Abstract
MicroRNA–protein complexes (microRNPs) can activate translation of target reporters and specific mRNAs in quiescent (i.e., G0) mammalian cell lines. Induced quiescent cells, like folliculated immature oocytes, have high levels of cAMP that activate protein kinase AII (PKAII) to maintain G0 and immature states. We report microRNA-mediated up-regulated expression of reporters in immature Xenopus laevis oocytes, dependent on Xenopus AGO or human AGO2 and on FXR1, as in mammalian cells. Importantly, we find that maintenance of cAMP levels and downstream PKAII signaling are required for microRNA-mediated up-regulated expression in oocytes. We identify an important, endogenous cell state regulator, Myt1 kinase, as a natural target of microRNA-mediated up-regulation in response to xlmiR16, ensuring maintenance of oocyte immaturity. Our data reveal the physiological relevance of cAMP/PKAII-controlled posttranscriptional gene expression activation by microRNAs in maintenance of the immature oocyte state.
MicroRNAs are 19- to 23-nt RNAs that serve as posttranscriptional regulators of gene expression when recruited into effector complexes with a core Argonaute protein, AGO2 (eIF2C2) in mammals. These microRNA–protein complexes (microRNPs) bind the target mRNA, normally within its 3′-UTR, and regulate translation and decay of mRNAs (1).
We previously demonstrated that microRNPs can effect translation activation of minimal target reporters and specific mRNAs in quiescent mammalian cells (2). Quiescence refers to nondividing G0 and G0-like states with specific gene expression programs that dividing cells can enter for extended periods of time in a reversible manner. The G0 state can be naturally programmed during differentiation or development or induced in cultured cells. DNA replication ceases and gene expression skews toward maintaining the G0 state and preventing promiscuous entry into other states (3).
Like G0 cells, the Xenopus laevis prophase I-arrested immature oocyte does not proliferate or replicate DNA (4). The immature oocyte is surrounded by follicle cells that maintain high cAMP levels and downstream protein kinase A (PKA) signaling, thereby inhibiting maturation (5). Defolliculation and progesterone treatment cause a loss of signaling through G protein-coupled receptors, leading to altered PKA signaling, loss of the nuclear membrane [called germinal vesicle (GV) breakdown], and maturation (5). The cAMP-inducible PKA holoenzyme acts as PKAI or PKAII as a result of modulation of the catalytic subunit by alternative cofactors, repressor I (RI) or II (RII) subunits (6); both RI and RII respond to cAMP levels, with RII requiring higher levels of cAMP. PKAI is present in proliferating cells and various tumors in which RI is overexpressed; PKAII is observed in arrested and nonproliferating cells in which RII predominates (6). Like immature oocytes, the G0 state in some mammalian cells can be elicited by increasing cAMP levels to induce PKAII (6, 7).
The oocyte up-regulates the expression of genes essential for maintaining the immature state (8). Among these is the cell state regulator, Myt1 kinase, which is up-regulated at the translational level as the immature oocyte advances from stages I–III to stages IV–VI (8). Myt1 is required for CDC2 phosphorylation and consequent inactivation of prematuration promoting factor (pre-MPF, comprised of cyclin B2 and CDC2) (8, 9), preventing maturation.
Here, we investigated whether microRNA-mediated activation occurs in naturally quiescent-like X. laevis immature oocytes. We find that activation is regulated by the G0-controlling cAMP/PKAII pathway and identify an endogenous microRNA in the immature oocyte required to increase expression of the cell state regulator Myt1. Thus, microRNA-mediated posttranscriptional up-regulation is relevant for maintenance of the immature oocyte state.
Results
Exogenous MicroRNAs Activate Expression of Target mRNA Reporters in the Immature Oocyte.
We tested microRNA-mediated expression in the G0-like immature X. laevis oocyte with luciferase reporters used in mammalian cells (2). We injected DNA constructs with a CMV promoter and bovine growth hormone polyadenylation sequence or in vitro-transcribed capped, unadenylated RNAs into the nucleus of folliculated stage IV–VI oocytes. We routinely included: (i) coinjection of a Renilla luciferase reporter (REN) bearing a nonspecific polylinker sequence in its 3′-UTR to normalize for injection and overall translation status; (ii) a Firefly luciferase reporter with either a mutated 3′-UTR or a polylinker sequence (control; CTRL) of the same size as the 3′-UTR of the test reporter; and (iii) assessment of RNA levels.
