescence using anti TPα antibody. Both WT-EC and TP null with transfected human TPα gene expressed abundant TPα protein as compared to that of TP null ECs. The faint expression of TPα in TP null EC may be due to the fact that TP null EC are functional but not genetic knockouts or it may be due to reaction of TPα antibody with a related protein as no primary antibody control staining could detect any background staining with TPα antibody (D).
Figure 2.
TP expression in WT-EC, TP null and TPα-EC by immunoblotting. (A) TP expression in these cells was not regulated by either infection with T. cruzi or when these cells were stimulated with a TP receptor against, IBOP (B) TP expressed in TP null and TPα EC were functional as ERK activation was observed when these cells were stimulated with IBOP (C) Beta actin was used as an equal loading control for all immunoblots and its expression was unaffected by experimental conditions (the controls for B and C not shown).
Significantly upregulated genes in T. cruzi infection and those that are also dependent on TP activation.
In order to evaluate significantly upregulated genes in the setting of T. cruzi infection and those that are also dependent on TP activation, we compared the entire data set including all target ECs (i.e., WT-EC, TP null EC and TPα-EC) and conditions (control vs. infected) at all time points. Data was normalized to mean values and statistical significance was determined using ANOVA (6,799 genes). In the selection process, the following criteria were used: first, genes were selected that were upregulated in infected TP null ECs by at least 1.5-fold when compared with the infected WT ECs, second, we selected only those genes from infected TPα-ECs that had expression values between 1–1.5-fold to that of infected WT ECs (445 genes). Third, we selected only those genes, which showed regulation when compared to the matched control (162 genes). Using these criteria we were able to isolate genes that were upregulated because of absence of the TXA2-TP signaling pathway during the course of T. cruzi infection. Comparing with the regulated genes observed in the reconstituted cells with TPα receptor expression resulted in the selection of those genes that were expressed at normal levels when the TXA2-TP signaling pathway was restored. Finally we used the Ingenuity Pathways Analysis to classify genes according to their function.
After trimming control and unknown genes from the list, we obtained 136 genes (Table 1) that were significantly upregulated because of TP null phenotype. These genes are believed to be otherwise downregulated if the TP pathway were intact as in the WT or when we reconstitute the cells with the TP receptor. Table 1 provides a list of upregulated genes and their functions.
Table 1.
The genes that were upregulated (>1.5-fold) a result of T. cruzi infection in the absence of thromboxane signaling.
| ID | Gene | Description | Location | Family |
| 1387316_at | CXCL2 | chemokine (C-X-C motif) ligand 2 | Extracellular Space | cytokine |
| 1380583_s_at | CSF1 | colony stimulating factor 1 (macrophage) | Extracellular Space | cytokine |
| 1379271_at | SOCS5* | suppressor of cytokine signaling 5 | Extracellular Space | cytokine |
| 1390555_at | SOCS5* | suppressor of cytokine signaling 5 | Extracellular Space | cytokine |
| 1387101_at | ACSL4 | acyl-CoA synthetase long-chain family member 4 | Cytoplasm | enzyme |
| 1384115_at | ACOT2 | acyl-CoA thioesterase 2 | Cytoplasm | enzyme |
| 1370902_at | AKR1B15 | aldo-keto reductase family 1, member B15 | Cytoplasm | enzyme |
| 1368916_at | ASL | argininosuccinate lyase | Cytoplasm | enzyme |
| 1375595_at | ARIH1 | ariadne homolog, ubiquitin-conjugating enzyme E2 binding protein, 1 (Drosophila) | Cytoplasm | enzyme |
| 1369967_at | CS | citrate synthase | Cytoplasm | enzyme |
| 1376754_at | CARS | cysteinyl-tRNA synthetase | Cytoplasm | enzyme |
| 1369984_at | COX17 | COX17 cytochrome c oxidase assembly homolog (S. cerevisiae) | Cytoplasm | enzyme |
| 1397304_at | IGTP | interferon gamma induced GTPase | Cytoplasm | enzyme |
| 1372599_at | MGST2 | microsomal glutathione S-transferase 2 | Cytoplasm | enzyme |
| 1372177_at | MOCS2 | molybdenum cofactor synthesis 2 | Cytoplasm | enzyme |
| 1370678_s_at | MAOA | monoamine oxidase A | Cytoplasm | enzyme |
| 1383899_at | NEDD4 | neural precursor cell expressed, developmentally downregulated 4 | Cytoplasm | enzyme |
| 1373418_at | EPRS | glutamyl-prolyl-tRNA synthetase | Cytoplasm | enzyme |
| 1382537_at | RRAGC* | Ras-related GTP binding C | Cytoplasm | enzyme |
| 1371723_at | RRAGC* | Ras-related GTP binding C | Cytoplasm | enzyme |
| 1394077_at | RND3 | Rho family GTPase 3 | Cytoplasm | enzyme |
| 1389468_at | RPIA | ribose 5-phosphate isomerase A | Cytoplasm | enzyme |
| 1383004_at | AHCYL1 | adenosylhomocysteinase-like 1 | Cytoplasm | enzyme |
| 1388574_at | WARS | tryptophanyl-tRNA synthetase | Cytoplasm | enzyme |
| 1373037_at | UBE2L6 | ubiquitin-conjugating enzyme E2L 6 | Cytoplasm | enzyme |
| 1371537_at | B4GALT5 | UDP-Gal:betaGlcNAc beta 1,4-galactosyltransferase, polypeptide 5 | Cytoplasm | enzyme |
