Abstract
It is thought that semantic memory represents taxonomic information differently from thematic information. This study investigated the neural basis for the taxonomic-thematic distinction in a unique way. We gathered picture-naming errors from 86 individuals with poststroke language impairment (aphasia). Error rates were determined separately for taxonomic errors (“pear” in response to apple) and thematic errors (“worm” in response to apple), and their shared variance was regressed out of each measure. With the segmented lesions normalized to a common template, we carried out voxel-based lesion-symptom mapping on each error type separately. We found that taxonomic errors localized to the left anterior temporal lobe and thematic errors localized to the left temporoparietal junction. This is an indication that the contribution of these regions to semantic memory cleaves along taxonomic-thematic lines. Our findings show that a distinction long recognized in the psychological sciences is grounded in the structure and function of the human brain.
A fundamental question in cognitive neuroscience is how semantic knowledge is represented in the brain. We pursued this question by analyzing picture-naming errors generated by individuals with acquired language impairment (aphasia), focusing on errors that reflect the structure of semantic memory. When one views object A with the intention to name it, semantically related concepts B, C, and D come to mind along with A. These related concepts compete for access to the mental lexicon and occasionally stimulate the production of an error. Insight into the organization of semantic memory comes from analyzing the relations that link the target-error pairs.
All types of speakers produce semantic errors when tasked with naming objects from pictures. In neurologically intact speakers, error rates are quite low unless special techniques are used to exaggerate the retrieval competition or force a speeded response (e.g., refs. 1 and 2). Across all types of speakers and all manner of testing, semantic naming errors overwhelmingly reflect taxonomic relations; that is, the predominant error is a category coordinate (apple named as “pear” or “grape”), superordinate (apple → “fruit”), or subordinate (apple → “Granny Smith”). A small subset are thematic errors, such as apple → “worm” or bone → “dog,” in which the target and error are from different taxonomic categories but frequently play complementary roles in the same actions or events (1, 3).
The distinction between taxonomic and thematic relations has been extensively researched in the psychology of concepts and conceptual relations (4, 5). Moreover, the distinction echoes the long-standing interest among philosophers in how similarity and contiguity organize the associative structure of the mind (e.g., ref. 6). Evidence from naming errors affords a rare opportunity to study how the taxonomic-thematic distinction manifests in language production. Most empirical research on this topic uses matching and sorting tasks that invoke explicit strategies and, consequently, are highly influenced by small variations in task demands (4). Perhaps for this reason, the few studies that have sought to differentiate the neural correlates of taxonomic and thematic relations have produced inconclusive results. For example, two studies used functional neuroimaging to identify brain regions involved in matching thematically or taxonomically related pictures. One study found that thematic and taxonomic matches recruited similar brain regions (7); the other identified dissociations within regions specialized for sensorimotor processing (8).
To date, there is no clear evidence that semantic systems in the brain are differentially specialized for taxonomic and thematic relations. It is thus unknown whether this distinction reflects a basic difference in neural representations or whether it instead emerges from differences in the strategies participants use to identify these relations in the standard laboratory tasks.
To answer this question, we collected numerous naming responses from a large sample of aphasic patients so as to have sufficient taxonomic and thematic errors to analyze. We then carried out advanced lesion analysis to determine whether the error types were associated with different sites of damage. In this type of lesion analysis, called voxel-based lesion-symptom mapping (VLSM) (9), uniform behavioral data and normalized lesion maps are collected on a large patient sample and statistical tests are then performed voxel by voxel to assess the association between the behavioral measure and damage in that voxel (10–12). Of particular relevance here is that the methods afforded statistical control over confounding factors, such as faulty picture comprehension and excessively large lesions, which would otherwise complicate the interpretation. The results show that taxonomic and thematic errors are generated from fundamentally different semantic systems in the brain.
Results
Behavioral Analysis.
On the 175-item picture-naming test, patients averaged 68% correct (range: 1–98%) and 20 age-matched controls averaged 97% correct (range: 91–100%) (t = 9.8, df = 93, P < 0.001). Patients produced a total of 645 taxonomic errors (mean = 7.5, SD = 5.2) and 134 thematic errors (mean = 1.6, SD 1.9). The ratio of taxonomic to thematic errors (4.8:1) was roughly comparable in the controls (5.5:1), although, of course, controls made many fewer errors (44 taxonomic and 8 thematic). Every patient made at least 1 taxonomic error. Fifty-five patients (64%) contributed thematic errors. All but 2 of these made more taxonomic than thematic errors, and the remaining 2 made few errors (<5) of either type. This confirms previous evidence that the predominant type of semantic error in naming is taxonomic. More specifically, in this large patient cohort, we observed no instances in which there were only or many more thematic errors; all the dissociations went the other way.
