Abstract
Ichthyosaurs were important marine predators in the Early Jurassic, and an abundant and diverse component of Mesozoic marine ecosystems. Despite their ecological importance, however, the Early Jurassic species represent a reduced remnant of their former significance in the Triassic. Ichthyosaurs passed through an evolutionary bottleneck at, or close to, the Triassic-Jurassic boundary, which reduced their diversity to as few as three or four lineages. Diversity bounced back to some extent in the aftermath of the end-Triassic mass extinction, but disparity remained at less than one-tenth of pre-extinction levels, and never recovered. The group remained at low diversity and disparity for its final 100 Myr. The end-Triassic mass extinction had a previously unsuspected profound effect in resetting the evolution of apex marine predators of the Mesozoic.
Keywords: Ichthyopterygia, cladistics, phylogeny, morphometrics
Marine reptiles arose in the Early Triassic, some 250 Ma, and dominated Mesozoic seas until their demise by the end of the Cretaceous, 65 Ma (1, 2). The emergence of diverse marine reptiles in the Triassic—the long-necked fish-eating eosauropterygians (pachypleurosaurs and nothosaurs), the mollusk-eating and armored placodonts, the serpentine thalattosaurs, and the streamlined ichthyosaurs—was part of the maelstrom of faunal recovery in the oceans following the devastation of the end-Permian mass extinction. These represented new apex predators, filling trophic levels that had not been widely exploited in the Permian. Most of these marine reptile groups disappeared in the Late Triassic, and were replaced by ichthyosaurs, plesiosaurs, and marine crocodilians as predators in Jurassic seas.
The Triassic-Jurassic (Tr-J) transition is marked by a mass extinction that may have been a single event or may incorporate earlier pulses of extinction in the Rhaetian (3–5), but its role in resetting the dominance of apex marine predators has not been fully recognized (1, 3). Indeed, it has been argued that the Tr-J event had little effect on marine reptilian evolution, because major clades such as the ichthyosaurs and the plesiosaurs passed through the mass extinction event, rapidly recovered to pre-extinction diversity levels, and retained many of their ecological roles (4, 5).
Ichthyosaurs offer an excellent case study: Fossils are abundant and of high quality, they occur in marine sediments and so may be dated against the international standard stratigraphic scale, and they passed through a significant bottleneck at the Tr-J boundary, ∼200 Ma. Although most commonly thought of as dolphin-shaped and dolphin-sized, ichthyosaurs ranged in length from 0.3 to 20 m, and in shape from long and slender to deep-bodied (Fig. 1). Most morphological variance is seen in the Triassic, with the Early Triassic lizard-like grippiids, small Middle Triassic mixosaurs, and whale-sized, deep-bodied shonisaurids in the Late Triassic (6, 7). Ichthyosaurs arose in the Early Triassic, perhaps 3–4 Myr after the devastating Permo-Triassic mass extinction, and they added new top carnivore trophic levels to the typical marine trophic pyramid, feeding mainly on fishes and cephalopods. Ichthyosaurs were diapsid reptiles, perhaps distantly related to modern lizards, and were supremely adapted to marine life, swimming with lateral undulations of the tail and steering with elongate fore paddles and producing live young at sea.
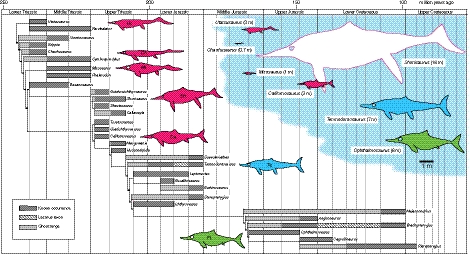
Fig. 1.
Phylogenetic tree of ichthyosaurs plotted against geological time. Times runs from left to right, showing known ranges (dark gray), ghost ranges (minimal implied phylogenetic gap; light gray), and Lazarus ranges (missing within-range representation; diagonal bars). Silhouette outlines (Ca., Californosaurus; Ch., Chaohusaurus; Mi., Mixosaurus; Pl., Platypterygius; Sh, Shonisaurus; Te., Temnodontosaurus; Ut., Utatsusaurus) indicate major body morphologies in Triassic (red), Early Jurassic (blue), and Middle Jurassic–Cretaceous (green). Relative sizes of the various major ichthyosaur morphotypes are indicated by scale drawings at top right, with a 1-m scale bar indicated in the blue cloud. Phylogeny is based on the majority-rule LE50 tree (SI Appendix, Fig. 3), similar to earlier findings (7, 13), and stratigraphic ranges are from a review of the literature (SI Appendix, Fig. 4). Ichthyosaur silhouettes are based on various sources (7).
