Abstract
Receptor kinases with leucine-rich repeat (LRR) extracellular domains form the largest family of receptors in plants. In the few cases for which there is mechanistic information, ligand binding in the extracellular domain often triggers the recruitment of a LRR-coreceptor kinase. The current model proposes that this recruitment is mediated by their respective kinase domains. Here, we show that the extracellular LRR domain of BRI1-ASSOCIATED KINASE1 (BAK1), a coreceptor involved in the disparate processes of cell surface steroid signaling and immunity in plants, is critical for its association with specific ligand-binding LRR-containing receptors. The LRRs of BAK1 thus serve as a platform for the molecular assembly of signal-competent receptors. We propose that this mechanism represents a paradigm for LRR receptor activation in plants.
Keywords: brassinosteroid signaling, flagellin signaling, plant innate immunity, Receptor-like kinase, signaling crosstalk
Leucine-rich repeat receptor kinases (LRR-RKs) form the largest family of receptors in plants (1). LRR-RKs bind a wide range of ligands, including small molecule hormones and peptides, and are involved in a variety of developmental and immune signaling processes (2, 3). In Arabidopsis, BAK1 (BRI1-ASSOCIATED KINASE1) is an LRR coreceptor kinase for several LRR-RKs, including the brassinosteroid (BR) receptor BRI1 (BRASSINOSTEROID-INSENSITIVE 1) and the flagellin receptor FLS2 (FLAGELLIN-SENSING 2) that are involved in growth and immune responses, respectively (3–5). Ligand perception at the cell surface by either BRI1 or FLS2 induces the subsequent recruitment of BAK1 to a ligand-bound receptor complex (6–10). This process triggers transphosphorylation at multiple serines and threonines of the respective kinase domains inside the cell (11–13). Perhaps because BRI1 is a long-lived protein that apparently cycles between the plasma membrane and endosomes (14), there are multiple mechanisms to maintain the kinase domain in a basal state. BRI1 kinase is auto-inhibited by its C-terminal tail (15), by auto-phosphorylation on threonine 872 (11), and by a protein, BRI1 KINASE INHIBITOR 1 (BKI1), which associates with BRI1’s kinase domain (10, 16). BKI1 inhibits BR signaling by binding to the BRI1’s kinase domain, thereby inhibiting the interaction between BRI1- and BAK1-kinase (10, 16). Upon ligand binding, BRI1 phosphorylates BKI1 on a tyrosine within its membrane-targeting region, which dissociates BKI1 from the cell membrane and targets it to the cytoplasm, where it is inactive (10). Dissociation of BKI1 from BRI1 allows formation of a stable BRI1-BAK1 complex that is competent to induce downstream signaling (17).
The interplay between BRI1 and BAK1 kinase domains is further regulated by BAK1 autophosphorylation on tyrosine 610 (tyr-610), which is required to stimulate BRI1 kinase activity in vitro and for proper BR signaling in vivo (18). Of note, BAK1 tyr-610 phosphorylation is not required for flagellin response and it is possible that tyr-610 phosphorylation might be involved in the proper interaction with its cognate receptors. However, tyr-610 mutations affect only BRI1 kinase activation but not its interaction with BRI1 intracellular domain (18). Therefore, a critical unanswered question is how ligand-bound LRR-RKs selectively recruit BAK1. Here, we report that the LRR domain of BAK1 is required for its recruitment to a ligand-bound LRR-RK and allows the kinase domains to be in physical contact for subsequent reciprocal transphosphorylation. Furthermore, our data indicate that the extracellular domain (ECD) of BAK1 is critical for the high affinity formation of the correct receptor/coreceptor pair.
Results and Discussion
Gain-of-Function Phenotype of bak1elg Allele in the Brassinosteroid Pathway.