Taxonomic and thematic errors were correlated with each other, and each was correlated with naming severity and size of lesion (all ρ values were between 0.38 and 0.42; P < 0.001). Philadelphia Naming Test (PNT) targets were 62% inanimate and 38% animate. Taxonomic errors followed a similar distribution: 58% were made to inanimate targets. Thematic errors were biased toward inanimate targets (72%) (χ2 = 8.6, P = 0.003).
Lesion Analysis.
For purposes of the lesion analysis, we further isolated thematic from taxonomic influences by regressing out the shared variance between the error scores. The measure we call “ResidTaxon” indexes the patient's tendency to generate taxonomic errors, with the variance attributable to thematic errors removed; “ResidTheme” is the analogous measure for thematic errors. In both of these measures, the variance attributable to performance on a picture-matching test of semantic comprehension was also regressed out (details are provided in Materials and Methods). This ensures that both ResidTaxon and ResidTheme reflect patients’ tendencies to err at the word retrieval stage of production rather than at the prior conceptual stage. In other analyses, described in SI Materials and Methods, we found that additionally regressing out variance attributable to word comprehension deficits did not alter the results of the lesion analysis.
The VLSM analysis was carried out in all voxels lesioned in at least 5 patients (a total of 504,228 voxels). No voxel was lesioned in more than 48 patients. Lesion coverage, and thus power, was good throughout the lateral portion of the left hemisphere exclusive of the occipital lobe and the medial and posterior inferior temporal lobe (Fig. S1).
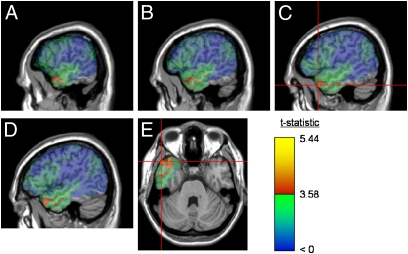
VLSM of ResidTaxon identified a total of 7,192 significant voxels. The largest cluster, containing 6,462 voxels (90%), occupied the anterior temporal lobe (ATL) (Fig. 1). A plurality of these [2,388 (33%)], including the 6 peak voxels (t = 5.44), were located in the temporal pole, Brodmann area (BA) 38. The anterior portions of BA 20 and BA 21, adjacent to the temporal pole, also contained a substantial portion of suprathreshold voxels [1,796 (25%) and 1,956 (27%), respectively]. Thirteen percent of suprathreshold voxels were in white matter, mostly underlying the ATL. The remaining 2% were scattered among other temporal and frontal BAs, with no obvious pattern of interest.
Fig. 1.
Maps of the reliability (t statistic) of the difference in ResidTaxon between patients with and without lesions in each voxel. Voxels exceeding the false discovery rate threshold (q = 0.02) are rendered in a red (t = 3.58) to yellow (t = 5.44) scale, whereas nonsignificant values are rendered on a scale from green (t just below threshold) to blue (t ≤ 0). Statistical maps are superimposed on the MNI (Montreal Neurological Institute) space Colin27 template. In all panels, a large cluster of significant voxels is visible in the anterior temporal lobe. A–D show sagittal slices at x = −60, x = −56, x = −52, and x = −48, respectively. The region of peak effect is marked with crosshairs. E shows an axial slice at z = −31, selected to intersect the peak region.
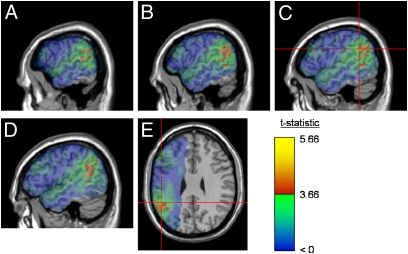
VLSM of ResidTheme identified a total of 5,513 significant voxels, with the vast majority [4,707 (85%)] occurring in and around the temporoparietal junction (TPJ) (Fig. 2). The largest concentration of suprathreshold voxels [1,916 (35%)] occupied the angular gyrus (BA 39). Another 306 voxels (6%) were located in the adjacent supramarginal gyrus, and these included 2 with peak t values (t = 5.66). Suprathreshold voxels in the temporal lobe clustered in contiguous regions of posterior BA 21 [1,094 (20%)], posterior BA 22 [818 (15%)], and superior BA 37 [281 (5%)]. Primary auditory cortex, BA 41 and BA 42, also contained significant voxels [570 (10%)]. Finally, a single voxel was identified in frontal BA 45. There were no significant voxels common to both the ResidTaxon and ResidTheme analyses. Table 1 presents a comparison of the effect size for each behavioral measure in key regions of the ATL and TPJ.