After the Tr-J bottleneck, ichthyosaurs apparently did not achieve such diversity of form but nonetheless recovered sufficiently to be the dominant marine predators of the Early Jurassic, after which they dwindled in diversity through the Middle and Late Jurassic and much of the Cretaceous until they disappeared at the end of the Cenomanian, ∼100 Ma, after having had a significant role in Mesozoic seas for 150 Myr.
In this study, we concentrate on disparity (morphological variance), which can be represented readily in morphospace plots. These show the relative distribution of different forms in space but give no indication of their phylogenetic relatedness or convergence (8). A complete fossil record is not required, rather merely a sufficient sample of morphologically diverse forms that include all bauplans (9). In studies of the evolutionary radiations of a wide range of organisms (8, 10–12), it has been found that diversity and disparity are generally decoupled, with disparity often increasing first and faster than diversity.
Disparity may be assessed from continuous morphological characters, often from landmark studies of body and organ shapes or from discrete characters. This latter approach is adopted here, benefiting from numerous recent detailed cladistic analyses of Ichthyopterygia (13–15). Ichthyosaurian osteology is known in great detail from thousands of exquisitely preserved specimens, and the species differ in cranial and postcranial proportions, skull bones, dentitions, vertebral columns, caudal fins, and pectoral and pelvic fin morphologies (16), and so are likely to have occupied different ecological niches. Ichthyosaurs evolved both hyperphalangy and hyperdactyly, some having up to 10 digits per forefin (Caypullisaurus) and others up to 20 phalanges per digit (Ichthyosaurus) (7).
Ichthyosaur diversity was drastically reduced during the Tr-J event to very few lineages, namely those leading to Suevoleviathan, Temnodontosaurus, and the clade Eurhinosauria + Thunnosauria. Although the Jurassic eurhinosaurid Leptonectes and thunnosaurid Ichthyosaurus are both reported from the latest Triassic in the pre-planorbis beds of the Rhaetian (16, 17), this is not evidence for their survival through the crisis, as these Triassic units fall within the ∼100,000 y after the mass extinction horizon and before the Tr-J boundary (3).
In this paper, we analyze morphological disparity through the recorded history of ichthyosaurs, and in particular across the critical Tr-J “bottleneck” period. The aims are to (i) compare disparity and diversity of ichthyosaurs through time, (ii) compare morphological disparity between different ichthyosaur clades, and (iii) investigate changes in disparity over time, particularly across the bottleneck event and the recovery.
Results
Phylogenetic Analysis.
We use a data matrix of ichthyosaurs, consisting of 32 genus-level taxa and 105 characters, based on an earlier study (13), modified by the addition of 6 subsequently described genera. The phylogenetic analysis resulted in 120 most-parsimonious trees with a length of 221 steps, a consistency index of 0.585 (excluding uninformative characters), and a retention index of 0.806. The strict consensus tree (SI Appendix, Fig. 1), agreement subtree (SI Appendix, Fig. 2), and majority rule consensus tree (SI Appendix, Fig. 3) are close to the phylogenetic hypothesis of Motani (13). The differences are: Six genera are added, relationships of basal Utatsusaurus and Parvinatator are better resolved, Grippia and Chaohusaurus no longer form a monophyletic group, and Leptonectidae and Temnodontosaurus are better resolved.
Diversity Through Time.
Ichthyosaurs existed throughout the Mesozoic, but their temporal distribution was patchy (SI Appendix, Fig. 4): There were numerous Triassic and Early Jurassic genera, but only one from the Middle Jurassic, and only three or four in the Late Jurassic and Cretaceous. The plot of the phylogenetic tree against geologic time (Fig. 1) shows concentrations of ghost ranges in the Early Triassic, early and late Late Triassic, early Early Jurassic, and late Middle to Late Jurassic, reflecting presumably a combination of sampling failure and rapid radiation of clades from low initial diversity. In view of the importance of the Tr-J event, it would be important to identify Rhaetian ichthyosaurs, assess global diversity, and see whether these terminal Triassic taxa might fall in the Late Triassic or Early Jurassic morphospaces; however, as noted, there are no Rhaetian taxa that are complete enough to be identified securely and coded for analysis.
Disparity.
