Abstract
Bispecific antibodies that bind cell-surface targets as well as digoxigenin (Dig) were generated for targeted payload delivery. Targeting moieties are IgGs that bind the tumor antigens Her2, IGF1R, CD22, or LeY. A Dig-binding single-chain Fv was attached in disulfide-stabilized form to C termini of CH3 domains of targeting antibodies. Bispecific molecules were expressed in mammalian cells and purified in the same manner as unmodified IgGs. They are stable without aggregation propensity and retain binding specificity/affinity to cell-surface antigens and Dig. Digoxigeninylated payloads were generated that retain full functionality and can be complexed to bispecific antibodies in a defined 2∶1 ratio. Payloads include small compounds (Dig-Cy5, Dig-Doxorubicin) and proteins (Dig-GFP). Complexed payloads are targeted by the bispecifics to cancer cells and because these complexes are stable in serum, they can be applied for targeted delivery. Because Dig bispecifics also effectively capture digoxigeninylated compounds under physiological conditions, separate administration of uncharged Dig bispecifics followed by application of Dig payload is sufficient to achieve antibody-mediated targeting in vitro and in vivo.
Keywords: dsFv, imaging, immunotoxin, protein engineering
Bispecific antibodies simultaneously bind two different antigens and can be applied to block two targets on cell surfaces to improve therapeutic efficacy (1–3). Recognition of two targets may also increase targeting specificity toward tissues or tumors that express both antigens (4–6). Bispecifics that bind tumor markers and effector cells (e.g., CD3) can also be used in immunotherapy to activate effector cells at tumors (7). Bispecifics are also applicable for payload delivery. In particular in oncology, antibodies can be combined with cytotoxic entities that have limited selectivity. Thereby, highly active but nonspecific payloads become enriched on desired tissues. Some immunotoxins, like the Her2 binding Trastuzumab-DM1 conjugate, show promising results in clinical trials (8). Antibody derivatives including bispecifics are also applied for radiotherapy or imaging (9, 10). So far, most targeting concepts base upon chemical conjugation of payloads or on generation of fusion proteins (11–14). Bispecific antibodies can also be applied for this task. One option to achieve targeted delivery is conjugation of haptens to payload, and subsequent complexion with hapten-binding bispecific antibodies. Complexion via antibody-antigen interaction AVOIDS chemical modification of antibodies and thereby reduces risks of inactivating the targeting entity or generating immunogenic sites within the protein. There is still a conjugation step of attaching hapten to payload, but this procedure can be performed by standard technologies. Bispecifics can bind various haptenylated payloads—or vice versa, one payload can be hooked up to hapten bispecs with different specificities. Thus, such targeting systems are highly versatile. One well-known hapten is digoxigenin. Coupling reagents/kits for digoxigeninylation are available for many applications (15–18), including coupling to low molecular weight compounds such as fluorophores, chelating agents, chemotherapeutics, nucleic acids, lipids, nanoparticles, or peptides and proteins (18). Furthermore, antibodies are available that bind digoxigenin with high affinity.
Results
Generation and Structure of a Humanized Digoxigenin-Binding Antibody.
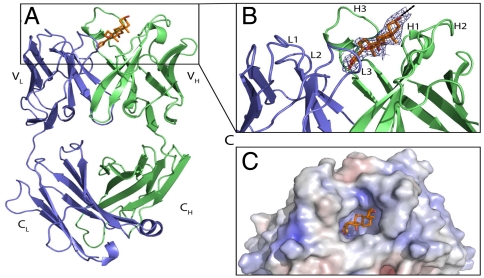
Recombinant modules that allow specific high-affinity binding to digoxigenin and digoxigeninylated compounds were derived from an antibody (IgG1/kappa) that is produced by a murine hybridoma (18). VH- and VL-encoding sequences were isolated by RT-PCR from hybridoma mRNA (details in SI Text), subsequently humanized and converted to disulfide-stabilized single-chain Fvs. Our humanization procedure based on complementarity determining region (CDR) grafting with back-mutations (19, 20) (see below). To support our humanization procedure and to optimize the design of digoxigenin (Dig) payloads, we obtained a structure of murine anti-Dig Fab bound to the fluorescent payload Dig-Cy5. The structure at 2.8-Å resolution (Fig. 1) shows that Dig is bound in a deep pocket that is in part formed by a long CDR-H3. Hydrophobic residues, in particular four VH tyrosines, are stacked toward Dig. Dig is almost completely buried in the binding pocket; its linker to Cy5 faces outward. The linker and Cy5 are not visible in the electron density map, indicating that these parts of Dig-Cy5 are flexible and not attached to the protein.
Fig. 1.
Structure of the digoxigenin-binding antibody. (A) Complex of the murine anti-Dig Fab (chain L in blue; chain H in green) with bound Dig. (B) Zoom in on CDR. The final 2FOFC electron density map around Dig moiety is shown as blue mesh countered at 1σ. The dashed line indicates the direction of the linker to Cy5. (C) Electrostatic surface potential of murine Fab with bound Dig. More details are provided in SI Text.
Digoxigeninylation of Payloads for Targeted Delivery.