Fig. 2.
Maps of the reliability (t statistic) of the difference in ResidTheme between patients with and without lesions in each voxel. Voxels exceeding the false discovery rate threshold (q = 0.02) are rendered in a red (t = 3.66) to yellow (t = 5.66) scale; green and blue are rendered as described in Fig. 1. Statistical maps are superimposed on the MNI (Montreal Neurological Institute) space Colin27 template. In all panels, a large cluster of significant voxels is visible in the temporoparietal junction. A–D show sagittal slices at x = −60, x = −56, x = −52, and x = −48, respectively. The region of peak effect is marked with crosshairs. E shows an axial slice at z = 28, selected to intersect the peak region.
Table 1.
Comparison of effect size
| Average t value |
|||
| BA | No. voxels | VLSM of taxonomic error* | VLSM of thematic error* |
| BA 38 (ATL) | 11,351 | 2.71 | 0.9 |
| BA 39 (TPJ) | 13,900 | −1.03 | 2.56 |
*Residualized on the other error type and the CCT.
In a further check on the independence of the anatomical correlates of the two error types from one another and from lesion size, we regressed each error type on five anatomical predictors: percentage of damage within BA 38, 21, 37, and 39 and total lesion volume.
Lesion volume is an appropriate proxy for aphasia severity, because standard behavioral measures, such as the Aphasia Quotient from the Western Aphasia Battery (13), are heavily influenced by naming performance, and therefore are unsuitable for the present purposes.
For ResidTaxon, the model was significant (R2 = 0.18, P = 0.01), with BA 38 making an independent contribution (β = 1.6, P = 0.05). For ResidTheme, the overall model was again significant (R2 = 0.15, P = 0.02), this time with BA 39 making an independent contribution (β = 1.6, P = 0.01). None of the other predictors in either model, including lesion volume, were significant (P > 0.1). Table 2 presents a comparison of thematic error scores in patients with and without damage to BA 39.
Table 2.
Thematic error scores for patients with damage to BA 39 (and not BA 38) are compared with those for patients without damage to BA 39 (t = −2.22, df = 28, two-tailed P = 0.03)
| +BA 39/−BA 38 | −BA 39 | |
| Mean thematic errors | 1.57 | 0.62 |
| No. patients | 23 | 34 |
It has recently been argued that thematic naming errors in aphasia are symptomatic of the inability to regulate retrieval from semantic memory in a task-appropriate manner (3) and that the semantic regulation impairment is attributable to lesions in either the prefrontal cortex or TPJ (3, 14). If lesions in TPJ voxels produce thematic errors attributable to loss of semantic control, they should also carry an association with other “off-task” naming errors. The canonical off-task error in object-picture naming is a nonnoun response that describes rather than names the target (e.g., horse → “it goes ‘neigh’”). We tabulated the frequency of these errors across patients and residualized them against the picture comprehension test to create a new dependent measure for VLSM, “ResidNonNoun.” One patient was excluded from this analysis because of an abnormally high rate of descriptions. As Fig. S2 shows, results for the VLSM of ResidNonNoun clearly patterned with ResidTaxon and not with ResidTheme. The 41,293 voxels that exceeded threshold, most occupying the ATL, overlapped with 77.6% of the voxels identified in the ResidTaxon analysis, including the peak voxels in BA 38. In contrast, they overlapped with only 0.014% of the voxels identified in the ResidTheme analysis, and these did not include the peak voxels.
Discussion
Semantic errors can be found in the naming performance of all speakers, both with and without neurological impairment. These errors are common, in part, because they have multiple causes. The controlled (residualized) analyses used in this study sought to tease these causes apart. As shown in the figures, the analyses successfully distinguished the anatomical correlates of two kinds of semantic errors. Specifically, they show maps in which regional injury predicts variance in taxonomic (Fig. 1) and thematic (Fig. 2) errors that cannot be explained either by variance in the other type of error or by variance in picture comprehension. Because of the specificity of these analyses, we were able to determine that the ATL and TPJ are particularly and differentially important for taxonomic and thematic errors, respectively. It is important to note that these conclusions would not be possible without the use of these controlled methods. VLSM analyses of raw taxonomic and thematic error rates in our sample implicate extensive overlapping swathes of the left hemisphere, and thus provide little insight into the anatomy of semantic memory.
Complementary Semantic Systems.
