Abstract
Drug pharmacokinetics can be altered in obese and diabetic subjects. In consideration of the prevalence of obesity and diabetes, characterization of transporter expression in mouse models of diabetes and obesity may be a useful tool to aid in prediction of altered drug pharmacokinetics or adverse drug reactions. It has been reported that ob/ob mice, which display a severe obesity and diabetes phenotype, exhibit multiple changes in drug transporter expression in liver and kidney. In the present study, the mRNA and protein expression of major drug transporters was determined in livers and kidneys of diet-induced obese (DIO) C57BL/6J male mice. The mice were fed a high-fat diet (HFD) (60% fat) from 6 weeks of age and display obesity, fatty liver, and mild hyperglycemia. The HFD diet increased expression of multidrug resistance-associated proteins Abcc3 and 4 mRNA and protein in liver by 3.4- and 1.4-fold, respectively, compared with that detected in control mice fed a low-fat diet (LFD). In contrast, Abcc1 mRNA and protein decreased by 50% in livers of DIO mice compared with those in livers to lean mice. The HFD did not alter transporter expression in kidney compared with the LFD. In summary, unlike ob/ob and db/db mice, DIO mice exhibited a selective induction of efflux transporter expression in liver (i.e., Abcc3 and 4). In addition, diet-induced obesity affects transporter expression in liver but not kidney in the C57BL/6J mouse model. These data indicate that hepatic transporter expression is only slightly altered in a model of mild diabetes and nonalcoholic fatty liver disease and obesity.
Introduction
According to the National Institutes of Health “Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity of Adults” (http://www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm), adults with a body mass index (BMI) of 30 kg/m2 are considered to be obese, and those with BMI of 25 to 29.9 kg/m2 are considered to be overweight. Worldwide, obesity affects more than 1 billion individuals (Redinger, 2009), causing approximately 300,000 deaths per year in the United States alone (Flegal et al., 2002). Obesity occurs because there is an imbalance between energy intake and expenditure and from the combined effects of lifestyle, heredity, and environment and their interactions (Uauy et al., 2001). According to several cohort studies, the prevalence of obesity is higher in individuals with no physical exercise who live a sedentary lifestyle (Lee et al., 2008; Rodríguez-Martín et al., 2009). High intake of macronutrients such as carbohydrates and proteins from meat and fruits and vegetables is also associated with general obesity (Lee et al., 2008). Diabetes mellitus is one of the commonly observed consequences of obesity. Obese adults present with increased peripheral insulin resistance and impaired nonesterified free fatty acid (FFA) oxidation (Schuster, 2009). Women with BMI of 23 to 25 kg/m2 have a 4-fold higher chance of becoming diabetic compared with women with BMI less than 20 kg/m2 (Colditz et al., 1995).
Along with obesity and diabetes, the prevalence of nonalcoholic fatty liver disease (NAFLD) is also increasing (Gentile and Pagliassotti, 2008). NAFLD is an obesity-related disorder characterized by fatty infiltration in hepatocytes, which is nonalcoholic in origin. It can progress into steatohepatitis (NASH) and finally end-stage liver disease (Gentile and Pagliassotti, 2008). According to the “two-hit” theory of NASH development (Day and James, 1998), the first hit is accumulation of triglycerides in hepatocytes because of obesity and insulin resistance, and the second hit is oxidative stress and inflammation.
To study obesity in rodents, the diet-induced obese (DIO) mouse is a commonly used research model. DIO mice are C57BL/6J male mice fed a high-fat (60% kcal) diet from 6 weeks of age. The mice gain weight faster than mice fed a standard rodent chow, which is a 10% kcal diet with the same protein content as the high-fat diet. By 17 weeks of age, DIO mice weigh approximately 20% more than mice fed the 10% kcal diet. Body fat content of DIO mice is more than 35% by 13 weeks of age, as opposed to 20% in mice of same age fed a 10% kcal diet (Bush et al., 2001). DIO mice are also used to model obesity-induced diabetes because these mice exhibit moderate hyperglycemia and mild to moderate hyperinsulinemia. Humans show the above-mentioned symptoms in a similar fashion, presenting with mild hyperinsulinemia and mild hyperglycemia and maturity onset of the diabetic phenotype, similar to that exhibited by DIO mice. Thus, DIO mice are a relevant model of maturity-onset diabetes (Bush et al., 2001).
Transporters are membrane proteins that function in the transport of chemicals into and out of cells. Hepatobiliary transporters assist in the extraction of chemicals from blood into hepatocytes, where the chemicals can undergo metabolism by phase I and II biotransformation enzymes to enhance excretion, whereas transporters present in kidney aid in renal clearance of chemicals and urinary excretion. As reviewed by Ho and Kim (2005), two transporter superfamilies predominantly mediate the translocation of most xenobiotics and endobiotics. Uptake transporters, such as the organic anion-transporting polypeptides, sodium/taurocholate-cotransporting polypeptide, and organic cation transporters are part of the solute carrier superfamily (Slc). They are present on the basolateral membrane and transport chemicals into hepatocytes. Members of the ATP-binding cassette (Abc) superfamily function as efflux transporters; they include multidrug resistance proteins, multidrug resistance-associated proteins, the bile salt-export pump, and breast cancer resistance protein. Efflux transporters localized to the canalicular membrane of hepatocytes mediate the vectorial excretion of chemicals from hepatocytes into bile, whereas other efflux transporters are localized to the basolateral membrane and mediate efflux of chemicals from hepatocytes to blood. In kidney, organic anion transporters and organic cation transporters are involved in the renal clearance of many drugs and toxicants.
Few studies have addressed how drug transporter expression is altered in models of obesity. Given the rising occurrence of obesity and the need to better predict adverse drug effects or efficacy, in the present study we sought to determine whether xenobiotic transporter expression is altered in the diet-induced obese mouse model, which presents with a mild-moderate phenotype of obesity and diabetes. The results presented herein demonstrate that there is selective transporter up-regulation in this model in liver, with no changed transporter expression in kidney.
Materials and Methods
Materials.
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and were of reagent grade or better. Antibody sources and concentrations used for Western blot and immunocytochemistry are detailed in the supplemental data. Oligonucleotides used for probe sets were generously donated by Dr. Curtis Klaassen (University of Kansas Medical Center, Kansas City, KS).
Diet-Induced Obese Mice and Lean Controls.
