Abstract
The extents to which small intestinal (SI) cytochrome P450 (P450) enzymes control the bioavailability of oral drugs are not well defined, particularly for drugs that are substrates for both P450 and the P-glycoprotein (P-gp). In this study, we have determined the role of SI P450 in the clearance of orally administered lovastatin (LVS), an anti-hypercholesterolemia drug, using an intestinal epithelium (IE)-specific P450 reductase knockout (IE-Cpr-null) mouse model. In the IE-Cpr-null mouse, which has little P450 activities in the IE, the oral bioavailability of LVS was substantially higher than that in wild-type (WT) mice (15 and 5%, respectively). In control experiments, the clearance rates were not different between the two strains, either for intraperitoneally dosed LVS, which bypasses SI metabolism, or for orally administered pravastatin, which is known to be poorly metabolized by P450. Thus, our results demonstrate a predominant role of SI P450 enzymes in the first-pass clearance of oral LVS. The absence of IE P450 activities in the IE-Cpr-null mice also facilitated the identification of the molecular targets for orally administered grapefruit juice (GFJ), which is known to inhibit LVS clearance in humans. We found that pretreatment of mice with oral GFJ enhanced the systemic exposure of LVS in WT, but not in IE-Cpr-null mice, a result suggesting that the main target of GFJ action in the small intestine is P450, but not P-gp.
Introduction
Cytochrome P450 (P450)-mediated drug metabolism in the small intestine could have a major impact on the bioavailability and, consequently, the therapeutic efficacy or toxicity of a given drug (Thummel et al., 1997; Lin et al., 1999; Suzuki and Sugiyama, 2000; Kaminsky and Zhang, 2003). Orally administered drugs are potentially subject to first-pass metabolism, initially in the small intestine, and then in the liver, before they reach systemic circulation. Knowledge of the relative organ contributions of liver and small intestine to the first-pass metabolism of a given drug is important, for improvements in drug bioavailability and for identification of sites of potential drug-drug interactions. Given the tissue differences between liver and small intestine in the expression of various P450 enzymes, other drug metabolism enzymes, and drug transporters (Suzuki and Sugiyama, 2000; Doherty and Charman, 2002; Ding and Kaminsky, 2003; Paine et al., 2006), the relative contributions of liver and small intestinal (SI) P450 enzymes to first-pass metabolism will likely vary for each drug.
It has been difficult to directly demonstrate the specific contributions of SI P450 enzymes to the first-pass clearance of orally administered drugs in vivo, until the recent generation of mouse models that have tissue-specific alterations of P450 activities in the intestine. These models include our intestinal epithelium (IE)-specific cytochrome P450 reductase (CPR) knockout mouse model (IE-Cpr-null), in which the activities of all microsomal P450s are suppressed in the intestinal epithelial cells (Zhang et al., 2009), and the Cyp3a(−/−)V model, in which human CYP3A4 is expressed specifically in IE cells of the Cyp3a-null mouse (van Herwaarden et al., 2007). Previously, we have used the IE-Cpr-null mouse to demonstrate the role of SI P450s in modulating the bioavailability of nifedipine (Zhang et al., 2009), a drug with high solubility and high permeability [Biopharmaceutics Classification System (BCS) class 1], and thus expected to be eliminated mainly through metabolism, rather than by the efflux transporter P-glycoprotein (P-gp) (Wu and Benet, 2005). In this study, we have further evaluated the role of SI P450 enzymes in the first-pass metabolism of lovastatin (LVS), as a representative of BCS class-2 drugs (with low solubility and high permeability), which are expected to be eliminated by both P450-mediated metabolism and efflux transport (Wu and Benet, 2005).
LVS belongs to the statin family of drugs that are widely used for the treatment of hypercholesterolemia (Hsu et al., 1995; Schachter, 2005). In a clinical environment, LVS is administered orally in its lactone form (as a prodrug), which is readily converted to the active, β-hydroxy acid form (LVA) in vivo. LVS has a very low oral bioavailability (∼5%) (Schachter, 2005). In the liver, LVS is mainly metabolized by CYP3A (Wang et al., 1991), and both LVS and LVA are CYP3A substrates (Ishigami et al., 2001). LVS and LVA are also substrates for P-gp; the latter has been suggested to influence the pharmacokinetic properties of various statins (Chen et al., 2005). However, the precise roles of SI P450 enzymes (including CYP3A), as well as P-gp, in the first-pass clearance of LVS had not been determined.
