Abstract
Although the functional significance of adult neurogenesis in hippocampal-dependent learning and memory has been well documented, the role of such neurogenesis in olfactory activity is rather obscure. To understand the significance of adult neurogenesis in olfactory functions, we genetically ablated newly born neurons by using tamoxifen-treated Nestin-CreERT2;neuron-specific enolase-diphtheria toxin fragment A (NSE-DTA) mice. In these mice, tamoxifen-inducible Cre recombinase allows the NSE (Eno2) gene to drive DTA expression in differentiating neurons, leading to the efficient ablation of newly born neurons in the forebrain. These mutant mice were capable of discriminating odors as competently as control mice. Strikingly, although control and mutant mice frequently showed freezing behaviors to a fox scent, a predator odor, mutant mice approached this odor when they were conditioned to associate the odor with a reward, whereas control mice did not approach the odor. Furthermore, although mutant males and females showed normal social recognition behaviors to other mice of a different sex, mutant males displayed deficits in male–male aggression and male sexual behaviors toward females, whereas mutant females displayed deficits in fertility and nurturing, indicating that sex-specific activities, which are known to depend on olfaction, are impaired. These results suggest that continuous neurogenesis is required for predator avoidance and sex-specific responses that are olfaction dependent and innately programmed.
Keywords: sex-specific response, innate olfactory response, maternal behavior, main olfactory bulb, accessory olfactory bulb
Neurogenesis occurs continuously in two brain regions of adult mammals, the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the hippocampus (1–3). Neurons born in the SVZ migrate into the olfactory bulb, whereas neurons born in the SGZ migrate into the hippocampal dentate gyrus. The functional significance of adult neurogenesis in hippocampal-dependent learning and memory has been well documented (1, 4–6). However, the role of such neurogenesis in olfactory activity is rather obscure (3, 7–9).
The olfactory bulb consists of two structures, the main and accessory olfactory bulbs. The former is involved in the cognitive response to chemical cues detected by the main olfactory epithelium, whereas the latter is involved in the instinctive response to pheromonal cues detected by the vomeronasal organ, although this functional difference is not absolute (10–12). To understand the functional significance of adult neurogenesis, ablation of newly born neurons has been attempted by treatments with X-ray irradiation or antimitotic drugs, which kill dividing neural stem/progenitor cells (13–17). Although these methods are effective, they also seem to cause side effects on neurons that may affect the mood and behavior of the animals. As an alternative method, we previously developed transgenic mice for the efficient ablation of newly born neurons, Nestin-CreERT2;neuron-specific enolase-diphtheria toxin fragment A (NSE-DTA) mice (18). In these mice, tamoxifen-inducible Cre is expressed by neural stem/progenitor cells under the control of the Nestin promoter/enhancer, whereas the loxP-STOP-loxP-IRES-diphtheria toxin fragment A (DTA) gene cassette was knocked into the 3′-noncoding region of the neuron-specific enolase (NSE, Eno2) gene. After tamoxifen administration, Cre is activated and deletes the STOP sequence in neural stem/progenitor cells, but DTA is not expressed in these cells because the NSE promoter is inactive. However, when the cells begin neuronal differentiation, the NSE promoter becomes active and induces the expression of DTA, killing the cells. Thus, in these mice, neurons born in the SVZ and SGZ are efficiently ablated after tamoxifen treatment (18). Inhibition of neurogenesis leads to severe disruption of the main olfactory bulb structure, but the odor sensing and discrimination ability is well preserved, suggesting that adult neurogenesis is mostly dispensable for olfaction to chemical cues (18).
To understand the significance of adult neurogenesis in olfactory activities, we further characterized the behavioral responses to various odors by using tamoxifen-treated Nestin-CreERT2;NSE-DTA mice. Strikingly, mutant mice approached a fox scent, a predator odor, when they were conditioned to associate it with a reward, whereas control mice did not do so. Furthermore, mutant mice displayed deficits in sex-specific innate activities that are known to depend on olfaction. These results indicate that continuous neurogenesis is required for innate olfactory responses.
Results
Continuous Neurogenesis Is Required for the Maintenance of the Accessory Olfactory Bulb.