Nineteen-week-old male C57BL/6J mice (n = 8/group) were purchased from The Jackson Laboratory (Bar Harbor, ME). These mice were fed a 60% kcal high-fat diet since 6 weeks of age. Lean control mice were C57BL/6J mice fed a 10% kcal diet with the same protein content as the high-fat diet. The mice were housed for 2 weeks under a constant 12-h dark/light cycle and given food and water ad libitum. The DIO mice were fed the same high-fat diet as administered in The Jackson Laboratory (60% kcal; Research Diets Inc., New Brunswick, NJ), and the control mice were fed the same 10% kcal fat diet (Research Diets Inc.). During acclimation, the body weight and blood glucose levels were checked weekly. Blood glucose levels were determined using a Bayer Contour glucose meter (Bayer Healthcare, Tarrytown, NY). After 2 weeks of acclimation, mice were anesthetized by isoflurane inhalation. Blood was collected by decapitation, and serum was obtained after centrifugation at 2300g for 5 min. Livers and kidneys were collected, snap-frozen using liquid nitrogen, and stored at −80°C for future analysis. The institutional animal care and use committee at the University of Rhode Island approved all animal experiments.
Human Liver Tissue.
Along with mouse tissues, preliminary data from human transporter protein quantification are also presented in this article. Human liver tissues from nonsteatotic and steatotic humans were obtained from the Liver Tissue Cell Distribution System (University of Minnesota, Minneapolis, MN, and XenoTech LLC, Lenexa, KS). Details for the human liver donors are described in Supplemental Table 2.
RNA Extraction and Quantification.
Total RNA from livers and kidneys was isolated by phenol-chloroform extraction using RNA Bee reagent (Tel-Test Inc., Friendswood, TX) according to the manufacturer's protocol. The RNA was quantified by measuring its absorbance at 280 nm in a UV-visible spectrophotometer (NanoDrop ND 1000; Thermo Fisher Scientific, Waltham, MA), and all the samples were diluted to 1 μg/μl concentration. Formaldehyde-agarose gel electrophoresis was used to check RNA integrity.
Oligonucleotide Probe Sets for the Branched DNA Signal Amplification Assay.
Probe sets for mouse Abcc1–6, Oat1 and 2, Oct1 and 2, and Slco1a1, 1a4, 1b2, 1a6, and 2b1 have been described previously (Cherrington et al., 2003; Buist and Klaassen, 2004; Aleksunes et al., 2005; Cheng et al., 2005a,b). Oligonucleotide probe sets required for the assay were graciously donated by Dr. Curtis Klaassen.
Branched DNA Signal Amplification Assay for mRNA Quantification.
All reagents for analysis including lysis buffer, amplifier/label probe diluent, and substrate solution were supplied in the QuantiGene HV signal amplification kit (Panomics, Fremont, CA). Oligonucleotide probes were diluted in lysis buffer. On day 1, RNA samples diluted to 1 μg/μl were added to each of the 96-well plates containing 50 μl of capture hybridization buffer and 100 μl of diluted probe set. The RNA was allowed to hybridize overnight with a probe set at 53°C. On day 2 of the assay, subsequent hybridization steps were followed as mentioned in the manufacturer's protocol, and luminescence was measured with a Quantiplex 320 branched DNA luminometer interfaced with Quantiplex Data Management Software (version 5.02; Bayer Corp., Diagnostics Div., Tarrytown, NY). The luminescence for each well was reported as relative light units per 10 μg of total RNA.
Preparation of Crude Membrane Fractions.
Approximately 50 mg of liver tissue was homogenized in 450 μl of sucrose-Tris (ST) buffer composed of 0.25 M sucrose and 10 mM Tris-HCl with pH 7.4 and containing protease inhibitor cocktail (2 μg/ml; Sigma-Aldrich). The homogenates were centrifuged at 100,000g for 60 min at 4°C. The resulting supernatant was discarded, and 200 μl of ST buffer was used to resuspend the resulting pellet, which was the crude membrane fraction. The total protein concentration of these preparations was determined by the assay of Lowry et al. (1951)using Bio-Rad protein assay reagents (Bio-Rad Laboratories, Hercules, CA).
Western Blotting Detection.
Western blots were used for identification and quantification of membrane proteins, and 50 μg of protein/well was electrophoretically resolved by SDS-polyacrylamide gel electrophoresis (8% resolving and 4% stacking for abcc and 12% resolving and 4% stacking for slc). Then proteins were transblotted on PVDF membrane (Millipore Corporation, Billerica, MA) at 100 V for 45 min. The membrane was blocked overnight at 4°C with 2% nonfat dry milk in phosphate-buffered saline with Tween 20. The membrane was then incubated with primary antibody in phosphate-buffered saline with Tween 20 for 3 h (room temperature). After washing, the membrane was incubated with species-specific peroxidase-labeled secondary antibody for 1 h at room temperature. Specific information about the source, dilution, type, and molecular weight of primary and secondary antibodies is detailed in Supplemental Table 1. After incubation with secondary antibody, the membranes were washed and incubated with an ECL+ fluorescence kit (GE Healthcare, Little Chalfont, Buckinghamshire, UK), and X-ray films were exposed to blots. The blots were quantified with Quantity One software (Bio-Rad Laboratories).
Hematoxylin and Eosin Staining.
At the time of necropsy, a section of the central lobe of liver was placed in buffered formalin for 24 h and then stored in 75% ethanol until further processing. Livers were processed by standard laboratory procedures for paraffin embedding and sectioning (AML Laboratories, Rockland, MD). Paraffin-embedded tissues were cut to approximately 5-μm sections and then stained with hematoxylin and eosin. Likewise, formalin-fixed kidney tissues were also sectioned and stained with hematoxylin and eosin.
Proinflammatory Cytokine Measurement in Liver.
Total RNA isolated from livers of lean and DIO mice was quantified for cytokine mRNA transcripts using a QuantiGene Plex 2.0 assay (Affymetrix, Santa Clara, CA) on a Bio-Plex multiple array system. Total RNA was allowed to hybridize with cytokine transcript-specific beads for 16 h at 53°C. After incubation, preamplifier was added to wells, allowing it to incubate for 1 h, followed by amplifier and label probe. After addition of the substrate, chemiluminescence was measured with Bioplex multiple array reader system. Data were collected with Bioplex manager 5.0 software and plotted as mean fluorescence intensity per 800 ng of total RNA.
Statistical Analysis.
The statistical significance of differences between groups was determined by analyzing log-transformed data with a Student's t test, assuming equal variance, and p ≤ 0.05 was considered statistically significant. Unless otherwise stated, all data are presented as mean ± S.E.M. for eight animals per group.