The Mantel test results (Table 1 and SI Appendix, Table 1) suggest that the Euclidean distance matrices (from the whole-body, cranial, and postcranial datasets) were all correlated, suggesting no differential in the broad patterns of disparity change through the Mesozoic. The results are more complex, however, when divided into time bins (Table 1 and SI Appendix, Table 1): The postcranial and whole-body distance matrices are strongly correlated throughout, cranial and postcranial matrices are not correlated for any time bins, and cranial and whole-body matrices are correlated only in the Late Triassic and Early Jurassic but not in the Early and Middle Triassic and Middle Jurassic–Cretaceous time bins.
Table 1.
Mantel tests, used to analyze correlation between the Euclidean distance matrices of each dataset
| Mesozoic |
Lower and Middle Triassic |
Upper Triassic |
Lower Jurassic |
Middle Jurassic–Cretaceous |
||||||
| Datasets | rho | P | rho | P | rho | P | rho | P | rho | P |
| Whole-body vs. cranial | 0.4804 | 0*** | 0.1375 | 0.1982 | 0.4214 | 0.0156* | 0.7042 | 0.0016** | 0.5403 | 0.0960 |
| Whole-body vs. postcranial | 0.9490 | 0*** | 0.6292 | 0.0010** | 0.8752 | 0*** | 0.6797 | 0.0066** | 0.9292 | 0.0018** |
| Cranial vs. postcranial | 0.3515 | 0*** | −0.0410 | 0.4298 | 0.2380 | 0.0680 | −0.0066 | 0.4498 | 0.2994 | 0.1812 |
The distances calculated from the whole-body dataset, the cranial dataset, and the postcranial dataset were compared over the whole of the Mesozoic and also over the four time-bin intervals using Spearman-rank rho values, where P is the probability that two datasets are correlated. *P < 0.05; **P < 0.005; ***P < 0.0005.
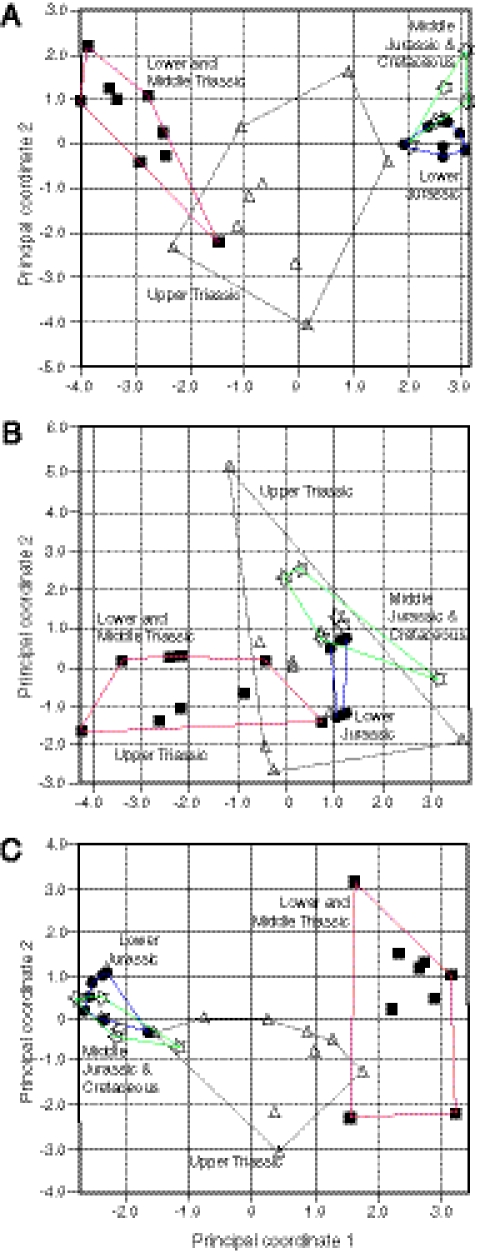
The key finding (Fig. 2) is that there were major shifts in morphospace occupation between all time bins, with the most dramatic changes taking place across the Tr-J boundary. Data from the whole skeleton (Fig. 2A) show that Early and Middle Triassic taxa overlap slightly with Late Triassic taxa in morphospace, but Triassic taxa are distinct from those of the Jurassic and Cretaceous. Further, Triassic taxa, particularly those from the Late Triassic, occupy a much larger area of morphospace than all Jurassic and Cretaceous taxa together, some 13 times greater. This confirms earlier qualitative observations (7, 16) that Late Triassic ichthyosaurs showed the widest range of body sizes and morphologies, whereas Early Jurassic ichthyosaurs, although hugely abundant within faunas and reasonably diverse (i.e., species rich), were conservative in morphology. The lack of overlap in morphospace from time bin to time bin could reflect small sample sizes. However, ichthyosaurs—especially those from the Early Jurassic—have been intensively sampled for 2 centuries and thousands of complete specimens have been collected. Crucially, all known morphotypes are included in the present study. Further, rarefaction (SI Appendix, Fig. 6) shows that disparity measures are not biased by within-study variations in sample size.