Whereas neurogenesis in the main olfactory bulb has been well studied, only short-term analysis was performed for neurogenesis in the accessory olfactory bulb (19). Therefore, we first assessed long-term neurogenesis in the accessory olfactory bulb of adult mice. To mark neural stem cells and their progeny, we used Nesin-CreERT2 mice, which express tamoxifen-inducible Cre recombinase in neural stem cells (20), and crossed these mice with Rosa26 reporter mice (R26-CFP). Nesin-CreERT2;R26-CFP mice were treated with tamoxifen at 2 mo of age, and neurons born from labeled neural stem cells (CFP+) were quantified. We found a gradual increase of the CFP+ granule cell number in the accessory olfactory bulb after tamoxifen treatment. The proportion of labeled granule cells (NeuN+CFP+) reached about 15% after 3 mo and about 25% after 12 mo (Fig. S1). The total neuronal number was not significantly changed during this period (Fig. 1M, control), indicating that continuous neurogenesis leads to substantial replacement of neurons in the accessory olfactory bulb, as observed in the main olfactory bulb (18).
Fig. 1.
Ablation of newly born neuron decreased the number of granule cells in the accessory olfactory bulb. (A and B) Experimental design. (C–L) Histology of the accessory olfactory bulb in oil- (control) and tamoxifen-treated (mutant) Nesin-CreERT2;NSE-DTA mice. Sections were stained for NenN. (M) Quantification of the number of NeuN+ granule cells in the accessory olfactory bulb of oil- (control) and tamoxifen-treated (mutant) Nesin-CreERT2;NSE-DTA mice. The average of three independent samples with a SD was shown at each time point. *P < 0.05, **P < 0.01, ***P < 0.001, t test. (Scale bar, 50 μm.)
To understand the functional significance of adult neurogenesis, we next examined Nesin-CreERT2 mice crossed with NSE-DTA mice, where diphtheria toxin fragment A (DTA) was expressed from the NSE locus after Cre became active (Nesin-CreERT2;NSE-DTA mice, Fig. 1 A and B). In these mice, newly born neurons in the SVZ and the SGZ were efficiently ablated after tamoxifen treatment (18). As a result, reduction of the neuronal number in the accessory olfactory bulb was detectable 1 mo after tamoxifen treatment (Fig. 1 F and M), although not all mice showed such reduction at this stage. The number of neurons in the accessory olfactory bulb of Nesin-CreERT2;NSE-DTA mice treated with tamoxifen was continuously decreased thereafter, compared with those treated with oil (Fig. 1 B–M). These results indicated that continuous neurogenesis is required for the maintenance of the neuronal number in the accessory olfactory bulb, as observed in the main olfactory bulb (18). However, mitral/tufted cells (Reelin+) in the main and accessory olfactory bulbs and sensory neurons (OMP+) in the vomeronasal organ and the main olfactory epithelium were not significantly affected (Figs. S2 and S3). Thus, in tamoxifen-treated Nesin-CreERT2;NSE-DTA mice, neurogenesis in the main and accessory olfactory bulbs is affected, but projection neurons (mitral/tufted cells) in these regions and sensory neurons in the peripheral tissues (the main olfactory epithelium and the vomeronasal organ) are not affected.
Learned Olfactory Responses to Chemical Cues Are Well Maintained in the Absence of Neurogenesis.
To understand the role of adult neurogenesis in olfactory activities, we examined NSE-DTA and Nestin-CreERT2;NSE-DTA mice that were treated with tamoxifen at 2 mo of age. These mice were subjected to behavioral tests at 2 mo after tamoxifen treatment, when the reduction of granule cells in the accessory olfactory bulb was invariably detectable, and at 10 mo after tamoxifen treatment, when inhibition of neurogenesis led to severe reduction of the neuronal number. Age-matched wild-type C57BL/6J mice were also examined, and we obtained the same results with wild-type C57BL/6J mice and tamoxifen-treated NSE-DTA mice. Therefore, hereafter tamoxifen-treated NSE-DTA mice are called control, whereas tamoxifen-treated Nestin-CreERT2;NSE-DTA mice are called mutant.