| 1385695_at | LOXL3 | lysyl oxidase-like 3 | Extracellular Space | enzyme |
| 1370144_at | GTPBP4 | GTP binding protein 4 | Nucleus | enzyme |
| 1374725_at | MOV10 | Mov10, Moloney leukemia virus 10, homolog (mouse) | Nucleus | enzyme |
| 1391608_at | PARN | poly(A)-specific ribonuclease (deadenylation nuclease) | Nucleus | enzyme |
| 1384157_at | ARL8B* | ADP-ribosylation factor-like 8B | Plasma Membrane | enzyme |
| 1397815_at | ARL8B* | ADP-ribosylation factor-like 8B | Plasma Membrane | enzyme |
| 1387925_at | ASNS | asparagine synthetase | unknown | enzyme |
| 1374489_at | GTPBP2 | GTP binding protein 2 | unknown | enzyme |
| 1399160_a_at | UBE2D3 (includes EG:66105) | ubiquitin-conjugating enzyme E2D 3 (UBC4/5 homolog, yeast) | unknown | enzyme |
| 1371710_at | ETNK1 | ethanolamine kinase 1 | Cytoplasm | kinase |
| 1380110_at | JAK2 | Janus kinase 2 | Cytoplasm | kinase |
| 1374550_at | MKNK1 | MAP kinase interacting serine/threonine kinase 1 | Cytoplasm | kinase |
| 1388521_at | ALDH18A1 | aldehyde dehydrogenase 18 family, member A1 | Cytoplasm | kinase |
| 1373943_at | STK4 | serine/threonine kinase 4 | Cytoplasm | kinase |
| 1382541_at | ALK | anaplastic lymphoma receptor tyrosine kinase | Plasma Membrane | kinase |
| 1367788_at | PHKG2 | phosphorylase kinase, gamma2 (testis) | unknown | kinase |
| 1387605_at | CASP12 (includes EG:12364) | caspase 12 | Cytoplasm | peptidase |
| 1387818_at | CASP4 | caspase 4, apoptosis-related cysteine peptidase | Cytoplasm | peptidase |
| 1398803_at | DYNC1H1 | dynein, cytoplasmic 1, heavy chain 1 | Cytoplasm | peptidase |
| 1367786_at | PSMB8 | proteasome (prosome, macropain) subunit, beta type, 8 (large multifunctional peptidase 7) | Cytoplasm | peptidase |
| 1378679_at | USP25 | ubiquitin specific peptidase 25 | unknown | peptidase |
| 1374447_at | USP9X | ubiquitin specific peptidase 9, X-linked | Plasma Membrane | peptidase |
| 1399125_at | INPP1 | inositol polyphosphate-1-phosphatase | Cytoplasm | phosphatase |
| 1367624_at | ATF4 | activating transcription factor 4 (tax-responsive enhancer element B67) | Nucleus | transcription regulator |
| 1379483_at | BHLHE40 | basic helix-loop-helix family, member e40 | Nucleus | transcription regulator |
| 1387800_at | DAXX | death-domain associated protein | Nucleus | transcription regulator |
| 1375205_at | KAT2B | K(lysine) acetyltransferase 2B | Nucleus | transcription regulator |
| 1372797_at | PQBP1 | polyglutamine binding protein 1 | Nucleus | transcription regulator |
| 1392828_at | MED12 | mediator complex subunit 12 | Nucleus | transcription regulator |
| 1383339_at | C19ORF2 | chromosome 19 open reading frame 2 | Nucleus | transcription regulator |
| 1399066_at | TMF1 | TATA element modulatory factor 1 | Cytoplasm | transcription regulator |
| 1393144_at | NMI | N-myc (and STAT) interactor | Cytoplasm | transcription regulator |
| 1393257_at | CUGBP1 | CUG triplet repeat, RNA binding protein 1 | Nucleus | translation regulator |
| 1368967_at | EIF2B3 | eukaryotic translation initiation factor 2B, subunit 3gamma, 58 kDa | Cytoplasm | translation regulator |
| 1373917_at | ETF1 | eukaryotic translation termination factor 1 | Cytoplasm | translation regulator |
| 1387202_at | ICAM1 | intercellular adhesion molecule 1 | Plasma Membrane | transmembrane receptor |
| 1388071_x_at | HLA-C* | major histocompatibility complex, class I, C | Plasma Membrane | transmembrane receptor |
| 1388071_x_at | HLA-C* | major histocompatibility complex, class I, C | Plasma Membrane | transmembrane receptor |
| 1369065_a_at | ATP2A2 | ATPase, Ca++ transporting, cardiac muscle, slow twitch 2 | Cytoplasm | transporter |
| 1387664_at | ATP6V1B2 | ATPase, H+ transporting, lysosomal 56/58 kDa, V1 subunit B2 | Cytoplasm | transporter |
| 1379255_at | ATP6AP2* | ATPase, H+ transporting, lysosomal accessory protein 2 | Cytoplasm | transporter |
| 1379255_at | ATP6AP2* | ATPase, H+ transporting, lysosomal accessory protein 2 | Cytoplasm | transporter |
| 1367503_at | BCAP31 | B-cell receptor-associated protein 31 | Cytoplasm | transporter |
| 1368881_at | BET1 | blocked early in transport 1 homolog (S. cerevisiae) | Cytoplasm | transporter |
| 1386934_at | SLC6A8 | solute carrier family 6 (neurotransmitter transporter, creatine), member 8 | Cytoplasm | transporter |
| 1368391_at | SLC7A1* | solute carrier family 7 (cationic amino acid transporter, y+ system), member 1 | Plasma Membrane | transporter |
| 1368392_at | SLC7A1* | solute carrier family 7 (cationic amino acid transporter, y+ system), member 1 | Plasma Membrane | transporter |
| 1368392_at | SLC7A1* | solute carrier family 7 (cationic amino acid transporter, y+ system), member 1 | Plasma Membrane | transporter |
| 1368391_at | SLC7A1* | solute carrier family 7 (cationic amino acid transporter, y+ system), member 1 | Plasma Membrane | transporter |
| 1370014_at | STX4 | syntaxin 4 | Plasma Membrane | transporter |
| 1371432_at | VAT1 | vesicle amine transport protein 1 homolog (T. californica) | Plasma Membrane | transporter |
| 1387026_at | SMC1A | structural maintenance of chromosomes 1A | Nucleus | transporter |