In the present study, we first confirmed the IE-specific suppression of LVS metabolism in the IE-Cpr-null mice. Then we compared, between IE-Cpr-null and wild-type (WT) mice, pharmacokinetic profiles and parameters for plasma LVA, after oral, intraperitoneal, or intravenous administration of LVS, to show the impact of the IE-specific loss of P450 activity toward LVS on the first-pass clearance and oral bioavailability of LVS. To further confirm the direct involvement of SI P450-mediated metabolism (rather than any unexpected changes resulting from the Cpr gene deletion and any associated genomic responses) in controlling LVS bioavailability, we further studied the clearance of pravastatin (PVS; as a negative control), which is known to be scarcely metabolized by P450 (e.g., Jacobsen et al., 1999a,b). PVS, a BCS class-3 drug (with high solubility, low permeability), is expected to be eliminated mainly through excretion into bile and urine without undergoing metabolism (Wu and Benet, 2005). In addition, by taking advantage of the absence of P450 activities in the small intestine of the IE-Cpr-null mice, we determined whether oral administration of grapefruit juice (GFJ), which is known to inhibit CYP3A-mediated metabolism of many drugs (Bailey et al., 1998), and to reduce LVS clearance in patients (Kantola et al., 1998), can further enhance LVS bioavailability in the IE-Cpr-null mice, through inhibition of P-gp. Our findings demonstrate the value of the IE-Cpr-null mouse model for identification of the specific roles of SI P450 enzymes in the first-pass clearance of BCS class-2 drugs.
Materials and Methods
Animals and Treatments.
Adult (2–4-month-old) female IE-Cpr-null (Zhang et al., 2009) mice and age-matched WT littermates were used. Animals were given food and water ad libitum. For LVS ((1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl}-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2S)-2-methylbutanoate; Cayman Chemical, Ann Arbor, MI) administration, mice were given a bolus dose (at 25 or 50 mg/kg) of LVS [dissolved in dimethyl sulfoxide/Tween 80/phosphate-buffered saline (1:2:7, v/v/v) at 2.5 or 5 mg/ml, respectively], either via oral gavage or through intraperitoneal injection, or they received intravenous injections of LVS (at 2.5 mg/kg; dissolved in the aforementioned vehicle at 0.25 mg/ml). For GFJ (Florida brand frozen juice concentrate; Wal-Mart) administration, GFJ (4×-strength juice, 20 ml/kg) was given via oral gavage 2 h before LVS (12.5 mg/kg) administration. For PVS ((3R,5R)-3,5-dihydroxy-7-((1R,2S,6S,8R, 8aR)-6-hydroxy-2-methyl-8-{[(2S)-2-methylbutanoyl]oxy}-1,2,6,7,8,8a-hexahydronaphthalen-1-yl)-heptanoic acid; Cayman Chemical) administration, mice were given PVS (dissolved in phosphate-buffered saline at 2.5 or 5 mg/ml) via intraperitoneal injection (25 mg/kg) or oral gavage (50 mg/kg). All animal studies were approved by the Institutional Animal Care and Use Committee of the Wadsworth Center.
Pharmacokinetic Analysis.
For mice (five to six in each group) treated with LVS or PVS via oral gavage or intraperitoneal injection, blood samples were collected from the tail vein at 0.25, 0.5, 1, 1.5, 2, and 4 h after drug administration; whereas, for mice treated with LVS intravenously, blood samples were obtained from the saphenous vein at 10 min, 0.5, 1, 1.5, and 2 h after dosing. LVA and PVS were extracted from plasma by solid-phase extraction. In brief, 10 μl of plasma was spiked with an internal standard, simvastatin [(Cayman Chemical) 40 ng/ml, in methanol], before dilution with 2 ml of deionized water; the mixture was vortex-mixed and then loaded onto a Waters Oasis HLB solid-phase extraction column (Waters, Milford, MA). The column was washed with 1 ml of H2O before the analytes were eluted with 250 μl of acetonitrile/H2O (8/2, v/v); 5 μl of the eluate was injected for liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis. Pharmacokinetic parameters were calculated from the plasma concentration-time data by a noncompartmental approach using WinNonlin software (version 5.1; Pharsight, Mountain View, CA). Statistical significance of differences between two groups for various parameters was analyzed using the Student's t test.
LC-MS/MS Analysis of LVA and PVS.