To identify a target for naming, a speaker first processes the visual array for relevant semantic information. Features that specify a target's appearance, function, taxonomic category, and other internal properties become activated. As this activation spreads from semantic features to the words that instantiate them, the target name is primed, along with the names of other entities that resemble it in, for example, appearance and function. The activation of these other words sets the stage for taxonomic errors. This is the familiar part of the story (e.g., ref. 15). The present behavioral evidence indicates that the set of coactivated words also includes some whose relation to the target is based less on feature similarity than on shared thematic context (16). The key question is whether this reflects the engagement of a semantic system that is distinct from the feature-based system just described.
The speech errors made by the patients do not by themselves point to a semantic system that is separate from the one responsible for taxonomic errors. The error data exhibit only a one-way dissociation, wherein taxonomic errors occurred without thematic errors but not the reverse. Such a result can be construed as consistent with a unitary account that aligns taxonomic and thematic information on a gradient of task appropriateness. For example, in a naming task, “pear” for apple is in the right ballpark; “worm” is less task-appropriate, and hence is less common. It is the neuroanatomical double dissociation between the ATL and TPJ that allows us to reject a unitary account in favor of complementary semantic systems. This evidence suggests that damage to the ATL disrupts successful naming by blurring distinctions among taxonomically related names, rendering “apple” confusable with “pear,” for example. In contrast, damage to the TPJ disrupts naming by blurring distinctions among thematically related names, rendering “apple” confusable with “worm,” for example.
Taxonomic Processing in the ATL.
In a prior VLSM study, we reported that aphasic patients’ semantic errors (controlling for semantic comprehension) localized to the left ATL, with peak voxels in the mid to anterior middle temporal gyrus (MTG) (17). The present results suggest that this ATL association was specifically carried by semantic taxonomic errors. This receives support from neuropsychological studies of patients with the progressive disorder known as semantic dementia, also known as the semantic variant of primary progressive aphasia. It has been shown that the profound disorder of naming that heralds this condition results from damage centered in the anterior inferolateral temporal region on the left (18, 19) and that the primary errors of commission in naming are taxonomic (category coordinate and superordinate) (20, 21). In contrast to aphasia, these patients hardly ever make thematic errors in naming (3). We suggest that the left ATL communicates feature and category information to posterior lexical-phonological systems, a specialization it derives from being part of the bilateral anteromedial and inferolateral network for visual object identification (22–25).
Thematic Processing in the TPJ.
The angular gyrus (BA 39) has a long-recognized role in picture naming, traditionally attributed to its status as a multimodality association area with interconnections to frontal and temporal speech areas (26). The inferior parietal lobule (IPL), encompassing the angular and supramarginal gyri, together with the posterior temporal regions that wrap around the posterior Sylvian fissure, is among the most consistently implicated in neuropsychological and neuroimaging studies of semantics (reviewed in ref. 27).* In particular, the left IPL (especially the supramarginal gyrus) and posterior MTG (pMTG) have been shown to be important substrates for action and tool semantics (27, 30–33). The proximity of semantic regions in the pMTG and IPL to those involved in motion perception and gesture production, respectively, has contributed to the theory that semantic processing is achieved by mental simulation or reenactment of one's own perceptual and motor experience (e.g., ref. 34; pertinent reviews and discussion are included in refs. 35–37).
Relevant to this theory, it has been proposed that the conceptualization of thematic relations is grounded in our experience of acting in the world, and particularly in object-centered action (38). Kalénine et al. (8) used functional MRI to measure brain activation while participants matched a target picture to one of two choice pictures that was related to it taxonomically or thematically. The targets’ semantic category (natural objects, artifacts) and manipulability (manipulable, nonmanipulable) were crossed factorially. Contrasting taxonomic against thematic matches, they found that taxonomic matches specifically activated bilateral visual areas (cuneus, BA 18), whereas thematic matches activated a bilateral temporoparietal network. Region-of-interest analyses derived from these contrasts showed that the effects were modulated by object category. In particular, activations in the left IPL (BA 39/40) and pMTG (BA 39/21/22) were especially strong for thematic matches with manipulable artifacts.
The cognitive requirements of choosing a match, as in the study by Kalénine et al. (8), are very different from those required to name an object; thus, it is impressive that with the more implicit measure of naming error, we identified thematic effects in those same left IPL and pMTG regions. Also consistent with that study is our finding that thematic errors have a greater affinity for inanimate targets. The specialization of the IPL/pMTG for tool and action semantics is thus likely to be part of the explanation for our findings. Although many thematic errors in our corpus do involve objects with complementary functions in action events (dog → “bone”; zipper → “jacket”), many others are linked by other types of relation, such as spatial relations (e.g., anchor → “sea”) or causal relations (e.g., ambulance → “fire”). This goes along with a broader role for this TPJ area in the representation of relational information (39), which may be what undergirds its essential contribution to sentence comprehension (40). We suggest that in the process of identifying an object for naming, relevant event representations are retrieved or simulated that create a momentary linkage between the target concept and others in the event context (41). This process probably takes place bilaterally in the TPJ, but it is the component on the left that conveys information about these linked concepts to left-lateralized lexical-phonological systems. Lesions here render this communication noisier or less precise, thereby reducing the natural advantage of the target concept over its contextual associates and encouraging an error in which one of these associates is named in place of the target.