| 1368732_at | TAP2 | transporter 2, ATP -binding cassette, sub-family B (MDR/TAP) | Cytoplasm | transporter |
| 1388903_at | DYNLT3 | dynein, light chain, Tctex-type 3 | Cytoplasm | other |
| 1372091_at | MID1IP1 | MID1 interacting protein 1 [gastrulation specific G12 homolog (zebrafish)] | Cytoplasm | other |
| 1371028_at | TGOLN2 (includes EG:10618) | trans-golgi network protein 2 | Cytoplasm | other |
| 1369031_at | IL18BP* | interleukin 18 binding protein | Extracellular Space | other |
| 1369031_at | IL18BP* | interleukin 18 binding protein | Extracellular Space | other |
| 1388983_at | C15ORF24 | chromosome 15 open reading frame 24 | Extracellular Space | other |
| 1389577_at | CIRH1A | cirrhosis, autosomal recessive 1A (cirhin) | Nucleus | other |
| 1382326_at | DEDD | death effector domain containing | Nucleus | other |
| 1368947_at | GADD45A | growth arrest and DNA-damage-inducible, alpha | Nucleus | other |
| 1388792_at | GADD45G | growth arrest and DNA-damage-inducible, gamma | Nucleus | other |
| 1372945_at | ING3 | inhibitor of growth family, member 3 | Nucleus | other |
| 1374551_at | IFI35 | interferon-induced protein 35 | Nucleus | other |
| 1372409_at | MAD2L1BP | MAD2L1 binding protein | Nucleus | other |
| 1376144_at | PARP9 | poly (ADP-ribose) polymerase family, member 9 | Nucleus | other |
| 1395523_at | RBMX | RNA binding motif protein, X-linked | Nucleus | other |
| 1390218_at | MEAF6 | MYST/Esa1-associated factor 6 | Nucleus | other |
| 1388436_at | SNRPA | small nuclear ribonucleoprotein polypeptide A | Nucleus | other |
| 1387824_at | SFRS12 | splicing factor, arginine/serine-rich 12 | Nucleus | other |
| 1390290_at | SURF6 | surfeit 6 | Nucleus | other |
| 1371968_at | TMBIM4 | transmembrane BAX inhibitor motif containing 4 | Nucleus | other |
| 1379249_at | WTAP | Wilms tumor 1 associated protein | Nucleus | other |
| 1393127_at | ZNF358 | zinc finger protein 358 | Nucleus | other |
| 1368921_a_at | CD44 | CD44 molecule (Indian blood group) | Plasma Membrane | other |
| 1371939_at | CAPRIN1 | cell cycle associated protein 1 | Plasma Membrane | other |
| 1373182_at | CLDN12 | claudin 12 | Plasma Membrane | other |
| 1387995_a_at | IFITM3 | interferon induced transmembrane protein 3 (1-8U) | Plasma Membrane | other |
| 1388347_at | LY6E | lymphocyte antigen 6 complex, locus E | Plasma Membrane | other |
| 1393915_at | LPCAT3 | lysophosphatidylcholine acyltransferase 3 | Plasma Membrane | other |
| 1388196_at | NCKAP1 | NCK-associated protein 1 | Plasma Membrane | other |
| 1374525_at | RAPH1 | Ras association (RalGDS/AF-6) and pleckstrin homology domains 1 | Plasma Membrane | other |
| 1372489_at | SLMAP | sarcolemma associated protein | Plasma Membrane | other |
| 1375697_at | MLEC | malectin | Plasma Membrane | other |
| 1375641_at | ARPC5L (includes EG:296710) | actin related protein 2/3 complex, subunit 5-like | unknown | other |
| 1382110_at | CNPY3 | canopy 3 homolog (zebrafish) | unknown | other |
| 1389573_at | CHAC1 | ChaC, cation transport regulator homolog 1 (E. coli) | unknown | other |
| 1372361_at | CCDC22 | coiled-coil domain containing 22 | unknown | other |
| 1375174_at | DPY19L1 | dpy-19-like 1 (C. elegans) | unknown | other |
| 1398925_at | FTSJD2 | FtsJ methyltransferase domain containing 2 | unknown | other |
| 1373956_at | FUNDC1 | FUN14 domain containing 1 | unknown | other |
| 1383255_at | GPKOW | G patch domain and KOW motifs | unknown | other |
| 1370975_at | KDM3A | lysine (K)-specific demethylase 3A | unknown | other |
| 1386478_at | MCART2 | mitochondrial carrier triple repeat 2 | unknown | other |
| 1389162_at | NFU1 | NFU1 iron-sulfur cluster scaffold homolog (S. cerevisiae) | unknown | other |
| 1393097_at | RPRD1A | regulation of nuclear pre-mRNA domain containing 1A | unknown | other |
| 1388900_at | RGD1566118 | RGD1566118 | unknown | other |
| 1372585_at | RGD1566254* | RGD1566254 | unknown | other |
| 1372585_at | RGD1566254* | RGD1566254 | unknown | other |
| 1375955_at | RNF114 | ring finger protein 114 | unknown | other |
| 1399070_at | SETD5 | SET domain containing 5 | unknown | other |
| 1389984_at | LOC681740 | similar to jumonji protein | unknown | other |
| 1371531_at | LOC678880 | similar to mammalian retrotransposon derived 8b | unknown | other |
| 1393096_at | HCG 21078 | ribosomal protein L27a pseudogene 6 | unknown | other |
| 1383793_at | TMCC1 | transmembrane and coiled-coil domain family 1 | unknown | other |
| 1394842_at | TMEM19 | transmembrane protein 19 | unknown | other |
| 1373136_at | ZUFSP | zinc finger with UFM1-specific peptidase domain | unknown | other |
Significantly, downregulated genes in T. cruzi infection that are also dependent on TP activation.
Using a similar approach, we found 106 genes to be significantly downregulated in TP null cells due to T. cruzi infection (Table 2). These genes are believed to be otherwise upregulated if the TP pathway were intact as in the wild-type when we reconstitute the cells with the TPα receptor.
Table 2.
The genes that were downregulated (>1.5-fold) due to T. cruzi infection in the absence of thromboxane signaling.