Fig. 2.
Morphospace occupation by ichthyosaurs. Plots are based on the first two axes from the principal coordinates analysis for each dataset: (A) whole-body characters; (B) cranial characters; and (C) postcranial characters. Taxa from the four time bins are distinguished by symbols, and minimal morphospace occupations by ichthyosaurs of each time bin are indicated by polygon outlines, differently colored for each time bin (red, Early–Middle Triassic; black, Late Triassic; blue, Early Jurassic; green, Middle Jurassic–Cretaceous).
Through the Mesozoic there was an overall unidirectional movement of morphospace occupation along the first morphospace axis [principal coordinates analysis (PCO) 1], which confirms the suggestion (7) of a change through time in body shape. The elongate “basal-grade” morphotype of the Early and Middle Triassic passed through an “intermediate grade” in the Middle and Late Triassic to a “fish-shaped grade” from the Late Triassic onward (Fig. 1).
Morphospace plots built from cranial and postcranial data only show overlaps between time-bin samples but no migration across the morphospace. The cranial plot (Fig. 2B) shows coincidence of the three Late Triassic to Cretaceous time bins but distinct morphospace occupation by the Early and Middle Triassic taxa. Early Jurassic forms occupy a tiny portion of the cranial morphospace occupied by Late Triassic forms, suggesting a considerable reduction in the variety of cranial shapes, but no novel skull morphologies, through the Tr-J bottleneck. The postcranial morphospace plot (Fig. 2C) shows greater separation of taxa from different time slices but still modest overlaps. In both data subsets, the morphospace areas occupied in the Triassic are much larger than those in the Jurassic and Cretaceous, but the Late Triassic region of morphospace is somewhat reduced in area when only postcranial characters are considered.
These visual comparisons are confirmed statistically: Nonparametric multivariate analysis of variance (NPMANOVA) tests show that Triassic taxa are significantly distinct from post-Triassic taxa in each case (Table 2 and SI Appendix, Table 2). Further, taxa from different time bins are significantly distinct when either the whole-skeleton or postcranial character subsets are used (Table 3 and SI Appendix, Table 3). However, for cranial characters alone, only Early and Middle Triassic taxa are significantly distinct from those in the other time bins (Fig. 2B).
Table 2.
NPMANOVA test for statistically significant differences in morphospace occupation between Triassic (n = 18) and post-Triassic (n = 13) taxa, based on PCO analysis output for the whole data matrix and the partitioned datasets
| P (same) overall | Pairwise comparisons, Bonferroni-corrected | |
| Whole body | <0.0001 | 0*** |
| Cranial | <0.0012 | 0.0004*** |
| Postcranial | <0.0001 | 0*** |
*P < 0.05; **P < 0.005; ***P < 0.0005.
Table 3.
NPMANOVA test for statistical significance between taxa from each of the four time bins, Lower and Middle Triassic (n = 9), Upper Triassic (n = 9), Lower Jurassic (n = 7), and Middle Jurassic–Cretaceous (n = 6), based on PCO analyses
 |
J, Jurassic; K, Cretaceous; L, Lower; M, Middle; Tr, Triassic; U, Upper. *P < 0.05; **P < 0.005; ***P < 0.0005.
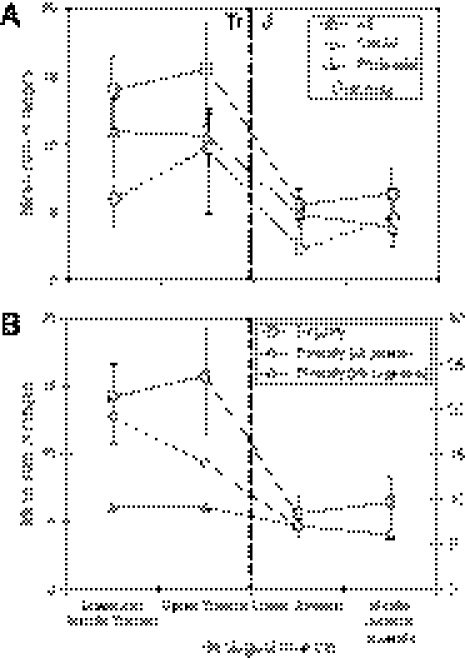
A massive drop in ichthyosaur disparity is seen at the end of the Triassic, falling from a mean value of 15.7 to 5.6 in the Early Jurassic (Fig. 3A). The cranial and postcranial data partitions show more modest drops across the Tr-J boundary, but all are statistically significantly different in that the error bars on the mean sums of ranges do not overlap (SI Appendix, Fig. 5). Many of the Late Triassic ichthyosaur extinctions appear to have taken place well before the Tr-J event itself (Fig. 1), although this could reflect incomplete sampling. Others survived to the end of the Triassic. Thus, the end-Triassic mass extinction was a crucial episode in the evolution of ichthyosaurs and other marine reptile groups, a time when 50% of all genera of marine organisms disappeared (3).