A LC-MS/MS system consisting of an Agilent 1200 Series high-performance liquid chromatography (Agilent Technologies, Santa Clara, CA) and an ABI 4000 Q-Trap mass spectrometer (Applied Biosystems, Foster City, CA), with a Waters X-Terra MS C18 column (100 × 3.0 mm i.d., 3.5 μm) was used. The mass spectrometer was set to the multiple-reaction monitoring mode and was operated with an electrospray ionization source. The method for the analysis of LVS is essentially the same as previous reported (Lodge et al., 2008). The solvent system comprised solvent A (0.05% formic acid) and solvent B (100% acetonitrile). A 4-min linear gradient from 55% B to 95% B was applied at a flow rate of 0.65 ml/min, followed by a 1-min isocratic elution at 95% B and then a 6-min wash at 55% B, before returning to the starting condition. LVS and simvastatin were monitored at m/z 423/303 and m/z 437/303, respectively, in positive ion mode.
For the analysis of PVS, the solvent system comprised solvent A (50 mM ammonium acetate) and solvent B (100% acetonitrile). A 4-min linear gradient from 25% B to 75% B was applied at a flow rate of 0.65 ml/min, followed by a 2-min isocratic elution at 75% B and then a 6-min wash at 25% B, before returning to the starting condition. PVS and simvastatin were monitored at m/z 423/321 and m/z 435/319, respectively, in negative ion mode (Jain et al., 2007). The MS instrumental parameters were the same as described previously (Lodge et al., 2008). Notably, PVS can rapidly isomerize to an isoform under acidic condition (Mulvana et al., 2000); consequently, two PVS peaks are detected when the drug was administered by oral dosing. The 3′α-hydroxy isomer of PVS was confirmed using a standard provided by Bristol-Myers Squibb (Wallingford, CT). The two peaks were combined for quantification.
LVA and PVS standard (4–5000 ng/ml), as well as the internal standard, were added to blank mouse plasma to construct the calibration curve. The recoveries of added standards in blank plasma were ∼85% at all concentrations tested.
Assays for In Vitro Metabolism of LVS.
Tissues from three to five mice were combined for each microsomal preparation. Epithelial cells from the small intestine were isolated, and microsomes were prepared as described previously (Zhang et al., 2003). Liver microsomes were prepared essentially as described previously (Fasco et al., 1993), but with use of protease inhibitors, as described for the preparation of SI microsomes (Zhang et al., 2003). Microsomes (0.1 mg) were incubated with LVS (100 μM) in a 200-μl reaction mixture containing 0.1 M potassium phosphate buffer, pH 7.4, 1.0 mM NADPH, and 3 mM MgCl2 for 30 min at 37°C. The reaction was initiated by the addition of NADPH, and it was terminated by the addition of 400 μl of acetonitrile to the reaction mixture. Control experiments were performed, in which NADPH was omitted. Simvastatin was added as the internal standard for monitoring extraction efficiency. After centrifugation at 1500g for 10 min, the organic layer was transferred to a new tube and spun at 1500g again. A 5-μl aliquot of the supernatant fraction was taken for LC-MS/MS analysis. For the detection of LVS metabolites, the same high-performance liquid chromatography column and solvent system as described above for LVS analysis were used; but a 6-min linear gradient from 30 to 90% B was applied at a flow rate of 0.65 ml/min, followed by a 1-min isocratic elution at 90% B and then a 6-min wash at 30% B, before returning to the starting condition. The two major metabolites of LVS, 6′β-hydroxy LVS and 6′-exomethylene LVS (Jacobsen et al., 1999a,b), were monitored at m/z 421 and m/z 403, respectively, in positive ion mode. Although metabolite standards were not available, the identities of the two metabolites were confirmed by detection of their unique UV spectra, as described in a previous report (Vyas et al., 1990). For quantitation, LVS was used as a surrogate for construction of a calibration curve (with simvastatin as the internal standard), and relative activities in different microsomal preparations were determined.
Results and Discussion
The Tissue-Specific Loss of CPR Led to a Substantial Reduction in the Rates of Microsomal LVS Metabolism in Enterocytes, but Not in Liver.