| ID | Symbol | Entrez gene name | Location | Type(s) |
| 1367629_at | COX7A2 | cytochrome c oxidase subunit VIIa polypeptide 2 (liver) | Cytoplasm | enzyme |
| 1380500_s_at | FKBP2 | FK506 binding protein 2, 13 kDa | Cytoplasm | enzyme |
| 1370829_at | FNTB | farnesyltransferase, CAAX box, beta | Cytoplasm | enzyme |
| 1375913_at | GALNT2 | UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 2 (GalNAc-T2) | Cytoplasm | enzyme |
| 1373675_at | GLRX2 | glutaredoxin 2 | Cytoplasm | enzyme |
| 1386982_at | MGAT2 | mannosyl (alpha-1,6-)-glycoprotein beta-1,2-N-acetylglucosaminyltransferase | Cytoplasm | enzyme |
| 1389288_at | NDUFA2 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex, 2, 8 kDa | Cytoplasm | enzyme |
| 1370012_at | PTGIS | prostaglandin I2 (prostacyclin) synthase | Cytoplasm | enzyme |
| 1389655_at | PTRH2 | peptidyl-tRNA hydrolase 2 | Cytoplasm | enzyme |
| 1376066_at | RND3 | Rho family GTPase 3 | Cytoplasm | enzyme |
| 1367668_a_at | SCD2 | stearoyl-Coenzyme A desaturase 2 | Cytoplasm | enzyme |
| 1386392_at | ANAPC10 | anaphase promoting complex subunit 10 | Nucleus | enzyme |
| 1397508_at | DDX18 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 18 | Nucleus | enzyme |
| 1371449_at | PIN1 | peptidylprolyl cis/trans isomerase, NIMA-interacting 1 | Nucleus | enzyme |
| 1372725_at | PLSCR2 | phospholipid scramblase 2 | Nucleus | enzyme |
| 1398899_at | POLR2C | polymerase (RNA) II (DNA directed) polypeptide C, 33 kDa | Nucleus | enzyme |
| 1377338_at | RAD1 | RAD1 homolog (S. pombe) | Nucleus | enzyme |
| 1372476_at | FADS3 | fatty acid desaturase 3 | Plasma Membrane | enzyme |
| 1399111_at | CYB561D2 | cytochrome b-561 domain containing 2 | unknown | enzyme |
| 1370075_at | DHFR | dihydrofolate reductase | unknown | enzyme |
| 1376314_at | UBE2Q2 | ubiquitin-conjugating enzyme E2Q family member 2 | unknown | enzyme |
| 1367631_at | CTGF | connective tissue growth factor | Extracellular Space | growth factor |
| 1368470_at | GGH | gamma-glutamyl hydrolase (conjugase, folylpolygammaglutamyl hydrolase) | Cytoplasm | peptidase |
| 1382385_at | PSMC6 | proteasome (prosome, macropain) 26 S subunit, ATPase, 6 | Nucleus | peptidase |
| 1378679_at | USP25 | ubiquitin specific peptidase 25 | unknown | peptidase |
| 1372685_at | CDKN3 | cyclin-dependent kinase inhibitor 3 | Nucleus | phosphatase |
| 1368917_at | NUDT1 | nudix (nucleoside diphosphate linked moiety X)-type motif 1 | Extracellular Space | phosphatase |
| 1386065_at | ANKRD57 | ankyrin repeat domain 57 | Nucleus | transcription regulator |
| 1384742_at | ATRX | alpha thalassemia/mental retardation syndrome X-linked (RAD54 homolog, S. cerevisiae) | Nucleus | transcription regulator |
| 1380558_at | DLX3 | distal-less homeobox 3 | Nucleus | transcription regulator |
| 1379969_at | FOXJ2 | forkhead box J2 | Nucleus | transcription regulator |
| 1383377_at | GABPA | GA binding protein transcription factor, alpha subunit 60 kDa | Nucleus | transcription regulator |
| 1377858_at | PRDM2 | PR domain containing 2, with ZNF domain | Nucleus | transcription regulator |
| 1391212_at | TCEAL1 | transcription elongation factor A (SII)-like 1 | Nucleus | transcription regulator |
| 1376197_at | TCF7 | transcription factor 7 (T-cell specific, HMG-box) | Nucleus | transcription regulator |
| 1394591_at | ZNF207 | zinc finger protein 207 | Nucleus | transcription regulator |
| 1379967_at | ZNF367 | zinc finger protein 367 | Nucleus | transcription regulator |
| 1367713_at | EIF2S1 | eukaryotic translation initiation factor 2, subunit 1 alpha, 35 kDa | Cytoplasm | translation regulator |
| 1375135_at | GCN1L1 | GCN1 general control of amino-acid synthesis 1-like 1 (yeast) | Cytoplasm | translation regulator |
| 1392888_at | GPC4 | glypican 4 | Plasma Membrane | transmembrane receptor |
| 1388416_at | LRP1 | low density lipoprotein-related protein 1 (alpha-2-macroglobulin receptor) | Plasma Membrane | transmembrane receptor |
| 1368869_at | AKAP12 | A kinase (PRKA) anchor protein 12 | Cytoplasm | transporter |
| 1398554_at | ATP6V0B | ATPase, H+ transporting, lysosomal 21 kDa, V0 subunit b | Cytoplasm | transporter |
| 1379730_at | ATP6V1H | ATPase, H+ transporting, lysosomal 50/57 kDa, V1 subunit H | Cytoplasm | transporter |
| 1368977_a_at | FXC1 | fracture callus 1 homolog (rat) | Cytoplasm | transporter |
| 1388796_at | GOSR1 | golgi SNAP receptor complex member 1 | Cytoplasm | transporter |
| 1370296_at | SCP2 | sterol carrier protein 2 | Cytoplasm | transporter |
| 1388519_at | SEC61B | Sec61 beta subunit | Cytoplasm | transporter |
| 1397740_at | SFXN1 | sideroflexin 1 | Cytoplasm | transporter |
| 1372834_at | VPS4B | vacuolar protein sorting 4 homolog B (S. cerevisiae) | Cytoplasm | transporter |
| 1370934_at | NUP153 | nucleoporin 153 kDa | Nucleus | transporter |
| 1382643_at | SNX16 | sorting nexin 16 | unknown | transporter |
| 1384339_s_at | CSNK2A1 | casein kinase 2, alpha1 polypeptide | Cytoplasm | kinase |
| 1378282_at | CSNK2A2 | casein kinase 2, alpha prime polypeptide | Cytoplasm | kinase |
| 1379433_at | PIK3C2A | phosphoinositide-3-kinase, class 2, alpha polypeptide | Cytoplasm | kinase |
| 1377832_at | PLK4 | polo-like kinase 4 (Drosophila) | Cytoplasm | kinase |
| 1382707_at | RPS6KA3 | ribosomal protein S6 kinase, 90 kDa, polypeptide 3 | Cytoplasm | kinase |
| 1383926_at | BUB1B | budding uninhibited by benzimidazoles 1 homolog beta (yeast) | Nucleus | kinase |
| 1389166_at | CIB2 | calcium and integrin binding family member 2 | unknown | kinase |
| 1380682_at | MEX3B | mex-3 homolog B (C. elegans) | unknown | kinase |
| 1389967_at | ARL6IP1 | ADP-ribosylation factor-like 6 interacting protein 1 | Cytoplasm | other |
| 1369588_a_at | ATPIF1 | ATPase inhibitory factor 1 | Cytoplasm | other |
| 1374449_at | CDCA3 | cell division cycle associated 3 | Cytoplasm | other |
| 1375186_at | DPH3 | DPH3, KTI11 homolog (S. cerevisiae) | Cytoplasm | other |
| 1388850_at | HSP90AA1 | heat shock protein 90 kDa alpha (cytosolic), class A member 1 | Cytoplasm | other |
| 1388851_at | HSPA9 | heat shock 70 kDa protein 9 (mortalin) | Cytoplasm | other |
| 1372389_at | IER2 | immediate early response 2 | Cytoplasm | other |
| 1392065_at | KIF18A | kinesin family member 18A | Cytoplasm | other |
| 1391063_at | KIF23 | kinesin family member 23 | Cytoplasm | other |
