Abstract
The flowering plants that dominate modern vegetation possess leaf gas exchange potentials that far exceed those of all other living or extinct plants. The great divide in maximal ability to exchange CO2 for water between leaves of nonangiosperms and angiosperms forms the mechanistic foundation for speculation about how angiosperms drove sweeping ecological and biogeochemical change during the Cretaceous. However, there is no empirical evidence that angiosperms evolved highly photosynthetically active leaves during the Cretaceous. Using vein density (DV) measurements of fossil angiosperm leaves, we show that the leaf hydraulic capacities of angiosperms escalated several-fold during the Cretaceous. During the first 30 million years of angiosperm leaf evolution, angiosperm leaves exhibited uniformly low vein DV that overlapped the DV range of dominant Early Cretaceous ferns and gymnosperms. Fossil angiosperm vein densities reveal a subsequent biphasic increase in DV. During the first mid-Cretaceous surge, angiosperm DV first surpassed the upper bound of DV limits for nonangiosperms. However, the upper limits of DV typical of modern megathermal rainforest trees first appear during a second wave of increased DV during the Cretaceous-Tertiary transition. Thus, our findings provide fossil evidence for the hypothesis that significant ecosystem change brought about by angiosperms lagged behind the Early Cretaceous taxonomic diversification of angiosperms.
Keywords: angiosperm evolution, plant evolution, transpiration, tropical rainforest, venation
Photosynthesis and transpiration by leaves fundamentally influence the cycling of carbon and water in the terrestrial realm. Consequently, evolutionary changes in the rates at which leaves exchange water for carbon bear on the origin and maintenance of biodiversity by varying the size and resource stoichiometry of the primary productivity base. How leaves exchange gases also shape climate and atmospheric gas composition by changing the amounts of water vapor and carbon in the atmosphere (1–3). Recent evidence has suggested that the evolution of flowering plants involved a sharp rise in the capacity of leaves to transport water and extract CO2 from the atmosphere (4, 5).
The evolution of unrivaled CO2 uptake and transpirational output by angiosperm leaves form the mechanistic cornerstone for a multitude of hypotheses citing angiosperms as agents of expansive ecosystem change during the Cretaceous (6–8). These hypotheses include (i) intensified mineral weathering by angiosperms that decreased global atmospheric CO2 concentration; (ii) heightened transpirational input to the atmosphere that increased regional rainfall and favored the spread and diversity of tropical rainforest vegetation; (iii) the nearly complete competitive exclusion by angiosperms of diverse gymnosperms and ferns from high-productivity sites worldwide; and (iv) the spread of novel fire regimes that entrained a positive feedback on angiosperm takeover (3, 4, 6, 9–15). In addition, increasing terrestrial productivity furnished by the rise of angiosperms with highly photosynthetically active leaves has been posited as a large-scale driver for the mid-Cretaceous turning point when terrestrial biodiversity eclipsed that of the marine realm (16). However, the actual timing for when angiosperms first evolved their high capacities for leaf photosynthetic gas exchange remains poorly resolved (4, 5, 17–19).
A fundamental shift in how angiosperm leaf venation functions has been proposed as a key mechanism in the evolution of high photosynthetic gas exchange potential (4, 5). Evidence for a shift in angiosperm venation structure was reconstructed from living phylogenetic diversity as a surge in the vein length density (DV) of angiosperm leaves that rose above the densities found across all other vascular plants during the mid-Cretaceous (5). In contrast to nonangiosperms, leaves of modern ecologically dominant angiosperms routinely exhibit tremendous vein lengths, which are evenly reticulated through the lamina (8). Leaves of tropical pioneer angiosperms, which operate at the upper limits of C3 gas exchange for woody vegetation, possess an average of 13 mm and a maximum of 20 mm of vein length within a single square millimeter of leaf area (4). By comparison, all known extant and extinct nonangiosperm leaves fall within the range of 0.1–6 mm of vein length per square millimeter (4). Increased vein branching furnishes greater photosynthetic capacity because adding more veins brings the xylem-based water supply closer to the sites of evaporation in the leaf. Hence, DV defines the hydraulic supply limit of water vapor exchange that secondarily constrains maximum CO2 assimilation by leaves (4, 5, 8, 20, 21).