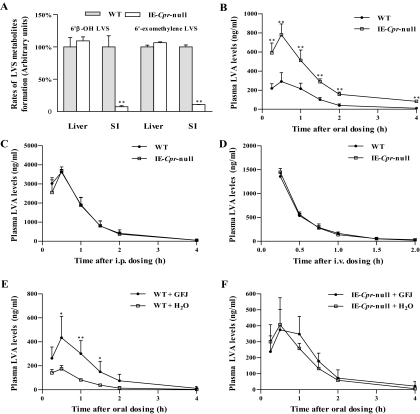
The impact of the abrogated intestinal epithelial CPR expression on intestinal and hepatic (as a control) microsomal P450 activities toward LVS was examined by comparing rates of in vitro metabolism between WT and IE-Cpr-null mice. The intestinal microsomal rates of formation of the two previously identified major LVS metabolites, 6′β-hydroxyLVS and 6′-exomethyleneLVS, were reduced by more than 90% in the IE-Cpr-null mice, compared with WT mice; whereas the hepatic microsomal rates of metabolites formation were similar between the two mouse strains (Fig. 1A). The extent of decrease in intestinal microsomal metabolic activity toward LVS was similar to the extent of reductions found previously for the metabolism of nifedipine and benzo(a)pyrene in the IE-Cpr-null mice (Zhang et al., 2009; Fang and Zhang, 2010). The residual activity detected in SI microsomes from the IE-Cpr-null mice can be explained by the presence of low levels of CPR (derived from nonenterocyte IE cells and contaminating submucosal cells) in the microsomal preparations (Zhang et al., 2009). The tissue-specific impact of the CPR loss on intestinal LVS metabolism confirms the validity of using the IE-Cpr-null mouse for studying the specific contributions of intestinal (versus liver) P450 enzymes to the first-pass clearance of LVS.
Fig. 1.
In vitro metabolism and in vivo clearance of LVS in IE-Cpr-null and WT mice. A, in vitro metabolism of LVS by SI and hepatic microsomes. Relative activities in the formation of LVS metabolites from LVS were determined for various microsomal preparations. Reaction mixtures contained phosphate buffer, pH 7.4, 100 μM LVS, and 0.5 mg/ml microsomal protein, in a final volume of 0.2 ml. Reactions were carried out at 37°C for 30 min, in the presence or absence of 1 mM NADPH. Each microsomal preparation was obtained from pooled tissues from two to three adult female mice; three microsomal preparations were analyzed for each group. The values reported are percentages of the rates determined in respective WT microsomes (means ± S.D., n = 3). **, P < 0.01, compared with WT mice (Student's t test). B–D, plasma levels of LVA in IE-Cpr-null and WT mice after a single oral, intraperitoneal, or intravenous dose of LVS. Adult female IE-Cpr-null and age-matched WT mice (5–6 mice per group) were given a single dose of LVS, via oral gavage at 25 mg/kg (B), through intraperitoneal injection at 25 mg/kg (C), or via intravenous injection at 2.5 mg/kg (D). Plasma samples were obtained at various times after dosing. The results shown are typical of two separate experiments. Values represent means ± S.D. (n = 5–6). **, P < 0.01, compared with WT mice (Student's t test). E and F, effects of pretreatment with GFJ on systemic bioavailability of oral LVS in IE-Cpr-null and WT mice. Adult female mice (5–6 mice per group) were given a single dose of 4× strength GFJ through oral gavage at 20 ml/kg, or they were given water (vehicle control), followed 2 h later by a single oral dose of LVS at 12.5 mg/kg. Plasma samples were obtained at various times after LVS dosing, for determination of LVA levels. Values shown represent means ± S.D. (n = 5–6). *, P < 0.05 and **, P < 0.01, compared with the corresponding vehicle-pretreated group (Student's t test).
Intestinal P450s Play an Important Role in the First-Pass Clearance of Orally Administered LVS.
The role of intestinal P450 enzymes in the clearance of orally administered LVS was assessed by comparing the pharmacokinetics of LVS between WT and IE-Cpr-null mice. The pharmacokinetic profiles and parameters are shown in Fig. 1, B to D, and in Table 1, respectively. Preliminary experiments (data not shown) indicated that there was no significant difference between male and female mice in LVS clearance; therefore, only female mice were used for the pharmacokinetic studies. Most notably, the mouse is known to differ substantially from humans for being able to rapidly hydrolyze LVS to produce LVA (Duggan et al., 1989); the LVS lactone is barely detected in mouse blood after LVS dosing, whereas LVA is the major circulating form of the drug (Lodge et al., 2008). Therefore, plasma LVA, rather than LVS, levels were determined for LVS pharmacokinetics studies in mice.
TABLE 1.