Although impaired processing in the TPJ can blunt the natural advantage of the target concept over its thematic associates, it must be recognized that object naming is first and foremost about an object's identification and name rather than its thematic properties. The complementary ATL system continues to provide relevant input on that score; consequently, the most probable semantic error, even with TPJ damage, will be a taxonomic one, consistent with our finding that these errors dominate in all patients. To give a specific example, suppose that the target picture shows an apple. The TPJ normally signals strongly for the word “apple,” and somewhat less strongly for the word “worm,” for example, whereas the ATL signals strongly for “apple” and somewhat less strongly for “pear” or “orange,” for example. If the TPJ is damaged, there will be a weaker signal for “apple” and perhaps a relatively stronger one for “worm.” The outcome of lexical selection is probabilistic; thus, under these circumstances, “worm” could be selected, but because feature and category input dominates in naming, it is more likely that the reduced TPJ support for “apple” would tip the balance toward “pear.”
Conclusions
It is known from patient research and functional neuroimaging studies of the intact brain that the TPJ and ATL are key components of the brain network for semantic processing in general and for naming concepts in particular. Our study shows that the contributions of these two areas to semantically driven naming are different and separable. Building on the influential theory holding that the ATL functions as a hub-like multimodal association area (25), we propose that the ATL and TPJ are each multimodal hubs that extract somewhat different relationships. The ATL extracts perceptual feature similarity for the purpose of object processing, whereas the TPJ extracts role relations for the purpose of event processing. The ATL system is the dominant one in naming, which explains why taxonomic errors predominate over thematic errors. Nevertheless, because the two systems make different and complementary contributions, we have succeeded in using naming-error data to show that a distinction long recognized in the psychological sciences is grounded in the structure and function of the human brain.
Materials and Methods
Participants.
Patients who had been diagnosed acutely with aphasia secondary to left hemisphere stroke were recruited from the Neuro-Cognitive Rehabilitation Research Patient Registry (42) at the Moss Rehabilitation Research Institute or from the Center for Cognitive Neuroscience Patient Database (43) at the University of Pennsylvania. Eighty-six passed the inclusion/exclusion criteria and gave informed consent to take part in a multisession language assessment under protocols approved by the Institutional Review Boards at the Albert Einstein Medical Center and University of Pennsylvania School of Medicine. All but 8 of these patients also gave informed consent to undergo structural MRI or computed tomography (CT) imaging of the brain to determine the precise localization of their lesion. For the remaining 8, a recently performed clinical imaging study judged to be of high quality was used instead. Imaging studies were conducted under the protocol of the University of Pennsylvania School of Medicine and were performed at that facility. Participants were paid for their participation and reimbursed for travel and related expenses.
In keeping with the inclusion criteria, the patients were all between 18 and 80 y of age, premorbidly right-handed, English-speaking, and without major psychiatric or neurological comorbidities or uncorrected vision or hearing impairment. We included only patients with imaging-confirmed focal unilateral left cortical lesions, excluding those in which the structural damage was diffuse, bilateral, or contained within the subcortex. We also excluded those patients who were unable to name at least one item or to understand or follow instructions.
The sample, consisting of 33 women and 53 men, averaged 58 y of age (range: 26–79 y), 14 y of education (range: 10–21 y), and 58 mo after onset of stroke (range: 1–381 mo). Eighty-five percent were in the chronic phase (>6 mo). The predominant subtype diagnosis was anomic aphasia (49% of patients), followed by Broca's (28% of patients) and conduction (17% of patients). All lived in the community at the time of testing.
For purposes of comparison, 20 healthy adults also performed the tasks that were assigned to the patients. They were recruited from the same community, and they were selected to match the age distribution of the patients, averaging 59 y of age (range: 25–75 y). All gave informed consent and were reimbursed for their time and expenses.
Language Measures.
PNT.