| 1392901_at | LRRC1 | leucine rich repeat containing 1 | Cytoplasm | other |
| 1371649_at | MRPS24 | mitochondrial ribosomal protein S24 | Cytoplasm | other |
| 1377779_at | PDCL3 | phosducin-like 3 | Cytoplasm | other |
| 1392983_at | PSMD12 | proteasome (prosome, macropain) 26 S subunit, non-ATPase, 12 | Cytoplasm | other |
| 1387064_at | PXMP3 | peroxisomal membrane protein 3, 35 kDa | Cytoplasm | other |
| 1384089_at | RABGEF1 | RAB guanine nucleotide exchange factor (GEF) 1 | Cytoplasm | other |
| 1384551_at | RANBP6 | RAN binding protein 6 | Cytoplasm | other |
| 1392232_at | RPS13 | ribosomal protein S13 | Cytoplasm | other |
| 1388705_at | SELM | selenoprotein M | Cytoplasm | other |
| 1390767_at | SSR1 | signal sequence receptor, alpha | Cytoplasm | other |
| 1368041_at | SYNJ2BP | synaptojanin 2 binding protein | Cytoplasm | other |
| 1383568_at | TUBE1 | tubulin, epsilon 1 | Cytoplasm | other |
| 1389533_at | FBLN2 | fibulin 2 | Extracellular Space | other |
| 1367912_at | LTBP1 | latent transforming growth factor beta binding protein 1 | Extracellular Space | other |
| 1384264_at | MYH14 | myosin, heavy chain 14, non-muscle | Extracellular Space | other |
| 1371873_at | ANP32E | acidic (leucine-rich) nuclear phosphoprotein 32 family, member E | Nucleus | other |
| 1374323_at | BCCIP | BRCA2 and CDKN1A interacting protein | Nucleus | other |
| 1371953_at | CCNG2 | cyclin G2 | Nucleus | other |
| 1389506_x_at | CDC20 | cell division cycle 20 homolog (S. cerevisiae) | Nucleus | other |
| 1383958_at | CDCA2 | cell division cycle associated 2 | Nucleus | other |
| 1374540_at | CDCA7 | cell division cycle associated 7 | Nucleus | other |
| 1388928_at | CFL2 | cofilin 2 (muscle) | Nucleus | other |
| 1377172_at | GPSM2 | G-protein signaling modulator 2 (AGS3-like, C. elegans) | Nucleus | other |
| 1390384_at | H2AFX | H2A histone family, member X | Nucleus | other |
| 1383292_at | INCENP | inner centromere protein antigens 135/155 kDa | Nucleus | other |
| 1374794_at | KIF15 | kinesin family member 15 | Nucleus | other |
| 1377689_at | KNTC1 | kinetochore associated 1 | Nucleus | other |
| 1374051_at | NCAPH2 | non-SMC condensin II complex, subunit H2 | Nucleus | other |
| 1393267_at | PSIP1 | PC4 and SFRS1 interacting protein 1 | Nucleus | other |
| 1383623_at | THYN1 | thymocyte nuclear protein 1 | Nucleus | other |
| 1389305_at | ANXA4 | annexin A4 | Plasma Membrane | other |
| 1389145_at | CDC42EP2 | CDC42 effector protein (Rho GTPase binding) 2 | Plasma Membrane | other |
| 1372300_at | DOK4 | docking protein 4 | Plasma Membrane | other |
| 1368255_at | NTM | neurotrimin | Plasma Membrane | other |
| 1370247_a_at | PMP22 | peripheral myelin protein 22 | Plasma Membrane | other |
| 1377089_a_at | TSPAN5 | tetraspanin 5 | Plasma Membrane | other |
Discussion
The pathogenesis of T. cruzi-induced cardiomyopathy and vasculopathy are not fully understood. Over the past decade there have been a number of microarray studies by our laboratory group and others examining the consequences of T. cruzi infection on murine heart,26–28 cultured cardiac myocytes,29 myoblasts,30 fibroblasts,31,32 HeLa cells33 and human coronary artery smooth muscle cells.34
In our previous microarray studies, we examined transcriptome changes in infected murine heart in C7BL/6 × 129sv (100 days) and CD-1,26,27 (a time course ranging from 30–180 days, encompassing both in the acute and chronic stages of infection) with the Brazil strain of T. cruzi. Among the genes that were upregulated in the previous studies and the current one includes secretary leukocyte protease inhibitor (SLPI) and Caspase-12. SLPI is an important modulator of inflammatory responses responsible for cardiac remodeling35,36 and was observed to be upregulated in the acute stage (six-fold increase), which waned as the infection evolved into the chronic stage.25 In the present study, we also observed overexpression of SLPI gene, however, we were unable to demonstrate this increase by immunoblotting (data not shown). Interestingly, both our group27 and Garg et al.37 observed that both in vitro and in vivo, among the most repressed genes includes those for oxidative phosphorylation complexes I and IV. In the current study, we also observed repressed cytochrome c oxidase VIIa and NADH dehydrogenase (ubiquinone-1-α subcomplex-1) genes.
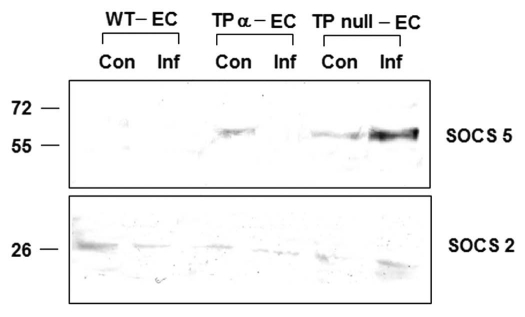
The current studies are an outgrowth of our interests in the role of eicosanoids in general and TXA2 signaling in particular in the pathogenesis of Chagas disease. Recently we identified the importance of SOCS (suppressor of cytokine signaling) proteins in T. cruzi infection with respect to arachidonic acid metabolic pathway in the host. We have observed that treatment of acutely infected mice (with Brazil strain) with aspirin (ASA) increased both mortality and parasitemia and this phenomenon was attributable to an increased expression of SOCS-2 in the spleens of infected, ASA-treated mice.38 There are eight SOCS proteins (1–7 and CIS) that negatively regulate cytokine signaling by a variety of mechanisms. In this analysis we found that SOCS-5 is upregulated in TP null ECs. The increase in SOCS-5 may explain the increased mortality found in TP null infected mice as in both the cases, reducing cytokine signaling has a profound effect in loss of innate immunity and hence host survival. SOCS-2 inhibits cytokine signaling by interleukin-6 (IL-6) and growth hormone while SOCS-5 binds to IL-4 receptor and phosphorylated insulin-like growth factor (IGF-I) and promotes in cellular growth and differentiation, and inhibits apoptosis via the Ras and PI3K signaling pathways. Interestingly, SOCS-2 protein was not overexpressed in TP null ECs as was observed in ASA-treated mice (unpublished data). However, there was an increased expression of SOCS-5 protein in the infected TP null ECs as compared to either TP null ECs or WT ECs (Fig. 3). This result indicates the importance of eicosanoid signaling in T. cruzi infection as potential immunemodulators. Inhibition of arachidonic acid metabolic pathway early in the infection, increases parasitemia and mortality by increasing SOCS-2 expression while on the other hand, failure to TP signaling shows similar phenotype by increasing SOCS-5 expression.