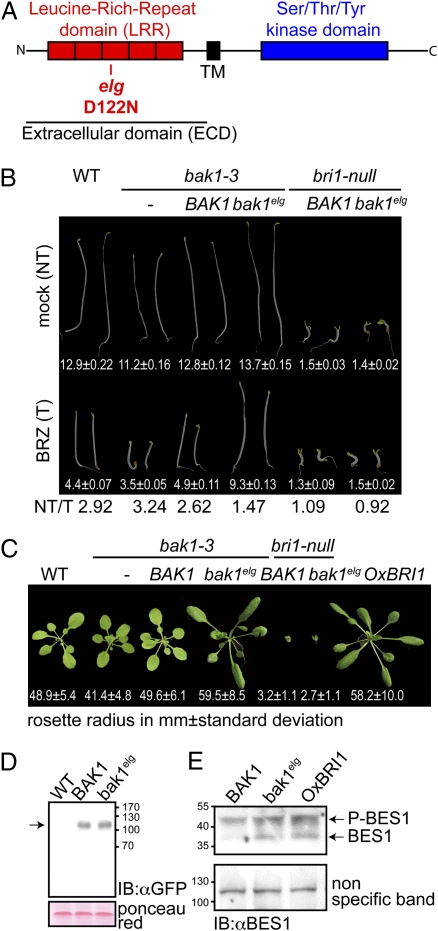
A previously described mutation in BAK1, elg (elongated), was originally identified as a suppressor of the gibberellin biosynthesis mutant, ga4 (19). The elg mutation results in a substitution of an aspartic acid to an asparagine (D122N) in the third LRR of BAK1 (20) (Fig. 1A and Fig. S1). The elg mutant is also hypersensitive to exogenous BR treatment (20). We found that both elg and transgenic lines of a null bak1 mutant (bak1-3) (9), expressing bak1elg fused with mCITRINE, a monomeric yellow variant of GFP (bak1elg::CITRINE), had slightly longer hypocotyls in the dark compared with control plants (Fig. 1B and Fig. S1). Cell elongation in etiolated seedlings is BRI1-dependent (4). Importantly, in the presence of brassinazole (BRZ), an inhibitor of BR biosynthesis, both elg and bak1-3 transgenic plants expressing a bak1elg::CITRINE fusion protein still displayed partially elongated hypocotyls compared with controls (Fig. 1B and Fig. S1). These phenotypes were not explained by differential protein accumulation (Fig. 1D). Moreover, when grown in the light, both elg and the bak1elg::CITRINE-expressing bak1-3 transgenic plants exhibited long twisted petioles and elongated leaf blades (Fig. 1C and Fig. S1), and a rosette phenotype reminiscent of plants either overexpressing BRI1 or treated exogenously with BR (21).
Fig. 1.
Gain-of-function phenotype of bak1elg allele for the brassinosteroid signaling pathway. (A) Schematic representation of BAK1 with its extracellular LRR domain in red and intracellular kinase domain in blue. TM: transmembrane segment. The position of the elg (D122N) mutation in BAK1 (LRR3) is indicated. (B) BAK1prom:BAK1::CITRINE expression complements the bak1-3 hypocotyl growth defect. BAK1prom:bak1elg::CITRINE expression in bak1-3 leads to an elongated hypocotyl phenotype in the dark that is BRI1-dependent. Note that BAK1prom:bak1elg::CITRINE-expressing hypocotyls still elongate when BR ligand is partially depleted by 1 μM brassinazole, BRZ. Hypocotyl length is in mm ± SD (n = 25), NT/T is the ratio of nontreated (NT) over BRZ-treated (T) hypocotyl length. (C) Pictures of rosette stage transgenic homozygous Arabidopsis (T3) expressing BAK1prom:BAK1::CITRINE or BAK1prom:bak1elg::CITRINE under the control of BAK1 promoter in the bak1-3 background. The phenotypes associated with the overexpression of BRI1 (on the right, for comparison), narrow leaf blades, elongated and twisting petioles were recapitulated by driving the expression of the bak1elg::CITRINE variant. Mean value of rosette radius is indicated in mm ± SD (n = 25). (D) BAK1::CITRINE accumulates to a similar extent as bak1elg::CITRINE. Microsomal protein extracts were prepared from wild-type Col-0, BAK1prom:BAK1::CITRINE in bak1-3 and BAK1prom:bak1elg::CITRINE in bak1-3 plants. These extracts were subjected to an anti-GFP protein immunoblot analysis to detect the accumulation of the CITRINE-tagged proteins. Equal loading was ensured by protein quantification before loading and by Ponceau red staining of the membrane postprotein transfer. (E) BES1 phosphorylation in BAK1::CITRINE/bak1-3, BAK1-bak1elg::CITRINE/bak1-3 and OxBRI1 lines. P-BES is phosphorylated BES1. Equal loading was ensured by protein quantification before loading and by the signal intensity of a nonspecific band.