Two related odors, one mixed with sugar (odor A) and the other without sugar (odor B), were given to food-restricted mice four times a day; after 4 d of training, each mouse was placed in a cage, in which both odors were placed at separate sites without sugar under the bedding (odor–reward association test, Fig. S4A). We previously found that control and mutant mice spent significantly more time digging at the site of sugar-associated odors than at the site of nonsugar-associated odors when two sets of enantiomers, (+)carvone versus (−)carvone and (d)limonene versus (l)limonene, were used (18). We tested other odors, (+)fenchone versus (−)fenchone, and found that control and mutant mice spent significantly more time digging at the site of sugar-associated odors than at the site of nonsugar-associated odors (Fig. S4B). These mice were also capable of differentiating between 80%(+)fenchone;20%(−)fenchone and 20%(+)fenchone;80%(−)fenchone mixtures but not between 60%(+)fenchone;40% (−)fenchone and 40%(+)fenchone;60%(−)fenchone mixtures (Fig. S4B). Furthermore, the threshold for the detection of various odors such as (−)carvone was not affected by the inhibition of neurogenesis (Fig. S4C). These results support our previous conclusion that learned olfactory responses to chemical cues are well maintained in the absence of neurogenesis (18).
Innate Avoidance Behaviors to a Predator Odor Are Reversed by Learning in the Absence of Neurogenesis.
We further screened other chemicals by performing odor–reward association tests and found that control and mutant mice displayed different responses to 2,4,5-trimethylthiazoline (TMT), a major component of fox scent (21). It has been shown that wild-type mice display aversive responses to TMT, which is an innate olfactory behavior (21–23). When exposed to TMT, control and mutant mice frequently showed freezing behaviors and did not approach this odor (Fig. 2A), suggesting that innate aversive responses are preserved in the absence of neurogenesis. When TMT paired with sugar and the nonaversive odor eugenol without sugar were used in an odor–reward association test, control mice frequently showed freezing behaviors and neither approached TMT nor ate the sugar during training (Fig. 2B and Movie S1). By contrast, under the same condition, mutant mice approached TMT and ate the sugar (Fig. 2B and Movie S2). Furthermore, after 4 d of training, when TMT and eugenol were placed without sugar under the bedding at separate sites, control mice frequently showed freezing behaviors and did not approach either site (Fig. 2C), whereas mutant mice spent significantly more time digging at the site of TMT, a sugar-associated odor, than at the site of eugenol, a nonsugar-associated odor (Fig. 2C). These mutant behaviors were similar, irrespective of duration of inhibition of neurogenesis (2 or 10 mo, Fig. 2 A and C). These results indicated that mutant mice were able to detect and avoid TMT, but failed to show aversive behaviors when they were conditioned to associate the odor with a reward, suggesting that adult neurogenesis is required to maintain innate aversive responses under any circumstance.
Fig. 2.
Ablation of newly born neurons affected aversive responses to a predator odor in odor–reward association tests. At 2 or 10 mo after tamoxifen treatment, NSE-DTA (control, n = 6) and Nestin-CreERT2;NSE-DTA (mutant, n = 6) mice were subjected to odor tests. (A) Habituation–dishabituation tests. Control and mutant mice were exposed to oil and TMT. When exposed to TMT, control and mutant mice frequently showed freezing behaviors and did not approach this odor. ns, not significant, t test. (B) Control and mutant mice were trained for 4 d to associate the reward (sugar grains) with TMT. On day 4, TMT with a sugar reward was presented under the bedding. A control mouse frequently showed freezing behaviors, whereas a mutant mouse was digging at the site of TMT. (C) Odor–reward association tests after 4 d of training. Mutant mice spent significantly more time digging at the site of TMT, a sugar-associated odor, than at the site of eugenol, a nonsugar-associated odor. By contrast, control mice did not approach either site. Mean digging time ± SEM during a 4-min test period is shown as a bar graph. ns, not significant; *P < 0.05, t test. Mutant behaviors were similar, irrespective of duration of inhibition of neurogenesis (A and C, at 2 or 10 mo after tamoxifen treatment).
Social Recognition Is Not Impaired in the Absence of Neurogenesis.
We next assessed social recognition behavior. It is well known that mice spend less time investigating (sniffing) familiar mice than unfamiliar mice of a different sex (24). Control and mutant males and females were individually caged, and an unfamiliar female or male was introduced, respectively. Control and mutant males and females spent similar lengths of time investigating the unfamiliar female and male, respectively: the sniffing time was noticeably decreased between the first and subsequent presentations of the same mouse, but increased significantly when a new female or male was introduced (Fig. S5). These results indicated that control and mutant mice differentiated between the first and second mouse, suggesting that social recognition was not impaired in the absence of neurogenesis.