Pharmacokinetic parameters for orally or intravenously administered LVS in IE-Cpr-null and WT mice
Data from Fig. 1, B and D, were used for the determination of pharmacokinetic parameters. Adult female IE-Cpr-null and age-matched WT mice (5–6 per group) were given a single dose of LVS, via oral gavage, at 25 mg/kg, or via intravenous injection, at 2.5 mg/kg. Values represent means ± S.D. (n = 5–6).
| Route | Strain | Tmax | Cmax | t1/2 | AUC0-∞ | CL/F |
|---|---|---|---|---|---|---|
| min | μg/ml | min | min · μg/ml | ml/min | ||
| p.o. | WT | 27 ± 7 | 0.3 ± 0.1 | 50 ± 9 | 23.5 ± 5.1 | 23.0 ± 4.6 |
| IE-Cpr-null | 30 ± 0 | 0.8 ± 0.1a | 68 ± 9b | 76.4 ± 5.1a | 7.0 ± 0.4a | |
| i.v. | WT | 10 ± 0 | 1.3 ± 0.1 | 17 ± 4 | 51.5 ± 4.4 | 1.0 ± 0.7 |
| IE-Cpr-null | 10 ± 0 | 1.4 ± 0.1 | 19 ± 4 | 53.5 ± 3.6 | 1.0 ± 0.1 |
CL/F, apparent clearance.
P < 0.01 and
P < 0.05, compared to the corresponding WT group (Student's t test).
When administered orally at 25 mg/kg, LVS was cleared quickly in WT mice; LVA (or LVS) was no longer detected in the blood at 4 h after dosing. However, LVS clearance in IE-Cpr-null mice was much slower than in WT mice. IE-Cpr-null mice had significantly higher blood LVA levels at each time point after oral dosing (Fig. 1B). The AUC0-∞, Cmax, and t1/2 values were 3.2-, 2.7-, and 1.4-fold, respectively, greater, whereas the clearance rate was 3.3-fold lower, in IE-Cpr-null than in WT mice (Table 1). These results indicated that intestinal P450 enzymes played an important role in the clearance of orally administered LVS. The latter conclusion was further supported by the finding that, when LVS was administered by an intraperitoneal injection, there was no significant difference between IE-Cpr-null and WT mice in either pharmacokinetic profiles (Fig. 1C) or in any of the calculated pharmacokinetic parameters (data not shown).
To determine the impact of intestinal CPR loss on the bioavailability of orally administered LVS, we further performed pharmacokinetic studies for intravenously administered LVS (at 2.5 mg/kg) in the IE-Cpr-null and WT mice. As expected, there was no significant difference between IE-Cpr-null and WT mice either in pharmacokinetic profiles (Fig. 1D) or in any of the calculated pharmacokinetic parameters (Table 1). The bioavailability of oral LVS (Foral) was calculated to be 5% in WT mice, and it was increased to 15% in the IE-Cpr-null mice. These findings indicate that intestinal P450 enzymes have a significantly role in lowering the bioavailability of oral LVS in WT mice.
Intestinal P450s Have Little Effect on the Clearance of Orally Administered PVS.
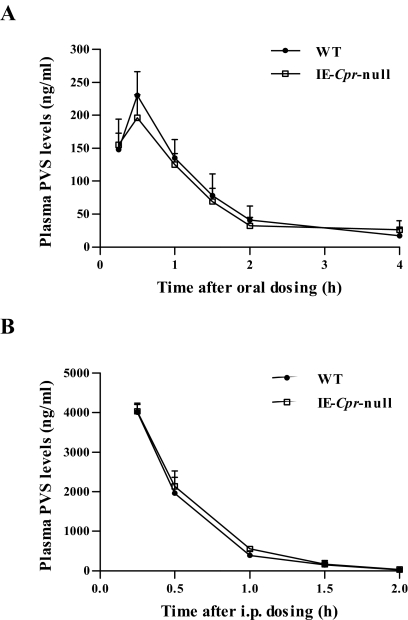
To confirm that the decrease in the first-pass clearance of oral LVS in the IE-Cpr-null mice was due to the loss of P450-mediated LVS metabolism, rather than to other unidentified events not related to P450s' function in drug metabolism, we performed pharmacokinetic studies for PVS, a drug that is similar (in chemical structure and drug target) to LVS but is in hydroxyl acid form and is not a good P450 substrate. As expected, when PVS was administered either orally at 50 mg/kg or through intraperitoneal injection at 25 mg/kg, there was no significant difference between WT and IE-Cpr-null mice in the pharmacokinetic profiles (Fig. 2), indicating that the loss of intestinal CPR did not alter the clearance of orally or intraperitoneally administered PVS. These data strongly support the notion that the increase in the oral bioavailability of LVS in IE-Cpr-null mice is due to the suppression of SI P450-mediated LVS metabolism.