The PNT is an object-naming test involving 175 black and white pictures (44). The targets represent entities from varied semantic categories, with the largest being manipulable objects (41%) and animals (15%). None of the targets represent known persons or other unique entities. Pictures have high familiarity, name agreement, and image quality. Names range in length from one to four syllables and in noun frequency from 1 to 2,110 tokens per million. The PNT has been used in many studies testing theories of production by investigating the distributions of aphasic picture-naming errors (e.g., refs. 45–47). Materials and scoring procedures can be downloaded from http://www.mrri.org.
The coding of naming errors took place in two stages. First, experts in PNT scoring coded each response using standard PNT procedures. On each trial, the first complete (i.e., nonfragment) response produced within 20 s was scored for accuracy and type of error. As is standard, a response was assigned the semantic error code if it was a noun that was related to the target by category, context, or both. If it also shared phonemes with the target, it was coded as “mixed.” At the next stage of coding, semantic and mixed errors were combined and then subdivided along taxonomic/thematic lines by expert consensus. The taxonomic code was applied to coordinate, superordinate, or subordinate noun substitutions, without regard to thematic relatedness. The thematic code was reserved for semantic errors that did not qualify as taxonomic and that named an object that co-occurred with the target in the context of an action, event, or sentence. Thus, we allowed for some intermixing of relations among the taxonomic errors, but the errors designated as thematic were purer instances of that type. A norming study with 77 undergraduates validated our coding decisions (SI Materials and Methods).
Camel and Cactus Test.
The Camel and Cactus Test (CCT) (48) is a nonverbal semantic comprehension test that requires the matching of pictured objects on the basis of their meaning. On each of the 64 trials, subjects choose which of four related objects goes best with a fifth, the probe. For example, with camel as the probe, the choices are cactus, tree, sunflower, and roses. Trials are untimed, and performance is scored for accuracy. Our patients averaged 77% correct (range: 25–95%), and 20 age-matched healthy controls averaged 89% correct (range: 77–97%) (t = 6.4, df = 78, P < 0.001).
Image Acquisition.
A total of 78 patients received MRI (n = 44) or CT (n = 34) brain scans at the Hospital of the University of Pennsylvania.† High-resolution whole-brain T1-weighted images [magnetization prepared rapid acquisition gradient echo (MPRAGE)] were acquired for all the patients undergoing MRI. Of these, 38 were scanned on a 3-T Siemens Trio scanner [repetition time (TR) = 1,620 ms, echo time (TE) = 3.87 ms, field of view (FOV) = 192 × 256 mm, 1 × 1 × 1-mm voxels]. Because medical implants were not approved for the higher strength magnetic field, 6 patients were scanned instead on a 1.5-T Siemens Sonata (TR = 3,000 ms, TE = 3.54 ms, FOV = 24 cm, 1.25 × 1.25 × 1.2-mm voxels). For those patients who were not eligible for MRI scanning, whole-brain CT scans without contrast (60 axial slices, 3 mm thick) were acquired. As noted earlier, 8 additional patients declined scanning; for these patients, recent clinical scans [CT (n = 7) and MRI (n = 1)] with clearly delineated lesion boundaries were substituted in the lesion tracing procedure. Details of lesion tracing and registration to a common template are provided in SI Materials and Methods.
VLSM.
Separate VLSM analyses were performed with each behavioral score: ResidTaxon and ResidTheme. ResidTaxon was computed by regressing taxonomic errors against thematic errors and CCT scores and using the residuals. ResidTheme was similarly computed.‡
In each analysis, one-tailed t tests were performed at every voxel lesioned in at least five patients, comparing behavioral scores for patients with and without damage in the voxel. The threshold for significance was calculated to control the false discovery rate (49) at q = 0.02, where q is the expected proportion of type I errors among suprathreshold voxels. Although the choice of q is somewhat arbitrary, we thought that a criterion of q = 0.02 made for the most readily interpretable figures. Applying a more stringent threshold of q = 0.01 reduced the number of significant voxels but did not alter the conclusions. For each analysis, maps of the t values were overlaid on the MNI space Colin27 template and figures were obtained from four sagittal slice planes at x coordinates of −60, −56, −52, and −48 (A–D in all figures). A single axial slice (E in all figures) displays the peak region, which is marked by crosshairs.
Supplementary Material
Acknowledgments
We thank Dr. Katherine Rawson for facilitating the collection of normative data. This work was supported by National Institutes of Health/ National Institute on Deafness and Other Communication Disorders Grant R01DC000191-29 (to M.F.S.).
Footnotes
The authors declare no conflict of interest.
An analysis of this study was presented at the Academy of Aphasia, October 24-26, 2010, Athens, Greece (50).
This article is a PNAS Direct Submission.
*The functional MRI literature has tended to underestimate the contribution of the ATL to semantics because of technical limitations and methodological factors (28, 29).
