Abstract
Administration of estrogen replacement therapy (ERT) decreases the incidence of breast cancer, as shown in a double-blind, placebo-controlled randomized trial of the Women’s Health Initiative (WHI) in 10,739 postmenopausal women with a prior hysterectomy. Though paradoxical because estrogen is recognized to stimulate breast cancer growth, laboratory data support a mechanism of estrogen-induced apoptosis under the correct environmental circumstances. Long-term antiestrogen treatment or estrogen deprivation causes the eventual development and evolution of antihormone resistance. Cell populations emerge with a vulnerability, as estrogen is no longer a survival signal but is an apoptotic trigger. The antitumor effect of ERT in estrogen-deprived postmenopausal women is consistent with laboratory models.
Introduction
It is widely held that estrogen can be carcinogenic in breast tissue (1) and is the “fuel for the fire” to stimulate the growth of estrogen receptor (ER)–positive breast cancer cells (2). This knowledge, supported by an enormous body of laboratory data, provides the conceptual basis for the successful development of antihormonal strategies to treat breast cancer (3). Selective ER modulators (SERMs), e.g. tamoxifen, block estrogen-stimulated tumor growth at the ER, and aromatase inhibitors prevent peripheral estrogen synthesis in postmenopausal patients, thereby creating estrogen deprivation to stop tumor growth (3). The successful treatment strategy for breast cancer with SERMs was subsequently translated into reducing the risk of breast cancer in high-risk women. SERMs are available to reduce the incidence of breast cancer in pre- and postmenopausal (tamoxifen) or postmenopausal (raloxifene) women (4–6). As predicted by the mechanism of action of SERMs as anticancer agents, only ER-positive breast cancer is reduced. In practice, preventing estrogen action prevents breast tumor initiation and growth. Paradoxically, the recent analysis of estrogen replacement therapy (ERT) in the Women’s Health Initiative (WHI) double-blind, placebo-controlled randomized trial in 10,739 postmenopausal women with a prior hysterectomy (ages 50–79; ref. 7) actually showed a decrease in invasive breast cancer, which was sustained for 5 years after ERT was stopped. This result seems to run counter to the perceived wisdom of the role of estrogen in breast carcinogenesis, was significant in women of all ages, and was similar in every age group.
When the WHI was initiated in 1993, their present clinical result of a reduction in breast cancer was unanticipated (7) but is consistent nevertheless with parallel laboratory studies completed over the past 20 years. Estrogen-induced apoptosis is a plausible molecular mechanism to support an antitumor action of physiologic estrogen (8). The key to our understanding of estrogen-induced apoptosis is the finding that breast cancer cell populations adapt to estrogen deprivation, but these populations are dynamic, and resistance to estrogen deprivation evolves over time (5 years). This evolution of resistance to estrogen deprivation causes a reconfiguration of cellular survival pathways, which in turn exposes a vulnerability of breast cancer cell survival. Physiologic estrogen causes apoptosis and does not act as a survival signal (8).
We will weigh the laboratory and clinical evidence to support the proposition that physiologic estrogen can cause apoptosis in breast cancer cells following long-term estrogen deprivation. Our objective is to make a case based on scientific observations to support our proposition that nascent breast cancer cells could have the same apoptotic response to ERT after estrogen deprivation caused by menopause. We will present the evidence in chronological order (Box 1).
Box 1. Cumulative evidence to support low dose estrogen-induced apoptosis in long term estrogen deprived nascent breast cancer.
Historical use of estrogens to treat breast cancer.
Physiologic estrogen as an antitumor agent in SERM resistant breast cancer models in vivo.
Estrogen-induced apoptosis in estrogen deprived ER-positive cell lines in vitro.
A current evaluation of estrogen to treat acquired antihormone resistance in metastatic breast cancer.
The extrapolation of the concept that physiologic estrogen kills breast cancer cells to adjuvant antihormone therapy.
Evidence from the Historical Use of Estrogens to Treat Metastatic Breast Cancer
The application of high-dose estrogen therapy for the treatment of metastatic breast cancer was the first use of a chemical therapy to treat any cancer successfully (9). Estrogen therapy became the standard of care to treat metastatic breast cancer in postmenopausal patients until the introduction of tamoxifen (late 1970s in the U.S.), a nonsteroidal antiestrogen (10). Tamoxifen became the “gold standard” for the treatment of ER-positive (estrogen stimulated) breast cancer for the next 20 years. Estrogen was all but abandoned as a treatment option, but Ingle et al. completed a provocative trial of tamoxifen versus the synthetic estrogen diethylstilbestrol (DES; high-dose) in metastatic breast cancer (11). Responses were equivalent with fewer side effects with tamoxifen, but a re-analysis years later demonstrated that survival was significantly improved with DES (12).