Fig. 2.
Plasma levels of PVS in IE-Cpr-null and WT mice after a single oral or intraperitoneal dose of PVS. Adult female IE-Cpr-null and age-matched WT mice were given a single dose of PVS through oral gavage at 50 mg/kg (A) or via intraperitoneal (i.p.) injection at 25 mg/kg (B). Plasma samples were obtained at various times after dosing. Values represent means ± S.D. (n = 5–6).
Utility of the IE-Cpr-Null Mouse Model for Identification of the Molecular Target of GFJ Inhibition in the Small Intestine.
GFJ can increase the oral bioavailability for a number of drugs known to be metabolized by CYP3A, including LVS (Mertens-Talcott et al., 2006). Orally administered GFJ can inhibit intestinal, but apparently not hepatic, CYP3A-mediated drug metabolism (Bailey et al., 1998). However, GFJ components can also inhibit efflux transport (Edwards et al., 1999; Honda et al., 2004), and it is not clear whether the in vivo effects of oral GFJ on the bioavailability of a given drug were strictly due to its inhibition of P450 enzymes. In this study, we have used the IE-Cpr-null mouse to determine whether the ability of GFJ to inhibit the first-pass clearance of oral LVS is mediated through its inhibition of SI P450 (including CYP3A) alone or through its inhibition of both P450 and P-gp in the small intestine.
As shown in Fig. 1E and in Table 2, when GFJ was administered orally (at 20 ml/kg) to WT mice at 2 h before LVS dosing, significant increases in both Cmax and AUC0–4h values were found, compared with the vehicle-treated group. In contrast, when GFJ was administered similarly to IE-Cpr-null mice (Fig. 1F; Table 2), no significant change was observed in either Cmax or AUC0–4h values, between GFJ-treated and vehicle-treated groups. These findings indicate that SI P450 was the main target, whereas SI P-gp was minimally involved, in the inhibition of oral LVS clearance by GFJ, under the experimental conditions used.
TABLE 2.
Pharmacokinetic parameters for orally administered LVS (12.5 mg/kg) in IE-Cpr-null and WT mice pretreated with GFJ
Data from Fig. 1, E–F, were analyzed for the determination of pharmacokinetic parameters. Adult female mice (5–6 per group) were given a single dose of 4× strength GFJ through oral gavage, at 20 ml/kg, or were given water (vehicle control), followed 2 h later by a single oral dose of LVS, at 12.5 mg/kg. Values represent means ± S.D. (n = 5–6).
| Strain | Pretreatment | Tmax | Cmax | t1/2 | AUC0–4 h | CL/F |
|---|---|---|---|---|---|---|
| min | μg/ml | min | min · μg/ml | ml/min | ||
| WT | Water | 27 ± 7 | 0.2 ± 0.0 | 36 ± 5 | 10.9 ± 1.5 | 22.3 ± 2.7 |
| WT | GFJ | 34 ± 11 | 0.5 ± 0.1a | 39 ± 13 | 34.4 ± 12.7a | 8.1 ± 2.9a |
| IE-Cpr-null | Water | 30 ± 0 | 0.4 ± 0.1 | 36 ± 9 | 30.0 ± 5.8 | 8.7 ± 1.7 |
| IE-Cpr-null | GFJ | 45 ± 17 | 0.4 ± 0.2 | 34 ± 12 | 34.2 ± 11.8 | 8.5 ± 4.2 |
CL/F, apparent clearance.
P < 0.01, compared to the corresponding water-pretreated group (Student's t test).
Summary.
We have demonstrated that intestinal P450-mediated metabolism plays an important role in the regulation of LVS oral bioavailability. Our findings further validate the IE-Cpr-null mouse as a powerful tool for determination of the specific in vivo contributions of intestinal P450 enzymes to the first-pass clearance of numerous orally ingested drugs and other xenobiotics, including drugs that are substrates for both P450 and P-gp. Through the utility of the IE-Cpr-null mouse, we have also demonstrated that the main target of GFJ action on LVS clearance in the small intestine is P450, but not P-gp. This latter experiment illustrates the additional value of the IE-Cpr-null mice for studying the mechanisms of drug-drug or drug-diet interactions.
Acknowledgments
We thank Dr. Xinxin Ding of the Wadsworth Center for helpful discussions and a critical reading of the manuscript. We also thank Weizhu Yang for assistance with animal breeding.
This study was supported in part by the National Institutes of Health National Institute of General Medical Sciences [Grant GM082978].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.037861.