†Imaging modality (CT vs. MRI) did not correlate with the production of taxonomic or thematic errors (r = 0.10 for both).
‡A comparable analysis using multiple regression instead of residualization to remove the effects of the two nuisance covariates yielded essentially the same results.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014935108/-/DCSupplemental.
References
- 1.Hodgson C, Lambon Ralph MA. Mimicking aphasic semantic errors in normal speech production: Evidence from a novel experimental paradigm. Brain Lang. 2008;104:89–101. doi: 10.1016/j.bandl.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Vitkovitch M, Humphreys GW. Perseverant responding in speeded naming of pictures—It's in the links. J Exp Psychol Learn. 1991;17:664–680. [Google Scholar]
- 3.Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: A case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- 4.Estes Z, Golonka S, Jones LL. Thematic thinking: The apprehension and consequences of thematic relations. Psychol Learn Motiv. 2011;54:249–294. [Google Scholar]
- 5.Lin EL, Murphy GL. Thematic relations in adults’ concepts. J Exp Psychol Gen. 2001;130:3–28. doi: 10.1037/0096-3445.130.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Bain A. The Senses and the Intellect. London: Parker & Sons; 1855. [Google Scholar]
- 7.Sachs O, Weis S, Krings T, Huber W, Kircher T. Categorical and thematic knowledge representation in the brain: Neural correlates of taxonomic and thematic conceptual relations. Neuropsychologia. 2008;46:409–418. doi: 10.1016/j.neuropsychologia.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Kalénine S, et al. The sensory-motor specificity of taxonomic and thematic conceptual relations: A behavioral and fMRI study. Neuroimage. 2009;44:1152–1162. doi: 10.1016/j.neuroimage.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 9.Bates E, et al. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- 10.Kimberg DY, Coslett HB, Schwartz MF. Power in Voxel-based lesion-symptom mapping. J Cogn Neurosci. 2007;19:1067–1080. doi: 10.1162/jocn.2007.19.7.1067. [DOI] [PubMed] [Google Scholar]
- 11.Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- 12.Rudrauf D, et al. Thresholding lesion overlap difference maps: Application to category-related naming and recognition deficits. Neuroimage. 2008;41:970–984. doi: 10.1016/j.neuroimage.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kertesz A. The Western Aphasia Battery. New York: Grune and Stratton; 1979. [Google Scholar]
- 14.Noonan KA, Jefferies E, Corbett F, Lambon Ralph MA. Elucidating the nature of deregulated semantic cognition in semantic aphasia: Evidence for the roles of prefrontal and temporo-parietal cortices. J Cogn Neurosci. 2010;22:1597–1613. doi: 10.1162/jocn.2009.21289. [DOI] [PubMed] [Google Scholar]
- 15.Belke E, Meyer AS, Damian MF. Refractory effects in picture naming as assessed in a semantic blocking paradigm. Q J Exp Psychol A. 2005;58:667–692. doi: 10.1080/02724980443000142. [DOI] [PubMed] [Google Scholar]
- 16.Abdel Rahman R, Melinger A. When bees hamper the production of honey: Lexical interference from associates in speech production. J Exp Psychol Learn Mem Cogn. 2007;33:604–614. doi: 10.1037/0278-7393.33.3.604. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz MF, et al. Anterior temporal involvement in semantic word retrieval: Voxel-based lesion-sympton mapping evidence from aphasia. Brain. 2009;132:3411–3427. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: Neuropsychological evidence and a computational model. J Cogn Neurosci. 2001;13:341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- 19.Mesulam M, et al. Neurology of anomia in the semantic variant of primary progressive aphasia. Brain. 2009;132:2553–2565. doi: 10.1093/brain/awp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers TT, et al. Structure and deterioration of semantic memory: A neuropsychological and computational investigation. Psychol Rev. 2004;111:205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- 21.Woollams AM, Cooper-Pye E, Hodges JR, Patterson K. Anomia: A doubly typical signature of semantic dementia. Neuropsychologia. 2008;46:2503–2514. doi: 10.1016/j.neuropsychologia.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Bright P, Moss HE, Stamatakis EA, Tyler LK. The anatomy of object processing: The role of anteromedial temporal cortex. Q J Exp Psychol B. 2005;58:361–377. doi: 10.1080/02724990544000013. [DOI] [PubMed] [Google Scholar]