We asked whether bak1elg::CITRINE growth promotion is BRI1-dependent. We introgressed both bak1elg::CITRINE and a complementing BAK1::CITRINE transgene into a bri1-null mutant. Both BAK1::CITRINE and bak1elg::CITRINE failed to induce hypocotyl and petiole elongation in bri1 plants (Fig. 1 B and C). Finally, we checked the phosphorylation status of the BRI1-EMS-SUPPRESSOR 1 (BES1) transcription factor in BAK1::CITRINE and bak1elg::CITRINE expressing bak1-3 transgenic plants (Fig. 1E). BES1 phosphorylation is a readout for BR activity, as phosphorylated BES1 (P-BES1) is a mark of low BR signaling and dephosphorylated BES1 is indicative of active BR signaling (22). We found that bak1elg::CITRINE but not BAK1::CITRINE plants accumulated dephosphorylated BES1 to a similar extent as plants overexpressing BRI1. We conclude that elg acts as a gain-of-function mutation that requires BRI1 to promote cell elongation.
Impaired Flagellin Signaling of bak1elg.
To address the phenotype of elg and bak1elg::CITRINE plants with respect to innate immune-response signaling, we monitored various readouts that include both early and late responses to flg22 (an elicitor peptide from bacterial flagellin) (3). Expression of BAK1::CITRINE, but not bak1elg::CITRINE, in the bak1-3 mutant almost completely rescued the induction of reactive oxygen species triggered by flg22, one of the earliest readouts for flagellin signaling (3) (Fig. 2A). Similarly, BAK1-CITRINE, but not bak1elg::CITRINE, rescued the bak1 phenotype with respect to loss of fresh weight and callose deposition triggered by flg22 late readouts of flagellin signaling (Fig. 2 B and C). The elg mutant was also insensitive to flg22 treatments with respect to loss of fresh weight and callose deposition (Fig. S1 D and E). Additionally, bak1elg::CITRINE bak1-3 plants did not exhibit protection from Pseudomonas syringae pv. tomato (Pto) DC3000 infection, which is normally induced in wild-type by cotreatment with flg22 (23) (Fig. 2D). Together, these results suggest that both early and late responses to flagellin are impaired by a single amino acid substitution in the ECD of BAK1. Importantly, bak1elg::CITRINE selectively affected innate immune responses triggered by various MAMPs (microbe-associated molecular patterns) (Fig. S2). Together, our results indicate that the bak1elg protein behaves differently with respect to BR signaling (gain-of-function) and flagellin responsiveness (loss-of-function).
Fig. 2.
bak1elg has impaired flagellin response. (A) Oxidative burst triggered by 100 nM flg22 in wild-type Col-0 (blue), fls2 (red), bak1-3 (green), BAK1prom:BAK1::CITRINE in bak1-3 (purple), and BAK1prom:bak1elg::CITRINE in bak1-3 (orange) leaf discs measured in relative light units (RLU). Result are mean ± SD (n = 24). (B) Average fresh-weight ratio of 14-d-old seedlings grown for 7 d in either water or water plus 1 μM flg22. The bar graph represents the average fresh-weight ratio from wild-type Col-0, bak1-3 mutant, BAK1prom:BAK1::CITRINE in bak1-3, and BAK1prom:bak1elg::CITRINE in bak1-3. Means and SDs were calculated from 48 seedlings (six random pools of eight seedlings). (C) Callose deposits stained with aniline blue from leaves of wild type Col-0, bak1-3, BAK1prom:BAK1::CITRINE in bak1-3 and BAK1prom:bak1elg::CITRINE in bak1-3 treated with 1 μM flg22. The number of leaves showing the displayed features over the total in a given genotype is indicated in parentheses. (D) Growth of Pseudomonas syringae pv. tomato (Pto DC3000) was measured on the genetic backgrounds indicated at bottom. Leaves from 4-wk-old plants were infiltrated with a bacterial inoculum of 105 cfu·mL−1 in the presence (orange) or absence (red) of 1 μM flg22 peptide. The number of bacteria per square centimeter of leaf was plotted on a log10 scale. Error bars represent two times the SE among four internal replicate samples from one of three experiments.
D122N Substitution in BAK1’s ECD Modifies its Interaction with Both BRI1 and FLS2 LRR-RKs.