Fig. 3.
Disparity and diversity of ichthyosaurs through the Triassic and Jurassic. The global disparity values are based on the sum-of-ranges metric, and diversity is the number of species. Error bars are 95% confidence intervals. (A) Disparity change is shown for all characters, cranial characters, and postcranial characters. (B) All-character disparity compared with genus-level diversity change, showing plots for study genera (those included in the disparity study) and for all genera (addition of incompletely known taxa that were excluded from the disparity study but are close relatives of included taxa). The Tr-J boundary is marked by a heavy dashed vertical line.
Changes in mean disparity values from Middle to Late Triassic, and from Early Jurassic to Middle Jurassic and later, are not statistically significant as the error bars overlap, and this is true for all data as well as for cranial and postcranial character partitions (Fig. 3A and SI Appendix, Fig. 5 B and C). Rarefaction analyses (SI Appendix, Fig. 6) show that these changes in mean disparity values are not biased by sample size.
Comparison of Disparity and Diversity.
Ichthyosaur disparity and diversity differ substantially (Fig. 3B). Whereas diversity (whether all genera, all species, or study genera alone are considered) declines continuously through the Mesozoic, disparity drops remarkably across the Tr-J boundary. Phylogenetic correction of taxic diversity estimates (i.e., addition of ghost ranges) adds only three taxa in the Late Triassic (Fig. 1), because time bins are broad. Further, species counts differ little from generic counts (2), because most genera are monospecific.
The rises in diversity and disparity in the Early and Middle Triassic reflect the initial diversification of ichthyosaurs as a whole, and the continuing rise in disparity in the Late Triassic corresponds to further increase in the range of body sizes and feeding strategies. However, this cannot be interpreted as an “early burst” in the evolutionary radiation (18), because it happened some 20–25 Myr after the origin of the clade. The subsequent drop in disparity was mediated by extinction events in the Late Triassic, most notably the Tr-J mass extinction. Further exploration and collection effort in the Rhaetian may reveal finer-scale detail of the latest Triassic diversity decline. The continuing low levels of generic diversity and disparity in the Early Jurassic (Fig. 3B) are not a simple continuation of the Triassic radiation.
The drop in disparity from Late Triassic to Early Jurassic may be linked to major changes in ecospace occupation. For example, Triassic ichthyosaurs may have been ambush predators, whereas Jurassic ichthyosaurs were mainly pursuit predators (6). Further, there seems to have been a reduction in dietary adaptation from the Triassic to the Jurassic: Early ichthyosaurs were probably more generalized in their prey preference, as suggested by heterodonty in many Early and Middle Triassic species (6). Some Middle Triassic forms such as Phalarodon and Omphalosaurus with large posterior teeth may have been mollusk-eaters. The giant Late Triassic shastasaurids, some lacking teeth, may have specialized in gulping large shoals of squid or belemnites (19). Most taxa specialized in pelagic prey including fishes and soft-bodied cephalopods, and this more specific diet was retained by the Jurassic ichthyosaurs. Therefore, two or three major feeding modes in Late Triassic ichthyosaurs reduced to one in the Early Jurassic.
Evolutionary Bottlenecks.
The reduction of ichthyosaur lineages across the Tr-J transition is an evolutionary bottleneck (20–22). In pattern terms, this may be regarded as a macroevolutionary analog of a population bottleneck, although the processes are quite different. Whereas in the more familiar population or genetic bottleneck (23) a species is reduced to a very small number of individuals, and these provide the basis of a much-reduced gene pool in the later expansion of the surviving population, in a macroevolutionary bottleneck (20–22) numerous lineages are reduced to one or a few. There is no gene pool and no sharing of information through time as in a genetic bottleneck. Following the dramatic decline, ichthyosaur diversity increased again to reach pre-extinction levels in the Early Jurassic, after which the group dwindled in diversity through the Middle and Late Jurassic and much of the Cretaceous until it disappeared at the end of the Cenomanian, ∼100 Ma, after 150 Myr of significant ecological dominance (Fig. 1).