When a Firefly luciferase plasmid bearing TNF-α AU-rich elements (AREs) in its 3′-UTR was coinjected with the corresponding microRNA, miR369-3p (2), a five- to sixfold increase in expression was observed relative to the control microRNA, let-7a, or the CTRL reporter (Fig. 1A). An increase in expression can arise from relief of repression, whereby the increased expression is equal to and not greater than the control (i.e., restoration of general translation), or activation, in which the increased expression is greater than the control (i.e., stimulation over general translation); the control is either a no-microRNA target reporter (i.e., CTRL) or a mismatched control microRNA added with the test reporter (2, 10). Expression of the ARE reporter with miR369-3p was higher than the reference controls, CTRL and the ARE reporter with a nonmatching microRNA, suggesting stimulation.
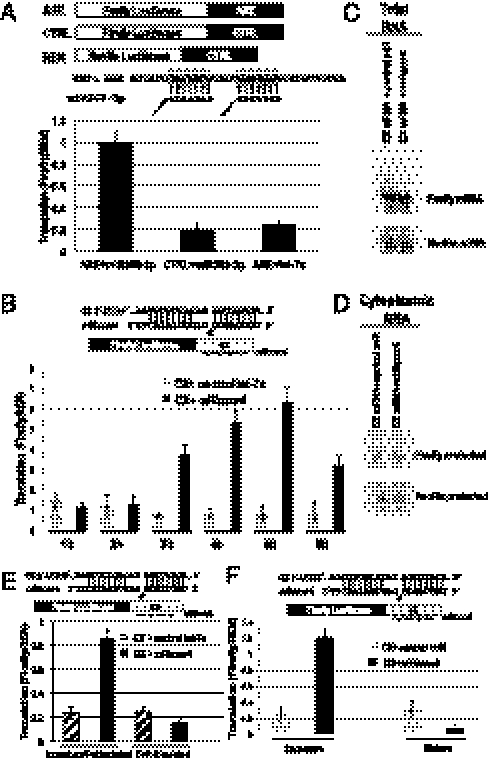
Fig. 1.
MicroRNAs mediate up-regulated expression of reporters coinjected into the GV of folliculated stage IV–VI oocytes. (A) MiR369-3p (but not control let-7a) up-regulates expression of the ARE reporter (but not of CTRL reporter), when coinjected into the GV with the ARE and CTRL luciferase plasmids along with REN. (B) MiRcxcr4 (but not control let-7a) up-regulates expression of the CX reporter (2, 11) over time when coinjected with the CX luciferase plasmid into GV. MiRcxcr4 and the control miR (let-7a) do not alter total oocyte (C) or cytoplasmic levels (D) of the CX reporter mRNA. Northern blots show the protected 250-nt band for the Firefly luciferase reporter and a 100-nt protected band for the coinjected REN reporter (SI Methods). (E) MiRcxcr4 (but not control let-7a) up-regulates expression of the CX transcript when coinjected into GVs of folliculated but not of defolliculated oocytes. (F) MiRcxcr4 represses translation of the CX reporter in progesterone-matured oocytes as opposed to up-regulated expression in immature, folliculated oocytes. MiR:mRNA base pairing shown in A and B and in E and F, as well as in subsequent figures. C–F used in vitro-transcribed, capped luciferase reporter RNAs. Firefly luciferase values were normalized to the cotransfected REN control.
We then injected the CX reporter plasmid, which harbors four copies of a target site for a synthetic microRNA, miRcxcr4, which had previously exhibited translation activation in G0 mammalian cells and translation repression in cycling cells (2, 11). A time course of luciferase activity revealed increased expression in the presence of miRcxcr4 compared with the control let-7a microRNA; a fourfold enhancement at 3 h after injection increased to sixfold at 5 h (Fig. 1B). Cytoplasmic levels of the mRNA did not exceed that of the microRNA until 6 h, when the exogenous microRNA levels with a half-life of approximately 30 min decreased (Fig. S1 A and C and SI Methods). Values increased only for the microRNA target, CX, and not for the control REN (Fig. S1D), suggesting specific activation of the microRNA target. The 4-h time point was chosen for comparison in subsequent experiments. Luciferase protein half-lives (Fig. S1B), CX total (Fig. 1C), and cytoplasmic mRNA (Fig. 1D and Fig. S1C) levels were comparable in the presence or absence of miRcxcr4. Comparable results were obtained by injecting the corresponding capped reporter mRNAs and microRNAs into the GV of folliculated oocytes (Fig. 1E). These results suggest that microRNA-mediated up-regulated expression at the translational level can be achieved in the natural, G0-like immature oocyte.