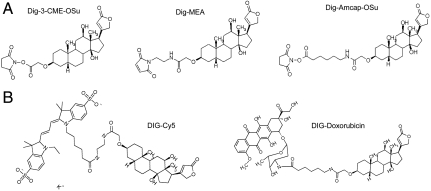
Digoxigeninylation of peptides, proteins, fluorophores, low molecular weight compounds, or nucleic acids has been achieved via N-hydroxysuccinimide (NHS) ester conjugation to amines, or via maleinemide conjugation to thiols (Fig. 2). The conjugation reagents also incorporate spacer elements of variable length. Sufficient length and flexibility of the spacer between Dig and payload is important to prevent the payload from interfering with Dig binding. The structure model (Fig. 1) indicates that Dig is buried in a deep pocket and that the atoms of Dig that are closest to the surface are still approximately 10 Å buried in the Fv. At least 10 Å must be bridged between Dig and payload to extend above the surface of the Fv; further extension by additional flexible spacers of 5 to 10 Å will facilitate payload access. These distances can be achieved by linear flexible linker elements of 10–13 C atoms (15–20 Å, Fig. 2). To analyze the influence of Dig labeling on the properties of the conjugated molecules, we generated digoxigenylated Cy5 and Doxorubicin (Dig-Cy5, Dig-Dox, Fig. 2), and Dig-eGFP. These compounds have fluorescent properties that are not affected by digoxigeninylation; neither did complexion with Dig-bispecs interfere with their fluorescence (Fig. S1). Because Doxorubicin is a compound that intercalates into DNA, we investigated if Dig-Dox retains this functionality. When nuclei were incubated with doxorubicin or Dig-Dox, both compounds intercalated into DNA (Fig. S2). We conclude from these results that payloads retain functionality upon conjugation to Digoxigenin and complexion with anti-Dig antibodies.
Fig. 2.
Digoxigeninylation of payloads. (A) NHS ester-based or maleinemide-based precursors with spacer elements of variable length for Dig conjugation to amines or thiols. (B) Dig-Cy5 and Dig-Dox. Details of digoxygeninylation procedures are provided in SI Text.
Bispecifics Combine Cell-Surface Targeting and Dig-Binding Entities.
The design of bispecific antibodies was based on tetravalent “Morrison” formats with single-chain Fv modules (scFvs) fused to C termini of IgG heavy chains (21). Cell-surface binding functionalities were located in the two normal Fab arms of the IgG, and anti-Dig modules were fused to the heavy chains as scFvs. VH and VL of scFvs were held together by a flexible linker peptide and additionally by an interchain disulfide bond between VH and VL (VHCys44 to VLCys100) to enhance stability (22–24) (Fig. 3 and Fig. S3). The cell-targeting entities were derived from IgGs that bind surface antigens: Her2 (25), IGF1-receptor (26, 27), CD22 (28), and the LeY carbohydrate antigen [B3 (29, 30)]. Modules for the Dig specificity were derived from the humanized anti-Dig antibody. Monospecific IgGs (Dig, LeY, IGF1R, Her2, and CD22) were generated as controls in the same manner as the bispecifics.
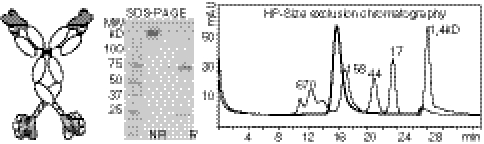
Fig. 3.
Composition and purification of bispecific antibody derivatives. The composition of Dig-binding bispecifics containing dsscFvs is schematically shown (Left): The scFv modules fused to C termini of H chains are connected by linkers composed of (Gly4Ser) modules; each square of the linker stands for one (Gly4-Ser) module. Protein homogeneity and correct size is demonstrated by SDS-PAGE of nonreduced (NR) and reduced (R) samples compared to MW standard (Center) and by HPLC-SEC (bold line, Right). Protein standards are overlayed in the HPLC run; sizes are indicated in kDa. Further details of purification and analytical procedures are provided in SI Text.
Expression, Purification, and Biophysical Properties of Mono- and Bispecific anti-Dig Antibodies.
Plasmids encoding L chains and H chains were transiently cotransfected into HEK 293 cells in suspension. Supernatants were harvested one week later. These supernatants could be frozen and stored at -20 °C before purification. Bispecifics were purified by protein A and size exclusion chromatography (SEC) in the same manner as conventional IgGs as they were fully competent to bind protein A. Expression yields of properly stabilized bispecs were similar to those of transiently expressed Dig IgG (7–40 mg/L, Table S1). The yield strongly depended on the stabilizing VHcys44-VLcys100 disulfide within the Dig-binding moieties; without disulfide stabilization, proteins had a high tendency to aggregate and yields were strongly reduced compared to parent antibody (Fig. S3).
Purified disulfide-stabilized bispecifics were obtained with yields between 6 and 36 mg homogenous protein (< 2–5% aggregates) per liter supernatant. Stability analyses did not indicate any unusual temperature-dependent disintegration or aggregation (Table S1). Size, homogeneity, and composition of all proteins were confirmed by high performance (HP) SEC and SDS-PAGE. HP-SEC elution profiles of anti-Dig IgG, IGF1R-Dig, and Her2-Dig (with disulfide-stabilized Dig scFv) show nonaggregated proteins of defined sizes and expected protein compositions on SDS-PAGE (Fig. 3). Mass spectrometry (Table S1) confirmed the correct composition of all proteins.
Dig-Bispecifics Bind Cell-Surface Antigens and Digoxigenin.
