Abstract
Photons in action: A five-step synthesis of a functionalized tricyclo[9.3.0.0]tetradecane ring system of the marine natural product bielschowskysin has been achieved through an exquisite intramolecular [2+2] photocycloaddition reaction of a macrocyclic precursor.
Keywords: [2+2] photocycloaddition, antimalarial agent, cytotoxic agent, natural product synthesis
Bielschowskysin (1, Figure 1a) is a recently discovered marine natural product possessing a novel molecular architecture and impressive biological properties.[1] Isolated from the Caribbean gorgonian octocoral Pseudopterogorgia kallos and characterized through spectroscopic and x-ray crystallographic analysis, bielschowskysin boasts an unprecedented tricyclo[9.3.0.0] tetradecane ring system (see 2, Figure 1a) decorated with a high degree of oxygenation and eleven stereogenic centers.[1] Its biological properties include antimalarial activity against Plasmodium falciparum (IC50 = 10 µg/mL) and potent and selective cytotoxicity against EKVX nonsmall lung cancer cells (GI50 < 10 nM), and CAKI-1 renal cancer cells (GI50 = 510 nM).[1]
Figure 1.
(a) Molecular structures of bielschowskysin (1) and its tricyclo[9.3.0.0]tetradecane ring system (2) and (b) functionalized tricyclo[9.3.0.0]tetradecane ring system (3), and its postulated macrocyclic precursor (4).
Due to its natural scarcity, the full biological profile of bielschowskysin remains unexplored, and its absolute configuration is unknown. This description leaves little doubt, if any, of the worthiness of bielschowskysin as a synthetic target, for an endeavor toward its total synthesis may provide an opportunity to develop new synthetic strategies and technologies, render the compound readily available for biological investigations, allow structure activity relationship studies, and reveal its absolute stereochemistry. In this communication, we report our preliminary forays toward the total synthesis of bielschowskysin that culminated in the construction of a functionalized tricyclo[9.3.0.0]tetradecane ring system 3 (Figure 1b) of the molecule and its 2-epi-enantiomer (2-epi-ent-3). The reported chemistry is notable for its cascade sequences, brevity, and efficiency.[2]
In contemplating a plan for the total synthesis of bielschowskysin (1), an intramolecular [2+2] photocycloaddition[3] similar to a postulated biosynthetic scheme[4] comes to mind. To test this hypothesis, we designed a functionalized tricyclo[9.3.0.0]tetradecane ring system 3 (Figure 1b) and its proposed macrocyclic precursor 4 (Figure 1b), the latter generated through a retro [2+2] photocycloaddition reaction as shown.
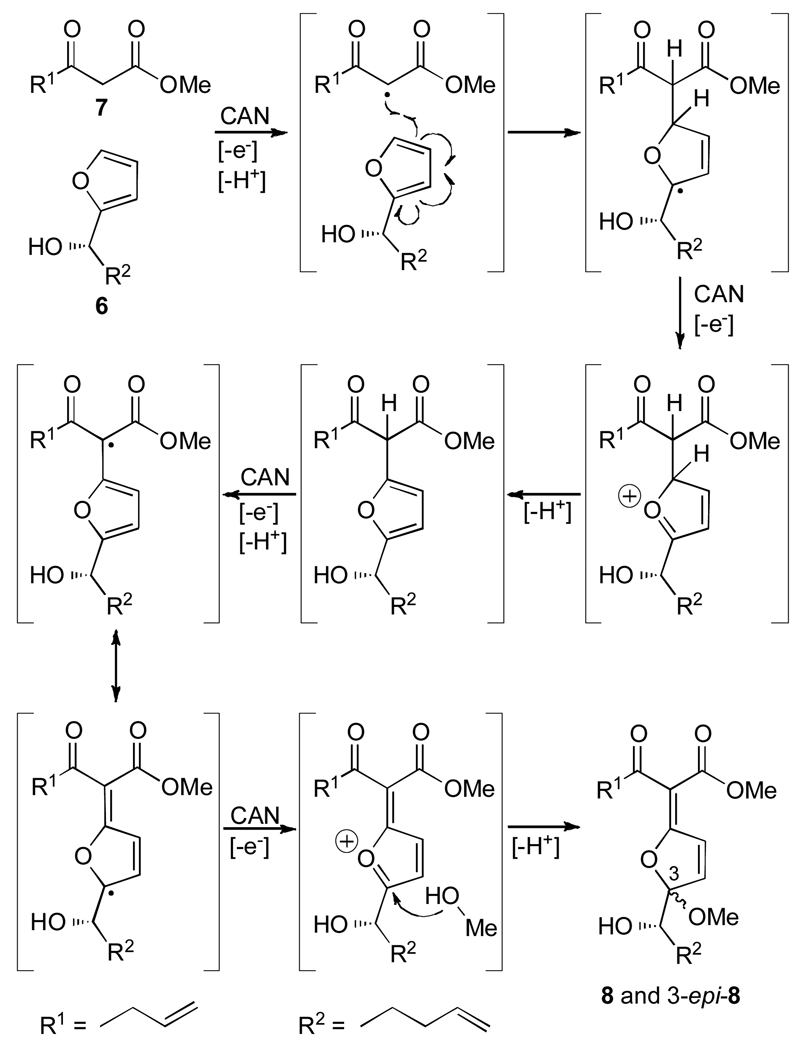