Up-Regulated Expression by MicroRNAs Occurs Only in Folliculated Immature Oocytes.
Up-regulated translation of the CX reporter by miRcxcr4 was observed in folliculated but not in defolliculated immature oocytes (Fig. 1E). Likewise, when oocytes were matured with progesterone, the sixfold stimulation of expression of the CX reporter was converted to a fourfold repressive effect (Fig. 1F). Thus, maturation, like defolliculation, correlates with a loss of up-regulated translation by microRNAs.
cAMP/PKA Pathway Is Required for Up-Regulated Translation in Xenopus Oocytes.
cAMP levels increase in some G0 mammalian cells (6, 7) and in immature folliculated oocytes (Fig. S2A) (5). Maturation can be prevented by adding inhibitors of phosphodiesterases and nondegradable cAMP analogues (5). Accordingly, the loss of translation activation of the CX reporter by miRcxcr4 upon defolliculation of immature oocytes can be reversed by addition of the phosphodiesterase inhibitor papaverine (Fig. 2A). Dibutyryl cAMP, an analogue that increases PKA activity (12), elicits increased translation of the CX reporter in the presence of miRcxcr4 or of an AGO2-tethered reporter (2) in defolliculated oocytes (Fig. S2B). In accord with observations of PKAII activity in arrested mammalian cells (6, 7), injection of RII (PKAII activation) or the catalytic subunit (C) plasmids into oocytes activated translation with miRcxcr4 and not with the control microRNA, let-7a, whereas RI (PKAI activation), associated with proliferation, did not (Fig. 2B and Fig. S2C). Overexpression of dominant negative PKA inhibited up-regulation in oocytes, confirming that PKA is required for translation activation (dnPKA; Fig. 2C) in immature Xenopus oocytes.
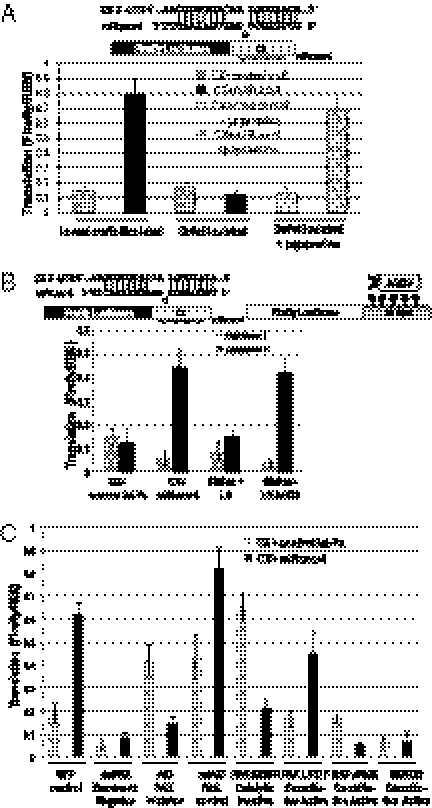
Fig. 2.
The cAMP/PKA pathway mediates up-regulated translation by microRNPs in oocytes. (A) Addition of 1.25 mM papaverine, a phosphodiesterase inhibitor that blocks turnover of cAMP, restores up-regulated translation of the CX reporter by miRcxcr4 (but not by control miR, let-7a) in defolliculated oocytes. (B) Expression of PKA repressor I (RI) abrogates, whereas repressor II (RII) activates, translation of the CX reporter in the presence of miRcxcr4 and of the AGO2-tethered reporter in oocytes. (C) Blocking the PKA pathway (dominant negative PKA, dnPKA) or the downstream PAK pathway (using a dominant negative inhibitor, AID) causes loss of up-regulation of CX by miRcxcr4 in folliculated immature oocytes compared with a mutant form of the inhibitor, mtAID, or a GFP control. Expression of a constitutively active PAK, L107F, enables up-regulated expression of CX by miRcxcr4 in defolliculated oocytes whereas a kinase inactive form, K299R, does not. Expression of a constitutively active RAFv600E or a constitutively active MEK, MEKDD (both are turned off by PAK), blocks up-regulated expression of CX by miRcxcr4 compared with the GFP control in folliculated immature oocytes. L107F and K299R samples used defolliculated oocytes, whereas all other samples were folliculated oocytes. All reporters were in vitro-transcribed, capped luciferase RNAs; the signaling proteins were expressed from plasmids. Firefly luciferase values were normalized against REN.