Surface plasmon resonance (SPR) was applied to compare the affinities of the bispecifics and parental IgGs to cellular targets and digoxigenin. These analyses revealed that the low nanomolar affinity of the parental humanized anti-Dig IgG (e.g., toward monodigoxigeninylated myoglobin, Table S2) was fully retained in bispecific fusions. Dig-binding functionality was independent of the additional targeting moiety that had been attached. Binding to the extracellular domain of IGF1R was determined to confirm functionality of surface targeting modules. In these experiments, no difference in affinity toward IGF1R between the parental IgG and the IGF1R-Dig bispecific was observed. Thus, the affinity of both binding entities of the bispecific IGF1R-Dig were comparable to their parent antibodies. Additional SPR experiments demonstrated simultaneous binding of both antigens. For example, Her2-Dig bispecifics can be captured to a Biacore chip by its Fc portion, thereafter bind the extracellular domain of Her2, and subsequently bind digoxigeninylated payload (Fig. S4). Finally, we addressed the Fc-receptor binding functionality of the Dig bispecifics via SPR and compared it with that of unmodified monospecific IgG. These experiments (anti-Her2 vs. Her2-Dig in Fig. S4) revealed that Dig bispecifics bind to FcRn and FcgRIIIa in the same manner as unmodified IgG.
Charging of Dig Bispecifics with Digoxigeninylated Payloads.
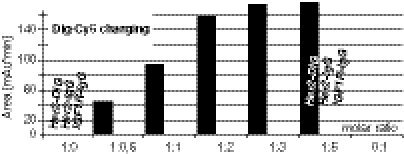
Because the anti-Dig modules bind Dig payloads under physiological conditions, we were able to apply a simple and robust payload-charging procedure to generate targeting complexes: Digoxigeninylated payload (Dig-Dox, Dig-Cy5, or Dig-proteins) and bispecifics were mixed to a 2∶1 molar ratio and incubated for 10 min at room temperature. The resulting complex was subsequently applied for analytical and functional assays without further modification. Tetravalency of our bispecifics causes two payload molecules to be bound, provided complete charging and sufficient complex stability after charging. A stoichiometry > 2 (payload over bispecific) would indicate nonspecific interactions between payload and antibody; ratios < 2 would indicate incomplete charging or complex instability. To analyze charging efficacy, we added various amounts of Dig-Cy5 to Her2-Dig bispecifics and analyzed the complexion reaction by size exclusion chromatography to separate small uncomplexed from large antibody-complexed Dig-Cy5 (Fig. 4): At charging ratios of less than two Dig payloads per antibody, all fluorescence binds the antibody and signals of proteinous fractions increase linearly in a dose-dependent manner. At a ratio of 2, charging reaches a plateau and raising the concentrations of Dig-Cy5 above the 2∶1 ratio only marginally increases IgG-complexed fluorescence signals (Fig. 4 and Fig. S4). Incubation of Dig-Cy5 with a monospecific anti-Her2 IgG or anti-IGF1R IgG did not result in any complex formation (Fig. 4). These results indicate charging with a defined stoichiometry of two payloads per antibody.
Fig. 4.
Stoichiometry of payload charging. Dig-Cy5 titrated against Her2-Dig bispecifics or Her2 or IGF1R monospecific IgG. Complexed Dig-Cy5 was detected by SEC with a fluorescence detector (experimental details in Fig. S4).
Delivery of Digoxigeninylated Fluorescent Compounds in Vitro.
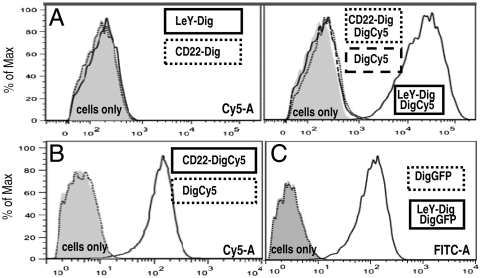
Dig-Cy5 is traceable upon accumulation on target cells and can be detected by FACS. Fig. 5A shows FACS analyses of MCF-7 breast cancer cells that express high levels of the carbohydrate antigen LeY, intermediate levels of IGF1R and Her2, and do not express the CD22 antigen (Fig. S5). Incubation of these cells with only bispecifics (LeY-Dig, CD22-Dig), or with uncomplexed Dig-Cy5, generated no significant Cy5-attributable signal. In contrast, exposure to antibody-complexed Dig-Cy5 revealed target-specific accumulation of fluorescence. MCF-7 cells become labeled upon exposure to Dig-Cy5 complexed with LeY-Dig. Similarly, cell-associated Cy5 signals were detected upon exposure of MCF-7 to Cy5-complexed Her2-Dig or IGF1R-Dig, which both recognize antigens that are present on MCF-7. Targeting toward these antigens results in reduced signals compared to LeY targeting because the antigen density of LeY is higher than those of Her2 and IGF1R. Dig-Cy5 complexed to CD22-Dig generated no cell-associated fluorescence because MCF-7 cells do not express detectable levels of CD22. In another experiment we applied CD22-Dig modules to Raji cells. Raji is a lymphoblastoid (Burkitt) cell line that expresses CD22 but does not express significant levels of LeY antigen. Upon exposure to Raji, the CD22-Dig/Dig-Cy5 complexes (negative on MCF-7) gave clear signals in the Cy5 channel (Fig. 5B). Thus, recruiting of payloads to target cells by the targeting bispecific is a general feature that can be applied to different antibodies, antigens, and cells. To demonstrate delivery of large molecules, digoxigeninylated eGFP (27-kDa protein) was complexed with LeY-Dig and applied to MCF-7. Fig. 5C shows that uncomplexed Dig-eGFP does not generate significant cell-associated signals. However, Dig-eGFP complexed with LeY-Dig bispecifics becomes targeted to and fluorescently labels MCF-7. These data prove that Dig bispecifics are applicable as a targeting platform for various cell-surface antigens, cell types, and payloads of different size.