Results
Liver, Kidney, Body Weights, Blood Glucose Levels, and Hepatic Fat Accumulation in Lean and DIO Mice.
Table 1 illustrates body, liver, and kidney weights, the liver/body weight ratio, and blood glucose levels in 21-week-old DIO and lean mice. The body and liver weights of DIO mice were approximately 40% higher than those of lean mice. The mean blood glucose levels for DIO mice were significantly higher than those measured in lean mice. The liver/body mass ratio was similar for both groups.
TABLE 1.
Body, liver, and kidney weight and blood glucose levels for DIO and lean mice
Male C57BL/6J mice were fed a 60% kcal diet (DIO) or a 10% kcal diet from 6 to 21 weeks of age. Livers, kidneys, and blood were collected at 21 weeks of age. All weights are expressed as the mean ± S.E.M.
| Strain | Liver Weight | Kidney Weight | Average Body Weight | Liver/Body Weight | Mean Blood Glucose Levels |
|---|---|---|---|---|---|
| g | % | mg/dl | |||
| Lean | 1.22 ± 0.031 | 0.32 ± 0.015 | 29.68 ± 0.15 | 4.12 ± 0.099 | 145.4 ± 6.06 |
| DIO | 1.65 ± 0.058 | 0.40 ± 0.017 | 41.50 ± 0.70* | 3.98 ± 0.093 | 198.6 ± 9.65* |
Statistically significant difference of parameters between control and DIO mice (p ≤ 0.05).
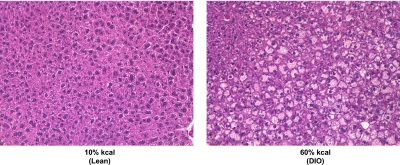
The 60% kcal diet caused moderate to severe hepatic steatosis in male C57BL/6J mice, which was not observed in mice fed the 10% kcal diet (Fig. 1). Centrally located parenchyma of liver showed moderate microvesicular and macrovesicular steatosis, whereas steatosis was severe in peripheral parenchyma. Liver sections for mice fed the 10% kcal diet appeared normal with no signs of steatosis. No necrotic lesions and few to no vacuolations were noted in kidney sections of DIO mice (data not shown).
Fig. 1.
Representative hematoxylin and eosin staining of liver sections from lean control and DIO mice. A small piece of liver from the central lobe was fixed, prepared for typical paraffin embedding, sectioned to 5 μM, and then stained with hematoxylin and eosin. It was observed that fat depositions were greater in liver sections from DIO mice than in those from lean mice. Original magnification, 200×.
Effect of Diet-Induced Obesity on Organic Anion-Transporting Polypeptide (Slco) Expression in Liver.
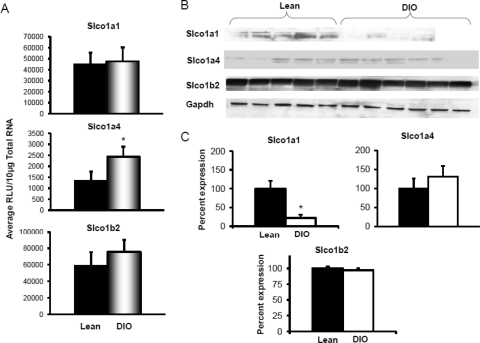
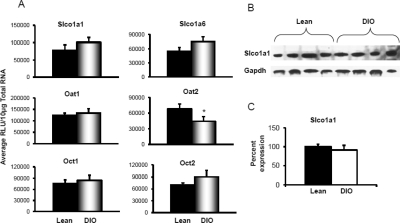
Uptake transporters are localized to the basolateral membrane of hepatocytes and extract chemicals from blood into hepatocytes. Slco1a1, 1a4, and 1b2 expression was analyzed by mRNA and protein quantification. Figure 2, A–C, illustrates Slco mRNA expression, protein expression by Western blot, and Western blot quantification. Diet-induced obesity did not significantly alter Slco1a1, 1a4, or 1b2 expression in liver compared with that in lean controls. The protein expression of uptake transporters also remained unchanged with diet-induced obesity.
Fig. 2.
Uptake transporter organic anion-transporting polypeptide Slco1a1, 1a4, and 2b1 expression in livers of lean control and DIO mice (n = 8). A, mRNA expression for Slco1a1, 1a4, and 2b1. Total RNA was isolated from mouse livers, and mRNA was quantified using a branched DNA signal amplification assay. The data are plotted as average relative light units (RLU) per 10 μg of total RNA ± S.E.M. *, statistically significant difference in expression between DIO mice and lean control mice (p ≤ 0.05). B, protein identification and quantification by Western blotting for Slco1a1, 1a4, and 2b1 in crude membrane fractions in livers of DIO and lean mice. Proteins (50 μg/lane) were isolated by electrophoresis on 4 to 20% acrylamide/bis gels, transblotted on a PVDF membrane, incubated with primary and secondary antibodies and substrate, and detected by chemiluminescence. C, quantification of Western blots with Quantity One software. The average band intensity for lean mice was considered to be 100%, and band intensity in the other group was compared with it to plot the graphs. *, statistically significant difference in band intensity average compared with that of lean mice (p ≤ 0.05). Slco1a4 mRNA expression was up-regulated in DIO mice compared with that in lean mice; however, protein levels remained unchanged for Slco1a4 and 1b2. Slco1a1 protein levels were significantly down-regulated in livers of DIO mice compared with those in lean mice. Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
Effect of Diet-Induced Obesity on Multidrug Resistance-Associated Protein Expression in Liver.