The PKA/cAMP pathway signals to the p21-activated kinase (PAK) to turn off the growth factor-stimulated MAPK proliferative pathway in mammalian cells; in oocytes, cAMP and PKA function with PAK to promote maintenance of the immature state (13). Injecting a dominant-negative inhibitor of PAK (AID) into immature folliculated oocytes prevents translation activation, whereas a mutant form of the inhibitor that cannot bind and block the active site of PAK (mtAID) does not (Fig. 2C; compare AID vs. mtAID). In defolliculated oocytes, a constitutively active form of PAK (L107F) promotes activation whereas a catalytically inactive form (K299R) does not (Fig. 2C; compare L107F vs. K299R samples, which are defolliculated oocytes), suggesting that the PAK pathway is required for microRNA-mediated up-regulation in oocytes. A constitutively active form of RAF, which activates MAPK, or a constitutively active MAPK prevents activation (Fig. 2C; RAFv600E and MEKDD), arguing that cAMP-mediated activation by microRNAs involves turning off downstream MAPK and involves PAK; additional pathways may also affect activation.
Up-Regulated Expression by MicroRNAs in Immature Oocytes Requires Human AGO2 or Xenopus AGO and FXR1.
We asked whether AGO and FXR1, factors essential for translation activation in G0 mammalian cells (2), are also involved in the oocyte. Western blotting with an anti-AGO2 antibody as well as an AGO antibody that recognizes AGOs 1 to 4 (Fig. 3A and Fig. S3 A and C) revealed a band in immature oocyte extracts. cDNA sequencing identified an allele, which matched one of two database entries for Xenopus eIF2C2 (NM_001093519) and is similar to a second Xenopus eIF2C2 sequence (EU338243). As the cDNA has not been characterized with known human AGO2 functions such as slicer activity (1), we refer to it as xlAGO based on its sequence identity to the Argonaute family.
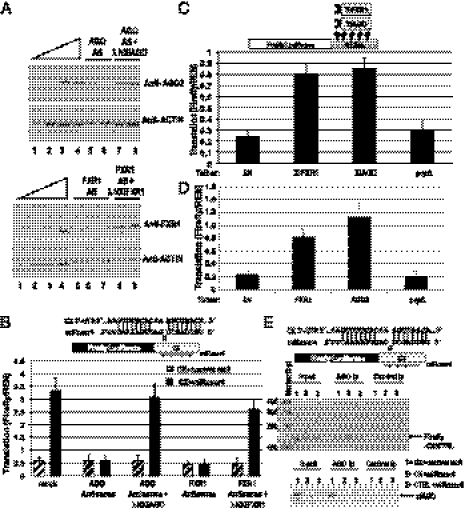
Fig. 3.
FXR1 and xlAGO are required for microRNA-mediated up-regulated translation in oocytes. (A) Sixteen hours of DNA antisense treatment decreases endogenous FXR1 and AGO protein levels in the oocyte, which were rescued by expression of in vitro-transcribed mRNAs encoding λN-tagged Xenopus FXR1 and AGO, respectively, as observed by Western blotting (SI Methods and Fig. S3D). Actin served as a loading control. Twofold dilutions (12.5–100%) of mock-treated immature oocyte extract (lanes 1–4) are shown: Upper (AGO) and Lower (FXR1). Lanes 5–6 and 7–8 (Upper) are duplicate samples, as are lanes 5–7 and 8–9 (Lower). (B) MiRcxcr4 activates translation of the CX reporter mRNA expressed from a plasmid when coinjected into the nuclei of immature, folliculated oocytes (mock). Antisense reduction of Xenopus AGO or FXR1 levels abrogates activation of the CX reporter, which can be rescued by expression of the λN-tagged resistant clones. (C) Expression of λN-tagged Xenopus FXR1 or AGO (XlFXR1 and XlAGO) mRNAs into immature oocyte nuclei causes translation activation of the coinjected tethered reporter. λN and the λNprpΔ mutant form of human AGO2 are controls. (D) Plasmids expressing λN-tagged human FXR1 or AGO2 coinjected into immature oocyte nuclei cause translation activation of the tethered reporter. In vitro-transcribed, capped XlFXR1 and XlAGO mRNAs (A–C), and plasmids expressing hAGO2 and hFXR1 (D) were used for protein expression; experiments in C and D used in vitro-transcribed capped tethered reporter. In B–D, Firefly luciferase values were normalized against REN. (E) RT-PCR analysis (Upper) of RNAs extracted from anti-AGO2 or control anti-FLAG coimmunoprecipitates (Ip) from oocyte nuclear extracts coinjected with the CX or CTRL reporters and the specific miRcxcr4 or the control microRNA, let-7a, reveals significant association of CX but not CTRL mRNA with xlAGO in the presence but not absence of miRcxcr4. (Lower) Western blot of AGO protein in input and immunoprecipitation (Ip) lanes.