Figure 3.
Increased SOCS-5 protein expression was observed in infected TP null EC as compared to WT EC and TPα EC by immunoblotting. However, SOCS-2 levels remained unchanged in TP null environment.
Although T. cruzi is known to produce PGH2, PGF2α and TXA2, we do not known whether the parasite possess a receptor for these eicosanoids. A deeper understanding of the mechanism of parasitic eicosanoid signaling may provide us clues to the differences between host response in the acute and chronic infection.
Materials and Methods
Parasites.
The Tulahuen strain of T. cruzi was maintained in A/J mice (Jackson Laboratories, Bar Harbor, ME). They were maintained in L6E9 myoblast cultures as previously.39
Materials.
Tissue culture reagents were purchased from Invitrogen (Carlsbad, CA). Plasticware was purchased from Costar (Cambridge, MA). The TP receptor agonist IBOP was obtained from Cayman Chemicals (128719-90-4).
Isolation of primary rat fat pad endothelial cells (RFPECs).
Primary endothelial cells (EC) were isolated from the epididymal fat pad of normal male Sprague Dawley rats as previously described.21 Reconstitution of TP-null RFPEC with the plasmids containing the human TPα coding sequence was performed as previously described,21 using antibiotic selection (150 µg/mL G418) to identify transfected cells. ECs were maintained in humidified incubator at 37°C and 5% CO2 in DMEM high glucose supplemented with 10% FBS and 100 µg/mL penicillin-streptomycin.
Infection of cells.
RFPECs (WT-EC, TP null EC and TPα EC) were infected with trypomastigotes at a multiplicity of infection of ∼2:1 for 2, 18 and 48 h and harvested as previously described.34 To visualize intracellular parasites cells were fixed in methanol and stained with Giemsa.
Genechip reaction.
The infected and the control uninfected cells were washed three times in PBS (pH 7.2) and total RNA were prepared using the TRIZOL (Invitrogen, 15596026) method. The purified RNA was quantitated in Nanodrop (Thermo Fisher Scientific, Waltham, MA) and used for GeneChip analysis. cDNA was synthesized using GeneChip Expression 3′ Amplification one-cycle cDNA synthesis kit (Affymetrix, 900431) using 5 µg of total RNA and T7 oligo (dT) primer. To monitor target labeling, a set of poly-A RNA controls were spiked into the total RNA using GeneChip Eukaryotic Poly-A RNA control kit (Affymetrix, 900433). The double stranded cDNAs were cleaned with the GeneChip sample clean up module (Affymetrix, 900371). Biotin labeled antisense cRNAs were generated by in vitro transcription of cDNA using T7RNA polymerase and biotynylated ribonucleatide analogs using GeneChip Expression 3′ Amplification reagents for IVT labeling (Affymetrix, 900449). The biotinylated cRNAs were further cleaned up using the GeneChip sample clean up module and fragmented to 35–200 bases using the fragmentation buffer as recommended by the manufacturer in the module. Finally, the labeled fragmented cRNAs were hybridized to GeneChip Rat genome 230 2.0 Array (Affymetrix, 900506) for 16 hrs and stained with Streptavidin-Phycoerythrin. Biotynylated anti streptavidin antibody for 1.5 hrs and scanned in GeneChip Scanner 3000, according to the manufacturer's protocol. Hybridization, washing, staining and scanning was performed in the Affymetrix Facility at Albert Einstein College of Medicine according to manufacturer's protocol.
Data analysis.
We analyzed gene expression using Gene Sifter (Geospiza, Inc., Seattle, WA). Briefly, all Affymetrix CHP files were uploaded in Gene Sifter and analysis of the data performed in either of the two following ways. When comparisons of either the conditions or time points were done, analysis was performed using pair wise tool, where the data were normalized with mean and statistical significance determined using t-test. When comparisons were done through all the conditions and all time points, we normalized the data with mean and statistical significance determined using one-way ANOVA. We used Ingenuity Pathways Analysis to classify genes according to their function.
Immunofluorescence.
WT-EC, TP null EC and TPα-EC were grown on coverslip for overnight. Cells were washed in TBS (pH 7.4), fixed in 1% paraformaldehyde (EMS, 15710) for 10 mins and blocked in 10% goat serum (Santa Cruz Biotechnology, sc-2043) in TBS containing 1% Triton X-100 (TBST) for 30 mins. The cells were immunostained with 2 µg/ml anti human TP antibody (Cayman Chemicals, 10004452) for one hour, washed three times in TBST and stained with Alexa 488 conjugated goat anti rabbit secondary antibody (Molecular probes, A11008) and DAPI (Molecular probes, D3571) for one hour in dark. Finally the cells were washed in TBST for three times and observed under an Olympus 1X71 Microscope with 60x oil immersion objective. Immunofluorescence images were captured with a CoolSnap HQ cooled charge-coupled device camera (Roper Scientific, Trenton, NJ) and Cy2 excitation and emission filters using Metamorph software (Molecular Devices, Sunnyvale, CA). Exposure times (100 ms) and brightness adjustments (image normalization) were kept constant for images from different cell types and only secondary antibody negative control.
Immunoblotting.
Whole cell lysates (30 µg protein per lane) were separated by SDS-PAGE under reducing conditions and transferred onto nitrocellulose membrane (Whatman, Dassel, Germany). Immunoblotting was performed using antibodies against human TP (Cayman Chemicals, 10004452), phospho ERK (Cell signaling Technology, 9101S), SOCS-2 (Santa Cruz Biotechnology, sc-9022), SOCS-5 (abcam, 3695) and Caspase 12 (Santa Cruz Biotechnology, sc-5627). Primary antibodies were used at a dilution of 1:500 and anti-rabbit AP-conjugated secondary antibodies (dilution of 1:5,000). For detection of equal loading (as a control), gels were used in parallel and probed with monoclonal β-actin antibody at a dilution of 1:1,000 and HRP-conjugated secondary antibodies at a dilution of 1:5,000.