Scheme 1 summarizes the short and enantioselective route to macrocyclic [2+2] photocycloaddition precursor 4 and its 2-epi-enantiomer 2-epi-ent-4. Thus, acyl furan 5 was enantioselectively reduced with Noyori’s catalyst A[5] to alcohol 6 (84% yield, 92% ee) and combined with β-ketoester 7 in the presence of CAN in MeOH to afford conjugated ketoester 8 as a mixture of α- and β-methoxy epimers 8 and 3-epi-8 (58% yield, ca. 1:1 ratio), which were chromatographically separated.[6] The geometry of the enol ether bond in these products was tentatively assigned at this stage on the basis of NMR spectroscopy, and subsequently proven by x-ray crystallographic analysis of a subsequent derivative (vide infra). Rapidly tailoring structures 8 and 3-epi-8, this highly productive and efficient process is presumed to proceed through the mechanism depicted in Scheme 2. The importance of exo enol ether–cyclic ketals (as in 8 and 3-epi-8) in natural products chemistry and the challenge of their construction have been recently noted by Pattenden through a series of elegant studies.[7] This CAN-mediated coupling process provides a rapid entry into the exo enol ether structural motif as exemplified in Scheme 1.
Scheme 1.
Construction of macrocyclic precursor 4. Reagents and conditions: a) Noyori cat. A (0.025 equiv), HCOONa (10 equiv), nBu4Cl (0.3 equiv), CH2Cl2:H2O (1:1), 25 °C, 15 h; then Noyori cat. A (0.01 equiv), 12 h, 84%, 92% ee; b) 6 (1.0 equiv), 7 (1.2 equiv), CAN (4.0 equiv), MeOH, 0 °C, 58% (ca. 1:1 dr); c) Grubbs I cat. (0.3 equiv for 8, 0.25 equiv for 3-epi-8), CH2Cl2, reflux, 4 h, 90% for 9 (trans:cis ca. 15:1), 71% for 3-epi-9 (trans:cis ca. 3:1); d) NaBH4 (6.0 equiv), THF:H2O (2:1), 0 °C, 30 min, 83% for 4, 63% for 2-epi-ent-4. CAN = ammonium cerium nitrate, THF = tetrahydrofuran, MeOH = methanol.
Scheme 2.
Proposed mechanism for the coupling reaction of 6 and 7 under the influence of CAN.
Treatment of 8 and 3-epi-8 separately with Grubbs I catalyst[8] resulted in ring closure to furnish the corresponding macrocyclic hydroxy ketones with the newly generated double bond predominantly trans (9, 90% yield, trans:cis ca. 15:1; 3-epi-9, 71% yield, trans:cis ca. 3:1, chromatographically separated) (see Scheme 1). X-Ray crystallographic analysis of the 3,5-dinitrobenzoate derivatives of 9 [(+)-9-DNB, mp = 196 – 197 °C (Et2O:MeOH:CH2Cl2, 1:1:1), see Figure 2][9] and 3-epi-9 [(+)-3-epi-9-DNB, mp = 215 – 217 °C (Et2O:MeOH:CH2Cl2, 1:1:2), see Figure 3][10] confirmed their structures, including the geometry of the enol ether double bond, and that of their precursors (i.e. 8 and 3-epi-8).
Figure 2.
X-Ray derived ORTEP representation of 3,5-dinitrobenzoate (+)-9-DNB (thermal ellipsoids at 30% probability); gray C, green H, blue N, red O.
Figure 3.
X-Ray derived ORTEP representation of 3,5-dinitrobenzoate (+)-(3-epi-9-DNB) (thermal ellipsoids at 30% probability); gray C, green H, blue N, red O.