Next, we addressed the mechanism by which the bak1elg protein induces BR signaling and blocks flagellin response. Control experiments showed that bak1elg::CITRINE accumulates to similar levels as BAK1::CITRINE (Fig. 1D) and had a similar subcellular localization (Fig. S3A). In addition, the elg mutant had normal accumulation of BRI1 (Fig. S1), and expression of bak1elg::CITRINE did not alter the accumulation of BRI1::CITRINE (Fig. 3A) or FLS2::GFP (Fig. 3B). Importantly, bak1elg::CITRINE did not modify BRI1::mCITRINE or FLS2-GFP subcellular localization (Fig. S3 B and C). Therefore, we hypothesized that the phenotypes ascribed to bak1elg in Fig. 1 are the result of alterations in the interaction between bak1elg and either BRI1 or FLS2.
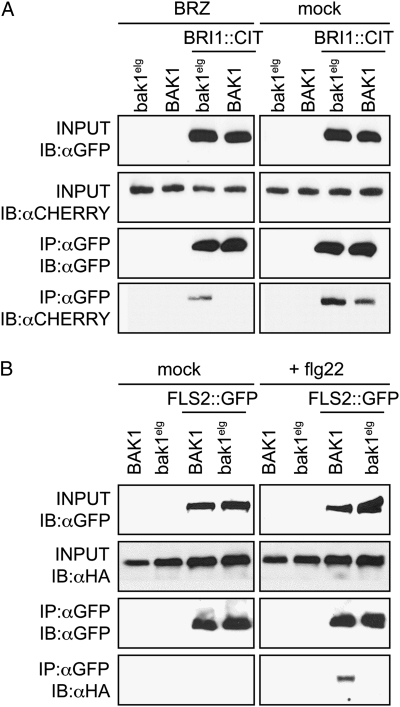
Fig. 3.
A mutation in the extracellular LRR domain of BAK1 modifies its interaction with BRI1 and FLS2. (A) Transgenic Arabidopsis plants expressing either BAK1prom:BAK1::CHERRY or BAK1prom:bak1elg::CHERRY alone or with BRI1prom:BRI1::CITRINE were grown with or without the BR biosynthesis inhibitor BRZ (5 μM added from sowing of seeds). Total membrane protein was immunoprecipitated (IP) with anti-GFP antibodies and subjected to immunoblot (IB) analysis, as indicated. (B) Transgenic plants expressing either BAK1prom:BAK1::6xHA or BAK1prom:bak1elg::6xHA alone or with FLS2prom:FLS2::GFP were grown on 1/2 LS media and treated 5 min before protein extraction with 10 μM flg22. Total membrane protein was immunoprecipitated (IP) with anti-GFP antibodies and subjected to immunoblot (IB) analysis as indicated.
Both BAK1::CHERRY and bak1elg::CHERRY coimmunoprecipitated with BRI1::CITRINE in the absence of the brassinosteroid biosynthesis inhibitor, BRZ (Fig. 3A). In contrast, only bak1elg::CHERRY coimmunoprecipitated with BRI1::CITRINE in the presence of BRZ (Fig. 3A). As described previously, flg22 treatment induced the recruitment of wild-type BAK1 to FLS2 (8, 9) (Fig. 3B). However, bak1elg::6xHA did not coimmunoprecipitate with FLS2::GFP under these conditions (Fig. 3B). We could immunoprecipitate only a fraction of BAK1 with FLS2 after flg22 treatment; therefore, we cannot exclude the possibility that BAK1elg can still bind to FLS2, albeit more weakly than wild-type BAK1. Taken together, our results indicate that the bak1elg variant interacts with BRI1, even when the BR concentration is very low, whereas its ligand-induced interaction with FLS2 is impaired. These differences in affinity likely explain the opposite gain- and loss-of-function phenotypes in BR and flagellin signaling, respectively.
BAK1 Kinase Activity Is Not Required for bak1elg Association with BRI1.