When oocytes depleted of FXR1 or AGO by antisense for 12 to 16 h (Fig. 3A, Fig. S3D, and SI Methods) are injected with the CX reporter and the corresponding miRcxcr4, no translation activation is observed (Fig. 3B) compared with a four- to fivefold translation up-regulation in mock-treated oocytes. In vitro-transcribed mRNAs of resistant forms of Xenopus FXR1 and AGO (λNXlFXR1 and λNXlAGO; Fig. 3 A and B) or of human FXR1 and AGO2 (Fig. S3B) rescue translation, suggesting a conserved function between humans and Xenopus; human AGO1, AGO3, and AGO4 have less significant effects on activation (Fig. S3B). When λN-tagged human or Xenopus AGO and FXR1 are individually expressed along with the 5B Box Firefly luciferase tethering reporter, either protein causes translation activation, whereas control mutant proteins and vectors do not (Fig. 3 C and D). Finally, to test whether association with the mRNA in Xenopus oocytes is microRNA-dependent, we analyzed AGO immunoprecipitates for the Firefly luciferase mRNA by RT-PCR. Fig. 3E shows that Xenopus AGO associates significantly with the CX reporter (but not CTRL mRNA) in the presence but not absence of miRcxcr4. These data argue that Xenopus AGO and FXR1 have retained at least the translation activation function of their mammalian counterparts.
Endogenous microRNA with a Heterogeneous 5′ End, xlmiR16, Up-Regulates Expression of Myt1 mRNA in the Oocyte.
We searched for potential targets reported to be regulated at the translation level during oocyte stages III–VI (8). Myt1 protein levels rise across the immature stages without a concomitant increase in mRNA levels and are required for CDC2 phosphorylation and inactivation of pre-MPF (8, 9). Myt1 mRNA harbors a 1758-nt 3′-UTR that contains seven potential xlmiR16 target sites (Fig. 4A) that might engage in noncanonical base pairing. An siRNA antisense to the loop region of precursor (pre-) xlmiR16 reduced mature xlmiR16 levels in oocytes (SI Methods and Fig. S4A). The endogenous xlmiR16 (14) appears more stable (Fig. S4B) than exogenous microRNAs (Figs. S1A, i, and S4C), requiring more than 6 h for siRNA-mediated antisense depletion, likely because of interference with processing or stability instead of cleavage. Reporters carrying the entire Myt1 3′-UTR or a 528-nt segment bearing the seven putative sites exhibit up-regulated expression in the presence of xlmiR16 (Fig. 4A) independent of RNA levels (Fig. 4B). We found that one site (1,284 nt from the stop codon) is necessary for up-regulation, as mutating this site (mt3Myt1; SI Methods) causes a loss of activation that is not further decreased by the loss of xlmiR16 (Fig. 4A). To determine whether the effect of xlmiR16 is direct, we made a correspondingly mutated xlmiR16, xlmt3miR16 (SI Methods), which should restore base pairing; we find that the compensatory microRNA restores translation activation (Fig. 4A) without altering mRNA levels (Fig. 4B).
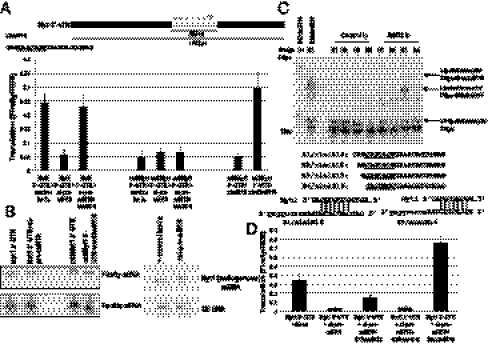
Fig. 4.
