Abstract
Abnormally low plasma concentrations of thyroid hormones during sepsis often occur in the absence of thyroidal illness; however, the mechanisms involved in the “euthyroid sick syndrome” remain poorly understood. Here, we describe a previously unrecognized interaction between the thyroid hormone thyroxine (T4) and the proinflammatory cytokine macrophage migration inhibitory factor (MIF), together with its clinical relevance in sepsis. We found that in both patients with severe sepsis, and our rodent model, low plasma T4 concentrations were inversely correlated with plasma MIF concentrations. The MIF molecule contains a hydrophobic pocket that is important for many of its proinflammatory activities. Binding of L-T4 (or its hormonally inert isomer D-T4) significantly, and dose-dependently, inhibited the catalytic activity of this pocket. Moreover, administration of exogenous D-T4 significantly improved survival in mice with severe sepsis. To examine the specificity of the MIF∶T4 interaction, wild-type and MIF knockout mice were subjected to the carrageenan-air pouch model of inflammation and then treated with D-T4 or vehicle. D-T4 significantly inhibited leukocyte infiltration in wild-type mice but not in MIF knockout mice, providing evidence that in vivo T4 may influence MIF-mediated inflammatory responses via inhibition of its hydrophobic proinflammatory pocket. These findings demonstrate a new physiological role for T4 as a natural inhibitor of MIF proinflammatory activity. The data may also, in part, explain the low plasma T4 concentrations in critically ill, euthyroid patients and suggest that targeting the imbalance between MIF and T4 may be beneficial in improving outcome from sepsis.
Sepsis is a critical illness with an important inflammatory component that occurs in millions of individuals each year and results in high mortality and morbidity. Low circulating thyroid hormone levels are common in critically ill patients with severe infections and sepsis. This phenomenon of low plasma thyroid hormone levels, occurring in the absence of thyroid illness, is often referred to as “euthyroid sick syndrome.” Approximately 60% of critically ill patients have abnormally low plasma T4 levels, with the lowest levels being observed in patients with sepsis. These low thyroid hormone levels are good indicators of disease severity and predictors of mortality (1, 2). Although the etiology of the euthyroid sick syndrome has been linked to stress responses, impaired tissue function, and altered peripheral thyroid hormone metabolism during sepsis (2–4), the mechanisms involved in its pathophysiology remain poorly understood.
Migration inhibitory factor (MIF) is a proinflammatory cytokine that plays a critical role in the pathogenesis of sepsis (5–7). Plasma MIF levels are significantly elevated in nonsurvivors, compared with survivors, of severe sepsis (8), and administration of antibodies against MIF improves survival in experimental sepsis (5). During sepsis, plasma MIF levels can be higher than 180 ng/mL (6, 9), and the increased accumulation of MIF can have a profound effect on organ function (10–14) and mortality (6, 8, 15, 16). MIF proinflammatory activity is mediated through signal transduction initiated by interaction between the MIF molecule and the major histocompatibility complex, class-II invariant chain, CD74 (17, 18). Three-dimensional X-ray crystallography demonstrates that the MIF molecule is homotrimeric with a hydrophobic pocket formed between each adjacent subunit (19, 20). The hydrophobic pocket of MIF likely plays an important role, as compounds [e.g., (S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester (ISO-1)] that bind this region decrease downstream MIF signaling, MIF biological activities, and MIF-associated clinical outcomes (11, 21, 22). Despite its importance in the pathogenesis of sepsis and other diseases, many of the endogenous mechanisms regulating MIF activity remain unclear.
We herein identify T4 as a potential endogenous inhibitor of the MIF proinflammatory activities involved in sepsis and thus indicate a previously unrecognized clinically relevant interaction between these two molecules in critically ill patients that can be exploited in future therapeutic approaches for the treatment of sepsis.
Results and Discussion
T4 and MIF Inversely Correlate in Sepsis.
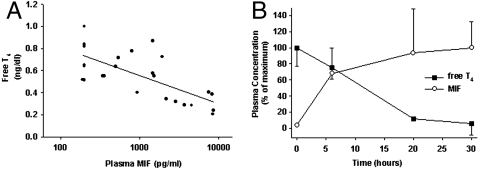
We first simultaneously determined T4 and MIF levels in severe sepsis by analyzing blood samples (n = 26) collected from patients in the medical intensive care unit within the first 72 h following diagnosis of sepsis. Plasma free-T4 levels (within the range 0.21–1.00 ng/dL) were significantly and inversely correlated with plasma MIF levels (within the range 200–8659 pg/mL) with a correlation coefficient of -0.71, (p = 0.0001) (Fig. 1A). Consistent with these human data, plasma free-T4 levels decreased and plasma MIF levels increased within hours after the onset of severe sepsis in rats induced by cecal ligation and puncture (CLP) (Fig. 1B). These results indicate that plasma T4 and MIF levels are inversely correlated during severe sepsis.