Fig. 5.
Delivery of Dig-payloads. (A) MCF-7 were incubated in the absence (cells only) or presence of Dig-bispecifics targeting LeY (LeY-Dig) or CD22 (CD22-Dig) and analyzed by FACS in absence or presence of Dig-Cy5. (B) Raji incubated in absence or presence of Dig-Cy5 or Dig-Cy5 complexed CD22-Dig. (C) MCF-7 incubated with Dig-eGFP or Dig-eGFP complexed with LeY-Dig analyzed in the FITC channel. Details of the detection procedures are provided in SI Text.
Internalization of Targeted Payloads.
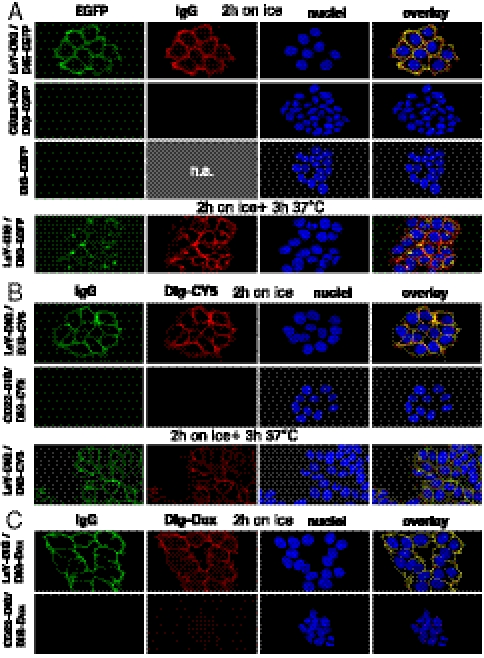
Confocal microscopy was applied to track the fate of Dig-eGFP, Dig-Cy5, and Dig-Dox upon targeting to the surface of MCF-7 cells. These payloads can be detected on and in cells by fluorescence (Fig. S1); simultaneously, the localization of bispecifics can be tracked by labeled secondary antibodies (SI Text). MCF-7 were exposed to LeY-Dig complexed with Dig-eGFP, Dig-Cy5, or Dig-Dox and subsequently subjected to confocal microscopy (Fig. 6 and Fig. S6): Exposure of MCF-7 to the antibody-complexed eGFP (on ice for 2 h to prevent internalization) generates initially a strong signal on the cell surface (Fig. 6A). At this stage, bispecifics (detected via labeled anti-human IgG) and the fluorescence of complexed Dig-eGFP colocalized on the cell surface. Further incubation at 37 °C demonstrated internalization of complexes into intracellular vesicular structures with a minor pool of surface-bound signal (Fig. 6A for 3 h and Fig. S6 for 6 h). This indicates that the payload was cointernalized with the antibody. Antigen specificity of these effects was demonstrated by microscopy of cells that were exposed to CD22-Dig complexed Dig-eGFP or to uncomplexed Dig-eGFP. As MCF-7 cells do not express detectable levels of the CD22, and because eGFP by itself does not bind to MCF-7, neither of these experiments resulted in significant fluorescent signals (Fig. 6A). The same results were obtained when the cell surface and intracellular distribution of Dig-Cy5 or Dig-Dox was analyzed: Dig-Cy5 is effectively delivered to LeY-antigen expressing MCF-7 via LeY-Dig bispecifics (Fig. 6B). In contrast, no fluorescent signals were detected on cells that were exposed to CD22-Dig complexes (CD22 is not present on MCF-7) or to uncomplexed Dig-Cy5. Fig. 6C shows the specific delivery of Dig-Dox to MCF-7. Doxorubicin is a cytotoxic compound that penetrates cells and intercalates into DNA (Fig. S2). Doxorubicin is cell permeable, but Dig-Dox does not effectively penetrate cell membranes probably due to its increased size and physicochemical properties. Because of that, exposure of cells to Dig-Dox displayed very little cellular uptake, virtually no intracellular or nuclear signal, and only a very small Dig-Dox signal was detectable in endocytic compartments (Fig. S2B). However, when Dig-Dox complexed with targeting bispecifics was applied, we observe surface association and strong vesicular staining. Again, accumulation was observed only with bispecifics that bound to the surface of MCF-7. These results are consistent with those of our FACS analyses: Payloads are targeted to cells by the bispecific antibodies and can become cointernalized. Furthermore, cellular uptake of Dig payload occurs independent of its chemical composition, charge, or size.
Fig. 6.
Targeting and internalization of payloads. (A) MCF-7 incubated on ice for 2 h with 40 nM LeY-Dig or 40 nM CD22-Dig complexed with Dig-eGFP, or with uncomplexed Dig-eGFP. Cells were fixed (Upper) or incubated for another 3 h at 37 °C (Lower). CY3-labeled goat anti-human IgG was used for visualization by confocal laser scanning microscopy. (B) MCF-7 incubated as in A with Dig-Cy5 as payload. (C) MCF-7 incubated as in A with Dig-Dox as payload. A detailed description of the procedures is provided in SI Text.