Previous reports indicated that the isolated kinase domains of BRI1 and BAK1 interact directly in vitro and in yeast (6, 7, 16, 18). It was therefore unexpected that the bak1elg ECD mutation modified its interaction with both BRI1 and FLS2. One simple explanation for this could be that the LRRs of BAK1 interact directly with LRRs of BRI1 and bak1elg enhances that interaction. Alternatively, bak1elg may indirectly activate BAK1 kinase activity, thus enhancing the binding affinity between the two kinase domains. To explore these possibilities, we took advantage of the fact that strong overexpression of kinase-dead BAK1 leads to a dwarf phenotype because of impaired BR signaling (7). This phenotype is likely caused by a dominant-negative effect of the kinase-dead BAK1 on BRI1 kinase activity. In contrast, expression of a BAK1 kinase-dead mutant (D434N) under the control of its own promoter in wild-type plants did not induce a dwarf phenotype, probably because at this lower expression level, bak1D434N is unable to compete with endogenous BAK1 to inhibit BRI1 activity (Fig. 4A). We reasoned that if bak1elg activates its own kinase activity, then a double-mutant bak1elg D434N would suppress any effect of the elg mutation. Alternatively, if the enhanced bak1elg interaction with BRI1 is mediated by their respective ECDs, then bak1elg D434N would bring the catalytically dead BAK1 kinase domain into proximity with the BRI1 kinase domain potentially enhancing any intrinsic dominant-negative effect on BRI1 activity, even at native bak1elgD434N expression levels. In fact, we found that at similar expression levels, bak1elg D434N::CITRINE but not bak1D434N::CITRINE resulted in a very strong dominant-negative phenotype; the plants were compact dwarfs that resembled mild to strong bri1 mutants (Fig. 4 A and B and Fig. S4). These results suggest that BAK1 is likely to interact with BRI1 through both its extracellular LRR domain, as well as its intracellular kinase domain, and that the bak1elg mutation enhances this interaction.
Fig. 4.
BRI1 is activated by a double-lock mechanism. (A) Rosette leaf phenotype of wild-type Col-0, BAK1prom:BAK1::CITRINE, BAK1prom:bak1D434N::CITRINE, BAK1prom:bak1elg::CITRINE, and BAK1prom:bak1elgD434N::CITRINE. Average rosette radius in mm ± SD (n = 25). (B) Expression level of transgenic proteins. (C) Rosette leaf phenotype of wild-type Col-0, BAK1prom:bak1elg::CITRINE, OxBKI1 and a cross between BAK1prom:bak1elg::CITRINE and OxBKI1 grown in short days. (D) Model for the formation of an active BR signaling complex. In the absence of ligand, BRI1 is maintained in an inactive state by its C-terminal tail as well as its inhibitory protein BKI1 and does not interact with BAK1 (Upper). Activation of BRI1 by BR triggers both the recruitment of BAK1 through its extracellular LRR domain as well as the BRI1-mediated phosphorylation of BKI1 inside the cell (Lower Left). This triggers dissociation of BKI1 from the plasma membrane and transphosphorylation between BRI1 and BAK1 kinase domain and leads to full activation of the receptor complex (Lower Right). BRI1 is represented as a monomer for simplicity but its isolated intracellular domain exist only as homodimers in solution (10) and 20% of the full-length receptor forms homodimers in vivo (30).
BRI1 Receptor Complex Formation Involves a “Double-Lock” Mechanism.
In conclusion, our study has identified a key role for the LRR ECD of the coreceptor BAK1 during recruitment to its receptors, BRI1 and FLS2. We propose a scenario in which LRR-containing coreceptors are recruited to their activated receptors by their ECDs, bringing the receptor and coreceptor together and thus facilitating subsequent conformational changes and transphosphorylation of their kinase domains. Our finding that a single substitution in the third LRR of BAK1-ECD leads to a modification in its binding to both BRI1 and FLS2, with opposite phenotypic consequences, suggests that specific interactions between the ECDs are critical for the formation of the correct receptor/coreceptor pair. It is unlikely that bak1elg hyperactivates the BR pathway by mass action because of its impaired interaction with FLS2. Indeed, BAK1 association with pattern recognition receptors is MAMP-dependent, but bak1elg ectopically associates with BRI1 in the absence of MAMPs. As such, our data further suggest a fine-tuning between BR and MAMP signaling, where BAK1’s affinity for the relevant receptors provides the cellular decision between elongation or defense. The third LRR of BAK1 plays a critical role in this decision as this region is involved in interaction with both BRI1 and FLS2.