Fig. 1.
Plasma T4 levels decrease and MIF levels increase in the course of sepsis. (A) MIF levels (log 10) are negatively correlated with free T4 levels in plasma of patients with sepsis (n = 26, Corr Coeff: -0.71, p = 0.0001). (B) Plasma-free T4 levels (solid squares) decrease, whereas MIF levels (open circles) increase (n = 5) during CLP-induced severe sepsis in rats [represented as percentage of vehicle control (n = 6)].
Molecular Modeling of MIF: T4 Interaction.
The intriguing inverse correlation between the MIF and T4 during sepsis prompted us to further investigate the intimate relationship between these two moieties at a molecular level. Three-dimensional X-ray crystallography has demonstrated that the MIF molecule is homotrimeric with a hydrophobic cavity formed between each adjacent subunit (19, 23). Previously, we have designed MIF antagonists that target this hydrophobic cavity and have shown that molecules that interact with this site block MIF proinflammatory activity (16, 19, 24–27) and improve the clinical outcome during severe inflammation (11, 15). One of these molecules, ISO-1, which has been used by several groups as a small molecule inhibitor of MIF (11, 15, 28–31), contains a hydroxylated aryl system. The thyroid hormone T4 also contains a structurally identical hydroxylated aryl system. Therefore, we reasoned that this hydroxylated aryl system may allow the T4 molecule to occupy the active site of MIF and thus T4 may be an endogenous MIF ligand. To examine this possibility we modeled the molecular interactions between MIF and T4, its hormonally inert dextro-rotary isomer D-T4, and the T4 metabolite triiodothyronine (T3), whose structures are shown in Fig. 2A. Molecular modeling studies suggested that the MIF hydrophobic pocket can accommodate T4 according to a binding mode where the hydroxylated aryl system occupies the deepest part of the pocket similar to ISO-1 and other known small molecule MIF inhibitors (Fig. 2 B–D). Based on this hypothesis, the hydroxyl group forms a hydrogen bond with Asn 97, and the aryl moiety occupies a hydrophobic pocket with two side extensions fitting the iodine residues. The distance between one of these iodine molecules and its electron acceptor suggests further I⋯O stabilization. The central aryl ring occupies a region of hydrophobic contact as illustrated by Fig. 2D, whereas one of the iodine moieties is ideally positioned for halogen bonding with the oxygen atom of Ser 63. Finally the polar head can form hydrogen bonds with polar functional groups of residues at the gate of the binding pocket, including Tyr 36 (Fig. 2 C and D) but also Lys 32 (not illustrated). This binding hypothesis is corroborated by the available structure-activity relationship data, which indicated (i) equal binding affinities for both T4 isomers, and (ii) a clear difference in affinity between T3 and T4, consistent with a loss of hydrophobic contact and possible loss of halogen bond stabilization. These molecular models and MIF:ISO-1 cocrystal structural data identify T4 as a potential endogenous ligand for MIF.
Fig. 2.
T4 binds to MIF hydrophobic cavity. (A) Chemical structures of L thyroxine (L-T4), D thyroxine (D-T4), triiodothyronine (T3), and ISO-1. (B) Pose of T4 in the binding site of MIF (chains A and C from crystal structure 1LJT) with highlight of H bonds (purple dashed lines) and I⋯O distances within reach for halogen bonding (plain green lines). (C) Highlight of contact preferences for hydrophobic moieties (green meshed surface) and for lone pair acceptors (purple meshed surface) overlaid to T4 posed in MIF. Ile 64 has not been represented for clarity in C and D. (D) Close view of bound T4 within MIF. The analytical solvent-accessible Connolly surface accenting the pocket is colored based on physico-chemical properties: hydrophobic (blue), hydrophilic (red), and solvent-exposed (gray). Lys 32 and Pro 33 have not been surfaced for clarity.
T4 Binding to MIF Inhibits MIF Tautomerase Activity.