- 23.Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- 24.Grabowski TJ, et al. A role for left temporal pole in the retrieval of words for unique entities. Hum Brain Mapp. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- 26.Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- 27.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binney RJ, Embleton KV, Jefferies E, Parker GJ, Ralph MA. The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: Evidence from a novel direct comparison of distortion-corrected fMRI, rTMS, and semantic dementia. Cereb Cortex. 2010;20:2728–2738. doi: 10.1093/cercor/bhq019. [DOI] [PubMed] [Google Scholar]
- 29.Rogers TT, et al. Anterior temporal cortex and semantic memory: Reconciling findings from neuropsychology and functional imaging. Cogn Affect Behav Neurosci. 2006;6:201–213. doi: 10.3758/cabn.6.3.201. [DOI] [PubMed] [Google Scholar]
- 30.Martin A, Chao LL. Semantic memory and the brain: Structure and processes. Curr Opin Neurobiol. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 31.Kable JW, Lease-Spellmeyer J, Chatterjee A. Neural substrates of action event knowledge. J Cogn Neurosci. 2002;14:795–805. doi: 10.1162/08989290260138681. [DOI] [PubMed] [Google Scholar]
- 32.Kalénine S, Buxbaum LJ, Coslett HB. Critical brain regions for action recognition: Lesion symptom mapping in left hemisphere stroke. Brain. 2010;133:3269–3280. doi: 10.1093/brain/awq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noppeney U. The neural systems of tool and action semantics: A perspective from functional neuroimaging. J Physiol Paris. 2008;102:40–49. doi: 10.1016/j.jphysparis.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Schubotz RI. Prediction of external events with our motor system: Towards a new framework. Trends Cogn Sci. 2007;11:211–218. doi: 10.1016/j.tics.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Barsalou LW. Simulation, situated conceptualization, and prediction. Philos Trans R Soc Lond B Biol Sci. 2009;364:1281–1289. doi: 10.1098/rstb.2008.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grafton ST. Embodied cognition and the simulation of action to understand others. Ann N Y Acad Sci. 2009;1156:97–117. doi: 10.1111/j.1749-6632.2009.04425.x. [DOI] [PubMed] [Google Scholar]
- 37.Buxbaum LJ, Kalénine S. Action knowledge, visuomotor activation, and embodiment in the two action systems. Ann N Y Acad Sci. 2010;1191:201–218. doi: 10.1111/j.1749-6632.2010.05447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson K. Making Sense: The Acquisition of Shared Meaning. Orlando, FL: Academic; 1985. [Google Scholar]
- 39.Wu DH, Waller S, Chatterjee A. The functional neuroanatomy of thematic role and locative relational knowledge. J Cogn Neurosci. 2007;19:1542–1555. doi: 10.1162/jocn.2007.19.9.1542. [DOI] [PubMed] [Google Scholar]
- 40.Baldo JV, Dronkers NF. Neural correlates of arithmetic and language comprehension: A common substrate? Neuropsychologia. 2007;45:229–235. doi: 10.1016/j.neuropsychologia.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Hare M, Jones M, Thomson C, Kelly S, McRae K. Activating event knowledge. Cognition. 2009;111:151–167. doi: 10.1016/j.cognition.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz MF, Brecher AR, Whyte JW, Klein MG. A patient registry for cognitive rehabilitation research: A strategy for balancing patients’ privacy rights with researchers’ need for access. Arch Phys Med Rehabil. 2005;86:1807–1814. doi: 10.1016/j.apmr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Fellows LK, Stark M, Berg A, Chatterjee A. Patient registries in cognitive neuroscience research: Advantages, challenges, and practical advice. J Cogn Neurosci. 2008;20:1107–1113. doi: 10.1162/jocn.2008.20065. [DOI] [PubMed] [Google Scholar]
- 44.Roach A, Schwartz MF, Martin N, Grewal RS, Brecher A. The Philadelphia Naming Test: Scoring and rationale. Clin Aphasiol. 1996;24:121–133. [Google Scholar]
- 45.Dell GS, Schwartz MF, Martin N, Saffran EM, Gagnon DA. Lexical access in aphasic and nonaphasic speakers. Psychol Rev. 1997;104:801–838. doi: 10.1037/0033-295x.104.4.801. [DOI] [PubMed] [Google Scholar]
- 46.Foygel D, Dell GS. Models of impaired lexical access in speech production. J Mem Lang. 2000;43:182–216. [Google Scholar]
- 47.Schwartz MF, Dell GS, Martin N, Gahl S, Sobel P. A case-series test of the interactive two-step model of lexical access: Evidence from picture naming. J Mem Lang. 2006;54:228–264. doi: 10.1016/j.jml.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- 49.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz M, et al. A behavioral and anatomical analysis of associative semantic errors in picture naming. Procedia Soc Behav Sci. 2010;6:134–136. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.