An evolutionary bottleneck may be detected by changes in taxic diversity (counts of species or genera), disparity (morphological variation), or ecological significance (abundance in faunas). All such data are subject to sampling error (2), but global diversity is most vulnerable to sampling bias.
The Tr-J bottleneck among ichthyosaurs is also seen among the other major group of Mesozoic marine reptiles, the sauropterygians (24); Triassic eosauropterygians (pachypleurosaurs, nothosaurs) and placodonts died out through the Late Triassic. Plesiosaurs survived as few lineages into the Jurassic, and then diversified (25). Some of the ecological roles of ichthyosaurs and sauropterygians in the Triassic were taken over by marine crocodilians, sharks, and bony fishes in the Jurassic, whereas other roles may have remained unoccupied, as in the case of the giant squid-gulping shastasaurids.
Our analysis highlights the previously unexpected role of the end-Triassic mass extinction event in imposing an evolutionary bottleneck on marine reptiles, most notably the ichthyosaurs. The extinction of Triassic taxa and rise of Jurassic taxa were mediated by an externally imposed crisis (3), and cannot be interpreted as a biotic replacement in which certain groups, say the Early Jurassic ichthyosaurs and plesiosaurs, demonstrated competitive superiority over their Triassic precursors. In this case, as with the initial rise of the dinosaurs (9) and the replacement of dinosaurs by mammals 65 Ma (26), external physical environmental factors were crucial. This confirms the need to assess carefully cases of purported competitive biotic replacements (27–29), and emphasizes the role of the Court Jester rather than the Red Queen in the larger patterns of macroevolution (30, 31).
Materials and Methods
Data Matrix.
Until 2003 (16), 235 species and 76 genera of ichthyosaurs had been named, but only 80 species and 36 genera were regarded as valid. We selected one of the several contemporary cladistic analyses, that by Motani (13), which consists of 105 characters and 27 ingroup taxa. Of these, 5 are species of Cymbospondylus and Mixosaurus, so in total 24 ichthyosaur genera are represented. For this study, only genera were coded, based on the type or sole specimen, and the data matrix (13) was updated to reflect the many new ichthyosaur descriptions and reclassifications published since 1999. Six new genera were added (Aegirosaurus, Callawayia, Guizhouichthyosaurus, Maiaspondylus, Qianichthyosaurus, Xinminosaurus) and one existing genus, Mixosaurus, was split in two, giving a final total of 31 ichthyosaur genera and a single outgroup, the basal diapsid Petrolacosaurus (see full details in SI Appendix1).
All 3,360 cells (32 taxa × 105 characters) in the data matrix were investigated to check the original character codings (13) and update and revise where necessary (see details in SI Appendix2). Of the 3,360 cells, 552 (16%) were revised. Excluding the 6 new genera, 208 (8%) of the 2,730 codings from Motani (13) have been revised or modified.
All 105 characters from Motani (13) are retained, no new ones are added, but 4 are amended, as follows.
Character 5. “Nasal/external naris contact—(0) absent; (1) present.” In the original paper (13), taxa were coded the other way round, so the description of this character was amended in this analysis so that a coding of 0 is for presence and of 1 is for absence.
Character 9. “Postfrontal postero-lateral process—(0) absent; (1) present, overlying the postorbital.” In one of the new genera, Aegirosaurus, the postero-lateral process does not clearly overlie the postorbital (32) and so cannot be coded using existing character states. Only the outgroup is coded 0, so this is an apomorphy of Ichthyopterygia. The character description was simplified to simple absence/presence, without reference to the postorbital, so Aegirosaurus can be coded with the derived condition.
Character 18. “Parietal supratemporal process—(0) short…; (1) long…,” which has been interpreted as the existence of either a short or long process from the parietal connecting with the supratemporal. However, an additional skull bone separates the parietal and supratemporal in Cymbospondylus (33), a different condition, which is accommodated by a further character state, “parietal and supratemporal separated by an additional skull bone.”
Character 84. “Pubis, obturator foramen—(0) completely enclosed in pubis…; (1) mostly in pubis but open on one side…; (2) part of obturator fossa….” However, in Aegirosaurus, the pubis and ischium are completely fused and no foramen is visible (32), so a fourth character state, “no foramen between the ischium and pubis,” was created.