We next examined the binding efficiency and the ability of T4 to interfere with the tautomerase activity of the MIF hydrophobic pocket, which also mediates many of MIF’s proinflammatory activities (15). Both T4 and its dextro-rotatory isomer (dextrothyroxine; D-T4) bind to and inhibit the enzymatic activity of the MIF hydrophobic cavity in a dose-dependent manner with an IC50 of 15.8 μM (Fig. 3). We found that T4 had similar potency in inhibiting MIF tautomerase activity compared with ISO-1 (IC50 25 μM), the gold standard synthetic inhibitor of MIF. In addition, T3, which differs from T4 in only a single iodine residue, was only a comparatively weak inhibitor of MIF (IC50 > 100 μM), demonstrating the high specificity of the T4∶MIF interaction. These results reveal that T4 (both L and D isomers) binds the MIF hydrophobic pocket with high affinity and inhibits its tautomerase enzymatic activity, thus suggesting that like ISO-1 (10), T4 could inhibit MIF cytokine proinflammatory activities.
Fig. 3.
L-T4 (solid circles), D-T4 (open circles), and ISO-1 (open triangles), but not T3 (solid triangles) produce dose-dependent inhibition of the MIF active site (tautomerase), which is associated with its proinflammatory activities.
T4 Inhibits MIF Proinflammatory Activity in Vivo.
To delineate MIF∶T4 interactions from possible T4 hormonal activities, we utilized the hormonally inert isomer D-T4 in these in vivo studies. In the widely used model of localized inflammation [carrageenan injection into a dorsal air pouch (32)] we found that D-T4 administration to male C57BL/6 mice (n = 21 per group) significantly reduced leukocyte accumulation in the pouch. Moreover, D-T4 inhibited leukocyte recruitment in wild-type mice, but not in MIF-deficient mice, indicating the specificity of T4 inhibition on MIF-associated inflammation (Fig. 4A). We next studied effects of exogenous T4 administration in mice with sepsis induced by CLP, a model of MIF-mediated systemic inflammation. In mice with CLP-induced peritonitis, a clinically relevant model of sepsis and lethal systemic inflammation, we found that D-T4 treatment (4 mg/kg) significantly improved survival (60% survival of D-T4-treated mice vs. 20% survival of vehicle-treated control mice p < 0.05) (Fig. 4B). Importantly, this significant survival improvement was achieved when D-T4 was first administered within a clinically relevant time frame, i.e., 24 h after the onset of sepsis. In additional studies, mice with thyroidectomy and parathyroidectomy (Thx) performed two weeks before CLP-sepsis induction had a decreased overall survival rate and succumbed to lethality faster than non-Thx mice, underscoring the involvement of thyroid hormones in the morbidity of sepsis. This result was first demonstrated in a study done by Moley et al. in which thyroidectomized rats showed decreased whole body oxygen consumption (VO2) and an increased mortality that could be reversed by T4 administration (33). Likewise, Thx mice given D-T4 in our study showed vast improvements in mortality rates (Fig. 4C) (p < 0.05), comparable to those of non-Thx mice given D-T4, thus indicating the protective effect is not due to effects of D-T4 on endogenous thyroid hormone levels or unknown feedback mechanisms.
Fig. 4.
D-T4 administration reduces local inflammation and improves survival in murine sepsis. (A) D-T4 inhibition of MIF-induced localized inflammation. D-T4 (solid bars) significantly suppressed leukocyte infiltration in wild-type mice (n = 17) in a carrageenan-induced airpouch model of localized inflammation compared to vehicle-treated controls (open bars). However, D-T4 administration had no effect on leukocyte migration in MIF knockout mice (n = 18). (B) D-T4 improves survival in murine sepsis. Administration of D-T4 (open circles) starting at 24 h after the onset of CLP-induced sepsis in mice significantly improves survival (n = ○17/•17, p < 0.01) compared with vehicle-treated controls (solid circles). (C) D-T4 improves survival in thyroidectomized mice subjected to CLP sepsis. Male C57BL/6 mice underwent thyroidectomy and parathyroidectomy as described in Materials and Methods and were then subject to CLP surgery. Mice were administered DMSO vehicle control (solid circles, n = 16) or D-T4 (solid triangles, n = 17), and survival was then monitored over two weeks. The survival, which was significantly increased in the D-T4 group, was 13 and 53%, respectively (p < 0.05).