Vein density evolution in the angiosperms has been argued to represent an innovation that transformed global terrestrial biogeochemistry and biodiversity (3–5, 15, 16). However, evidence for the timing of this Earth-changing shift in leaf function remains untested in the fossil record and is limited to phylogenetically based reconstruction of vein evolution from living angiosperms (5). Clarifying when densely veined angiosperm leaves became the dominant fixture of the vegetation is critical for disentangling how Mesozoic environmental change affected angiosperm evolution and how innovations in early angiosperm function restructured Cretaceous ecosystem function (6, 11–16). Fossil angiosperm leaves of Cretaceous age often faithfully preserve the fine details of leaf venation (17, 22). Thus, vein densities of fossil leaves may offer signatures for ancient maximal leaf hydraulic limits and their evolutionary changes during the early angiosperm radiation (4). Interestingly, there are reports that early angiosperms possessed low vein densities, but when high DV leaves that dominate modern angiosperm forests first appear remains unknown (22). In this paper, we analyze fossil angiosperm leaves through the Cretaceous to determine the evolutionary timing of angiosperms’ DV innovation. We measured vein densities of 307 taxa (approximate morphotypes) of fossil early angiosperm leaves as well as diverse gymnosperms and ferns from the early phases of angiosperm diversification and ecological expansion (Early Cretaceous to earliest Tertiary) and plotted vein density patterns through this crucial period.
Results
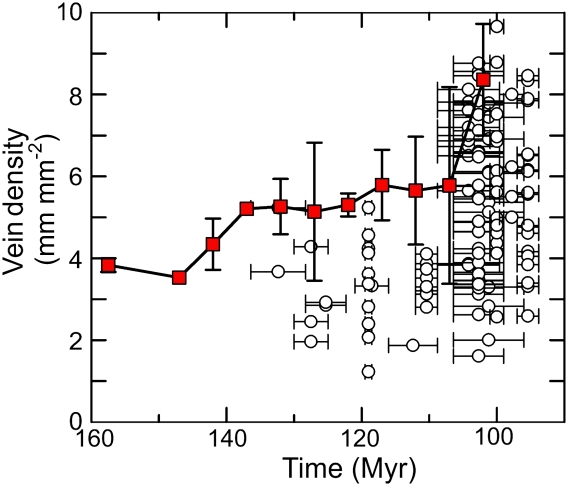
DV values of the oldest angiosperm fossil leaves sampled (Hauterivian to Early Albian, 136–112 Ma) averaged 3.31 mm mm−2 ± 0.93 SD (n = 29). These angiosperm leaf fossils nested within the range of diverse co-occurring and coeval gymnosperms and ferns (Fig. 1). In contrast with nonangiosperm fossil vein densities, which remained uniform through the Cretaceous to Paleogene (mean = 2.43 mm mm−2 ± 1.19 SD; n = 107), angiosperm DV values first increased above the nonangiosperm range by the Middle-Late Albian (106–100 Ma; Fig. 1). At 108–94 Ma (Late Albian-Cenomanian), mean angiosperm DV (5.55 mm mm−2 ± 1.79 SD, n = 72) became ∼2× greater than that of nonangiosperms. Maximum DV values for Late Albian-Early Cenomanian angiosperms increased to ∼40% greater than the highest DV values (∼6 mm mm−2) measured for nonangiosperms and were nearly 4× greater than the nonangiosperm mean. The timing and magnitude for increased fossil angiosperm DV during the mid-Cretaceous compared well to previous ancestral state reconstructions of DV evolution across extant angiosperms (Fig. 2).
Fig. 1.