Towards the end of his distinguished career, Professor Sir Alexander Haddow FRS reflected (during the inaugural Karnofsky Memorial Lecture; ref. (13) on the remarkable responses noted with estrogen in some tumors, often when treatment was more than a decade past menopause: “The extraordinary extent of tumour regression observed in perhaps 1% of post-menopausal cases (with oestrogen) has always been regarded as of major theoretical importance, and it is a matter for some disappointment that so much of the underlying mechanisms continues to elude us.”
Although laboratory research to address Haddow’s estrogen paradox essentially ceased for the next 20 years, at least one animal model transplanted with a human breast tumor replicated the antitumor action of high-dose estrogen therapy for breast cancer (14, 15). The question could have been addressed. However, the breakthrough in our understanding of a mechanism for estrogen-induced apoptosis came with a study of continuous long-term SERM treatment in transplantable SERM-resistant breast cancer in athymic mice. As often happens in science, a discovery in an apparently unrelated area becomes the required breakthrough to create transparency in nature.
Physiologic Estrogen Is an Antitumor Agent in SERM-Resistant Breast Cancer In Vivo
In the 1980s, the first athymic animal models of tamoxifen-induced antihormone resistance were reported, but the acquired resistance surfaced within 2 years as tamoxifen-stimulated growth (2). This replicated the use of tamoxifen in the treatment of metastatic ER-positive breast cancer but did not explain the astonishing success of 5 years of adjuvant tamoxifen therapy in reducing recurrences by 50% and mortality by 30%. Most important, the gains obtained during therapy are maintained (and mortality further reduced) for the next 15 years. We were missing a vital clue about the evolution of antihormone resistance in micrometastatic breast cancer.
Five years of re-transplantation of tumors into tamoxifen-treated athymic mice revealed a vulnerability in breast cancer that would subsequently be exploited in clinical trial. Physiologic estradiol does not promote tumor growth, but small tumors undergo rapid and complete regression (16). It was suggested (16) that following the cessation of adjuvant tamoxifen, a woman’s own estrogen would exert an antitumor action and enhance survivorship. Further studies (17) subsequently demonstrated that following tumor regression with physiologic estradiol, any remaining tumor that re-grows in the estrogen environment is again responsive to tamoxifen as an antitumor agent. Continuing studies demonstrated that the principle of physiological estrogen therapy causing apoptosis in SERM-resistant disease was also true for raloxifene (18, 19). These data provided a scientific rationale for subsequent clinical studies.
Estrogen Induces Apoptosis in Estrogen-deprived ER-positive Breast Cancer Cell Lines
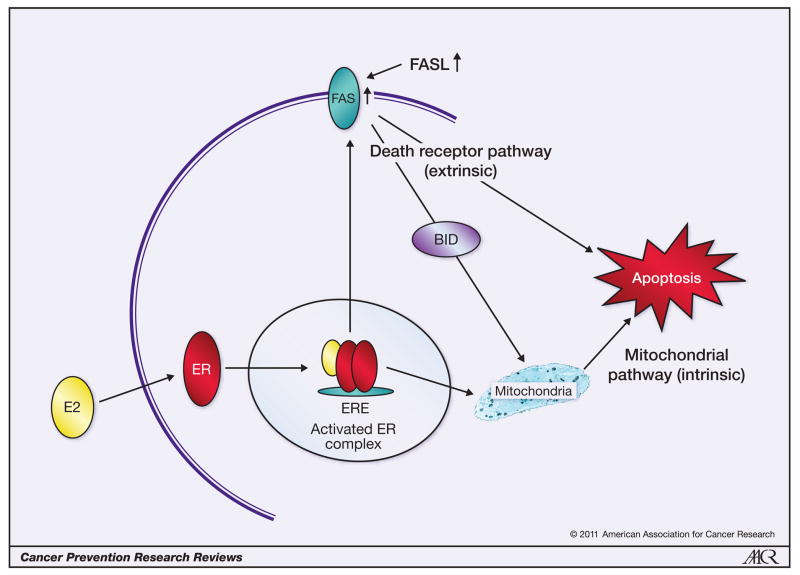
Song and co-workers (20) first showed in cell culture that high concentrations of estrogen could induce cellular apoptosis directly through a FAS/FASL pathway. However, the discovery that physiologic concentrations of estradiol could induce apoptosis (21) in both cell culture and animal models was the advance pertinent to the clinical observation that ERT reduces the incidence of breast cancer in postmenopausal women (7). This is now a consistent experimental observation with new knowledge emerging about the molecular mechanisms of estrogen-induced apoptosis. Figure 1 summarizes much of the current data on molecular mechanisms of estrogen-induced apoptosis, the topic of a forthcoming mini-review in Cancer Prevention Research later this year.