- P450
- cytochrome P450
- SI
- small intestinal
- IE
- intestinal epithelium
- CPR
- cytochrome P450 reductase
- IE-Cpr-null
- intestinal epithelium-specific Cpr-null
- BCS
- Biopharmaceutics Classification System
- P-gp
- P-glycoprotein
- LVS
- lovastatin
- LVA
- lovastatin hydroxy acid
- WT
- wild-type
- PVS
- pravastatin
- GFJ
- grapefruit juice
- LC-MS/MS
- liquid chromatography tandem mass spectrometry
- AUC
- area under the curve.
Authorship Contributions
Participated in research design: Zhu, D'Agostino, and Zhang.
Conducted experiments: Zhu, D'Agostino, and Zhang.
Performed data analysis: Zhu, D'Agostino, and Zhang.
Wrote or contributed to the writing of the manuscript: Zhu and Zhang.
References
- Bailey DG, Malcolm J, Arnold O, Spence JD. (1998) Grapefruit juice-drug interactions. Br J Clin Pharmacol 46:101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Mireles RJ, Campbell SD, Lin J, Mills JB, Xu JJ, Smolarek TA. (2005) Differential interaction of 3-hydroxy-3-methylglutaryl-coa reductase inhibitors with ABCB1, ABCC2, and OATP1B1. Drug Metab Dispos 33:537–546 [DOI] [PubMed] [Google Scholar]
- Ding X, Kaminsky LS. (2003) Human extrahepatic cytochromes P450: function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu Rev Pharmacol Toxicol 43:149–173 [DOI] [PubMed] [Google Scholar]
- Doherty MM, Charman WN. (2002) The mucosa of the small intestine: how clinically relevant as an organ of drug metabolism? Clin Pharmacokinet 41:235–253 [DOI] [PubMed] [Google Scholar]
- Duggan DE, Chen IW, Bayne WF, Halpin RA, Duncan CA, Schwartz MS, Stubbs RJ, Vickers S. (1989) The physiological disposition of lovastatin. Drug Metab Dispos 17:166–173 [PubMed] [Google Scholar]
- Edwards DJ, Fitzsimmons ME, Schuetz EG, Yasuda K, Ducharme MP, Warbasse LH, Woster PM, Schuetz JD, Watkins P. (1999) 6′,7′-Dihydroxybergamottin in grapefruit juice and Seville orange juice: effects on cyclosporine disposition, enterocyte CYP3A4, and P-glycoprotein. Clin Pharmacol Ther 65:237–244 [DOI] [PubMed] [Google Scholar]
- Fang C, Zhang QY. (2010) The role of small-intestinal P450 enzymes in protection against systemic exposure of orally administered benzo[a]pyrene. J Pharmacol Exp Ther 334:156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasco MJ, Silkworth JB, Dunbar DA, Kaminsky LS. (1993) Rat small intestinal cytochromes P450 probed by warfarin metabolism. Mol Pharmacol 43:226–233 [PubMed] [Google Scholar]
- Honda Y, Ushigome F, Koyabu N, Morimoto S, Shoyama Y, Uchiumi T, Kuwano M, Ohtani H, Sawada Y. (2004) Effects of grapefruit juice and orange juice components on P-glycoprotein- and MRP2-mediated drug efflux. Br J Pharmacol 143:856–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu I, Spinler SA, Johnson NE. (1995) Comparative evaluation of the safety and efficacy of HMG-CoA reductase inhibitor monotherapy in the treatment of primary hypercholesterolemia. Ann Pharmacother 29:743–759 [DOI] [PubMed] [Google Scholar]
- Ishigami M, Honda T, Takasaki W, Ikeda T, Komai T, Ito K, Sugiyama Y. (2001) A comparison of the effects of 3-hydroxy-3-methylglutaryl-coenzyme a (HMG-CoA) reductase inhibitors on the CYP3A4-dependent oxidation of mexazolam in vitro. Drug Metab Dispos 29:282–288 [PubMed] [Google Scholar]
- Jacobsen W, Kirchner G, Hallensleben K, Mancinelli L, Deters M, Hackbarth I, Baner K, Benet LZ, Sewing KF, Christians U. (1999a) Small intestinal metabolism of the 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor lovastatin and comparison with pravastatin. J Pharmacol Exp Ther 291:131–139 [PubMed] [Google Scholar]
- Jacobsen W, Kirchner G, Hallensleben K, Mancinelli L, Deters M, Hackbarth I, Benet LZ, Sewing KF, Christians U. (1999b) Comparison of cytochrome P-450-dependent metabolism and drug interactions of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors lovastatin and pravastatin in the liver. Drug Metab Dispos 27:173–179 [PubMed] [Google Scholar]