Attempts to induce photolytically the desired intramolecular [2+2] cycloaddition with 9 or 3-epi-9 proved unproductive with starting material persisting and partial enol ether isomerization. This failure was presumably due to the conjugation of the enol ether olefinic bond with the two carbonyl groups and/or the presence of the sp2 carbon of the macrocycle carbonyl group, resulting in a prohibitive strain in the expected polycyclic product. This carbonyl group was, therefore, reduced within each of the two methoxy epimers (hydroxy ketones 9 and 3-epi-9) with NaBH4 to produce, in each case, a single diastereoisomeric diol (4, 83% yield, and 2-epi-ent-4, 63% yield) (see Scheme 1). The configuration of the newly formed hydroxy group in 4 and 2-epi-ent-4 was tentatively assigned at this stage on the basis of manual molecular models that indicated a favored external hydride delivery by the reducing agent (see 9a and 3-epi-9a, Scheme 1), and was later confirmed through x-ray crystallographic analysis of downstream intermediates (vide infra). Note that an internal hydroxyl directed hydride delivery in this reduction (i.e. 9 and 3-epi-9 to 4 and 2-epi-ent-4, respectively) may also explain this stereochemical outcome as seen by manual molecular modelling.
Pleasantly, irradiation of a benzene or chloroform solution of macrocycle 4 with UV light (450 Watt Hanovia, >254 nm or Rayonet, >254 nm) for 48 h resulted in the formation of tetracycle 3 (90% yield) as a single diastereoisomer. The structure of compound 3 was unambiguously proven by x-ray crystallographic analysis of its racemic bis-3,5-dinitrobenzoate ester [(±)-3-bDNB, mp = 203 – 205 °C (Et2O:CH2Cl2, slow diffusion), see Figure 4].[11] The [2+2] photocycloaddition of 4 is presumed to proceed through a radical mechanism and the intermediacy of its transient enol ether geometrical isomer 4a, as shown in Scheme 3. Thus, photoexcitation of the chromophore of substrate 4 under the influence of light may furnish diradical 10, which apparently undergoes C–C bond rotation to afford the less strained diradical 10a. The latter species suffers, according to the rule of fives,[12] facile and regioselective ring closure to afford cyclopentane intermediate diradical 11a, which then undergoes a second ring closure, facilitated by the newly acquired rigidity and proximity, to afford the observed tetracyclic product 3. Indeed, NMR spectroscopic monitoring of the reaction reveals initial formation of the enol ether isomer of 4, compound 4a, which dissipates with time to the final product, presumably through radical species 10a and 11a. Interestingly, isolated geometrical isomer 4a only partially reverts back to the original geometrical isomer 4 under the irradiation conditions, suggesting the equilibrium between 10 and 10a lies far to the right, favoring 10a. The alternative pathway of diradical 10 to 12 through 11 is apparently shut, most likely due to unfavorable geometrical constraints imposed by strain in the macrocycle.
Figure 4.
X-Ray derived ORTEP representation of (±)-3-bDNB (thermal ellipsoids at 30% probability); gray C, green H, blue N, red O.
Scheme 3.
Photocycloaddition of macrocyclic precursor 4 and 2-epi-ent-4. Conditions: e) C6H6 or CHCl3, ambient temperature, hν (Hanovia 450 Watt, >254 nm or Rayonet, >254 nm), 48 h for 3, 90%; 16 h for 2-epi-ent-3, 88%.
Macrocycle 2-epi-ent-4 was also converted to a [9.3.0.0] tetracyclic core structure (2-epi-ent-3, 88% yield, Scheme 3) employing the same photoirradiation conditions (see Supplementary Information). Although no olefin isomerization of 2-epi-ent-4 (as with 4) was observed by NMR spectroscopic or TLC analysis, the transient existence of the corresponding geometrical isomer may be inferred by examination of the structure of the product 2-epi-ent-3. The structure of 2-epi-ent-3 was confirmed by x-ray crystallographic analysis of its racemic bis-4-methoxybenzoate derivative [(±)-(2-epi-ent-3-bMB), mp = 207 – 209 °C (MeOH:CH2Cl2, 5:1), see Figure 5].[13] Racemic bis-dinitrobenzoate (±)-3-bDNB and racemic bis-methoxybenzoate (±)-(2-epi-ent-3-bMB) were prepared from racemic alcohol (±)-6 (obtained by Grignard addition into furfural). Interestingly, their enantiomeric counterparts obtained from enantiopure 3 and 2-epi-ent-3, respectively, did not yield suitable crystals for x-ray crystallographic analysis. It should be noted that the asymmetric creation of the first stereocenter of the molecule in step one (i.e. 6) allows the construction of a complex structure (i.e. 3) containing the [9.3.0.0] ring framework, seven stereogenic centers, and five functional groups from two simple starting materials (i.e. acylfuran olefin 5 and β-ketoester olefin 7).