Phylogenetic Analysis.
The amended and updated data matrix (SI Appendix4) was subjected to cladistic analysis using PAUP* 4b10 (34). All characters were treated as unordered and given equal weight. The analysis was performed in two stages, using two heuristic searches with default settings. The first search (swap = tbr; addseq = random; nreps = 10000; multrees = no) saved one tree per replicate, and the second search (swap = tbr; addseq = random; nreps = 10000; multrees = yes; start = current) used the existing trees from the first search as a starting point and allowed multiple trees to be saved per replicate. The strict consensus tree, agreement subtree, and majority rule consensus tree were constructed.
Diversity Through Time.
The phylogenetic tree was plotted against geological time, based on summary data (2), with revisions (SI Appendix, pp 1 and 2). Lazarus and ghost taxa were added. Diversity was calculated for four time intervals: Early and Middle Triassic; Late Triassic; Early Jurassic; and Middle Jurassic–Cretaceous. Smaller time intervals could not be used because of small sample sizes. Three measures of diversity were used: observed taxa; observed plus Lazarus taxa; and observed plus Lazarus and ghost taxa.
Disparity.
Disparity measures were calculated from the cladistic character data matrix using standard methods (9, 11, 12). Pairwise (taxon to taxon) Euclidean distance matrices were calculated using Matrix (35) and then subjected to PCO using GINKGO (36), from which morphospace plots were produced. Multivariate statistical tests (NPMANOVA), with Bonferroni correction, were performed to ascertain statistical significance of overlap and separation of groups. The PCO data were subjected to rarefaction analysis, using RARE (35) to normalize for sample size.
Supplementary Material
Acknowledgments
We thank Ryosuke Motani for early discussions, Michael Maisch for correspondence and papers, and anonymous referees for very helpful comments.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018959108/-/DCSupplemental.
References
- 1.Motani R. The evolution of marine reptiles. Evol Educ Outreach. 2009;2:224–235. [Google Scholar]
- 2.Benson RBJ, Butler RJ, Lindgren J, Smith AS. Mesozoic marine tetrapod diversity: Mass extinctions and temporal heterogeneity in geological megabiases affecting vertebrates. Proc R Soc Lond Biol Sci. 2010;277:829–834. doi: 10.1098/rspb.2009.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deenen MHL, et al. A new chronology for the end-Triassic mass extinction. Earth Planet Sci Lett. 2010;291:113–125. [Google Scholar]
- 4.Hallam A. How catastrophic was the end-Triassic mass extinction? Lethaia. 2002;35:147–157. [Google Scholar]
- 5.Tanner LH, Lucas SG, Chapman MG. Assessing the record and causes of Late Triassic extinctions. Earth Sci Rev. 2004;65:103–139. [Google Scholar]
- 6.Massare JA, Callaway JM. The affinities and ecology of Triassic ichthyosaurs. Bull Geol Soc Am. 1990;102:409–416. [Google Scholar]
- 7.Motani R. Ichthyosauria: Evolution and physical constraints of fish-shaped reptiles. Annu Rev Earth Planet Sci. 2005;33:395–420. [Google Scholar]
- 8.Erwin DH. Disparity: Morphological pattern and developmental context. Palaeontology. 2007;50:57–73. [Google Scholar]
- 9.Brusatte SL, Benton MJ, Ruta M, Lloyd GT. Superiority, competition, and opportunism in the evolutionary radiation of dinosaurs. Science. 2008;321:1485–1488. doi: 10.1126/science.1161833. [DOI] [PubMed] [Google Scholar]
- 10.Foote M. Discordance and concordance between morphological and taxonomic diversity. Paleobiology. 1993;19:185–204. [Google Scholar]
- 11.Wills M, Briggs DEG, Fortey R. Disparity as an evolutionary index: A comparison of Cambrian and Recent arthropods. Paleobiology. 1994;20:93–130. [Google Scholar]
- 12.Wesley-Hunt GD. The morphological diversification of carnivores in North America. Paleobiology. 2005;31:35–55. [Google Scholar]