Escalation in angiosperm DV from Cretaceous to earliest Tertiary angiosperm fossil leaves (140–58 Ma). Angiosperm (○) and nonangiosperm (●) fossils represent mean DV values per morphotype. Angiosperm DV first increases above the nonangiosperm maximum at ∼100 Ma (Late Albian). A second phase of angiosperm DV increase occurred during the latest Cretaceous/earliest Tertiary (68–58 Ma). The fossil angiosperm DV values during the second phase represent the earliest known densities comparable to the range of extant megathermal rainforest (Right box plot represents 25 species of woody plants across diverse regeneration guilds from Madang, Papua New Guinea). Representative fossil angiosperm leaves represent the pre-Late Albian (sparse DV, Ficophyllum crassinerve = 3.5 mm mm−2), mid-Cretaceous (moderately high DV, Sapindopsis magnolifolia = 7.8 mm mm−2), and Paleocene (dense DV, morphotype CJ40 = 13.9 mm mm−2) vein density shifts through time. Horizontal error bars around all data points represent the SD around the mean values for fossil ages. Geological ages: Ber, Berriasian; V, Valanginian; H, Hauterivian; B, Barremian; Ap, Aptian; Al, Albian; Cen, Cenomanian; T, Turonian; Co, Coniacian; S, Santonian; Camp, Campanian; M, Maastrichtian; Paleo, Paleocene. The shaded pink area denotes the range of DV for all nonangiosperms, living and extinct (4). (Scale bar, 1 mm.)
Fig. 2.
The mid-Cretaceous increased angiosperm vein density based on fossil leaves (open circles) is comparable to the reconstructed vein density evolution based on ancestral states across extant angiosperm phylogeny (red squares) (5). Ancestral state values were averaged over 10-Myr slices with SD illustrated. Horizontal error bars around the fossil angiosperm data represent the SD around the mean values for fossil ages.
By the latest Cretaceous (Maastrichtian)-Paleocene, mean DV values of angiosperms (mean = 9.76 mm mm−2 ± 3.0 SD; n = 78) increased 74% above the nonangiosperm sample through time, and the mean was 66% greater than the Hauterivian-Early Albian angiosperm mean. Several angiosperm DV values from this timeframe became comparable to the vein densities found across extant canopy-dominants of the megathermal rainforest biome (Fig. 1).
Discussion
Fossil Evidence for a Cretaceous Surge in Angiosperm Vein Density.
Our measurements show fossil evidence that leaf hydraulic function changed dramatically during the Cretaceous radiation of angiosperms. We show that Early Cretaceous fossil angiosperm leaves shifted from possessing low vein densities to very high vein densities by the close of the Cretaceous. During the first 30 Myr of angiosperm leaf evolution seen in the fossil leaves from Brazil, China, and Europe, as well as North and South America across a large paleolatitudinal gradient (Dataset S1), angiosperm leaves exhibited uniformly low vein densities that fell in the range of dominant Early Cretaceous ferns and gymnosperms. These results support the conclusion that a widespread pattern of low leaf hydraulic potential existed for pre-Early Albian angiosperms (22).
By the latest Albian-Cenomanian (108–94 Ma), a rapid and sharp increase in angiosperm DV became evident. Angiosperm DV surpassed the upper bound of the physiological vein morphospace for living and fossil nonangiosperm vascular plant lineages during the mid-Cretaceous surge (4, 5). The highest fossil angiosperm DV measured during the Late Albian approached 8 mm mm−2 and occurred in extinct platanoids (Sapindopsis) and Laurales. That these taxa defined the upper limits of mid-Cretaceous angiosperm vein densities supports earlier paleoecological evidence that platanoids and lauroids functioned with the most weedy, nonherbaceous ecologies at the time (17, 23). Interestingly, the timing and the amount of DV increase found in mid-Cretaceous angiosperm fossils compared well to the pattern reconstructed from age-calibrated ancestral state analyses of DV evolution based on living angiosperms (5) (Fig. 2). Thus, our fossil evidence shows that time-calibrated phylogenies of living angiosperms offer accurate evolutionary signals for an ancient hydraulic transition during the early angiosperm radiation.
Our results indicate that after the Late Albian-Cenomanian, angiosperm DV continued to increase. By the latest Cretaceous-Paleocene, angiosperm DV exhibited peak values comparable with the very high vein densities typical of hyperproductive modern megathermal rainforest angiosperm trees, averaging ∼10 (and up to 16) mm mm−2. The magnitude of vein branching measured for Late Cretaceous angiosperm leaf fossils represents the arrival of leaves capable of very high gas exchange capacities that today characterize hyperproductive megathermal rainforest angiosperms (4, 5, 7, 8).
Escalation of Leaf Angiosperm Hydraulic Potential.