Myt1 mRNA translation is up-regulated by a 5′ variant xlmiR16. (A) Myt1 3′-UTR has seven putative xlmiR16 sites (dashes). Mutation of the site at 1,284 nt (mt3Myt1 3′-UTR) in the 528-nt or full-length Myt1 3′-UTR failed to demonstrate translation activation and was unaffected by xlmiR16 presence or depletion (SI Methods; siRNAs antisense to pre-miR16 reduced mature xlmiR16 after 6 h, likely because of interference with processing or stability instead of cleavage activity; Fig. S4A). Translation activation is restored by addition of a compensatorily mutated xlmt3miR16 that can base pair to the mutated site (SI Methods). Both B1/xlmiR16 (shown) and B3/xlmiR16 (Fig. 4C shows base pairing) are able to rescue. (B) Northern analyses of Firefly reporters containing the 528-nt Myt1 3′-UTR and mt3Myt1 3′-UTR and REN RNA levels after treatment with si-pre-miR16 with or without mt3xlmiR16 add-back. (Right) Endogenous Myt1 mRNA levels with or without si-pre-miR16 treatment; U6 RNA is a loading control. (C) Splint ligation reactions (SI Methods) containing the labeled 14-nt acceptor oligo and RNAs isolated from anti-AGO2 or -FLAG (control) immunoprecipitates (Ip). XlmiR16 variants that are successively truncated by one nucleotide at the 5′ end (B1/xlmiR16-B4/xlmiR16) were detected using DNA bridge oligos B1-B4, complementary to the first 16 nt of the predicted microRNAs. The predicted full-length form, B1/xlmiR16, is only faintly visible in the sample immunoprecipitated with anti-AGO2, suggesting that it is less abundant than B3/xlmiR16, which is revealed by the B3 bridge oligo to be an abundant microRNA in anti-AGO2 immunoprecipitates. Lanes marked B1/xlmiR16 and B3/xlmiR16 show synthetic miR16 RNAs as size controls. Underlined nucleotides are required for interaction with the Myt1 3′-UTR. Base pairing between Myt1 target site and B1/xlmiR16 or B3/xlmiR16 are shown. (D) Oocytes treated with mock (let-7a) or si-pre-miR16 were coinjected with synthetic xlmiR16 forms to assess rescue of full-length Myt1 3′-UTR reporter translation. Firefly luciferase values were normalized to REN. B1/xlmiR16 undergoes trimming to the B3/xlmiR16 form and rescues translation to a lesser extent than B3/xlmiR16.
The xlmiR16 target sequence in the Myt1 3′-UTR defined earlier is complementary to nucleotides 4 to 10 (or 4-12) of the microRNA rather than to nucleotides 2 to 7/8, as expected from the seed rules (15). Therefore, either the Myt1 target site uses noncanonical base pairing (16, 17) or the endogenous microRNA has an altered 5′ end that enables functional interactions (Fig. 4C, potential base pairing with Myt1 shown). We determined the 5′ end of the microRNA by splint ligation by using oligonucleotides designed to capture stepwise shortened versions of the 5′ end of xlmiR16: B1 is the full 5′ end, and B2, B3, and B4 are successively 1, 2, and 3 nt shorter. The B3 version (truncated by 2 nt at the 5′ end of xlmiR16) is abundant in both total oocyte RNA and in AGO immunoprecipitates (Fig. 4C), suggesting that B3/xlmiR16 is the predominant functional form of the microRNA, whereas the nontruncated version of xlmiR16 (B1/xlmiR16) is only faintly detectable. To prove that B3/xlmiR16 can up-regulate translation by acting on the full-length Myt1 3′-UTR (potential base pairing with Myt1 shown in Fig. 4C), luciferase assays were repeated after depleting endogenous xlmiR16 and adding back various 5′-truncated forms. Addition of B3/xlmiR16 restores translation activation (Fig. 4D) more robustly than B1/xlmiR16.
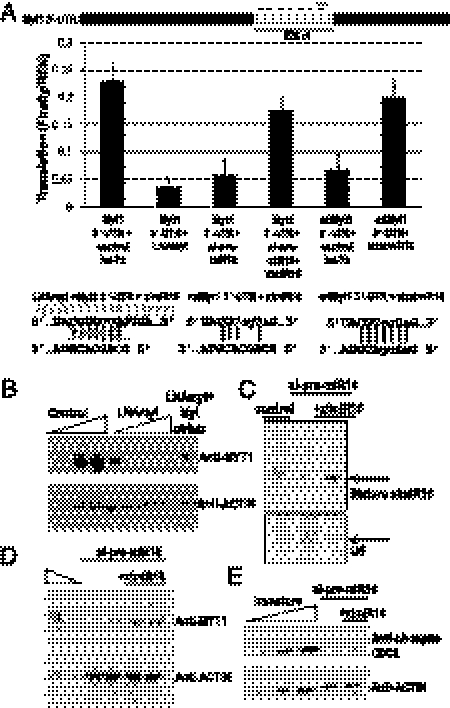
To confirm that the site at 1284 in Myt1 3′-UTR is critical for translation up-regulation, we designed an LNA oligonucleotide antisense to this site and its adjacent sequences, similar to the protectors developed earlier (18). When LNAmyt is injected into oocytes (Fig. 5A), translation activation of luciferase reporters bearing the 528-nt Myt1 3′-UTR (or full length) is abrogated. Loss of translation activation is also observed when endogenous xlmiR16 levels are depleted by si-pre-miR16 (Fig. 5A); activation is rescued by add-back of synthetic xlmiR16 (B3/xlmiR16). Conversely, the loss of translation up-regulation observed with the mtMyt1 reporter is rescued by addition of a B3/xlmiR16 form, xlmtmiR16, harboring compensatory mutations that restore base pairing with the mutant mtMyt1 3′-UTR (Fig. 5A).