Delivery of Digoxigeninylated Payloads in Vivo.
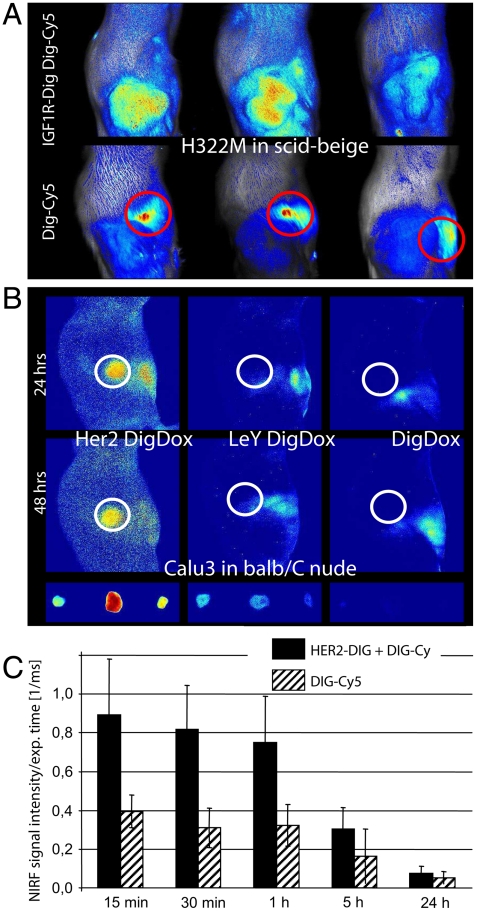
Cy5 emits light in the near-infrared spectrum, and it can therefore be visualized in vivo by near-infrared fluorescence (NIRF) imaging. We tested the in vivo applicability of our delivery platform by NIRF imaging of antibody-targeted Dig-Cy5 in tumor xenografts. Models that we applied were H322M [nonsmall cell lung cancer (NSCLC)] cells subcutaneously implanted in SCID beige mice that express high levels of IGF1R, orthotropic implanted KPL-4 (breast cancer) cells with high expression of Her2, and Calu3 (lung adenocarcinoma) cells that also display high levels of Her2. We injected 50-μg preformed 2∶1 complexes of Dig-Cy5 and bispecific intravenously followed by near-infrared fluorescence (NIRF) imaging. Uncomplexed Dig-Cy5 was applied as a control. The results of these studies are shown in Fig. 7A: 30 min after injection of 50 μg 2∶1 complexes into mice with H322M xenografts, specific accumulation of Dig-CY5 at the tumor was observed. Bound complexes are detectable until at least 4 h after injection (Fig. S7). Tumor accumulation was antibody-mediated because uncomplexed Dig-Cy5 did not cause signal increases at the location of the xenograft. Instead, Dig-Cy5 became eliminated rapidly, indicated by staining of the liver (circles in Fig. 7A, Lower). Comparable results were achieved by application of Dig-Cy5 complexed Her2-Dig into Her2 expressing KPL4 xenografts. NIRF imaging performed 24 h after injection revealed targeted accumulation of Dig-Cy5 (Fig. S7). Dig-Dox can be detected by NIRF in a similar manner as Dig-Cy5. Targeting complexes containing Dig-Dox (Dig-Dox with Her2-Dig or LeY-Dig in 2∶1 ratios) were injected into mice with Calu3 xenografts and subjected to NIRF imaging 24 h thereafter. The animals were sacrificed 48 h after injection for ex vivo imaging of the tumors. This study (Fig. 7B) revealed targeted accumulation of Dig-Dox at the Her2-positive xenografts. Explanted tumors from the group that was treated with Her2-Dig/Dig-Dox confirmed tumor accumulation (Fig. 7B, Lower). Tumor accumulation of Dig-Dox was antibody-mediated because injection of Dig-Dox without bispecifics did not generate significant signal increases at the xenograft in vivo or in explanted tumors. Antibody-mediated targeting specificity was further confirmed by application of Dig-Dox complexed with LeY-Dig. The LeY antigen is not present in detectable levels in the Calu3 xenograft, and, consequently, application of Dig-Dox complexed with LeY-Dig did not generate significant signal increases at the xenograft in vivo or in the explanted tumors.
Fig. 7.
Payload targeting in vivo. (A) IGF1R-Dig bispecifics charged with Dig-Cy5 were applied to H322M xenografts. IGF1R-Dig carries Dig-Cy5 to the xenograft, Dig-Cy5 without antibody appears in liver (circles) and becomes eliminated (Fig. S7). (B) Her2-Dig bispecifics specifically targets Dig-Dox to Calu3 xenografts. (C) Preapplication of bispecifics followed by Dig-Cy5 leads to specific capture of Dig-Cy5 at the tumor.
In Vivo Recruitment of a Dig-Cy5 by Pretargeted Antibodies.