Figure 1.
The two main pathways involved in estrogen-induced apoptosis regulation. This apoptosis can be triggered either through the extrinsic death-receptor pathway with an increase in Fas ligand (20) or Fas (27) or via the intrinsic pathway of mitochondrial disruption and release of cytochrome C (28). E2, estradiol (the most potent estrogen in women); ERE, estrogen response element; BID, Bcl-2–interacting domain.
Despite the significant body of laboratory data to support the proposition that physiologic estrogen can induce apoptosis in long-term estrogen-deprived breast cancer cells, only the translation to patients tests the veracity of the experimental approach as a conversation with nature and a general principle.
Current Evaluation of Estrogen to Treat Acquired Antihormone Resistance in Metastatic Breast Cancer
Lonning and co-workers (22) studied the efficacy of high dose of DES on the responsiveness of metastatic breast cancer following exhaustive treatment with antihormone therapies (tamoxifen, aromatase inhibitors, etcetera). A remarkable 4 of 32 patients had complete responses (22), and one patient, who was treated for 5 years, had no recurrence of her disease 6 years after stopping DES (23). The question, however, is whether physiologic estrogen has efficacy as an antitumor agent in the appropriately prepared estrogen-deprived breast tumor. Ellis and co-workers (24) addressed this question and found an equivalent clinical benefit for high (30 mg daily) and low (6 mg daily) dose of estradiol in metastatic breast cancer patients who had failed aromatase inhibitor therapy, i.e., long-term estrogen deprivation. Their clinical advance was that low-dose estrogen was as efficacious as high-dose estrogen for antitumor therapy in breast cancer (for the appropriate tumor that had been estrogen deprived), but there were fewer side effects with low-dose therapy. The target, estrogen-deprived breast cancer, is vulnerable to physiologic estrogen.
The Extrapolation of the Concept that Physiologic Estrogen Kills Breast Cancer to Adjuvant Antihormone Therapy
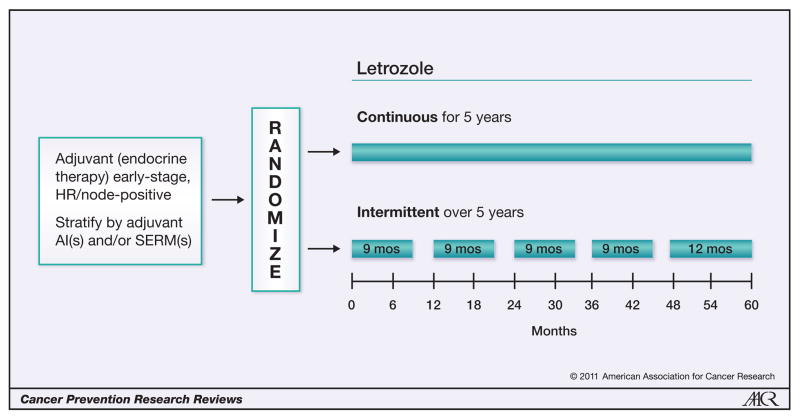
The result from the WHI Trial of ERT in hysterectomized women (7), which showed a sustained reduction in the incidence of breast cancer, provides additional evidence that the strategy to decipher the mechanism of physiologic estrogen to induce apoptosis (8, 25, 26) has significance for both treatment and prevention. Indeed, the idea that a woman’s own estrogen was responsible for enhanced survivorship by causing apoptosis of the appropriately prepared and vulnerable micrometastases (16) followed the completion of long-term adjuvant tamoxifen therapy and now is incorporated into the Study of Letrozole Extension (SOLE) Trial. This extended adjuvant antihormone treatment study (Figure 2) is addressing the question of whether regular drug holidays will decrease recurrence rates compared with continuous therapy. For initial safety reasons, a women’s own estrogen during the drug holiday is hypothesized to be adequate as an apoptotic trigger because rigorous prior antihormone therapy will have selected vulnerable cell populations as the waiting target. Subsequent trials may have to use ERT for a few weeks to trigger apoptosis.