Neurogenesis Is Required for Sex-Specific Responses That Are Olfaction Dependent and Innately Programmed.
To examine sex-specific responses that are known to be olfaction dependent and innately programmed, we evaluated the significance of neurogenesis in male-specific activities. To assess male–male intraspecies aggression behaviors, wild-type, control, and mutant males were individually caged for 7 d (resident), and then a wild-type male was introduced as an intruder into each cage. Aggressive behaviors by residents toward intruders were monitored (frequency and duration) in a 30-min trial. Although control resident males were aggressive, mutant males displayed virtually no aggressive behaviors toward male intruders (Fig. 3 A and B). We next assessed male sexual behaviors toward females by scoring the frequency and duration of mounting during the first 30 min of exposure. Although control males displayed robust mounting behaviors toward females, mutant males did not (Fig. 3 C and D). Furthermore, although control males efficiently produced vaginal plugs in wild-type females within a week, mutant males did not (Fig. 3E). These defects of mutant males were similar, irrespective of duration of inhibition of neurogenesis (2 or 10 mo, Fig. 3). These results indicated that male–male aggression and male sexual behaviors toward females are severely impaired in the absence of continuous neurogenesis.
Fig. 3.
Ablation of newly born neurons affected male-specific activities. At 2 or 10 mo after tamoxifen treatment, NSE-DTA (control, n = 13) and Nestin-CreERT2;NSE-DTA (mutant, n = 14) mice, as well as wild-type mice (n = 10), were subjected to behavior tests. (A and B) Male–male aggression behavior tests. (C and D) Mounting behaviors of wild-type, control, and mutant males toward wild-type females. (E) Proportions of vaginal plug formation in wild-type females within 1 wk. The average with a SE (A–D) or the proportions (E) are shown. *P < 0.05, **P < 0.01, one-way ANOVA.
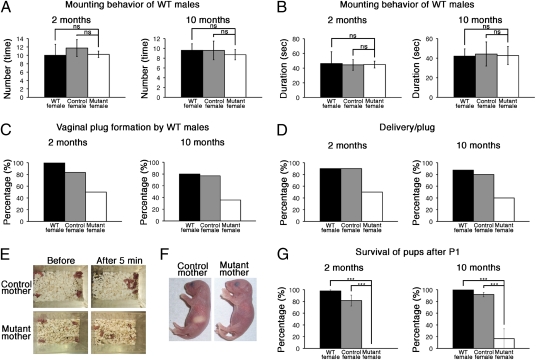
We next evaluated the significance of neurogenesis in female-specific activities. Control and mutant females showed normal estrous cycles (Fig. S6). These females were housed with wild-type males, and the frequency and duration of mounting were scored during the first 30 min of exposure. Although wild-type males displayed mounting behaviors toward control and mutant females at a similar frequency (Fig. 4 A and B), vaginal plugs were produced at a much lower frequency in mutant females than in controls (Fig. 4C). Furthermore, although most control females with vaginal plugs became pregnant, the ratios were significantly reduced in mutant females (Fig. 4D), suggesting that neurogenesis is important for the maintenance of pregnancy. Strikingly, after delivery, although control females retrieved and nursed their offspring, mutant females did not (Fig. 4E). It was reported that when neurogenesis was reduced by treatment with an antimitotic drug during pregnancy, these mice showed impaired maternal behaviors in novel cages, but not in their home cages (25). However, mutant females displayed abnormal maternal behaviors even in their home cages (Fig. 4E). Furthermore, although the stomachs of the offspring of control females were full of milk, there was no milk in the stomachs of most offspring of mutant females (Fig. 4F), and these offspring mostly died within 24 h of delivery (Fig. 4G). There was some milk in the stomachs of some offspring of mutant females, but these neonates also died within 3 d of delivery. These defects of mutant females were similar, irrespective of duration of inhibition of neurogenesis (2 or 10 mo, Fig. 4). These results indicated that pregnancy and nursing behaviors were severely impaired in the absence of continuous neurogenesis.
Fig. 4.