The bound primary antibodies were visualized by ECL chemiluminescence (Amersham Biosciences, Buckinghamshire, UK) when HRP-conjugated secondary antibodies were used or by the BCIP/NBT color detection system (Promega, Madison, WI) for AP-conjugated secondary antibodies. For these experiments, a representative gel is shown and a Student's t-test was performed and significance of difference was determined as p < 0.05.
Acknowledgements
This work was supported by Scientist Development Grant from the National affiliate of the American Heart Association (SDG 0735252N to S.M.), NIH grant (AI-076248 to H.B.T.). We acknowledge the assistance Vickie Braunstein for cell culture and maintenance.
Abbreviations
- AA
arachidonic acid
- BFT
blood form trypomastigotes
- MT
metacyclic trypomastigotes
- PGF2α
prostaglandin F2α
- PGH2
prostaglandin H2
- TXA2
thromboxane A2
- TP
TXA2 receptor
- TXA2S
TXA2 synthase
- RFP-EC
rat fat pad endothelial cells
- SOCS
suppressor of cytokine signaling
- ERK
extracellular signal-regulated kinase
- IBOP
[1S-1α,2α(Z),3β(1E,3S*),4α]-7-[3-[3-hydroxy-4-(4-iodophenoxy)-1-butenyl]-7-oxabicyclo[2.2.1]hept-2-yl]-5-heptenoic acid
- SLPI
secretary leukocyte protease inhibitor
- ASA
aspirin
References
- 1.Haeggström JZ, Rinaldo-Matthis A, Wheelock CE, Wetterholm A. Advances in eicosanoid research, novel therapeutic implications. Biochem Biophys Res Commun. 2010;396:135–139. doi: 10.1016/j.bbrc.2010.03.140. [DOI] [PubMed] [Google Scholar]
- 2.Noverr MC, Erb-Downward JR, Huffnagle GB. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin Microbiol Rev. 2003;16:517–533. doi: 10.1128/CMR.16.3.517-533.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belley A, Chadee K. Eicosanoid production by parasites: from pathogenesis to immunomodulation. Parasitol Today. 1995;11:327–334. doi: 10.1016/0169-4758(95)80185-5. [DOI] [PubMed] [Google Scholar]
- 4.Kilunga Kubata B, Eguchi N, Urade Y, Yamashita K, Mitamura T, Tai K, et al. Plasmodium falciparum produces prostaglandins that are pyrogenic, somnogenic and immunosuppressive substances in humans. J Exp Med. 1998;88:1197–1202. doi: 10.1084/jem.188.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubata BK, Duszenko M, Kabututu Z, Rawer M, Szallies A, Fujimori K, et al. Identification of a novel prostaglandin F2α synthase in Trypanosoma brucei. J Exp Med. 2000;192:1327–1338. doi: 10.1084/jem.192.9.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu LX, Weller PF. Arachidonic acid metabolism in filaria parasites. Exp Parasitol. 1990;71:496–501. doi: 10.1016/0014-4894(90)90076-o. [DOI] [PubMed] [Google Scholar]
- 7.Tanowitz HB, Kirchhoff V, Simon D, Morris SA, Weiss LM, Wittner M. Chagas' disease. Clin Microbiol Rev. 1992;5:400–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantarovich F, Vazquez M, Garcia WD, Abbud Filho M, Herrera C, Villegas Hernandez A. Special infections in organ transplantation in South America. Transplant Proc. 1992;24:1902–1908. [PubMed] [Google Scholar]
- 9.Leiby DA, Herron RM, Jr, Read EJ, Lenes BA, Stumpf RF. Trypanosoma cruzi in Los Angeles and Miami blood donors: impact of evolving donor demographics on seroprevalence and implications for transfusion transmission. Transfusion. 2002;42:549–555. doi: 10.1046/j.1537-2995.2002.00077.x. [DOI] [PubMed] [Google Scholar]
- 10.Cantarovich F, Vazquez M, Garcia WD, Abbud Filho M, Herrera C, Villegas Hernandez A. Special infections in organ transplantation in South America. Transplant Proc. 1992;24:1902–1908. [PubMed] [Google Scholar]
- 11.Pereira KS, Schmidt FL, Barbosa RL, Guaraldo AM, Franco RM, Dias VL, et al. Transmission of Chagas disease (American trypanosomiasis) by food. Adv Food Nutr Res. 2010;59:63–85. doi: 10.1016/S1043-4526(10)59003-X. [DOI] [PubMed] [Google Scholar]
- 12.Vaidian AK, Weiss LM, Tanowitz HB. Chagas' disease and AIDS. Kinetoplastid Biol Dis. 2004;3:2. doi: 10.1186/1475-9292-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira D, Cortez M, Atayde VD, Yoshida N. Actin cytoskeleton-dependent and -independent host cell invasion by Trypanosoma cruzi is mediated by distinct parasite surface molecules. Infect Immun. 2006;74:5522–5528. doi: 10.1128/IAI.00518-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang H, Chan J, Wittner M, Jelicks LA, Morris SA, Factor SM, et al. Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J Mol Cell Cardiol. 1999;31:75–88. doi: 10.1006/jmcc.1998.0848. [DOI] [PubMed] [Google Scholar]
- 15.Teixeira MM, Gazzinelli RT, Silva JS. Chemokines, inflammation and Trypanosoma cruzi infection. Trends Parasitol. 2002;18:262–265. doi: 10.1016/s1471-4922(02)02283-3. [DOI] [PubMed] [Google Scholar]
- 16.Petkova SB, Huang H, Factor SM, Pestell RG, Bouzahzah B, Jelicks LA, et al. The role of endothelin in the pathogenesis of Chagas' disease. Int J Parasitol. 2001;31:499–511. doi: 10.1016/s0020-7519(01)00168-0. [DOI] [PubMed] [Google Scholar]
- 17.Petkova SB, Tanowitz HB, Magazine HI, Factor SM, Chan J, Pestell RJ, et al. Myocardial expression of endothelin-1 in murine Trypanosoma cruzi infection. Cardiovasc Pathol. 2000;9:257–265. doi: 10.1016/s1054-8807(00)00045-4. [DOI] [PubMed] [Google Scholar]
- 18.Huang H, Calderon TM, Berman JW, Braunstein VL, Weiss LM, Wittner M, et al. Infection of endothelial cells with Trypanosoma cruzi activates NFκB and induces vascular adhesion molecule expression. Infect Immun. 1999;67:5434–5440. doi: 10.1128/iai.67.10.5434-5440.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durand JL, Mukherjee S, Commodari F, De Souza AP, Zhao D, Machado FS, et al. Role of NO synthase in the development of Trypanosoma cruzi-induced cardiomyopathy in mice. Am J Trop Med Hyg. 2009;80:782–787. [PMC free article] [PubMed] [Google Scholar]