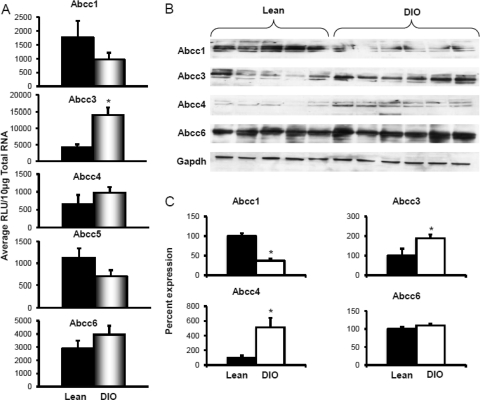
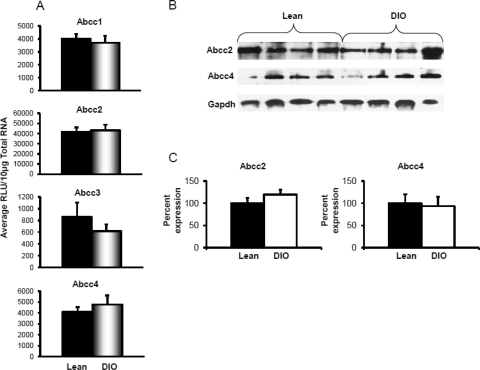
Efflux transporters are localized to the basolateral and canalicular membranes and transport drugs/chemicals or their conjugates to blood or bile. Along with exogenous chemicals, these transporters also facilitate transport of endogenous substances such as bile acids and conjugated hormones and bilirubin. Figure 3A depicts Abcc1 and Abcc3–6 mRNA expression in livers of lean and DIO mice. Feeding the 60% kcal diet decreased Abcc1 mRNA expression in liver, with expression being approximately half of that detected in lean mice. In contrast, diet-induced obesity significantly increased Abcc3 mRNA expression in liver. Feeding the 60% kcal diet increased Abcc3 and 4 mRNA expressions in liver by 3.4-fold, respectively, compared with that detected in lean mice fed the 10% kcal diet. Abcc6 mRNA expression did not differ between the groups.
Fig. 3.
Basolateral efflux transporters multidrug resistance-associated protein Abcc1, 3, 4, 5, and 6 expression in livers of lean control and DIO mice. A, mRNA expression for Abcc1, 3, 4, 5, and 6. Total RNA was isolated from mouse livers, and mRNA was quantified using a branched DNA signal amplification assay. The data are plotted as average relative light units (RLU) per 10 μg of total RNA ± S.E.M. *, statistically significant difference of expression between control and DIO mice (p ≤ 0.05). B, protein identification and quantification by Western blotting for Abcc1, 3, 4, and 6 in the crude membrane fractions from livers of DIO and lean mice. Proteins (50 μg/lane) were isolated by electrophoresis on 10% acrylamide/bis gels, transblotted, incubated with primary and secondary antibodies and substrate, and detected by chemiluminescence. C, quantification of Western blots with Quantity One software. The average band intensity for lean mice was considered to be 100%, and band intensity in the other group was compared with it to plot the graphs. *, statistically significant difference between band intensity average compared with that of control mice (p ≤ 0.05). mRNA expression for Abcc1, 4, 5, and 6 remained unchanged, whereas Abcc3 expression was up-regulated in DIO mice compared with that in lean mice. Abcc3 and 4 protein expression was up-regulated, whereas abcc1 was down-regulated in livers of DIO mice compared with that in livers of lean controls. Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
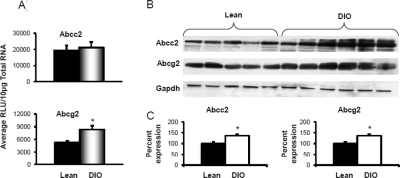
Figure 3, B and C, depicts abcc protein expression in crude membrane fractions isolated from livers of mice fed the 10 and 60% kcal diets. Diet-induced obesity decreased liver abcc1 protein expression by half compared with expression in livers of lean controls. Liver Abcc3 and 4 protein expression was also up-regulated in DIO mice. Diet-induced obesity increased Abcc3 protein expression by 2-fold in liver, which was consistent with the observed increase in mRNA expression. Abcc4 protein expression increased by almost 5-fold in livers of DIO mice compared with that detected in livers of lean mice. Protein expression for Abcc6 was similar in mice fed the 10 or 60% kcal diet. Figure 4 illustrates Abcc2 and Abcg2 mRNA and protein expression in livers from mice fed the 10 or 60% kcal diet. Abcc2 mRNA expression remained unchanged with diet-induced obesity. Abcg2 mRNA, in contrast, increased in DIO mice by more than 1.5-fold compared with that in lean mice. Protein expression for both Abcc2 and Abcg2 was up-regulated by approximately 35% in livers of DIO mice compared with that in lean controls.
Fig. 4.
Canalicular efflux transporters multidrug resistance-associated protein Abcc2 and breast cancer resistance protein Abcg2 expression in livers of lean control and DIO mice. A, mRNA expression for Abcc2 and Abcg2. Total RNA was isolated from mouse livers, and mRNA was quantified using a branched DNA signal amplification assay. The data are plotted as average relative light units (RLU) per 10 μg of total RNA ± S.E.M. *, statistically significant difference of expression between lean control and DIO mice (p ≤ 0.05). B, protein identification and quantification by Western blotting for Abcc2 and Abcg2 in the crude membrane fraction from livers of lean control and DIO mice. The proteins were isolated by electrophoresis on 10% acrylamide/bis gels, transblotted, incubated with primary and secondary antibodies and substrate, and detected by chemiluminescence. C, quantification of Western blots with Quantity One software. The average band intensity for lean mice was considered to be 100%, and band intensity in the other groups were compared with it to plot the graphs. *, statistically significant difference between band intensity average compared with that of lean control mice (p ≤ 0.05). Abcc2 mRNA remained unchanged, but protein expression was up-regulated, whereas for Abcg2, both mRNA and protein expression was up-regulated in livers of DIO mice compared with that in livers of lean mice. Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
Effect of Diet-Induced Obesity on Transporter Expression in Kidney.
In kidney, major classes of uptake transporters are organic anion transporters and organic cation transporters, along with organic anion-transporting polypeptides. Slco1a1 and 1a6, Oat1 and 2, Oct1 and 2, and Abcc1–4 mRNA expression in DIO and control mice was determined. Figure 5 illustrates uptake transporter expression in kidneys of DIO and lean mice. Uptake transporters Slco1a1 and 1a6 did not show any change in expression in kidneys of DIO mice. Likewise, Oat1, Oct1, and Oct2 also remained unchanged in kidneys of DIO mice compared with that in kidneys of lean mice. Oat2 was the only solute carrier transporter with changed expression in kidney. Diet-induced obesity decreased Oat2 mRNA expression to approximately 65% of that detected in kidneys of lean mice. Figure 6 demonstrates efflux transporter expression in kidneys of DIO and lean mice. As for uptake transporters, efflux transporter expression also remained virtually unchanged with diet-induced obesity in kidneys of mice.
Fig. 5.