The interaction between the activated BRI1 and BAK1 kinase domains is also critical, because a kinase-dead BRI1 does not interact with BAK1 in planta (12). Notably, we found that bak1elg cannot reverse the phenotype induced by overexpression of BRI1 KINASE INHIBITOR1 (BKI1) (Fig. 4C), an inhibitory protein that prevents the interaction between BRI1 and BAK1 kinase domains (10). Therefore, we propose that receptor/coreceptor heterodimerization is regulated by a double-lock mechanism, in which both the ECDs and the kinase domains participate and which is a critical step for full receptor activation and downstream signaling (Fig. 4D). This strategy would provide room for multiple levels of regulation, coming both from outside and within the cell, in the form of noncell autonomous signals (e.g., ligand) and cell-autonomous regulators [e.g., inhibitory proteins like BKI1 (10)]. This double-lock mechanism would ensure both specificity and robustness in receptor complex formation and might represent a paradigm for LRR-RK activation. Of note, similar but not identical, strategies are used during activation of receptor tyrosine kinases (RTKs) in metazoans. Indeed, the ECDs of RTKs, such as the EGF receptor or the stem-cell factor receptor (KIT) homodimerize following ligand perception, which brings the kinases in the right orientation for trans-phosphorylation (24). In plants, the system is somewhat different in that receptor activation does not require ligand-induced homodimerization but heterodimerization with a coreceptor (3, 21). Because these coreceptors do not directly bind ligands, they are extremely labile and can be recruited to a variety of receptors. This invention allows one coreceptor, such as BAK1, to promote cell growth and innate immunity and, therefore, to be at a critical decision node as a plant determines to use resources to defend itself against microorganisms or to grow toward new resources[e.g., light, water, nutrients (25)]. Future challenges will be to understand the molecular basis of the recognition between receptor and coreceptor to better our understanding of signaling crosstalk.
Experimental Procedures
Plant Material and Growth Conditions.
The wild-type used in all experiments was A. thaliana accession Columbia (Col-0) (except in Fig. S1, in which the wild-type control was accession Landsberg erecta, Ler). Plants were grown on either soil or Petri dishes containing 0.5× Linsmaier and Skoog medium (Caisson Laboratories) in long-day light conditions (16 h light/8 h dark). For bacterial assays, plants were grown in short-day conditions (8 h light/16 h dark). The mutants used in this study are the null bak1-3 (9), the null bri1 allele GABI_134E10, and the null fls2 allele SALK_026801c. The insertion sites of the two T-DNA lines (GABI_134E10 and SALK_026801c) were located in the first exon of BRI1 and FLS2, respectively. The homozygous mutations of BRI1 and FLS2 and the sequence of the insertion site were confirmed by PCR and sequencing. The bri1 mutant was confirmed to be a null allele by Western blot using native anti-BRI1 polyclonal antibody against the C terminus of BRI1 (26). The functional FLS2prom:FLS2::GFP in the Col-0 background is a gift from Silke Robatzek (The Sainsbury Laboratory, Norwich, UK) (9).
Confocal Microscopy, Hormone, and Inhibitor Treatments.
Microscopy and drug treatments were performed as described previously (27). Confocal microscopy was performed with a Leica SP/2 inverted microscope and image analysis was done as described previously (28). BRZ (Chemiclones; 10 mM stock in DMSO) was used at the indicated concentration and was supplemented into the agar medium from the onset of germination.
Protein Extraction from Plants and Immunoprecipitation.
Monoclonal anti-GFP HRP-coupled (Miltenyi Biotech), anti-HA-HRP coupled (Miltenyi Biotech), anti-ACTIN (clone C4; MP Biomedicals), and polyclonal anti-CHERRY (DsRed polyclonal; Clontech) were used at 1:5,000. Polyclonal anti-BRI1 [raised against BRI1 C terminus in rabbit) (26)] was used at 1:1,000. Flg22 treatment before protein extraction was done in liquid medium (0.5× Linsmaier and Skoog medium) for 5 min under vacuum. The immunoprecipitaiton extraction buffer was supplemented with 10 μM flg22; the mock condition corresponds to addition of the same volume of water. Similarly, BRZ was supplied in the immunoprecipitation extraction buffer at a concentration of 5 μM in the BRZ-treated condition; the mock condition corresponds to the addition of the same volume of DMSO (BRZ solvent). All immunoprecipitations were performed as previously described (28). Approximately 100 mg of 14-d-old light-grown seedlings were harvested for Western blot experiments. Immunoprecipitation experiments required from 1 to 3 g of seedlings (14-d-old). Tissues were ground at 4 °C in a 15-mL tube containing 2-mL of ice-cold sucrose buffer [20 mM Tris, pH 8; 0.33M Sucrose; 1 mM EDTA, pH 8; protease inhibitor (Roche)] using a polytron (Brinkman). Samples were centrifuged for 10 min at 5,000 × g at 4 °C or until the supernatants were clear. This total protein fractions were centrifuged at 4 °C for 45 min at 20,000 × g to pellet microsomes. The pellet was resuspended in 1 mL of immunoprecipitation buffer (50 mM Tris pH 8, 150 mM NaCl, 1% Triton X-100) using a 2-mL potter-Elvehjem homogenizer (Wheaton) and left on a rotating wheel for 30 min at 4 °C. Samples were then pelleted for 10 min at 20,000 × g and 4 °C. The supernatant corresponded to the fraction enriched in microsomal associated proteins. The proteins were quantified and immunoprecipitates were performed on 1 mg of microsomal proteins. Each experiment was repeated at least three times and showed consistent results.