In FACS experiments, targeted delivery was achieved by application of preformed complexes, but also by separate application of targeting vehicles and payload. Can this principle also be applied in vivo? That is, can we apply bispecifics and Dig payloads as separate entities and still achieve payload targeting? To address these questions, we injected i.v. 50 μg of uncomplexed bispecifics followed 48 h later with Dig payload. This approach enables pretargeting of bispecifics to the xenograft and subsequent capture and enrichment of the payload by the bispecific antibody. This option was evaluated with Her2-Dig bispecifics and Dig-Cy5 in KPL4 xenografts: One group of tumor-bearing mice received Her2-Dig, another (control) group buffer only. Forty-eight hours later, a tenfold molar surplus of Dig-Cy5 was applied to both groups, and the fluorescence of the xenograft was monitored at different times. The results of this study (Fig. 7C) demonstrate that preapplication of bispecifics followed by separate application of Dig-Cy5 mediates tumor accumulation of Dig-Cy5. In control animals that were injected with Dig-Cy5 but not with bispecifics, tumor accumulation was not observed. This demonstrates that payload complexion with targeting vehicles prior to application is not an essential requirement. Pretargeted antibodies are capable to capture at the tumor the small entities that are separately applied. Our results show that Dig bispecifics deliver complexed payloads to target tissues in vivo and can also mediate site-specific recruitment of systemically applied payloads.
Discussion
We have demonstrated payload delivery in vitro and in vivo with bispecifics that contain target- and Dig-binding modules. Payloads that are attached to these bind to antigen-expressing cells and can become cointernalized with the targeting vehicle. This, as well as the option to complex a wide variety of payloads to different targeting vehicles, makes anti-Dig bispecifics a versatile platform for diagnostic applications and targeted therapy. The Dig-binding module is a humanized derivative of a murine IgG that is applied as an ingredient in laboratory kits and diagnostic assays (15–18). Availability of its protein structure (Fig. 1) now gives insight into its high-affinity binding mode. Structural information also paves the way for further modification or improvements of this antibody. A delivery platform that bases on an antibody system that has been around for more than 20 years has many advantages, including commercial availability of reagents and existence of robust procedures for digoxigeninylation of chemical compounds, peptides, lipids, proteins, and nucleic acids (18).
The field of antibody-drug conjugates and immunotoxins has evolved beyond niche indications in the last few years and will most likely be part of the next wave of advanced therapeutics. For broad applicability, however, overcoming limiting technical hurdles is essential. The components of the bispecific delivery platform that use full-length IgG’s as the “backbone” can be produced and handled with processes that are fully compatible with existing industrial production and analytic technologies for antibody-based drugs and drug payloads. For targeted therapy, it is also essential to identify optimal combinations of targeting antibodies and payloads and to provide scientific rationales for those choices. For such tasks, a modular targeting platform that allows instant switching of different targeting modules and payloads (without any additional steps) is an ideal approach. Similarly, switching or coapplication of payloads or targeting vehicles can easily be achieved.
Compared to other technologies, such as chemical conjugation to lysines, cysteines, or carbohydrates, highlights of the favorable features of our platform follow: (i) payloads can be connected to the targeting moieties by a simple generic charging reaction, which results in defined charging ratios with regard to number of attached payloads and positions of attachment. Thus, production and handling of targeting complexes is easier than chemical coupling; (ii) payload charging occurs under physiological conditions without covalent alteration of the targeting vehicle. Thereby, the risk to interfere with the target binding functionality of the remainder of the molecule is minimized; (iii) payloads are complexed without covalent modification of the targeting vehicle, which minimizes the risk to generate immunogenic epitopes within targeting vehicles (covalently linked nonnatural payloads do not fully disintegrate upon proteolytic processing and are presented via major histocompatibility complex class II).
Digoxigenin is pharmacologically active: It is a plant toxin used to treat heart conditions, but which at higher doses also interferes with heart functionality (31). Despite that, we have not observed any adverse effects of our system in vivo, which can be explained by a combination of parameters: (i) Digoxigenin is an aglycon and less potent than digoxin; (ii) Dig is always conjugated to another payload that further decreases its remaining activity or tissue accessibility; (iii) payload complexes are applied in rather low molar concentrations; (iv) Dig is stably bound and completely covered by the capture modules and therefore not available as active entity. It is well known that the activity of Digoxin is blocked by complexation with antibodies; digoxin-specific antibody fragments are clinically applied to treat Dig overdoses (32).
Payload complexation under physiological conditions enables us to use our delivery platform not only with preformed entities, but also via consecutive delivery of separate modules. This mode of application (not applicable to covalent conjugates) may be useful for imaging, radiotherapy, or targeted therapy with vehicles of limited tissue penetration that require sufficient time for accumulation on target tissues. For such applications, FcRn-binding functionality assures long half-lives of the bispecific antibody derivatives. Small fluorescent, radioactive, or cytotoxic payloads with short serum half-life can be applied thereafter; unbound material will be rapidly cleared. This minimizes systemic exposure, e.g., to radioactivity, in comparison to covalent antibody conjugates that have a long half-life. Of particular interest may also be the option of repeated in vivo “recharging” of targeting vehicles with small payloads that may be applied orally. Stability and long half-lifes of IgG-based bispecific targeting vehicles can also be advantageous for targeting antigens that are rapidly internalized from cell surfaces because (i) they are available days after injection and (ii) they may recycle after internalization to the cell surface (with or without antigen). Therefore, sufficient antibody will be available for hapten binding a subsequent targeting also at later time points.