Uptake transporters Slco1a1 and 1a6, Oat1 and 2, and Oct1 and 2 expression in kidneys of lean control and DIO mice. A, mRNA expression for uptake transporters organic anion-transporting polypeptide Slco1a1 and 1a6 and organic anion and cation transporters Oat1 and 2 and Oct1 and 2 in kidneys of lean control and DIO mice. Total RNA was isolated from kidneys of mice by phenol chloroform extraction, and mRNA was quantified using a branched DNA signal amplification assay. The data are plotted as average relative light units (RLU) per 10 μg of total RNA ± S.E.M. B, protein identification and quantification by Western blotting for Slco1a1 in the crude membrane fraction from kidneys of lean control and DIO mice. The proteins were isolated by electrophoresis on 10% acrylamide/bis gels, transblotted, incubated with primary and secondary antibodies and substrate, and detected by chemiluminescence. C, quantification of Western blots with Quantity One software. The average band intensity for lean mice was considered to be 100%, and band intensities in the other groups were compared with it to plot the graphs. *, statistically significant difference of expression between lean control and DIO mice (p ≤ 0.05). Only Oat2 mRNA expression was down-regulated in DIO mice; Slco1a1 and 1a6, Oat1, and Oct1 and 2 mRNA remained unchanged compared with that in lean mice. Slco1a1 protein expression also remained unchanged. Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
Fig. 6.
Efflux transporter expression in kidneys of lean control and DIO mice. A, mRNA expression for efflux transporters multidrug resistance-associated protein Abcc1, 2, 3, and 4 in kidneys of lean control and DIO mice. Total RNA was isolated from mouse kidneys, and mRNA was quantified using a branched DNA signal amplification assay. The data are plotted as average relative light units (RLU) per 10 μg of total RNA ± S.E.M. B, protein identification and quantification by Western blotting for Abcc2 and 4 in the crude membrane fraction from kidneys of lean control and DIO mice. The proteins were isolated by electrophoresis on 10% acrylamide/bis gels, transblotted, incubated with primary and secondary antibodies and substrate, and detected by chemiluminescence. C, quantification of Western blots with Quantity One software. The average band intensity for lean mice was considered to be 100%, and band intensities in the other groups were compared with it to plot the graphs. *, statistically significant difference of expression between lean control and DIO mice (p ≤ 0.05). Abcc1, 2, 3, and 4 mRNA, as well as Abcc2 and 4 protein expression remained unchanged in kidneys of DIO mice. Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
Cytokine mRNA Expression in Livers of Lean and DIO Mice.
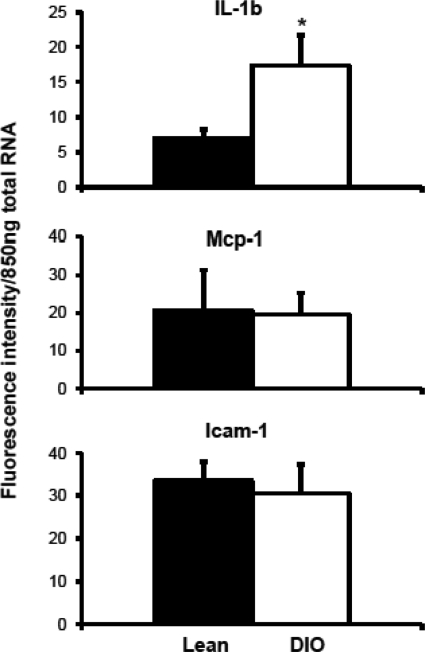
Proinflammatory cytokine interleukin-1β mRNA levels were increased significantly in livers of DIO mice compared with those in livers of lean mice (Fig. 7). Expression of monocyte chemoattractant protein-1 (Mcp-1) and intracellular adhesion molecule-1 (Icam-1) did not change in livers of mice with diet-induced obesity.
Fig. 7.
mRNA expression of proinflammatory cytokines interleukin (IL)-1β, Mcp-1, and Icam-1 in livers of lean and DIO mice. Total RNA was isolated from liver tissues and quantified by the QuantiGene Plex 2.0 assay for cytokines. Data are plotted as average fluorescence intensity per 850 ng of total RNA. *, statistically significant difference of expression between lean control and DIO mice (p ≤ 0.05). IL-1β mRNA expression was up-regulated in DIO mice compared with that in lean mice. Mcp-1 and Icam-1 remained unchanged.
Multidrug Resistance-Associated Protein Expression in Human Liver with Steatosis.
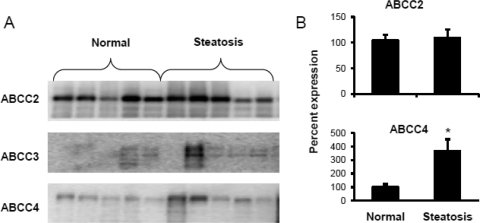
ABCC2, ABCC3, and ABCC4 protein expression in the livers of humans with steatosis was studied and compared with that in nonsteatotic human livers (Fig. 8). ABCC4 protein expression in steatotic livers was significantly higher compared with that in normal nonsteatotic livers. ABCC2 expression remained unchanged in steatotic livers compared with that in nonsteatotic human livers. ABCC3 also displayed an increasing trend with steatosis.
Fig. 8.
Protein expression of efflux transporters ABCC2, 3, and 4 in steatotic and nonsteatotic human liver tissues. Human liver tissues were obtained from the Liver Tissue Cell Distribution System. A, protein expression by Western blotting. The membrane fractions were isolated from these tissues and subjected to SDS-polyacrylamide gel electrophoresis, followed by transfer to a PVDF membrane and incubations with primary and secondary antibodies. B, quantification of Western blots. The Western blots were quantified by Quantity One software. It was observed that steatosis significantly up-regulated ABCC4 protein expression. ABCC2 protein levels remained unchanged. ABCC3 also showed an increasing trend, but the blot was not quantified, as some bands were not detectable by software.
Discussion
The present study demonstrates that DIO mice, which are a model of obesity-induced diabetes, have altered expression of abcc transporters in liver. In addition, we report that diet-induced obesity in mice only caused some changes in transporter expression in liver and virtually none in kidney. Taken together, the present data along with recent publications indicate that transporter expression is less dysregulated in a mouse model of mild obesity and diabetes but is more dysregulated in models of severe obesity and diabetes.
Transporters play a vital role in disposition of certain endogenous compounds such as bile acids (Dawson et al., 2009) and steroid hormones (Janvilisri et al., 2003). Our results demonstrate that Abcc3 and 4 protein expression was increased in livers of DIO mice. Abcc3 and 4 are basolateral efflux transporters that pump various endogenous and exogenous chemicals from hepatocytes to blood; multiple drug and drug metabolites have been identified as substrates for human and mouse ABCC3/Abcc3 and ABCC4/Abcc4 (Kruh and Belinsky, 2003). Induction of Abcc3 and Abcc4 by chemical inducers or nonalcoholic steatohepatitis is associated with altered vectorial excretion of acetaminophen glucuronide (Lickteig et al., 2007a). In contrast with Abcc3 and 4, Abcc1 mRNA expression was decreased in livers of DIO mice. Human ABCC1 transports certain leukotrienes, such as leukotriene C4 (Zhou et al., 2008). Perhaps NAFLD may decrease leukotriene C4 excretion, such that hepatic inflammation is increased.