- Jain DS, Subbaiah G, Sanyal M, Jain VK, Shrivastav P. (2007) A rapid and specific approach for direct measurement of pravastatin concentration in plasma by LC-MS/MS employing solid-phase extraction. Biomed Chromatogr 21:67–78 [DOI] [PubMed] [Google Scholar]
- Kaminsky LS, Zhang QY. (2003) The small intestine as a xenobiotic-metabolizing organ. Drug Metab Dispos 31:1520–1525 [DOI] [PubMed] [Google Scholar]
- Kantola T, Kivistö KT, Neuvonen PJ. (1998) Grapefruit juice greatly increases serum concentrations of lovastatin and lovastatin acid. Clin Pharmacol Ther 63:397–402 [DOI] [PubMed] [Google Scholar]
- Lin JH, Chiba M, Baillie TA. (1999) Is the role of the small intestine in first-pass metabolism overemphasized? Pharmacol Rev 51:135–158 [PubMed] [Google Scholar]
- Lodge JW, Fletcher BL, Brown SS, Parham AJ, Fernando RA, Collins BJ. (2008) Determination of lovastatin hydroxy acid in female B6C3F1 mouse serum. J Anal Toxicol 32:248–252 [DOI] [PubMed] [Google Scholar]
- Mertens-Talcott SU, Zadezensky I, De Castro WV, Derendorf H, Butterweck V. (2006) Grapefruit-drug interactions: can interactions with drugs be avoided? J Clin Pharmacol 46:1390–1416 [DOI] [PubMed] [Google Scholar]
- Mulvana D, Jemal M, Pulver SC. (2000) Quantitative determination of pravastatin and its biotransformation products in human serum by turbo ion spray LC/MS/MS. J Pharm Biomed Anal 23:851–866 [DOI] [PubMed] [Google Scholar]
- Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. (2006) The human intestinal cytochrome P450 “pie.” Drug Metab Dispos 34:880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter M. (2005) Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol 19:117–125 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sugiyama Y. (2000) Role of metabolic enzymes and efflux transporters in the absorption of drugs from the small intestine. Eur J Pharm Sci 12:3–12 [DOI] [PubMed] [Google Scholar]
- Thummel KE, Kunze KL, Shen DD. (1997) Enzyme-catalyzed processes of first-pass hepatic and intestinal drug extraction. Adv Drug Deliv Rev 27:99–127 [DOI] [PubMed] [Google Scholar]
- van Herwaarden AE, Wagenaar E, van der Kruijssen CM, van Waterschoot RA, Smit JW, Song JY, van der Valk MA, van Tellingen O, van der Hoorn JW, Rosing H, et al. (2007) Knockout of cytochrome P450 3A yields new mouse models for understanding xenobiotic metabolism. J Clin Invest 117:3583–3592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas KP, Kari PH, Pitzenberger SM, Halpin RA, Ramjit HG, Arison B, Murphy JS, Hoffman WF, Schwartz MS, Ulm EH. (1990) Biotransformation of lovastatin. I. Structure elucidation of in vitro and in vivo metabolites in the rat and mouse. Drug Metab Dispos 18:203–211 [PubMed] [Google Scholar]
- Wang RW, Kari PH, Lu AY, Thomas PE, Guengerich FP, Vyas KP. (1991) Biotransformation of lovastatin. IV. Identification of cytochrome P450 3A proteins as the major enzymes responsible for the oxidative metabolism of lovastatin in rat and human liver microsomes. Arch Biochem Biophys 290:355–361 [DOI] [PubMed] [Google Scholar]
- Wu CY, Benet LZ. (2005) Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res 22:11–23 [DOI] [PubMed] [Google Scholar]
- Zhang QY, Dunbar D, Kaminsky LS. (2003) Characterization of mouse small intestinal cytochrome P450 expression. Drug Metab Dispos 31:1346–1351 [DOI] [PubMed] [Google Scholar]
- Zhang QY, Fang C, Zhang J, Dunbar D, Kaminsky L, Ding X. (2009) An intestinal epithelium-specific cytochrome P450 (P450) reductase-knockout mouse model: direct evidence for a role of intestinal P450s in first-pass clearance of oral nifedipine. Drug Metab Dispos 37:651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]