Fig. 5.
Translation activation of Myt1 mRNA by xlmiR16 is essential for maintenance of the immature state. (A) Coinjection of an LNA antisense oligonucleotide complementary to the xlmiR16 target site (LNAmyt, gray dash) in the 528-nt Myt1 3′-UTR abrogates translation activation, as does knockdown of xlmiR16 with si-pre-miR16, which can be rescued by B1/xlmiR16. Mutation of this target site (mtMYT1) abrogates translation activation, but add-back of the compensatorily mutated microRNA, xlmtmiR16, restores activation. Firefly luciferase values were normalized to REN. Mutations are in lowercase. (B) LNAmyt (increasing twofold, 0.25–4 pmol) blocks accumulation of endogenous Myt1 protein in the oocyte, whereas Myt1 protein levels are restored at the highest LNAmyt concentration by coinjecting a Myt1 expression vector lacking its 3′-UTR (Mytctrlutr), compared with twofold dilutions (25–100%) of control untreated immature oocyte extract. (C) Northern blot demonstrating that depletion of mature xlmiR16 in the oocyte can be reversed by injecting synthetic mature B3/xlmiR16. U6 controlled for loading. (D) Pretreatment with si-pre-miR16, which lowers endogenous Myt1 protein levels in the oocyte (duplicate lanes), can be reversed by injection of synthetic mature B3/xlmiR16 (duplicate lanes) compared with untreated immature oocyte extract (100% and 50%). (E) Pretreatment with si-pre-miR16 leads to decreased levels of Myt1 protein and consequent loss of phosphorylated CDC2, which can be restored by addition of synthetic mature B3/xlmiR16, as judged by comparison with twofold dilutions (25–100%) of untreated immature oocyte extract. Actin served as the loading control for Western analyses (B, D, and E).
MicroRNA-Activated Expression Is Required to Maintain the Oocyte Immature State.
Ablation of Myt1 kinase in the oocyte leads to CDC2 dephosphorylation and loss of the immature state (8, 9). We asked whether translation activation via xlmiR16 is essential for maintenance of the immature state. First, we treated oocytes with the LNA protector, LNAmyt, to block translation activation of the endogenous Myt1 mRNA. As shown in Fig. 5B, increasing levels of LNAmyt reduce endogenous Myt1 protein. Injection of a Myt1 gene construct with a polylinker as its 3′-UTR (Mytctrlutr), which should not bind LNAmyt, partially restores Myt1 levels (Fig. 5B), demonstrating that the loss of translation activation by LNAmyt is through the Myt1 3′-UTR. Second, we depleted endogenous xlmiR16 levels by using si-pre-miR16 (Fig. 5C) and observed a corresponding loss of Myt1 protein levels (Fig. 5D). We then asked whether xlmiR16-mediated translation activation of Myt1 is physiologically essential by examining the marker of immaturity, phosphorylated CDC2. Phosphorylated CDC2 levels dramatically decrease (Fig. 5E) in concert with the decrease in Myt1 levels. Significantly, addition of synthetic B3/xlmiR16 (Fig. 5C) restores Myt1 protein levels (Fig. 5D) and phosphorylated CDC2 increases (Fig. 5E). Importantly, loss of MYT1 expression by blocking its 3′-UTR miR16 target site with LNAmyt (Fig. 5B) also leads to loss of immaturity, marked by loss of CDC2 phosphorylation (Fig. S5A, lane 3). Decreased levels of phosphorylated CDC2 and loss of immaturity upon xlmiR16 depletion (Fig. 5E and Fig. S5A, lane 6) or upon LNAmyt protection of the miR16 target site on endogenous Myt1 mRNA (Fig. S5A, lane 3) are rescued by injecting the Myt1 construct without its regulatory 3′-UTR (Mytctrlutr; Fig. S5A, lanes 4 and 8; and Fig. S5B). These data argue that xlmiR16 is required to maintain the immature state in part via up-regulating Myt1 expression.