Transporter expression in ob/ob mice has been described previously (Cheng et al., 2008). ob/ob mice, in general, exhibited decreased uptake of transporter and increased efflux of transporter expression in liver compared with that in C57BL/6 mice (Cheng et al., 2008). Because transporters are responsible for a significant phase of chemical handling, this observed change in transporter expression implies that obese mice should have differential patterns of chemical absorption and elimination. Multiple drugs show altered pharmacokinetics and toxicokinetics in obese patients and the underlying reasons for altered pharmacokinetics in obese patients have not been well defined, but alterations in drug transporter expression could contribute to these observations (Bergman et al., 2007; Edelman et al., 2009). Abernethy et al. (1986) reported altered disposition of nitrazepam in obese human subjects. The elimination half-life of nitrazepam increased significantly in obese individuals compared with that in nonobese subjects. In addition, gentamicin disposition was studied in obese and lean human subjects (Sketris et al., 1981). The volume of distribution was significantly different in obese individuals compared with that in lean individuals. Disposition of vancomycin was also studied in obese and lean patients (Vance-Bryan et al., 1993). Last, antihyperlipidemic therapy by atorvastatin (ATV) was found to increase serum alanine aminotransferase levels more in obese than in lean patients, indicating a possibility for ATV-mediated liver injury (Kiortsis et al., 2003). Multiple transporters are involved in disposition of ATV, including SLCO1B1, SLCO1B3, and ABCC2 (Lau et al., 2006), and perhaps alterations in human transporters with NAFLD or NASH contribute to the latter observation. Given the increased number of persons presenting with NAFLD and NASH and emerging evidence that fatty liver affects transporter expression in humans and rodents (Lickteig et al., 2007a), there is a growing need to better predict drug absorption, distribution, metabolism, and elimination, efficacy, and drug-induced liver injury using rodent models of obesity and diabetes.
The data presented herein demonstrate that transporter expression in livers and kidneys of DIO mice are markedly different from that observed previously with ob/ob and db/db mice. A major difference between the DIO model versus the ob/ob and db/db models is the presence of a functional leptin axis. Leptin is a hormone responsible for maintaining energy homeostasis in the body and hepatic metabolic function. Along with appetite signaling, it also affects insulin secretion from the pancreas. Lack of leptin causes increased secretion of insulin, which leads to insulin resistance, marked obesity, and diabetes (Rabe et al., 2008). DIO mice become overweight/obese because of consumption of a diet high in fat and calories, which causes increased adiposity in adipose and liver tissues, eventually resulting in insulin resistance, but they still possess a functional leptin axis. In contrast, ob/ob mice have a spontaneous mutation in the gene responsible for production of leptin (Ingalls et al., 1950), and db/db mice lack a functional leptin receptor (Hummel et al., 1966). ob/ob and db/db mice also exhibit marked onset of obesity, fatty liver, and hyperglycemia, with serum glucose and insulin levels being approximately double that observed for DIO mice (Lam et al., 2010). Although it is not clear whether leptin has a primary or secondary effect on transporter expression in the liver, it is clear that lack of a functioning leptin pathway results in marked changes in transporter expression compared with that in mice, which possess functional leptin and leptin receptors.
A notable difference in the data presented in this study compared with the results of Cheng et al. (2008) is the lack of alterations observed in kidney transporter expression in DIO mice. Cheng et al. (2008) reported multiple changes in renal expression of slco1a1 and abcc4 transporters in kidneys of ob/ob mice. However, in the current study, we observed a single slight decrease in one transporter (oat2) in kidneys of DIO mice. Few studies have addressed renal expression in models of obesity or NAFLD. One could hypothesize that there are several differences between the DIO and ob/ob models that could contribute to regulation of renal expression, such as circulating glucose, leptin, insulin, and glucagon levels. First, a functional leptin pathway may act on renal cells to regulate gene expression directly. There is some evidence that circulating leptin is important for regulating enzyme expression in kidney. For example, renal 25-hydroxyvitamin D3-24-hydroxylase (CYP24) mRNA synthesis also is markedly elevated in ob/ob and db/db mice, and administration of leptin attenuates this effect (Matsunuma and Horiuchi, 2007; Tsuji et al., 2010). Second, the ob/ob and db/db models typically exhibit higher levels of insulin and glucagon compared with those in DIO mice, and insulin is known to interact with various nuclear hormone receptor-activated pathways (Pandey et al., 2007; Davis et al., 2010).
Abcc transporters are known to be regulated by several nuclear receptors, constitutive androstane receptor (CAR), pregnane X receptor, peroxisome proliferator-activated receptor-α, and nuclear factor E2-related factor 2 (Nrf2, Nfe2l2) (Maher et al., 2008; Gao and Xie, 2010). With regard to NASH, it has been demonstrated that CAR activation plays a role in development of liver injury (Yamazaki et al., 2007). In consideration of certain Abcc transporter regulation by CAR and the relationship between NASH and CAR, there is the possibility of CAR playing a role in altered transporter expression in DIO mice. In addition, circulating and hepatic triglycerides and FFAs are elevated in the DIO model (Masaki et al., 2001), and it is known that the FFAs are ligands for nuclear hormone receptors such as peroxisome proliferator-activated receptor α, which can up-regulate Abcc3 and 4 mRNA expression (Maher et al., 2008). In a previous study, Cheng et al. (2008) demonstrated that nuclear levels of Nrf2 are increased in livers of ob/ob mice. Thus, one possibility for up-regulation of abcc is increased Nrf2 activity in mice with fatty liver. In liver, Nrf2 regulates the expression of abcc3 and 4 (Maher et al., 2008; Okada et al., 2008).
Along with the above-mentioned transcription factors, certain cytokines have also been shown to affect transporter expression. Lipopolysaccharide-induced inflammation was found to increase Abcc1, 3, and 5 expression but decrease Abcc2 and 6 expression in wild-type mice (Lickteig et al., 2007b). In addition, in some studies using human hepatocytes, it was seen that treatment with interleukin-6 decreased expression of ABCC2, ABCG2, and ABCB1 (Vee et al., 2009). In livers of DIO mice, interleukin-1β was increased compared with that in lean mice, indicating the presence of inflammation, which might affect transporter expression.