- 20.Nakahata N. Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol Ther. 2008;118:18–35. doi: 10.1016/j.pharmthera.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Tanowitz HB, Burns ER, Sinha AK, Kahn NN, Morris SA, Factor SM, et al. Enhanced platelet adherence and aggregation in Chagas' disease: a potential pathogenic mechanism for cardiomyopathy. Am J Trop Med Hyg. 1990;43:274–281. doi: 10.4269/ajtmh.1990.43.274. [DOI] [PubMed] [Google Scholar]
- 22.Ashton AW, Mukherjee S, Nagajyothi FN, Huang H, Braunstein VL, Desruisseaux MS, et al. Thromboxane A2 is a key regulator of pathogenesis during Trypanosoma cruzi infection. J Exp Med. 2007;204:929–940. doi: 10.1084/jem.20062432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashton AW, Ware GM, Kaul DK, Ware JA. Inhibition of tumor necrosis factor α-mediated NFκB activation and leukocyte adhesion, with enhanced endothelial apoptosis, by G protein-linked receptor (TP) ligands. J Biol Chem. 2003;278:11858–11866. doi: 10.1074/jbc.M210766200. [DOI] [PubMed] [Google Scholar]
- 24.Gao Y, Yokota R, Tang S, Ashton AW, Ware JA. Reversal of angiogenesis in vitro, induction of apoptosis and inhibition of AKT phosphorylation in endothelial cells by thromboxane A2. Circ Res. 2000;87:739–745. doi: 10.1161/01.res.87.9.739. [DOI] [PubMed] [Google Scholar]
- 25.Bao Y, Weiss LM, Ma YF, Lisanti MP, Tanowitz HB, Das BC, et al. Molecular cloning and characterization of mitogen-activated protein kinase 2 in Trypanosoma cruzi. Cell Cycle. 2010;9:2888–2896. doi: 10.4161/cc.9.14.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukherjee S, Nagajyothi F, Mukhopadhyay A, Machado FS, Belbin TJ, Campos de Carvalho A, et al. Alterations in myocardial gene expression associated with experimental Trypanosoma cruzi infection. Genomics. 2008;91:423–432. doi: 10.1016/j.ygeno.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukherjee S, Belbin TJ, Spray DC, Iacobas DA, Weiss LM, Kitsis RN, et al. Microarray analysis of changes in gene expression in a murine model of chronic chagasic cardiomyopathy. Parasitol Res. 2003;91:187–196. doi: 10.1007/s00436-003-0937-z. [DOI] [PubMed] [Google Scholar]
- 28.Soares MB, de Lima RS, Rocha LL, Vasconcelos JF, Rogatto SR, dos Santos RR, et al. Gene expression changes associated with myocarditis and fibrosis in hearts of mice with chronic chagasic cardiomyopathy. J Infect Dis. 2010;202:416–426. doi: 10.1086/653481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldenberg RC, Iacobas DA, Iacobas S, Rocha LL, da Silva de Azevedo Fortes F, Vairo L, et al. Transcriptomic alterations in Trypanosoma cruzi-infected cardiac myocytes. Microbes Infect. 2009;11:1140–1149. doi: 10.1016/j.micinf.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adesse D, Iacobas DA, Iacobas S, Garzoni LR, Meirelles Mde N, Tanowitz HB, et al. Transcriptomic signatures of alterations in a myoblast cell line infected with four distinct strains of Trypanosoma cruzi. Am J Trop Med Hyg. 2010;82:846–854. doi: 10.4269/ajtmh.2010.09-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imai K, Mimori T, Kawai M, Koga H. Microarray analysis of host gene-expression during intracellular nests formation of Trypanosoma cruzi amastigotes. Microbiol Immunol. 2005;49:623–631. doi: 10.1111/j.1348-0421.2005.tb03654.x. [DOI] [PubMed] [Google Scholar]
- 32.Moore-Lai D, Rowland E. Microarray data demonstrate that Trypanosoma cruzi downregulates the expression of apoptotic genes in BALB/c fibroblasts. J Parasitol. 2004;90:893–895. doi: 10.1645/GE-146R. [DOI] [PubMed] [Google Scholar]
- 33.Shigihara T, Hashimoto M, Shindo N, Aoki T. Transcriptome profile of Trypanosoma cruzi-infected cells: simultaneous up and downregulation of proliferation inhibitors and promoters. Parasitol Res. 2008;102:715–722. doi: 10.1007/s00436-007-0819-x. [DOI] [PubMed] [Google Scholar]
- 34.Nde PN, Johnson CA, Pratap S, Cardenas TC, Kleshchenko YY, Furtak VA, et al. Gene network analysis during early infection of human coronary artery smooth muscle cells by Trypanosoma cruzi and Its gp83 ligand. Chem Biodivers. 2010;7:1051–1064. doi: 10.1002/cbdv.200900320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geraghty P, Greene CM, O'Mahony M, O'Neill SJ, Taggart CC, McElvaney NG. Secretory leucocyte protease inhibitor inhibits interferon-gamma-induced cathepsin S expression. J Biol Chem. 2007;282:33389–33395. doi: 10.1074/jbc.M706884200. [DOI] [PubMed] [Google Scholar]
- 36.Doumas S, Kolokotronis A, Stefanopoulos P. Anti-inflammatory and antimicrobial roles of secretory leukocyte protease inhibitor. Infect Immun. 2005;73:1271–1274. doi: 10.1128/IAI.73.3.1271-1274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garg N, Popov VL, Papaconstantinou J. Profiling gene transcription reveals a deficiency of mitochondrial oxidative phosphorylation in Trypanosoma cruzi-infected murine hearts: implications in chagasic myocarditis development. Biochim Biophys Acta. 2003;1638:106–120. doi: 10.1016/s0925-4439(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 38.Mukherjee S, Machado FS, Huang H, Oz HS, Jelicks LA, Prado CM, et al. Aspirin treatment of mice infected with Trypanosoma cruzi and implications for the pathogenesis of Chagas disease. PLoS One. 2011;6:e16959. doi: 10.1371/journal.pone.0016959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukherjee S, Huang H, Petkova SB, Albanese C, Pestell RG, Braunstein VL, et al. Trypanosoma cruzi infection activates extracellular signal-regulated kinase in cultured endothelial and smooth muscle cells. Infect Immun. 2004;72:5274–5282. doi: 10.1128/IAI.72.9.5274-5282.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]