In conclusion, we have provided evidence for an inverse correlation between plasma T4 and MIF levels during the progression of sepsis and identify T4 as a potential endogenous antagonist of MIF inflammatory activity. The data suggest that low T4 and high MIF may be primary effectors in the lethal systemic inflammatory response during severe sepsis as modulation of either (T4 replacement or MIF antagonism) can improve survival. It is likely that the low plasma free T4 levels could result from a significant endogenous T4 fraction binding the hydrophobic pocket within the MIF molecule. However, because MIF production and release are markedly up-regulated during sepsis, this binding may not be sufficient to effectively inhibit MIF proinflammatory activity and prevent an overwhelming inflammatory response. Interestingly, in our study, D-T4 (the nonphysiological isomer of T4) supplementation was able to prevent the increased mortality rate in a murine sepsis model, indicating that the therapeutic levels of this compound were likely binding and inhibiting the elevated endogenous MIF. Our findings indicate a previously unrecognized clinically relevant interaction between T4 and MIF, two key molecules in the critically ill patient. This interaction may be vital for sepsis outcome and should generate interest in the development of previously undescribed therapeutic strategies targeting the imbalance between MIF and T4 in sepsis.
Materials and Methods
Animals.
Male Sprague Dawley rats (300–350 g, Taconic), male BALB/c mice (25–28 g, Taconic), and WT or MIF knockout (KO) C57BL/6 were used for in vivo studies. MIF KO mice were backcrossed eight generations onto a C57BL/6 background and bred using homozygous MIF KO animals. The genotype of the MIF locus of all progeny was determined by genomic PCR. Animals were housed in standard conditions (room temperature 22 °C with a 12-h light:dark cycle) with free access to regular chow and water. Animals were allowed to acclimate for at least 14 d before the corresponding experiment. All animal experiments were performed in accordance with the National Institutes of Health Guidelines under protocols approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research, North Shore-LIJ Health System.
Determination of MIF and Free T4 in Plasma of Patients with Sepsis.
Clinical sample and data collection were performed after approval by the Institutional Review Board. Heparinised blood (n = 26) was collected, between 12–120 h postdiagnosis of severe sepsis, from patients in the hospital intensive care unit. Blood was centrifuged at 2,000 × g for 10 min and plasma was used for MIF and free T4 determinations by using enzyme linked immunosorbent assays (R&D Systems and BioQuant, respectively).
Determination of MIF and T4 in Plasma of Rats with CLP-Induced Sepsis.
Peritonitis and severe sepsis was induced in male rats (319 ± 37 g) by CLP as described previously (11). Animals (n = 5/group) were euthanized at 6, 20, or 30 h post-CLP and heparinised blood samples collected via cardiac puncture. Blood was also collected from sham operated animals (n = 6) immediately postsurgery. Blood was centrifuged at 2,000 × g for 10 min and plasma was assessed for MIF and free T4 using enzyme linked immunosorbent assays (Chemicon International and BioQuant, respectively).
Inhibition of the MIF Tautomerase Activity Associated with the Hydrophobic Cavity.
MIF tautomerase activity was determined as previously described (25). Dopachrome Tautomerase Assay: L-Dopachrome methyl ester was prepared at 2.4 mM through oxidation of L-3,4-dihydroxyphenylalanine methyl ester with sodium periodate as previously described (25). Activity was determined at room temperature by adding dopachrome methyl ester (0.3 mL) to a cuvette containing 50 nM MIF in 50 mM potassium phosphate buffer, pH 6, 0.5 mM EDTA and measuring the decrease in absorbance from 2 to 20 s at 475 nm spectrophotometrically. T4, D-T4, T3, and ISO-1 were dissolved in Me2SO at various concentrations and added to the cuvette with the MIF prior to the addition of the dopachrome.
Carrageenan Air Pouch Model and D-T4 Administration.
The carrageenan air pouch model was performed as previously described (32). Briefly, dorsal air pouches were generated in female C57BL/6 mice and MIF KO mice (C57BL/6) by injecting 5 mL of sterile air s.c. on days 0 and 3. On day 6, mice were given D-T4 (4 mg/kg, i.p.) or vehicle and then challenged with an intrapouch injection of 1% carrageenan (1 mL) 15 min later. Animals were euthanized by CO2 asphyxiation 5 h post carrageenan injection, and cellular infiltrates were collected following the injection of 3 mL of PBS containing 2 mM EDTA. RBC-free cellular infiltrates were counted using a hemocytometer.
D-T4 Administration in Mice with CLP-Induced Severe Sepsis.