Because certain transporters are regulated in rodents and humans by similar nuclear receptors and share similar substrates, data from this study as well as from others (Cheng et al., 2008; Lam et al., 2010) suggest that humans who are obese and have diabetes may have altered expression of certain transporters. As is seen in Fig. 8, ABCC4 protein expression was up-regulated in livers of humans with steatosis, which agrees with increased Abcc4 mRNA and protein expression in livers of DIO mice. This result indicates that obese humans, who are more prone to steatosis, might have altered expression of other transporters mentioned in this study. Differences in rodent obesity models and the effect of a functional leptin axis on gene and protein expression should be considered in the selection of rodent models for predicting drug absorption, distribution, metabolism, and elimination and drug-induced liver injury.
Supplementary Material
Acknowledgments
We thank Drs. Michael Goedken, Lauren Aleksunes, Maureen Drisoll, and Jialin Xu for providing valuable input in editing the manuscript. We also thank Dr. Michael Goedken for pathological evaluation of hematoxylin and eosin-stained liver and kidney sections.
This work was supported by the National Institutes of Health National Institute of Environmental Health Sciences [Grants 1R01-ES016042, 5K22-ES013782]; the National Institutes of Health National Center for Research Resources [Grant P20-RR016457-10] (Rhode Island IDeA Network of Biomedical Research Excellence); and the Rhode Island Foundation.
Parts of this work were previously presented at the following conference: More VR and Slitt AL (2010) Transporter expression in db/db and diet-induced obese (DIO) mice. 49th Annual Meeting & ToxExpo; 2010 Mar 7–11, Salt Lake City, UT. Society of Toxicology, Reston, VA.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.110.037507.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
- BMI
- body mass index
- FFA
- free fatty acid
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- DIO
- diet-induced obesity
- SLC/Slc
- solute carrier superfamily
- ABC/Abc
- ATP-binding cassette
- oat
- organic anion-transporting polypeptide
- oct
- organic cation transporter
- PVDF
- polyvinylidene difluoride
- Mcp-1
- monocyte chemoattractant protein-1
- Icam-1
- intracellular adhesion molecule-1
- ATV
- atorvastatin
- CAR
- constitutive androstane receptor
- Nrf2
- nuclear factor E2-related factor 2.
Authorship Contributions
Participated in research design: More and Slitt.
Conducted experiments: More.
Performed data analysis: More.
Wrote or contributed to the writing of the manuscript: More and Slitt.
References
- Abernethy DR, Greenblatt DJ, Locniskar A, Ochs HR, Harmatz JS, Shader RI. (1986) Obesity effects on nitrazepam disposition. Br J Clin Pharmacol 22:551–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes LM, Slitt AM, Cherrington NJ, Thibodeau MS, Klaassen CD, Manautou JE. (2005) Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol Sci 83:44–52 [DOI] [PubMed] [Google Scholar]
- Bergman SJ, Speil C, Short M, Koirala J. (2007) Pharmacokinetic and pharmacodynamic aspects of antibiotic use in high-risk populations. Infect Dis Clin North Am 21:821–846, x [DOI] [PubMed] [Google Scholar]
- Buist SC, Klaassen CD. (2004) Rat and mouse differences in gender-predominant expression of organic anion transporter (Oat1–3; Slc22a6–8) mRNA levels. Drug Metab Dispos 32:620–625 [DOI] [PubMed] [Google Scholar]
- Bush EN, Shapiro R, Nuss M, Kaszubska W, Trevillyan J, Knourek-Segel V, Kennedy M, Adler A, Jirousek M, Jacobson P. (2001) Adiposity, leptin resistance, hyperrphagia, hyperglycemia, glucose intolerance and insulin resistance in C57BL/6J mice fed high fat diets, in Proceedings of the 83rd Annual Meeting and Expo of the Endocrine Society; 2001 Jun 20–23; Denver, CO The Endocrine Society, Chevy Chase, MD [Google Scholar]
- Cheng Q, Aleksunes LM, Manautou JE, Cherrington NJ, Scheffer GL, Yamasaki H, Slitt AL. (2008) Drug-metabolizing enzyme and transporter expression in a mouse model of diabetes and obesity. Mol Pharm 5:77–91 [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher J, Chen C, Klaassen CD. (2005a) Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (Oatps). Drug Metab Dispos 33:1062–1073 [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher J, Dieter MZ, Klaassen CD. (2005b) Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos 33:1276–1282 [DOI] [PubMed] [Google Scholar]
- Cherrington NJ, Slitt AL, Maher JM, Zhang XX, Zhang J, Huang W, Wan YJ, Moore DD, Klaassen CD. (2003) Induction of multidrug resistance protein 3 (mrp3) in vivo is independent of constitutive androstane receptor. Drug Metab Dispos 31:1315–1319 [DOI] [PubMed] [Google Scholar]
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. (1995) Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 122:481–486 [DOI] [PubMed] [Google Scholar]
- Davis RC, Castellani LW, Hosseini M, Ben-Zeev O, Mao HZ, Weinstein MM, Jung DY, Jun JY, Kim JK, Lusis AJ, et al. (2010) Early hepatic insulin resistance precedes the onset of diabetes in obese C57BLKS-db/db mice. Diabetes 59:1616–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Lan T, Rao A. (2009) Bile acid transporters. J Lipid Res 50:2340–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CP, James OF. (1998) Steatohepatitis: a tale of two “hits”? Gastroenterology 114:842–845 [DOI] [PubMed] [Google Scholar]
- Edelman AB, Carlson NE, Cherala G, Munar MY, Stouffer RL, Cameron JL, Stanczyk FZ, Jensen JT. (2009) Impact of obesity on oral contraceptive pharmacokinetics and hypothalamic-pituitary-ovarian activity. Contraception 80:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. (2002) Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288:1723–1727 [DOI] [PubMed] [Google Scholar]
- Gao J, Xie W. (2010) Pregnane X receptor and constitutive androstane receptor at the crossroads of drug metabolism and energy metabolism. Drug Metab Dispos 38:2091–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile CL, Pagliassotti MJ. (2008) The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. J Nutr Biochem 19:567–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho RH, Kim RB. (2005) Transporters and drug therapy: implications for drug disposition and disease. Clin Pharmacol Ther 78:260–277 [DOI] [PubMed] [Google Scholar]