Broadly, the pattern of angiosperm DV evolution supplies fossil evidence that the maximum potential for hydraulic capacity by angiosperm leaves escalated tremendously during the Cretaceous (6, 9, 16, 24). Although connecting changes in maximal leaf hydraulic capacity to competitive ability and ecosystem productivity remains a thorny issue for Cretaceous angiosperm macroevolution (13, 19, 24), the dynamics of fossil angiosperm DV through time adds a new quantitative functional dimension to several critical turning points in early angiosperm ecological evolution.
For example, the Late Albian-Cenomanian pulse of increased angiosperm DV coincides with a landmark ecological event—the first appearance of large angiosperm trees. Fossilized logs up to 1 m in diameter with the xylem plumbed by large-diameter (≥200 μm) vessels and simple perforation plates are first found in the mid-Cretaceous (23, 25–28). That a large shift in angiosperm body size, wood hydraulic efficiency, and, as we now show, moderately high DV emerged synchronously points to a coordinated whole plant transformation in angiosperm hydraulic capacity during the mid-Cretaceous (19–21). Although angiosperms first entered the forest canopy and diversified at moderately high DV by the Cenomanian, global inventories of angiosperm fossil abundance and diversity indicate that mid-Cretaceous angiosperms occupied subordinate ecological roles relative to conifers and ferns (13, 14, 23). Indeed, fossilized wood depositional environments, inferred from facies analyses, suggested that mid-Cretaceous angiosperm trees remained restricted to early successional floodplain zones (17, 27).
The second Maastrichtian-Paleocene surge in angiosperm DV corresponds with the key time interval for when (i) angiosperms radiated into and dominated diverse environments, including late successional zones of mesothermal forests and megathermal peat- and coal-forming environments; and (ii) when angiosperm ecological abundance first eclipsed ferns and conifers across several biomes (13, 14, 23, 24, 28, 29). These fossil patterns are consistent with a major expansion in angiosperm competitive ability, which is supported by our finding that DV values associated with the most productive extant angiosperm forests first emerge during the Late Cretaceous (1). In light of recent research that tropical angiosperm trees can strongly modify their climates by virtue of their densely veined leaves (3), the second wave of increased angiosperm DV provides new time constraints for a possible major transition in vegetation-atmosphere interactions. High leaf transpiration, enabled by high DV values in the range of 12–16 mm mm−2 and coupled to high forest cover, intensifies the hydrological cycle through the recycling of precipitation in ways that fostered the origin and diversity of disparate tropical organisms (2–4). Thus, our findings on fossil angiosperm veins through time show that the leaf hydraulic basis for how megathermal rainforest climate, transpiration, and productivity operate today was not explored by angiosperm leaf evolution until the latest Cretaceous (1, 3).
Though our findings indicate a biphasic rise in angiosperm DV change through the Cretaceous, these results should be regarded as a hypothesis requiring future testing. For example, sampling of fossil angiosperm leaves from Turonian to Campanian sediments, where our sampling is sparse and limited to one middle paleolatitude site, may fill in the gap to reveal a steady rise in DV. Nevertheless, the appearance of fossil DV close to the modern limits of angiosperm leaf hydraulic capacity during the Cretaceous-Tertiary transition was consistently found among three fossil sites across a large paleolatitude gradient (11 °N to 36 °S) (30, 31) (Dataset S1). Such a pattern suggests that by the Maastrichtian-Paleocene, angiosperms bearing high DV leaves became geographically significant. In addition, our measurements of very high DV in the Cerrejon flora of Colombia extends systematic and paleoclimatic evidence in showing that the fossil flora functioned as the earliest known equatorial neotropical megathermal rainforest (31). The appearance of high angiosperm vein densities first in this zone during the Maastrichtian (Guaduas, Colombia) generate important questions about the ecophysiological modernization of the equatorial vegetational belt: (i) Do the high DV values reflect colonization of a new megathermal wet environment by angiosperms that was opened up by tectonic and oceanic circulation change? or (ii) Did invading angiosperms with high DV generate a hydrological feedback in situ to increase the geographic spread of tropical humid biomes (3).
Tuning the Angiosperm Venation Pipeline: Atmospheric and Conduit Change.