Peritonitis and severe sepsis were induced in male BALB/c mice by CLP as previously described (15). Mice were administered D-T4 (4 mg/kg, n = 17) or vehicle (•, n = 17) intraperitoneally 24 h after CLP. Single doses of D-T4 or vehicle were administered again on days 2 and 3. Survival was monitored for 7 d.
For some experiments, the animals had undergone thyroid and parathyroid removal approximately 2 wk before undergoing CLP. These mice were supplemented with calcium chloride in the drinking water.
Statistical Analyses.
Data are expressed as mean ± SEM. Regression analysis was applied to assess correlations. Significant differences were assessed by using one way analysis of variance (ANOVA) followed by a Student t test. The statistical significance of differences between groups of animals in survival experiments was analyzed by the log-rank test using the all pairwise multiple comparison procedure (Holm–Sidak method). Differences with P < 0.05 were considered statistically significant.
Acknowledgments.
Funding for these studies was supported by National Institutes of Health Grants R01 HL081655 (to E.J.M.) and R01 GM070727 (to C.N.M.) and the American Heart Association (Y.A.).
Footnotes
Conflict of interest statement: Y.A. is an inventor on a patent for the use of D-T4 in the treatment of sepsis.
*This Direct Submission article had a prearranged editor.
References
- 1.Plikat K, et al. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism. 2007;56:239–244. doi: 10.1016/j.metabol.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Bello G, et al. Nonthyroidal illness syndrome and prolonged mechanical ventilation in patients admitted to the ICU. Chest. 2009;135:1448–1454. doi: 10.1378/chest.08-1816. [DOI] [PubMed] [Google Scholar]
- 3.Mebis L, van den Berghe G. The hypothalamus-pituitary-thyroid axis in critical illness. Neth J Med. 2009;67:332–340. [PubMed] [Google Scholar]
- 4.Umpierrez GE. Euthyroid sick syndrome. South Med J. 2002;95:506–513. [PubMed] [Google Scholar]
- 5.Bernhagen J, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 6.Calandra T, et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat Med. 2000;6:164–170. doi: 10.1038/72262. [DOI] [PubMed] [Google Scholar]
- 7.Beishuizen A, Thijs LG, Haanen C, Vermes I. Macrophage migration inhibitory factor and hypothalamo-pituitary-adrenal function during critical illness. J Clin Endocrinol Metab. 2001;86:2811–2816. doi: 10.1210/jcem.86.6.7570. [DOI] [PubMed] [Google Scholar]
- 8.Bozza FA, et al. Macrophage migration inhibitory factor levels correlate with fatal outcome in sepsis. Shock. 2004;22:309–313. doi: 10.1097/01.shk.0000140305.01641.c8. [DOI] [PubMed] [Google Scholar]
- 9.Gando S, et al. Macrophage migration inhibitory factor is a critical mediator of systemic inflammatory response syndrome. Intensive Care Med. 2001;27:1187–1193. doi: 10.1007/s001340000818. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, et al. Macrophage CD74 contributes to MIF-induced pulmonary inflammation. Respir Res. 2009;10:33. doi: 10.1186/1465-9921-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakuragi T, et al. Lung-derived macrophage migration inhibitory factor in sepsis induces cardio-circulatory depression. Surg Infect (Larchmt) 2007;8:29–40. doi: 10.1089/sur.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnelly SC, et al. Regulatory role for macrophage migration inhibitory factor in acute respiratory distress syndrome. Nat Med. 1997;3:320–323. doi: 10.1038/nm0397-320. [DOI] [PubMed] [Google Scholar]
- 13.Chagnon F, Metz CN, Bucala R, Lesur O. Endotoxin-induced myocardial dysfunction: Effects of macrophage migration inhibitory factor neutralization. Circ Res. 2005;96:1095–1102. doi: 10.1161/01.RES.0000168327.22888.4d. [DOI] [PubMed] [Google Scholar]
- 14.Garner LB, et al. Macrophage migration inhibitory factor is a cardiac-derived myocardial depressant factor. Am J Physiol Heart Circ Physiol. 2003;285:H2500–2509. doi: 10.1152/ajpheart.00432.2003. [DOI] [PubMed] [Google Scholar]
- 15.Al-Abed Y, et al. ISO-1 binding to the tautomerase active site of MIF inhibits its pro-inflammatory activity and increases survival in severe sepsis. J Biol Chem. 2005;280:36541–36544. doi: 10.1074/jbc.C500243200. [DOI] [PubMed] [Google Scholar]