- Hummel KP, Dickie MM, Coleman DL. (1966) Diabetes, a new mutation in the mouse. Science 153:1127–1128 [DOI] [PubMed] [Google Scholar]
- Ingalls AM, Dickie MM, Snell GD. (1950) Obese, a new mutation in the house mouse. J Hered 41:317–318 [DOI] [PubMed] [Google Scholar]
- Janvilisri T, Venter H, Shahi S, Reuter G, Balakrishnan L, van Veen HW. (2003) Sterol transport by the human breast cancer resistance protein (ABCG2) expressed in Lactococcus lactis. J Biol Chem 278:20645–20651 [DOI] [PubMed] [Google Scholar]
- Kiortsis DN, Nikas S, Hatzidimou K, Tsianos E, Elisaf MS. (2003) Lipid-lowering drugs and serum liver enzymes: the effects of body weight and baseline enzyme levels. Fundam Clin Pharmacol 17:491–494 [DOI] [PubMed] [Google Scholar]
- Kruh GD, Belinsky MG. (2003) The MRP family of drug efflux pumps. Oncogene 22:7537–7552 [DOI] [PubMed] [Google Scholar]
- Lam JL, Jiang Y, Zhang T, Zhang Y, Smith BJ. (2010) Expression and functional analysis of hepatic cytochrome P450s, nuclear receptors, and membrane transporters in 10-week and 25-week old db/db mice. Drug Metab Dispos 38:2252–2258 [DOI] [PubMed] [Google Scholar]
- Lau YY, Okochi H, Huang Y, Benet LZ. (2006) Multiple transporters affect the disposition of atorvastatin and its two active hydroxy metabolites: application of in vitro and ex situ systems. J Pharmacol Exp Ther 316:762–771 [DOI] [PubMed] [Google Scholar]
- Lee SA, Wen W, Xu WH, Zheng W, Li H, Yang G, Xiang YB, Shu XO. (2008) Prevalence of obesity and correlations with lifestyle and dietary factors in Chinese men. Obesity (Silver Spring) 16:1440–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickteig AJ, Fisher CD, Augustine LM, Aleksunes LM, Besselsen DG, Slitt AL, Manautou JE, Cherrington NJ. (2007a) Efflux transporter expression and acetaminophen metabolite excretion are altered in rodent models of nonalcoholic fatty liver disease. Drug Metab Dispos 35:1970–1978 [DOI] [PubMed] [Google Scholar]
- Lickteig AJ, Slitt AL, Arkan MC, Karin M, Cherrington NJ. (2007b) Differential regulation of hepatic transporters in the absence of tumor necrosis factor-α, interleukin-1β, interleukin-6, and nuclear factor-κB in two models of cholestasis. Drug Metab Dispos 35:402–409 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275 [PubMed] [Google Scholar]
- Maher JM, Aleksunes LM, Dieter MZ, Tanaka Y, Peters JM, Manautou JE, Klaassen CD. (2008) Nrf2- and PPARα-mediated regulation of hepatic Mrp transporters after exposure to perfluorooctanoic acid and perfluorodecanoic acid. Toxicol Sci 106:319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki T, Yoshimatsu H, Chiba S, Watanabe T, Sakata T. (2001) Central infusion of histamine reduces fat accumulation and upregulates UCP family in leptin-resistant obese mice. Diabetes 50:376–384 [DOI] [PubMed] [Google Scholar]
- Matsunuma A, Horiuchi N. (2007) Leptin attenuates gene expression for renal 25-hydroxyvitamin D3-1α-hydroxylase in mice via the long form of the leptin receptor. Arch Biochem Biophys 463:118–127 [DOI] [PubMed] [Google Scholar]
- Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, Ohtsuki M, Goto N, Takeda K, Utsunomiya H, et al. (2008) Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am J Physiol Gastrointest Liver Physiol 295:G735–G747 [DOI] [PubMed] [Google Scholar]
- Pandey SK, Yu XX, Watts LM, Michael MD, Sloop KW, Rivard AR, Leedom TA, Manchem VP, Samadzadeh L, McKay RA, et al. (2007) Reduction of low molecular weight protein-tyrosine phosphatase expression improves hyperglycemia and insulin sensitivity in obese mice. J Biol Chem 282:14291–14299 [DOI] [PubMed] [Google Scholar]
- Rabe K, Lehrke M, Parhofer KG, Broedl UC. (2008) Adipokines and insulin resistance. Mol Med 14:741–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinger RN. (2009) Fat storage and the biology of energy expenditure. Transl Res 154:52–60 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Martín A, Novalbos Ruiz JP, Martínez Nieto JM, Escobar Jiménez L. (2009) Life-style factors associated with overweight and obesity among Spanish adults. Nutr Hosp 24:144–151 [PubMed] [Google Scholar]
- Schuster DP. (2009) Changes in physiology with increasing fat mass. Semin Pediatr Surg 18:126–135 [DOI] [PubMed] [Google Scholar]
- Sketris I, Lesar T, Zaske DE, Cipolle RJ. (1981) Effect of obesity on gentamicin pharmacokinetics. J Clin Pharmacol 21:288–293 [DOI] [PubMed] [Google Scholar]
- Tsuji K, Maeda T, Kawane T, Matsunuma A, Horiuchi N. (2010) Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1α,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J Bone Miner Res 25:1711–1723 [DOI] [PubMed] [Google Scholar]
- Uauy R, Albala C, Kain J. (2001) Obesity trends in Latin America: transiting from under- to overweight. J Nutr 131:893S–899S [DOI] [PubMed] [Google Scholar]
- Vance-Bryan K, Guay DR, Gilliland SS, Rodvold KA, Rotschafer JC. (1993) Effect of obesity on vancomycin pharmacokinetic parameters as determined by using a Bayesian forecasting technique. Antimicrob Agents Chemother 37:436–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vee ML, Lecureur V, Stieger B, Fardel O. (2009) Regulation of drug transporter expression in human hepatocytes exposed to the proinflammatory cytokines tumor necrosis factor-α or interleukin-6. Drug Metab Dispos 37:685–693 [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Kakizaki S, Horiguchi N, Sohara N, Sato K, Takagi H, Mori M, Negishi M. (2007) The role of the nuclear receptor constitutive androstane receptor in the pathogenesis of non-alcoholic steatohepatitis. Gut 56:565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SF, Wang LL, Di YM, Xue CC, Duan W, Li CG, Li Y. (2008) Substrates and inhibitors of human multidrug resistance associated proteins and the implications in drug development. Curr Med Chem 15:1981–2039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.