Ablation of newly born neurons affected female-specific activities. At 2 or 10 mo after tamoxifen treatment, NSE-DTA (control, n = 13) and Nestin-CreERT2;NSE-DTA (mutant, n = 14) mice, as well as wild-type mice (n = 10), were subjected to behavior tests. (A and B) Mounting behaviors of wild-type males toward wild-type, control, and mutant females. (C) Proportions of vaginal plug formation by wild-type males within 1 wk. (D) Proportions of delivery by females that had vaginal plugs. The average with a SE (A and B) or the proportions (C and D) are shown. (E) Control females retrieved their offspring within 5 min, whereas mutant females treated with tamoxifen at 2 or 10 mo before did not. (F) The stomachs of the offspring of control females were full of milk, but this was not observed in the offspring of mutant females treated with tamoxifen at 2 or 10 mo before. (G) Proportions of survival of pups after postnatal day 1 (P1). ns, not significant; ***P < 0.001, one-way ANOVA.
Discussion
The role of neurogenesis in olfactory activity is rather obscure (3, 7–9), although it has been demonstrated that neurogenesis is important for some odor-dependent responses, such as fine discrimination and short-term spontaneous memory of chemical cues (13–17, 26). Our previous (18) and present data indicated that tamoxifen-treated Nestin-CreERT2;NSE-DTA mice were able to discriminate similar odors and retain short- and long-term odor-associated memory, suggesting that learned responses to chemical cues are well preserved in the absence of adult neurogenesis. However, although mutant mice showed innate aversive behaviors to TMT, a predator odor, they failed to do so, or rather approached the odor when they were conditioned to associate it with sugar. By contrast, control mice did not approach TMT even when the odor was presented with sugar. These results suggested that innate avoidance behaviors to a predator odor are reversed by learning in the absence of continuous neurogenesis. It was previously shown that the glomeruli in the dorsal domain of the olfactory bulb are required for aversive responses, whereas those in the ventral domain are involved in associative olfactory learning (23). Mice that lacked glomeruli in the dorsal domain failed to show innate avoidance behaviors without conditioning with reward (23). Because neurogenesis contributes similarly to dorsal and ventral regions of the olfactory bulb, it is unlikely that inhibition of neurogenesis differentially affects the activities of glomeruli in different domains. Furthermore, our preliminary data by optical imaging of the olfactory bulb suggested that no apparent difference was detectable in the distribution of activated glomeruli between control and mutant mice. These results suggest that the abnormal behavior due to the lack of neurogenesis does not result from defects at the glomerular level but further upstream, most likely at projection neurons (mitral/tufted cells), because granule cells, major neurons born in the adult brain, modulate the output of mitral and tufted cells. It has been shown that aversive responses to TMT depend on the activity of the bed nucleus of the stria terminalis (23, 27), and it remains to be determined whether loss of neurogenesis affects the activity of this nucleus.
We also showed that although mutant males and females can recognize different individuals, mutant males displayed deficits in male–male aggression and male sexual behaviors toward females, whereas mutant females displayed deficits in fertility and nurturing, which are known to depend on olfactory activities (28–30). Accumulating evidence suggested an association between neurogenesis and sex-specific responses, but the exact roles of neurogenesis in such responses are still controversial. It has been shown that pheromones of dominant males, but not of other males induce neurogenesis in females (19, 31). Furthermore, pregnancy stimulates neurogenesis in the SVZ, leading to an increase of newly born neurons in the olfactory bulb (32). This induction of neurogenesis is mediated by prolactin, which directly activates proliferation and neuronal differentiation of neural stem cells in the SVZ (25, 32). Reduced prolactin signaling following the administration of a dopamine D2-receptor agonist or inactivation of the prolactin receptor gene led to decreased neurogenesis and impaired maternal behaviors (25, 32, 33). However, it was not clear whether these defects are due to reduced prolactin-dependent neurogenesis or other prolactin-dependent activities. Here, we showed that the reduction of neurogenesis not only affects pregnancy but also impairs maternal behaviors. Blocking neurogenesis was also attempted by the administration of an antimitotic drug during pregnancy, but these mice underwent normal pregnancy and showed defects in maternal behaviors only in novel cages but not in their home cages (25). These defects are rather mild compared with those of our mutant mice that had defects in pregnancy and maternal behaviors even in their home cages. The difference in the severity of defects is probably due to the duration and extent of the inhibition of neurogenesis. Antimitotic drug treatment was performed for only several days during pregnancy (25), whereas neurogenesis was continuously inhibited in our mutant mice. Another study showed that inhibition of neurogenesis in the SVZ by γ-ray irradiation did not affect maternal behaviors (34). The discrepancy between this study and the others remains to be clarified, but prolactin-dependent neurogenesis could be induced during pregnancy in these mice, because substantial neurogenesis still occurred after γ-ray irradiation (34). Another possibility is that newly born neurons in the dentate gyrus might be involved in maternal behaviors, because neurogenesis was blocked in the SVZ and SGZ of our mutant mice and the mice treated with an antimitotic drug but only in the SVZ of the mice treated with γ-ray irradiation.