The processes that drove the transformation of angiosperm venation during the Cretaceous remain unknown. However, an interplay of Cretaceous environmental opportunities and evolutionary novelty in angiosperm leaf hydraulics likely influenced the shifts in angiosperm leaf hydraulics through time (5, 19). A long-term decline in CO2 and a rise in O2 atmospheric concentrations during the Cretaceous indicate environmental pressures for increasing vein reticulation because both directions of atmospheric change favor increased vein density by increasing the transpirational cost for carbon uptake (5, 14, 32, 33). In this light, the broad decline in global CO2 and rise in O2 during the Cretaceous suggests an environmental opportunity that angiosperms were able to seize (5, 33). Indeed, an abrupt drawdown in global CO2 associated with an ocean anoxic event (OAE2) during the mid-Cretaceous occurred at the same time as the first wave of increased angiosperm DV (34). However, the possibility of a second wave of angiosperm DV during the latest Cretaceous does not mirror current understanding of CO2/O2 dynamics through the Late Mesozoic (35, 36), which suggests that intrinsic innovations also bear on the evolution of angiosperm leaf gas exchange capacity.
Two functional conundrums of increased DV by angiosperms are as follows: (i) only angiosperms evolved high DV despite the fact that diverse nonangiosperm vascular plants also evolved through periods of low CO2, and several of these clades also possessed complex multiple-vein order designs or single-vein systems with accessory lignified tissues that are convergent with angiosperms (4, 8); and (ii) increasing DV would, all else being equal, elevate leaf construction costs because veins, being lignified, are expensive (19, 21, 33). Therefore, gains in leaf photosynthetic potential by increasing DV could be offset by inflated vascular plumbing costs (33). A hypothesis underlying the angiosperm monopoly of high DV that requires future evaluation is that evolution of xylem vessels in angiosperm leaf veins provided an essential cost-mitigating mechanism (5, 19, 21). Vessels, through furnishing less-resistive flow compared with ancestral tracheids, may enable more vein reticulation and therefore faster rates of hydraulic supply for a given amount of vein volume (5, 19).
Conclusions
Our results indicate that a several-fold escalation in angiosperm leaf hydraulic capacity occurred during the Cretaceous. Increasing leaf hydraulic potential and linked high rates of transpiration by evolution of angiosperm vein densities exceeding those of nonangiosperms would have set in motion novel changes in the flows of carbon, water, and nutrients through the biosphere beginning in the mid-Cretaceous (3, 5, 15). However, the range of leaf hydraulic capacities representing the modern limits of woody angiosperm photosynthesis, which are those of capable of changing climates and foster today's enormous megathermal rainforest diversity and productivity, emerged long after angiosperm origin (3, 10, 13, 16, 19, 22, 23, 36, 37). Thus, our findings support previous interpretations of angiosperm paleoecology that despite tremendous phylogenetic diversification in the Early Cretaceous, transformative ecosystem change brought about by increased angiosperm productivity unfolded much later (24, 29).
Materials and Methods
Dv is the length of veins per unit leaf area (mm mm−2). Dv was measured only on well-preserved compression fossils by tracing veins on fossil images using ImageJ (http://rsb.info.nih.gov/ij/) on 5–12 mm2 of leaf area (typically 2–4 areoles). We made three DV measurements on each sampled fossil taxon or morphotype. We obtained images either by using a digital camera and macrolens or by digital measurements on previously figured fossils (Dataset S1). Fossils sampled included low, middle, and high paleolatitude belts from both hemispheres throughout much of the Cretaceous (Dataset S1). To examine how fossil angiosperms compare with megathermal lowland rainforest species, which operate at the modern limits of maximal leaf carbon/water exchange, we sampled 25 species across a range of regeneration guilds from a diverse lowland tropical rainforest in Madang, Papua New Guinea (mean annual temperature, 28 °C; annual rainfall, 4,500 mm with no significant dry season). We measured DV of extant species on three samples per species using previously described techniques (4).