- 13.Motani R. Phylogeny of the Ichthyopterygia. J Vertebr Paleontol. 1999;19:472–495. [Google Scholar]
- 14.Maisch MW, Matzke AT. The Ichthyosauria. Stuttgarter Beitr Ser B. 2000;298:1–159. [Google Scholar]
- 15.Sander PM. Ichthyosauria: Their diversity, distribution, and phylogeny. Paläontol Z. 2000;74:1–35. [Google Scholar]
- 16.McGowan C, Motani R. Ichthyopterygia. München: Pfeil; 2003. [Google Scholar]
- 17.McGowan C. A Revision of the Latipinnate Ichthyosaurs of the Lower Jurassic of England (Reptilia: Ichthyosauria) Toronto: Royal Ontario Museum; 1974. Life Sciences Contribution 100. [Google Scholar]
- 18.Gavrilets S, Losos JB. Adaptive radiation: Contrasting theory with data. Science. 2009;323:732–737. doi: 10.1126/science.1157966. [DOI] [PubMed] [Google Scholar]
- 19.Nicholls EL, Manabe M. Giant ichthyosaurs of the Triassic—A new species of Shonisaurus from the Pardonet Formation (Norian: Late Triassic) of British Columbia. J Vertebr Paleontol. 2004;24:838–849. [Google Scholar]
- 20.Raup DM. Biases in the fossil record of species and genera. Bull Carnegie Mus Nat Hist. 1979;13:85–91. [Google Scholar]
- 21.McGowan AJ. The effect of the Permo-Triassic bottleneck on Triassic ammonoid morphological evolution. Paleobiology. 2002;30:369–395. [Google Scholar]
- 22.Sallan LC, Coates MI. End-Devonian extinction and a bottleneck in the early evolution of modern jawed vertebrates. Proc Natl Acad Sci USA. 2010;107:10131–10135. doi: 10.1073/pnas.0914000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gherman A, et al. Population bottlenecks as a potential major shaping force of human genome architecture. PLoS Genet. 2007;3:e119. doi: 10.1371/journal.pgen.0030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rieppel P. Sauropterygia I—Placodontia, Pachypleurosauria, Nothosauroidea, Pistosauroidea. München: Pfeil; 2000. [Google Scholar]
- 25.Ketchum HF, Benson RB. Global interrelationships of Plesiosauria (Reptilia, Sauropterygia) and the pivotal role of taxon sampling in determining the outcome of phylogenetic analyses. Biol Rev Camb Philos Soc. 2010;85:361–392. doi: 10.1111/j.1469-185X.2009.00107.x. [DOI] [PubMed] [Google Scholar]
- 26.Smith FA, et al. The evolution of maximum body size of terrestrial mammals. Science. 2010;330:1216–1219. doi: 10.1126/science.1194830. [DOI] [PubMed] [Google Scholar]
- 27.Gould S, Calloway C. Clams and brachiopods—Ships that pass in the night. Paleobiology. 1980;6:383–396. [Google Scholar]
- 28.Benton MJ. Dinosaur success in the Triassic: A noncompetitive ecological model. Q Rev Biol. 1983;58:29–55. [Google Scholar]
- 29.Benton MJ. Progress and competition in macroevolution. Biol Rev Camb Philos Soc. 1987;62:305–338. [Google Scholar]
- 30.Barnosky AD. Distinguishing the effects of the Red Queen and Court Jester on Miocene mammal evolution in the northern Rocky Mountains. J Vertebr Paleontol. 2000;21:172–185. [Google Scholar]
- 31.Benton MJ. The Red Queen and the Court Jester: Species diversity and the role of biotic and abiotic factors through time. Science. 2009;323:728–732. doi: 10.1126/science.1157719. [DOI] [PubMed] [Google Scholar]
- 32.Bardet N, Fernández M. A new ichthyosaur from the Upper Jurassic lithographic limestones of Bavaria. J Paleontol. 2000;74:503–511. [Google Scholar]
- 33.Fröbisch NB, Sander PM, Rieppel P. A new species of Cymbospondylus (Diapsida, Ichthyosauria) from the Middle Triassic of Nevada and a re-evaluation of the skull osteology of the genus. Zool J Linn Soc. 2006;147:515–538. [Google Scholar]
- 34.Swofford DL. 2002. PAUP: Phylogenetic Analysis Using Parsimony, PAUP* (Sinauer, Sunderland, MA), 4.0 beta 4b. [Google Scholar]
- 35.Wills M. Crustacean disparity through the Phanerozoic: Comparing morphological and stratigraphic data. Biol J Linn Soc Lond. 1998;65:455–500. [Google Scholar]
- 36.De Cáceres M, Oliva F, Font X, Vives S. GINKGO, a program for non-standard multivariate fuzzy analysis. Adv Fuzzy Sets Syst. 2007;2:41–56. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.