MAMP Response Assays.
Flg22 (QRLSTGSRINSAKDDAAGLQIA) and elf18 (acetyl-MSKEKFERTKPHVNVGTI) peptides were synthesized at >95% purity by ezbiolab and dissolved to a 10-mM stock in water. A pectidoglycan (Sigma-Aldrich) stock solution was prepared at 10 mg/mL in water. A 10 mg/mL chitin from shrimp shell (Sigma-Aldrich) stock solution was prepared as follows. Chitin powder was suspended in sterile PBS and sonicated at 25% output power three times for 5 min with a sonicator. The suspension was then filtered with 100-, 70-, and 40-μm sterile cell strainers. Following centrifugation (2,800 × g, 10 min), chitin fragments from the 40- to 70-μm fraction were suspended in the desired volume of sterile PBS and autoclaved. Oxidative burst assays were performed as described previously (9, 23), except that luminescence was measured using a Tecan Saphire plate reader. Loss of fresh-weight ratio was calculated on 14-d-old seedlings grown for 7 d in either water or 1 μM flg22 (n = 48, six random pools of eight seedlings). For callose deposition assays, 14-d-old plants were completely submerged in individual 0.5-mL Eppendorf tube containing the elicitor at the indicated concentration. A vacuum was applied for 15 min and plants remained in the elicitor solution for another 16 h. Next, seedlings were fixed in a 3:1 ethanol:acetic acid solution for several hours. Seedlings were rehydrated in 70% ethanol for 2 h, 50% ethanol for an additional 2 h, and then with water overnight. Seedlings were then incubated in 150 mM K2HPO4, pH 9.5, and 0.01% Aniline blue (Sigma-Aldrich) for several hours. Individual leaves were mounted on slides in 50% glycerol, and callose was observed immediately using a Leica DM5000B under UV (excitation, 390 nm; emission, 460 nm). Bacterial assays were performed as described earlier (23, 29) except that bacterial count were assayed at 3 d postinfection. Each experiment was repeated at least three times and showed consistent results.
Supplementary Material
Acknowledgments
We thank W. Chen and T. Dabi for technical assistance; M. Dreux , E. Kaiserli, U. Pedmale, G. Vert, and M. Hothorn, and M. Nishimura and P. Epple for providing critical feedback on the manuscript; The Max Planck Society and the Salk Institute for providing the insertion mutant lines; S. Robatzek for providing the FLS2prom:FLS2::GFP in Col-0; N. Geldner for providing pNIGEL; J. Long for providing pBJ36; R. Tsien for providing mCHERRY and mCITRINE fluorescent tags; Y. Yin for providing anti-BES1 antibody; and the Nottingham Arabidopsis Stock Centre and Arabidopsis Biological Resource Center for providing material. These studies were supported by the Howard Hughes Medical Institute and Grant IOS-0649389 from the National Science Foundation (to J.C.), and by Grants GM057171 and GM066025 from the National Institutes of Health and Grant IOS-0929410 from the National Science Foundation's Arabidopsis 2010 Program (to J.L.D.); and National Science Foundation Grant IOS-0649389 (to J.C.). Y.B. was a Howard Hughes Medical Institute fellow of the Life Sciences Research Foundation and also received support from the Philippe Foundation; Y.J. was supported by a fellowship from the European Molecular Biology Organization and in part by the Marc and Eva Stern Foundation; E.B-P was supported by a PhD fellowship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and an SWE (SANDWICHE) fellowship from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
Footnotes
See Commentary on page 8073.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103556108/-/DCSupplemental.