Delivery of cytotoxics is one application that may benefit from a bispecific targeting platform. Stable noncovalent payload connections may pose less immunogenicity risks compared to covalent antibody conjugates. Noncovalent connection also supports intracellular payload release. Many covalently linked IgG-toxin conjugates require either degradation of the antibody moiety (33) or complex cleavage reactions to release active payload. Cleavable linkers can be associated with systemic instability and hence undesired premature payload release (34). Dig-payload complexes provide an attractive alternative to conventional antibody drug conjugates. Stable under physiological conditions with low probability of premature release, they may effectively release payloads upon internalization (which follows the same mode and kinetics as that of the corresponding unmodified antibody). Another advantage of bispecifics derived from full-length IgGs is that their FcgRIIIa-binding functionality is fully retained. Thereby, the vehicles by themselves fulfill the RcgRIIIa binding requirements for antibody-dependent cell mediated cytotoxicity (ADCC) competence. This may further enhance therapeutic efficacy.
Release of cytotoxic payloads can be a first step for intracellular activity, but by itself does not guarantee potency if the target for delivered compounds is cytoplasmic or nuclear. In such cases, compounds must escape from vesicular compartments. Our Dig-Dox targeting experiments demonstrate that this is a major hurdle: Doxorubicin is a potent chemotherapeutic that enters cells and intercalates into DNA. In contrast, Dig-Dox looses its drug-like character [as of Lipinsky’s “rule of 5” (35)]. It accumulates on and in cells (Fig. S2), but remains in intracellular compartments, most likely because of its size. In consequence, targeted Dig-Dox is not cytotoxic.
Our platform is applicable for in vivo targeting of fluorescent or radioactive payloads, and for payloads that are active at cell surfaces or within accessible internalized compartments. In its current format, our platform has limitations for payloads that address cytoplasmic or nuclear targets but cannot penetrate membranes. For such applications, further optimization is needed to add functionalities that enable entry into the cytoplasm of target cells.
Methods
Bispecific antibodies were generated, purified, and characterized as described in SI Text. Dig payloads were generated by chemically coupling NHS- or maleimide-Dig (SI Text). Crystals for structure determination of anti-Dig-Fab were obtained by hanging drop vapor diffusion. Diffraction data were collected at X06SA (Swiss Light Source), integrated, and scaled with X-ray diffraction spectroscopy. Crystals diffracted to a resolution of 2.8 Å (details in SI Text). In vitro targeting was detected by FACS or confocal microscopy (details in SI Text). In vivo experiments with SCID beige or balbC/nude mice haboring KPL4, H322M, or Calu3 xenografts were performed in an Association for Assessment and Accreditation of Laboratory Animal Care International approved facility as described in SI Text.
Supplementary Material
Acknowledgments.
We thank M. Antony, K. Mayer, C. von Schwerin, A. Adlberger, D. Matscheko, M. Mehwald, F. Osl, S. Schneid-Müller, H. Seul, and C. Wagner for excellent technical assistance.
Footnotes
Conflict of interest statement: All authors except K.-P.H. and A.L. are employed by Roche, and the contributions of K.-P.H. and A.L. were supported by Roche.
*This Direct Submission article had a prearranged editor.
Data deposition: The crystallography, atomic coordinates, and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3RA7).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018565108/-/DCSupplemental.
References
- 1.Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol. 2010;10:345–352. doi: 10.1038/nri2747. [DOI] [PubMed] [Google Scholar]
- 2.Joosten LA, Helsen MM, van de Loo FA, van den Berg WB. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice: A comparative study using anti-TNFalpha, anti-IL-1alpha/beta and IL-1Ra. Arthritis Rheum. 2008;58:S110–S122. doi: 10.1002/art.23363. [DOI] [PubMed] [Google Scholar]
- 3.van den Berg WB, Joosten LA, Helsen M, van de Loo FA. Amelioration of established murine collagen-induced arthritis with anti-IL-1 treatment. Clin Exp Immunol. 1994;95:237–243. doi: 10.1111/j.1365-2249.1994.tb06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu D, et al. Simultaneous blockade of both the epidermal growth factor receptor and the insulin-like growth factor receptor signaling pathways in cancer cells with a fully human recombinant bispecific antibody. J Biol Chem. 2004;279:2856–2865. doi: 10.1074/jbc.M310132200. [DOI] [PubMed] [Google Scholar]
- 5.Lu D, et al. A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J Biol Chem. 2005;280:19665–19672. doi: 10.1074/jbc.M500815200. [DOI] [PubMed] [Google Scholar]