Taken together, these data indicated that although adult neurogenesis is mostly dispensable for learned responses to chemical odors, it is indispensable for predator avoidance and sex-specific responses that are olfaction dependent and innately programmed. However, the possibility that some of the observed defects might be secondary to impaired adult neurogenesis is not totally excluded, and further analyses will be required to determine the role of new neurons in predator avoidance and sex-specific responses. It was previously shown that male–male aggression and male–female sexual behaviors are altered when the pathway from the vomeronasal organ to the accessory olfactory bulb was inactivated (35–37). These defects are somewhat similar to those of our mutant mice, suggesting that newly born neurons in the accessory olfactory bulb may be involved in sex-specific behaviors. However, because all available ablation methods, which inhibit neurogenesis in the SVZ, should affect both the main and accessory olfactory bulbs, it is difficult to determine which of the two structures is more important for predator avoidance and sex-specific behaviors. More restricted ablation methods will be required to answer this question.
Materials and Methods
Animals.
Nestin-CreERT2 line 4 mice and NSE-DTA mice were described previously (18, 20, 23). Nestin-CreERT2 and NSE-DTA mice were maintained on a C57BL/6J background. R26-CFP mice (38) were kindly provided by Frank Costantini (Columbia University, New York). Genotype of each mouse was determined by PCR, as previously described (20). Mice were housed in a room with 12-h light/dark cycle (light on at 6:00 AM). All animals were handled in accordance with the Kyoto University Guide for the Care and Use of Laboratory Animals.
Tamoxifen Treatment.
For activation of CreERT2, 10 mg of tamoxifen (Sigma) in corn oil (Sigma) was orally administered to 2-mo-old mice once a day for 4 consecutive days. In behavioral experiments, tamoxifen-treated NSE-DTA mice were apparently normal and used as controls. At 2 or 10 mo after tamoxifen treatment, mice were subjected to behavioral tests.
Statistical Analysis.
Statistical analysis was performed with Prism 5.0 software (GraphPad). Statistical differences were tested with Student's t test or one-way ANOVA with Tukey's multiple comparison test. Any P values less than 0.05 were considered to be significant.
Immunohistochemistry.
The dissected brains were fixed in 4% PFA/PBS overnight at 4 °C, followed by transcardial perfusion, washed in PBS, and equilibrated in 20% sucrose/PBS before mounting in OCT compound (Sakura Finetek). For sectioning the olfactory epithelium, the tissue was decalcified with 0.5 M EDTA at 4 °C for 2 d before sucrose treatment. Frozen sections (16 μm thick) were stained with antibodies against GFP (Invitrogen), NeuN (Chemicon), OMP (Wako), and Reelin (kindly provided by Masaharu Ogawa, RIKEN, Wako, Japan). Goat or donkey antispecies IgG conjugated with Alexa 488 or Alexa 594 were used as secondary antibodies. Sections were analyzed with confocal microscopy (Zeiss).
Odor–Reward Association Test.
The test was performed as described (39) with minor modifications. Mice were food restricted to maintain 80–85% of their free-feeding weights and trained to associate one of two odors with a sugar reward for 4 d. During the training, mice received four 10-min trials a day: two trials for an odor paired with a sugar reward and two for an unpaired odor. On days 1 and 2, an odor paired with sugar was presented in front of mice so that they could see the reward. On days 3 and 4, an odor paired with sugar was presented under the bedding. An unpaired odor was also presented in the same way. On day 5, both test odors were placed at separate sites, without sugar, in a cage (26 × 40 × 18 cm) under bedding (5-cm depth). The mouse behavior was recorded with a digital video camera for the analysis. The time (in seconds) spent digging for each odor was measured during a 4-min test period. Odors used were: (+)fenchone, (−)fenchone (Tokyo Chemical Industry), eugenol (Nacalai Tesque), and 2,4,5-TMT (Phero Tech). For each odor, 25 μL was used.