References
- 1.Shiu SH, Bleecker AB. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci USA. 2001;98:10763–10768. doi: 10.1073/pnas.181141598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Smet I, Voss U, Jürgens G, Beeckman T. Receptor-like kinases shape the plant. Nat Cell Biol. 2009;11:1166–1173. doi: 10.1038/ncb1009-1166. [DOI] [PubMed] [Google Scholar]
- 3.Boller T, Felix G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- 4.Vert G, Nemhauser JL, Geldner N, Hong F, Chory J. Molecular mechanisms of steroid hormone signaling in plants. Annu Rev Cell Dev Biol. 2005;21:177–201. doi: 10.1146/annurev.cellbio.21.090704.151241. [DOI] [PubMed] [Google Scholar]
- 5.Gómez-Gómez L, Boller T. FLS2: An LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell. 2000;5:1003–1011. doi: 10.1016/s1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 6.Nam KH, Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/s0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- 7.Li J, et al. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. doi: 10.1016/s0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- 8.Heese A, et al. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA. 2007;104:12217–12222. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinchilla D, et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- 10.Jaillais Y, et al. Tyrosine phosphorylation controls brassinosteroid receptor activation by triggering membrane release of its kinase inhibitor. Genes Dev. 2011;25:232–237. doi: 10.1101/gad.2001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, et al. Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 receptor kinase. Plant Cell. 2005;17:1685–1703. doi: 10.1105/tpc.105.031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, et al. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev Cell. 2008;15:220–235. doi: 10.1016/j.devcel.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Schulze B, et al. Rapid heteromerization and phosphorylation of ligand plant transmembrane receptors and their associated kinase BAK1. J Biol Chem. 2010;285:9444–9451. doi: 10.1074/jbc.M109.096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geldner N, Hyman DL, Wang X, Schumacher K, Chory J. Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev. 2007;21:1598–1602. doi: 10.1101/gad.1561307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, et al. Autoregulation and homodimerization are involved in the activation of the plant steroid receptor BRI1. Dev Cell. 2005;8:855–865. doi: 10.1016/j.devcel.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Chory J. Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling, from the plasma membrane. Science. 2006;313:1118–1122. doi: 10.1126/science.1127593. [DOI] [PubMed] [Google Scholar]
- 17.Kim T-W, et al. Brassinosteroid signal transduction from cell-surface receptor kinases to nuclear transcription factors. Nat Cell Biol. 2009;11:1254–1260. doi: 10.1038/ncb1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh M-H, et al. Autophosphorylation of Tyr-610 in the receptor kinase BAK1 plays a role in brassinosteroid signaling and basal defense gene expression. Proc Natl Acad Sci USA. 2010;107:17827–17832. doi: 10.1073/pnas.0915064107. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Halliday K, Devlin PF, Whitelam GC, Hanhart C, Koornneef M. The ELONGATED gene of Arabidopsis acts independently of light and gibberellins in the control of elongation growth. Plant J. 1996;9:305–312. doi: 10.1046/j.1365-313x.1996.09030305.x. [DOI] [PubMed] [Google Scholar]
- 20.Whippo CW, Hangarter RP. A brassinosteroid-hypersensitive mutant of BAK1 indicates that a convergence of photomorphogenic and hormonal signaling modulates phototropism. Plant Physiol. 2005;139:448–457. doi: 10.1104/pp.105.064444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belkhadir Y, Chory J. Brassinosteroid signaling: A paradigm for steroid hormone signaling from the cell surface. Science. 2006;314:1410–1411. doi: 10.1126/science.1134040. [DOI] [PubMed] [Google Scholar]
- 22.Vert G, Chory J. Downstream nuclear events in brassinosteroid signalling. Nature. 2006;441:96–100. doi: 10.1038/nature04681. [DOI] [PubMed] [Google Scholar]
- 23.Zipfel C, et al. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428:764–767. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- 24.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaillais Y, Chory J. Unraveling the paradoxes of plant hormone signaling integration. Nat Struct Mol Biol. 2010;17:642–645. doi: 10.1038/nsmb0610-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belkhadir Y, et al. Intragenic suppression of a trafficking-defective brassinosteroid receptor mutant in Arabidopsis. Genetics. 2010;185:1283–1296. doi: 10.1534/genetics.109.111898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaillais Y, Fobis-Loisy I, Miège C, Rollin C, Gaude T. AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature. 2006;443:106–109. doi: 10.1038/nature05046. [DOI] [PubMed] [Google Scholar]
- 28.Jaillais Y, et al. The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell. 2007;130:1057–1070. doi: 10.1016/j.cell.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 29.Zipfel C, et al. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–760. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 30.Hink MA, Shah K, Russinova E, de Vries SC, Visser AJWG. Fluorescence fluctuation analysis of Arabidopsis thaliana somatic embryogenesis receptor-like kinase and brassinosteroid insensitive 1 receptor oligomerization. Biophys J. 2008;94:1052–1062. doi: 10.1529/biophysj.107.112003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.