- 6.Muller D, Kontermann RE. Bispecific antibodies for cancer immunotherapy: Current perspectives. BioDrugs. 2010;24:89–98. doi: 10.2165/11530960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Linke R, Klein A, Seimetz D. Catumaxomab: Clinical development and future directions. MAbs. 2010;2:129–136. doi: 10.4161/mabs.2.2.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krop IE, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28:2698–2704. doi: 10.1200/JCO.2009.26.2071. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg DM, et al. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J Clin Oncol. 2006;24:823–834. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg DM, et al. Cancer imaging and therapy with bispecific antibody pretargeting. Update Cancer Ther. 2007;2:19–31. doi: 10.1016/j.uct.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldsmith SJ. Radioimmunotherapy of lymphoma: Bexxar and Zevalin. Semin Nucl Med. 2010;40:122–135. doi: 10.1053/j.semnuclmed.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Kreitman RJ. Immunotoxins in cancer therapy. Curr Opin Immunol. 1999;11:570–578. doi: 10.1016/s0952-7915(99)00005-9. [DOI] [PubMed] [Google Scholar]
- 13.Ronca R, Sozzani S, Presta M, Alessi P. Delivering cytokines at tumor site: The immunocytokine-conjugated anti-EDB-fibronectin antibody case. Immunobiology. 2009;214:800–810. doi: 10.1016/j.imbio.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Wu AM, Senter PD. Arming antibodies: Prospects and challenges for immunoconjugates. Nat Biotechnol. 2005;23:1137–1146. doi: 10.1038/nbt1141. [DOI] [PubMed] [Google Scholar]
- 15.Holtke HJ, et al. Non-radioactive labeling and detection of nucleic acids. II. Optimization of the digoxigenin system. Biol Chem Hoppe Seyler. 1990;371:929–938. doi: 10.1515/bchm3.1990.371.2.929. [DOI] [PubMed] [Google Scholar]
- 16.Holtke HJ, et al. The digoxigenin (DIG) system for non-radioactive labelling and detection of nucleic acids—An overview. Cell Mol Biol. 1995;41:883–905. [PubMed] [Google Scholar]
- 17.Kessler C, et al. Non-radioactive labeling and detection of nucleic acids. I. A novel DNA labeling and detection system based on digoxigenin: Anti-digoxigenin ELISA principle (digoxigenin system) Biol Chem Hoppe Seyler. 1990;371:917–927. doi: 10.1515/bchm3.1990.371.2.917. [DOI] [PubMed] [Google Scholar]
- 18.Kessler C. The digoxigenin:anti-digoxigenin (DIG) technology—A survey on the concept and realization of a novel bioanalytical indicator system. Mol Cell Probe. 1991;5:161–205. doi: 10.1016/0890-8508(91)90041-h. [DOI] [PubMed] [Google Scholar]
- 19.Lo BKC. Antibody Engineering Methods and Protocols. Vol 248. Weinheim, Totowa, NJ: Humana Press; 2007. Methods in molecular biology; pp. 135–159. [Google Scholar]
- 20.Saldanha JW. Handbook of therapeutic antibodies. In: Duebel S, editor. Weinheim, Germany: Wiley-VCH; 2007. pp. 119–144. [Google Scholar]
- 21.Coloma MJ, Morrison SL. Design and production of novel tetravalent bispecific antibodies. Nat Biotechnol. 1997;15:159–163. doi: 10.1038/nbt0297-159. [DOI] [PubMed] [Google Scholar]
- 22.Jung SH, Pastan I, Lee B. Design of interchain disulfide bonds in the framework region of the Fv fragment of the monoclonal antibody B3. Proteins. 1994;19:35–47. doi: 10.1002/prot.340190106. [DOI] [PubMed] [Google Scholar]
- 23.Reiter Y, et al. Disulfide stabilization of antibody Fv: Computer predictions and experimental evaluation. Protein Eng. 1995;8:1323–1331. doi: 10.1093/protein/8.12.1323. [DOI] [PubMed] [Google Scholar]
- 24.Reiter Y, Brinkmann U, Lee B, Pastan I. Engineering antibody Fv fragments for cancer detection and therapy: Disulfide-stabilized Fv fragments. Nat Biotechnol. 1996;14:1239–1245. doi: 10.1038/nbt1096-1239. [DOI] [PubMed] [Google Scholar]
- 25.Molina MA, et al. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–4749. [PubMed] [Google Scholar]
- 26.Beuger V, et al. Short-hairpin-RNA-mediated silencing of fucosyltransferase 8 in Chinese-hamster ovary cells for the production of antibodies with enhanced antibody immune effector function. Biotechnol Appl Biochem. 2009;53:31–37. doi: 10.1042/BA20080220. [DOI] [PubMed] [Google Scholar]
- 27.Chitnis MM, et al. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364–6370. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- 28.Mansfield E, Amlot P, Pastan I, FitzGerald DJ. Recombinant RFB4 immunotoxins exhibit potent cytotoxic activity for CD22-bearing cells and tumors. Blood. 1997;90:2020–2026. [PubMed] [Google Scholar]
- 29.Brinkmann U, et al. B3(Fv)-PE38KDEL, a single-chain immunotoxin that causes complete regression of a human carcinoma in mice. Proc Natl Acad Sci USA. 1991;88:8616–8620. doi: 10.1073/pnas.88.19.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pastan I, et al. Characterization of monoclonal antibodies B1 and B3 that react with mucinous adenocarcinomas. Cancer Res. 1991;51:3781–3787. [PubMed] [Google Scholar]
- 31.Reichstein T. Chemie der herzaktiven Glykoside. Angew Chem. 1951;63:412–421. [Google Scholar]
- 32.Flanagan RJ, Jones AL. Fab antibody fragments: Some applications in clinical toxicology. Drug Safety. 2004;27:1115–1133. doi: 10.2165/00002018-200427140-00004. [DOI] [PubMed] [Google Scholar]
- 33.Doronina SO, et al. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: Effects of linker technology on efficacy and toxicity. Bioconjug Chem. 2006;17:114–124. doi: 10.1021/bc0502917. [DOI] [PubMed] [Google Scholar]
- 34.Tolcher AW, et al. Cantuzumab mertansine, a maytansinoid immunoconjugate directed to the CanAg antigen: A phase I, pharmacokinetic, and biologic correlative study. J Clin Oncol. 2003;21:211–222. doi: 10.1200/JCO.2003.05.137. [DOI] [PubMed] [Google Scholar]
- 35.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliver Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.