- 16.Dabideen DR, et al. Phenolic hydrazones are potent inhibitors of macrophage migration inhibitory factor proinflammatory activity and survival improving agents in sepsis. J Med Chem. 2007;50:1993–1997. doi: 10.1021/jm061477+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leng L, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–1476. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leng L, et al. A small-molecule macrophage migration inhibitory factor antagonist protects against glomerulonephritis in lupus-prone NZB/NZW F1 and MRL/lpr mice. J Immunol. 2011;186:527–538. doi: 10.4049/jimmunol.1001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lubetsky JB, et al. The tautomerase active site of macrophage migration inhibitory factor is a potential target for discovery of novel anti-inflammatory agents. J Biol Chem. 2002;277:24976–24982. doi: 10.1074/jbc.M203220200. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki M, et al. Crystal structure of the macrophage migration inhibitory factor from rat liver. Nat Struct Biol. 1996;3:259–266. doi: 10.1038/nsb0396-259. [DOI] [PubMed] [Google Scholar]
- 21.Cvetkovic I, et al. Critical role of macrophage migration inhibitory factor activity in experimental autoimmune diabetes. Endocrinology. 2005;146:2942–2951. doi: 10.1210/en.2004-1393. [DOI] [PubMed] [Google Scholar]
- 22.Al-Abed Y, VanPatten S. MIF as a disease target: ISO-1 as a proof-of-concept therapeutic. Future Med Chem. 2011;3:45–63. doi: 10.4155/fmc.10.281. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto H, et al. Crystallization of rat liver macrophage migration inhibitory factor for MAD analysis. J Struct Biol. 1995;115:331–334. doi: 10.1006/jsbi.1995.1057. [DOI] [PubMed] [Google Scholar]
- 24.Bendrat K, et al. Biochemical and mutational investigations of the enzymatic activity of macrophage migration inhibitory factor. Biochemistry. 1997;36:15356–15362. doi: 10.1021/bi971153a. [DOI] [PubMed] [Google Scholar]
- 25.Dios A, et al. Inhibition of MIF bioactivity by rational design of pharmacological inhibitors of MIF tautomerase activity. J Med Chem. 2002;45:2410–2416. doi: 10.1021/jm010534q. [DOI] [PubMed] [Google Scholar]
- 26.Senter PD, et al. Inhibition of macrophage migration inhibitory factor (MIF) tautomerase and biological activities by acetaminophen metabolites. Proc Natl Acad Sci USA. 2002;99:144–149. doi: 10.1073/pnas.011569399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crichlow GV, et al. Alternative chemical modifications reverse the binding orientation of a pharmacophore scaffold in the active site of macrophage migration inhibitory factor. J Biol Chem. 2007;282:23089–23095. doi: 10.1074/jbc.M701825200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F, et al. Spinal macrophage migration inhibitory factor contributes to the pathogenesis of inflammatory hyperalgesia in rats. Pain. 2010;148:275–283. doi: 10.1016/j.pain.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Hou XQ, et al. Role of macrophage migration inhibitory factor in influenza H5N1 virus pneumonia. Acta Virol. 2009;53:225–231. doi: 10.4149/av_2009_04_225. [DOI] [PubMed] [Google Scholar]
- 30.Piette C, et al. The dexamethasone-induced inhibition of proliferation, migration, and invasion in glioma cell lines is antagonized by macrophage migration inhibitory factor (MIF) and can be enhanced by specific MIF inhibitors. J Biol Chem. 2009;284:32483–32492. doi: 10.1074/jbc.M109.014589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He XX, et al. Macrophage migration inhibitory factor promotes colorectal cancer. Mol Med. 2009;15:1–10. doi: 10.2119/molmed.2008.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saeed RW, et al. Ethanol blocks leukocyte recruitment and endothelial cell activation in vivo and in vitro. J Immunol. 2004;173:6376–6383. doi: 10.4049/jimmunol.173.10.6376. [DOI] [PubMed] [Google Scholar]
- 33.Moley JF, Ohkawa M, Chaudry IH, Clemens MG, Baue AE. Hypothyroidism abolishes the hyperdynamic phase and increases susceptibility to sepsis. J Surg Res. 1984;36:265–273. doi: 10.1016/0022-4804(84)90097-0. [DOI] [PubMed] [Google Scholar]