Habituation–Dishabituation Tests.
Mice were habituated to a cage (20 × 15 × 13 cm) for 30 min, and then a sheet of filter paper (2 × 2 cm) with 20 μL of mineral oil (Sigma) was presented for 3 min. This procedure was repeated with 15-min intervals. Filter paper with 20 μL of TMT was presented for 3 min at the fifth trial. Nasal contacts with the filter paper within a 1-mm distance were judged as “investigating.” Investigation time during a 3-min test period was measured.
Resident–Intruder Assay (Male–Male Aggressive Behavior).
Control and mutant male mice were individually caged (10 × 17 × 11 cm) for 7 d. A wild-type male mouse (8–12 wk old) was introduced as an intruder to each cage for 30 min. The frequency and the duration of aggressive behaviors toward intruders were measured. Wrestling and biting were defined as aggressive behaviors.
Mating Behavior.
For a male behavior test, control and mutant male mice were individually caged (10 × 17 × 11 cm) 1 d before the experiment. An estrous wild-type female (8–12 wk old and sexually naive) was then introduced into each cage. The frequency and duration of mounting behaviors were measured during the first 30 min. Vaginal plug formation was checked every morning, and the proportions of vaginal plug formation in a wild-type female within a week were calculated.
For a female behavior test, wild-type males (8–12 wk old and sexually naive) were individually caged (10 × 17 × 11 cm) 1 d before the experiment. A sexually naive control or mutant female was introduced into each cage. The frequency and duration of mounting behaviors were measured during the first 30 min. Vaginal plug formation was checked every morning, and the proportions of vaginal plug formation within a week were calculated. All females were housed individually once pregnant and the proportions of delivery among females with vaginal plugs were calculated.
Pups Retrieving.
This experiment was performed after delivery. Control and mutant mothers were separated from their home cages for 15 min. After pups were scattered, their mothers were returned. Their behaviors were recorded for 5 min.
Supplementary Material
Acknowledgments
We thank F. Costantini, T. Ikeda, S. Itohara, and M. Ogawa for materials. This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan. M.S. was supported by research fellowships from the Japan Society for the Promotion of Science for Young Scientists.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018782108/-/DCSupplemental.
References
- 1.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 2.Lledo P-M, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 3.Whitman MC, Greer CA. Adult neurogenesis and the olfactory system. Prog Neurobiol. 2009;89:162–175. doi: 10.1016/j.pneurobio.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kempermann G. The neurogenic reserve hypothesis: What is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31:163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 6.Lagace DC, et al. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci USA. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gheusi G, et al. Importance of newly generated neurons in the adult olfactory bulb for odor discrimination. Proc Natl Acad Sci USA. 2000;97:1823–1828. doi: 10.1073/pnas.97.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schellinck HM, Arnold A, Rafuse VF. Neural cell adhesion molecule (NCAM) null mice do not show a deficit in odour discrimination learning. Behav Brain Res. 2004;152:327–334. doi: 10.1016/j.bbr.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Kim WR, et al. Impaired migration in the rostral migratory stream but spared olfactory function after the elimination of programmed cell death in Bax knock-out mice. J Neurosci. 2007;27:14392–14403. doi: 10.1523/JNEUROSCI.3903-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dulac C, Wagner S. Genetic analysis of brain circuits underlying pheromone signaling. Annu Rev Genet. 2006;40:449–467. doi: 10.1146/annurev.genet.39.073003.093937. [DOI] [PubMed] [Google Scholar]
- 11.Baum MJ, Kelliher KR. Complementary roles of the main and accessory olfactory systems in mammalian mate recognition. Annu Rev Physiol. 2009;71:141–160. doi: 10.1146/annurev.physiol.010908.163137. [DOI] [PubMed] [Google Scholar]