Figure 5.
X-Ray derived ORTEP representation of (±)-(2-epi-ent-3-bMB) (thermal ellipsoids at 30% probability); gray C, green H, red O.
The described chemistry provides a five-step enantioselective entry into the [9.3.0.0] novel carbocyclic core structure of bielschowskysin (from simple building blocks 6 and 7), bearing substantial functionality that may endow it with the potential to serve as a scaffold for building further molecular complexity and possibly imparting biological activity. Such studies may prove useful in the total synthesis of the natural product and its mimics for biological investigations.
Supplementary Material
Footnotes
We thank Drs. D.-H. Huang and R. Chadha for spectroscopic and X-ray crystallographic assistance, respectively, and Dr. G. Siuzdak and Ms. Doris Tan for mass spectrometric assistance. Financial support for this work was provided by A*STAR, Singapore, the Skaggs Institute for Chemical Research, the National Institutes of Health (grant AI 055475-09) and the National Science Foundation (Graduate Research Fellowship to C.R.H.H.).
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
K. C. Nicolaou, Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA) and Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093 (USA).
Vikrant A. Adsool, Chemical Synthesis Laboratory@Biopolis, Institute of Chemical and Engineering Sciences (ICES), Agency for Science, Technology, and Research (A*STAR), 11 Biopolis Way, The Helios Block, # 03–08, Singapore 138667 (Singapore)
Christopher R. H. Hale, Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037 (USA) and Department of Chemistry and Biochemistry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093 (USA)
References
- 1.Marrero J, Rodríguez AD, Baran P, Raptis RG, Sánchez JA, Ortega-Barria E, Capson TL. Org. Lett. 2004;6:1661. doi: 10.1021/ol049495d. [DOI] [PubMed] [Google Scholar]
- 2. For previous model studies toward bielschowskysin, see: Doroh B, Sulikowski GA. Org. Lett. 2006;8:903. doi: 10.1021/ol0530225. Miao R, Gramani SG, Lear MJ. Tetrahedron Lett. 2009;50:1731..
- 3.Bach T, Hehn JP. Angew. Chem. 2011;123:1032. doi: 10.1002/anie.201002845. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2011;50:1000. [Google Scholar]
- 4.Roethle PA, Trauner D. Nat. Prod. Rep. 2008;25:298. doi: 10.1039/b705660p. [DOI] [PubMed] [Google Scholar]
- 5.Fujii A, Hashiguchi S, Uematsu N, Ikariya T, Noyori R. J. Am. Chem. Soc. 1996;118:2521. [Google Scholar]; b) Ferrie L, Reymond S, Capdevielle P, Cossy J. Org. Lett. 2007;9:2461. doi: 10.1021/ol070670a. [DOI] [PubMed] [Google Scholar]
- 6. For a precedent of this reaction involving a simple furan and a malonate diester, see: Weinstock LM, Corley E, Abramson NL, King AO, Karady S. Heterocycles. 1988;27:2627. Simone J-M, Loiseau F, Carcache D, Bobal P, Jeanneret-Gris J, Neier R. Monatshefte für Chemie. 2007;138:131. For related electrochemically induced reactions, see: Wu H, Moeller KD. Org. Lett. 2007;9:4599. doi: 10.1021/ol702118n. Mihelcic J, Moeller KD. J. Am. Chem. Soc. 2003;125:36. doi: 10.1021/ja029064v. Mihelcic J, Moeller KD. J. Am. Chem. Soc. 2004;126:9106. doi: 10.1021/ja048085h. and references cited therein.
- 7.Li Y, Pattenden G, Rogers J. Tetrahedron Lett. 2010;51:1280. [Google Scholar]
- 8.Schwab P, France MB, Ziller JW, Grubbs RH. Angew. Chem. 1995;107:2179. [Google Scholar]; Angew. Chem. Int. Ed. Engl. 1995;34:2039. [Google Scholar]; b) Schwab P, Grubbs RH, Ziller JW. J. Am. Chem. Soc. 1996;118:100. [Google Scholar]
- 9.CCDC 813043 [(+)-9-DNB] contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 10.CCDC 813041 [(+)-(3-epi-9-DNB)] contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 11.CCDC 813042 [(±)-3-bDNB] contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 12.a) Liu RSH, Hammond GS. J. Am. Chem. Soc. 1967;89:4936. [Google Scholar]; b) Srinivasan R, Carlough KH. J. Am. Chem. Soc. 1967;89:4932. [Google Scholar]
- 13.CCDC 814212 [(±)-(2-epi-ent-3-bMB)] contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.