Figure 2.
Schema for the Study of Letrozole Extension (SOLE; IBCSG 35-07) conducted by the International Breast Cancer Study Group (IBCSG). Upon completing 4 to 6 years of prior adjuvant endocrine therapy with a SERM(s) and/or aromatase inhibitor(s) (AI), patients were randomly assigned to continuous or intermittent letrozole (3-month drug holidays per year) for 5 years. The rationale for this approach was that the woman’s own estrogen in the intermittent arm would trigger apoptosis in long-term estrogen-deprived breast cancer and reduce recurrence rates.
We have presented an integrated approach to support the proposition that ERT could induce apoptosis and reduce the incidence of breast cancer. The important issue for the decision of breast cancer cells to survive or die in response to estradiol depends entirely on the cell populations present in an estrogenized environment or following estrogen deprivation. Based on laboratory data, the decision is survival or death, respectively. The role of estrogen deprivation, either pharmacologic with antihormones or physiologic with menopause, is to select populations of cells that can survive without physiologic estrogen. These cells choose to die through a natural process when re-exposed to pharmacologic or physiologic estrogen. The genetics are the same, but different epigenetic events based on the well-established property of cancer cells to be able to adapt to any environment and survive remains true. As the WHI study of ERT shows (7), physiologic estrogen has delivered what the scientific database would now predict.
Acknowledgments
Grant Support
This work was supported by the following grants of VC Jordan: Department of Defense Breast Program under Award number BC050277 Center of Excellence (this interdisciplinary research grant supports research into estrogen-induced apoptosis in breast cancer); subcontract under the SU2C (AACR) grant number SU2C-AACR-DT0409); the Susan G Komen for the Cure Foundation under Award number SAC100009 and the Lombardi Comprehensive Cancer Center Support Grant (CCSG) Core Grant NIH P30 CA051008 from the National Cancer Institute.
Footnotes
Disclosure of Potential Conflicts of Interest
No conflicts of interest were reported.
Authors’ Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The views and opinions of the author(s) do not reflect those of the US Army or the Department of Defense.
References
- 1.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–82. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 2.Levenson AS, Jordan VC. MCF-7: the first hormone-responsive breast cancer cell line. Cancer Res. 1997;57:3071–8. [PubMed] [Google Scholar]
- 3.Jordan VC. A century of deciphering the control mechanisms of sex steroid action in breast and prostate cancer: the origins of targeted therapy and chemoprevention. Cancer Res. 2009;69:1243–54. doi: 10.1158/0008-5472.CAN-09-0029. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–62. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 5.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 6.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Croix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305:1305–14. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan VC. The 38th David A. Karnofsky lecture: the paradoxical actions of estrogen in breast cancer--survival or death? J Clin Oncol. 2008;26:3073–82. doi: 10.1200/JCO.2008.17.5190. [DOI] [PubMed] [Google Scholar]
- 9.Haddow A, Watkinson JM, Paterson E, Koller PC. Influence of Synthetic Oestrogens on Advanced Malignant Disease. Br Med J. 1944;2:393–8. doi: 10.1136/bmj.2.4368.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lerner LJ, Jordan VC. Development of antiestrogens and their use in breast cancer: eighth Cain memorial award lecture. Cancer Res. 1990;50:4177–89. [PubMed] [Google Scholar]
- 11.Ingle JN, Ahmann DL, Green SJ, Edmonson JH, Bisel HF, Kvols LK, et al. Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N Engl J Med. 1981;304:16–21. doi: 10.1056/NEJM198101013040104. [DOI] [PubMed] [Google Scholar]