- 12.Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- 13.Lazarini F, et al. Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PLoS ONE. 2009;4:e7017. doi: 10.1371/journal.pone.0007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sultan S, et al. Learning-dependent neurogenesis in the olfactory bulb determines long-term olfactory memory. FASEB J. 2010;24:2355–2363. doi: 10.1096/fj.09-151456. [DOI] [PubMed] [Google Scholar]
- 15.Valley MT, Mullen TR, Schultz LC, Sagdullaev BT, Firestein S. Ablation of mouse adult neurogenesis alters olfactory bulb structure and olfactory fear conditioning. Front Neurosci. 2009;3:51. doi: 10.3389/neuro.22.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moreno MM, et al. Olfactory perceptual learning requires adult neurogenesis. Proc Natl Acad Sci USA. 2009;106:17980–17985. doi: 10.1073/pnas.0907063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breton-Provencher V, Lemasson M, Peralta MR, 3rd, Saghatelyan A. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J Neurosci. 2009;29:15245–15257. doi: 10.1523/JNEUROSCI.3606-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imayoshi I, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 19.Oboti L, et al. Integration and sensory experience-dependent survival of newly-generated neurons in the accessory olfactory bulb of female mice. Eur J Neurosci. 2009;29:679–692. doi: 10.1111/j.1460-9568.2009.06614.x. [DOI] [PubMed] [Google Scholar]
- 20.Imayoshi I, Ohtsuka T, Metzger D, Chambon P, Kageyama R. Temporal regulation of Cre recombinase activity in neural stem cells. Genesis. 2006;44:233–238. doi: 10.1002/dvg.20212. [DOI] [PubMed] [Google Scholar]
- 21.Vernet-Maury E. Trimethyl-thiazoline in fox feces: A natural alarming substance for the rat. Olfaction Taste. 1980;7:407. [Google Scholar]
- 22.Hebb ALO, et al. Odor-induced variation in anxiety-like behavior in mice is associated with discrete and differential effects on mesocorticolimbic cholecystokinin mRNA expression. Neuropsychopharmacology. 2002;27:744–755. doi: 10.1016/S0893-133X(02)00354-8. [DOI] [PubMed] [Google Scholar]
- 23.Kobayakawa K, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- 24.Jin D, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- 25.Larsen CM, Grattan DR. Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology. 2010;151:3805–3814. doi: 10.1210/en.2009-1385. [DOI] [PubMed] [Google Scholar]
- 26.Enwere E, et al. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23:23–28. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruce HM. An exteroceptive block to pregnancy in the mouse. Nature. 1959;184:105. doi: 10.1038/184105a0. [DOI] [PubMed] [Google Scholar]
- 29.Kaba H, Hayashi Y, Higuchi T, Nakanishi S. Induction of an olfactory memory by the activation of a metabotropic glutamate receptor. Science. 1994;265:262–264. doi: 10.1126/science.8023145. [DOI] [PubMed] [Google Scholar]
- 30.Serguera C, Triaca V, Kelly-Barrett J, Banchaabouchi MA, Minichiello L. Increased dopamine after mating impairs olfaction and prevents odor interference with pregnancy. Nat Neurosci. 2008;11:949–956. doi: 10.1038/nn.2154. [DOI] [PubMed] [Google Scholar]
- 31.Mak GK, et al. Male pheromone-stimulated neurogenesis in the adult female brain: Possible role in mating behavior. Nat Neurosci. 2007;10:1003–1011. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- 32.Shingo T, et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- 33.Lucas BK, Ormandy CJ, Binart N, Bridges RS, Kelly PA. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology. 1998;139:4102–4107. doi: 10.1210/endo.139.10.6243. [DOI] [PubMed] [Google Scholar]
- 34.Feierstein CE, et al. Disruption of adult neurogenesis in the olfactory bulb affects social interaction but not maternal behavior. Front Neurosci. 2010;4:176. doi: 10.3389/fnbeh.2010.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 36.Leypold BG, et al. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci USA. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Punta K, et al. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature. 2002;419:70–74. doi: 10.1038/nature00955. [DOI] [PubMed] [Google Scholar]
- 38.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schellinck HM, Forestell CA, LoLordo VM. A simple and reliable test of olfactory learning and memory in mice. Chem Senses. 2001;26:663–672. doi: 10.1093/chemse/26.6.663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.