Supplementary Material
Acknowledgments
We thank Lawong Balun for assistance during field work. Helpful comments were provided by Patrick Baker, Kevin Boyce, David Beerling, Claire Feild, Peter Wilf, and Hubert Feild. We thank Mauro Passalía, Griselda Puebla, Alejandra Gandolfo, and Elizabeth Hermsen for providing fossil leaf photography for DV measurements. This work was supported by National Science Foundation Grant DEB-0919071, Consejo Nacional de Investigaciones Científicas y Técnicas (A.I.), and National Science Foundation Grant IOB-0714156 (to T.S.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014456108/-/DCSupplemental.
References
- 1.Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science. 1998;281:237–240. doi: 10.1126/science.281.5374.237. [DOI] [PubMed] [Google Scholar]
- 2.Kreft H, Jetz W. Global patterns and determinants of vascular plant diversity. Proc Natl Acad Sci USA. 2007;104:5925–5930. doi: 10.1073/pnas.0608361104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyce CK, Lee JE. An exceptional role for flowering plant physiology in the expansion of tropical rainforests and biodiversity. Proc Biol Sci. 2010;277:3437–3443. doi: 10.1098/rspb.2010.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyce CK, Brodribb TJ, Feild TS, Zwieniecki MA. Angiosperm leaf vein evolution was physiologically and environmentally transformative. Proc Biol Sci. 2009;276:1771–1776. doi: 10.1098/rspb.2008.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodribb TJ, Feild TS. Leaf hydraulic evolution led a surge in leaf photosynthetic capacity during early angiosperm diversification. Ecol Lett. 2010;13:175–183. doi: 10.1111/j.1461-0248.2009.01410.x. [DOI] [PubMed] [Google Scholar]
- 6.Bond WJ. The tortoise and the hare – ecology of angiosperm dominance and gymnosperm persistence. Biol J Linn Soc Lond. 1989;36:227–249. [Google Scholar]
- 7.Körner C. In: Ecophysiology of Photosynthesis. Schulze ED, Caldwell MM, editors. Heidelberg: Springer; 1995. pp. 463–490. [Google Scholar]
- 8.Brodribb TJ, Feild TS, Jordan GJ. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol. 2007;144:1890–1898. doi: 10.1104/pp.107.101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knoll AH. In: Community Ecology. Diamond J, Case T, editors. New York: Harper and Row; 1986. pp. 126–144. [Google Scholar]
- 10.Lidgard S, Crane PR. Quantitative analyses of the early angiosperm radiation. Nature. 1988;331:344–346. [Google Scholar]
- 11.Taylor LL, et al. Biological weathering and the long-term carbon cycle: Integrating mycorrhizal evolution and function into the current paradigm. Geobiology. 2009;7:171–191. doi: 10.1111/j.1472-4669.2009.00194.x. [DOI] [PubMed] [Google Scholar]
- 12.Robinson JM. Speculations on carbon dioxide starvation, Late Tertiary evolution of stomatal regulation and floristic modernization. Plant Cell Environ. 1994;17:1–10. [Google Scholar]
- 13.Lupia R, Lidgard S, Crane PR. Comparing palynological abundance and diversity: Implications for biotic replacement during the Cretaceous angiosperm radiation. Paleobiology. 1999;25:305–340. [Google Scholar]
- 14.McElwain JC, Willis KJ, Lupia R. In: A History of Atmospheric CO2 and Its Effects on Plants, Animals, and Ecosystems. Ehlering JR, Cerling TE, Dearing MD, editors. Heidelberg: Springer; 2005. pp. 133–165. [Google Scholar]
- 15.Bond WJ, Scott AC. Fire and the spread of flowering plants in the Cretaceous. New Phytol. 2010;188:1137–1150. doi: 10.1111/j.1469-8137.2010.03418.x. [DOI] [PubMed] [Google Scholar]
- 16.Vermeij GJ, Grosberg RK. The Great Divergence: When did diversity on land exceed that in the sea? Integr Comp Biol. 2010;50:1–8. doi: 10.1093/icb/icq078. [DOI] [PubMed] [Google Scholar]
- 17.Hickey LJ, Doyle JA. Early Cretaceous fossil evidence for angiosperm evolution. Bot Rev. 1977;43:3–104. [Google Scholar]