- 12.Peethambaram PP, Ingle JN, Suman VJ, Hartmann LC, Loprinzi CL. Randomized trial of diethylstilbestrol vs. tamoxifen in postmenopausal women with metastatic breast cancer. An updated analysis. Breast Cancer Res Treat. 1999;54:117–22. doi: 10.1023/a:1006185805079. [DOI] [PubMed] [Google Scholar]
- 13.Haddow A. David A. Karnofsky memorial lecture. Thoughts on chemical therapy. Cancer. 1970;26:737–54. doi: 10.1002/1097-0142(197010)26:4<737::aid-cncr2820260402>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Brunner N, Spang-Thomsen M, Vindelov L, Nielsen A. Effect of 17 beta-oestradiol on growth curves and flow cytometric DNA distribution of two human breast carcinomas grown in nude mice. Br J Cancer. 1983;47:641–7. doi: 10.1038/bjc.1983.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunner N, Bastert GB, Poulsen HS, Spang-Thomsen M, Engelholm SA, Vindelov L, et al. Characterization of the T61 human breast carcinoma established in nude mice. Eur J Cancer Clin Oncol. 1985;21:833–43. doi: 10.1016/0277-5379(85)90223-8. [DOI] [PubMed] [Google Scholar]
- 16.Wolf DM, Jordan VC. A laboratory model to explain the survival advantage observed in patients taking adjuvant tamoxifen therapy. Recent Results Cancer Res. 1993;127:23–33. doi: 10.1007/978-3-642-84745-5_4. [DOI] [PubMed] [Google Scholar]
- 17.Yao K, Lee ES, Bentrem DJ, England G, Schafer JI, O’Regan RM, et al. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:2028–36. [PubMed] [Google Scholar]
- 18.Liu H, Lee ES, Gajdos C, Pearce ST, Chen B, Osipo C, et al. Apoptotic action of 17beta-estradiol in raloxifene-resistant MCF-7 cells in vitro and in vivo. J Natl Cancer Inst. 2003;95:1586–97. doi: 10.1093/jnci/djg080. [DOI] [PubMed] [Google Scholar]
- 19.Balaburski GM, Dardes RC, Johnson M, Haddad B, Zhu F, Ross EA, et al. Raloxifene-stimulated experimental breast cancer with the paradoxical actions of estrogen to promote or prevent tumor growth: a unifying concept in anti-hormone resistance. Int J Oncol. 2010;37:387–98. doi: 10.3892/ijo_00000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song RX, Mor G, Naftolin F, McPherson RA, Song J, Zhang Z, et al. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17beta-estradiol. J Natl Cancer Inst. 2001;93:1714–23. doi: 10.1093/jnci/93.22.1714. [DOI] [PubMed] [Google Scholar]
- 21.Jordan VC, Liu H, Dardes R. Re: Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17 beta-estradiol and the two faces of Janus: sex steroids as mediators of both cell proliferation and cell death. J Natl Cancer Inst. 2002;94:1173–5. doi: 10.1093/jnci/94.15.1173. [DOI] [PubMed] [Google Scholar]
- 22.Lonning PE, Taylor PD, Anker G, Iddon J, Wie L, Jorgensen LM, et al. High-dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine therapy. Breast Cancer Res Treat. 2001;67:111–6. doi: 10.1023/a:1010619225209. [DOI] [PubMed] [Google Scholar]
- 23.Lonning PE. Additive endocrine therapy for advanced breast cancer - back to the future. Acta Oncol. 2009;48:1092–101. doi: 10.3109/02841860903117816. [DOI] [PubMed] [Google Scholar]
- 24.Ellis MJ, Gao F, Dehdashti F, Jeffe DB, Marcom PK, Carey LA, et al. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302:774–80. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maximov PY, Lewis-Wambi JS, Jordan VC. The Paradox of Oestradiol-Induced Breast Cancer Cell Growth and Apoptosis. Curr Signal Transduct Ther. 2009;4:88–102. doi: 10.2174/157436209788167484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis-Wambi JS, Jordan VC. Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit? Breast Cancer Res. 2009;11:206. doi: 10.1186/bcr2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osipo C, Gajdos C, Liu H, Chen B, Jordan VC. Paradoxical action of fulvestrant in estradiol-induced regression of tamoxifen-stimulated breast cancer. J Natl Cancer Inst. 2003;95:1597–608. doi: 10.1093/jnci/djg079. [DOI] [PubMed] [Google Scholar]
- 28.Lewis JS, Meeke K, Osipo C, Ross EA, Kidawi N, Li T, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97:1746–59. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]