- 18.Feild TS, et al. Dark and disturbed: A new image of early angiosperm ecology. Paleobiology. 2004;30:82–107. [Google Scholar]
- 19.Feild TS, Chatelet DS, Brodribb TJ. Ancestral xerophobia: A hypothesis on the whole plant ecophysiology of early angiosperms. Geobiology. 2009;7:237–264. doi: 10.1111/j.1472-4669.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 20.Sack L, Frole K. Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology. 2006;87:483–491. doi: 10.1890/05-0710. [DOI] [PubMed] [Google Scholar]
- 21.McKown AD, Cochard H, Sack L. Decoding leaf hydraulics with a spatially explicit model: Principles of venation architecture and implications for its evolution. Am Nat. 2010;175:447–460. doi: 10.1086/650721. [DOI] [PubMed] [Google Scholar]
- 22.Feild TS, et al. Fossil evidence for low gas exchange capacities for Early Cretaceous angiosperm leaves. Paleobiology. 2011;37:195–213. [Google Scholar]
- 23.Upchurch GR, Wolfe JA. Cretaceous vegetation of the Western Interior and adjacent regions of North America. Geo Assoc Canada Spec Pub. 1993;39:243–281. [Google Scholar]
- 24.Wing SL, Boucher LD. Ecological aspects of the Cretaceous flowering plant radiation. Annu Rev Earth Planet Sci. 1998;26:379–421. [Google Scholar]
- 25.Wheeler EA, Baas P. A survey of the fossil record for dicotyledonous wood and its significance for evolutionary and ecological wood anatomy. IAWA J. 1991;12:271–332. [Google Scholar]
- 26.Philippe M, et al. Woody or not woody? Evidence for early angiosperm habit from the Early Cretaceous fossil wood record of Europe. Palaeoworld. 2008;17:142–152. [Google Scholar]
- 27.Oakley D, Falcon-Lang HJ. Morphometric analysis of Cretaceous (Cenomanian) angiosperm woods from the Czech Republic. Rev Palaeobot Palynol. 2009;153:375–385. [Google Scholar]
- 28.Wheeler EA, Lehman TM. New Late Cretaceous and Paleocene dicot woods of Big Bend National Park, Texas and review of Cretaceous wood characteristics. IAWA J. 2009;30:293–318. [Google Scholar]
- 29.Wing SL, Hickey LJ, Swisher CC. Implications of an exceptional fossil flora for Late Cretaceous vegetation. Nature. 1993;363:342–344. [Google Scholar]
- 30.Iglesias A, et al. A Paleocene lowland macroflora from Patagonia reveals significantly greater richness than North American analogs. Geology. 2007;35:947–950. [Google Scholar]
- 31.Wing SL, et al. Late Paleocene fossils from the Cerrejon Formation, Colombia, are the earliest record of Neotropical rainforest. Proc Natl Acad Sci USA. 2009;106:18627–18632. doi: 10.1073/pnas.0905130106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gale J, Rachmilevitch S, Reuveni J, Volokita M. The high oxygen atmosphere toward the end-Cretaceous; a possible contributing factor to the K/T boundary extinctions and to the emergence of C(4) species. J Exp Bot. 2001;52:801–809. doi: 10.1093/jexbot/52.357.801. [DOI] [PubMed] [Google Scholar]
- 33.Beerling DJ, Franks PJ. Plant science: The hidden cost of transpiration. Nature. 2010;464:495–496. doi: 10.1038/464495a. [DOI] [PubMed] [Google Scholar]
- 34.Barclay RS, McElwain JC, Sageman BB. Carbon sequestration activated by a volcanic CO2 pulse during Ocean Anoxic Event 2. Nat Geosci. 2010;3:205–208. [Google Scholar]
- 35.Royer DL. Fossil soils constrain ancient climate sensitivity. Proc Natl Acad Sci USA. 2010;107:517–518. doi: 10.1073/pnas.0913188107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glasspool IJ, Scott AC. Phanerozoic concentrations of atmospheric oxygen reconstructed from sedimentary charcoal. Nat Geosci. 2010;3:627–630. [Google Scholar]
- 37.Smith SA, Beaulieu JM, Donoghue MJ. An uncorrelated relaxed-clock analysis suggests an earlier origin for flowering plants. Proc Natl Acad Sci USA. 2010;107:5897–5902. doi: 10.1073/pnas.1001225107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.