Discussion
MicroRNPs exhibit versatility in their control of gene expression, dependent on the specific mRNA 3′-UTR (10, 19). Translational activation in alternative cell states, such as the immature oocyte, provides a means of gene expression to maintain the state. We previously demonstrated that microRNPs can activate translation of minimal target reporters in G0 mammalian cells (2). Here we have documented microRNA-mediated translation activation in the naturally G0-like X. laevis immature oocyte. xlAGO and FXR1 proteins are required, as in mammalian G0-induced activation. We further demonstrate that microRNA-dependent activation is dependent on cAMP/PKA signaling in X. laevis oocytes. Our data argue that xlmiR16 is required in part for oocyte immaturity and that microRNA-mediated activated expression of endogenous mRNAs in the X. laevis oocyte is physiologically relevant for maintenance of the immature state.
A common feature of the immature Xenopus oocyte and mammalian G0 state is that cAMP signaling is required to sustain immaturity (5, 20) and G0 in some mammalian cells (7). Loss of the oocyte immature state leads to loss of microRNA-mediated activation (Fig. 1 E and F), as does loss of the mammalian G0 state (2). cAMP binds the R subunit and activates PKA dependent on the predominance of RI, associated with proliferation or RII, associated with arrested cells (6). Overexpression of RII/PKAII (but not RI) in oocytes leads to activated expression in the presence of microRNAs (Fig. 2B). PKAII signaling is induced by a relative increase in cAMP levels (21) observed in immature oocytes and G0, rather than the total levels of cAMP; therefore, there is a loss of activation controlled by PKAII upon defolliculation (Fig. 1E) and loss of adenyl cyclase stimulation. However, the effect is more pronounced in mature oocytes (5) in which GV breakdown increases phosphodiesterase activity and loss of adenyl cyclase activity and MPF activation perpetuates the decreased cAMP response via a feedback loop (5). Stabilized persistence of the low cAMP state upon oocyte maturation (5) and in proliferating cells is sufficient for RI/PKA I activity (12, 21), which correlates with translation repression (Fig. 2B) after maturation (Fig. 1F).
A second common feature of oocytes and G0 cells is that the repressive microRNP cofactor GW182 (22) appears compromised: GW182 interaction with AGO is not detected in immature or mature oocytes and in mouse oocytes, which fail to show significant repression (23), and is reduced in G0 mammalian cells (24), in which activation is observed (2). AGO that cannot interact with GW182 can fail to repress translation (23, 25), cause activation of unadenylated target reporters, or repress via the cap (26), likely decided by additional determinants. Specific mRNAs may adapt to the absence of the repressive AGO–GW182 interaction in oocytes and G0 (2, 23, 24) and instead recruit a translation activating AGO–FXR1 complex (which relocalizes to polysomes in G0) (2). Regulation by cAMP may give AGO–FXR1 an advantage in oocytes and G0, in which AGO–GW182 interaction is reduced (2, 23, 24), consistent with a recent report that FXR1 function in zebrafish requires PAK (27), a kinase downstream of cAMP required for microRNA-mediated activation in oocytes (Fig. 2C).
The potential mechanisms of enhanced expression, including mRNA stability and translation up-regulation, remain to be explored. Although the total and cytoplasmic levels of the mRNAs (Fig. 1 C and D and Fig. S1C) do not change with the presence of interacting microRNA, effects of the cap and the poly(A) tail remain to be defined. The experiments were carried out with nonadenylated mRNAs, as enhanced expression compared with the increased levels observed with polyadenylated reporters could not be discerned. The need to inject into the nucleus for activation and the absence of activation in mature oocytes, which lack intact nuclei (Fig. 1F), may result from the fact that mRNA translation effects are often decided by nuclear events (28) and that microRNP functions can involve a nuclear phase for AGO (29).
MicroRNAs are less abundant relative to other small RNAs in Xenopus oocytes (14, 30). The range of targets regulated may be expanded by trimming microRNAs in a flexible manner. A 5′-truncated version of B3/xlmiR16 (20 nt) in AGO complexes (Fig. 4C) triggered more robust translation activation of Myt1 mRNA than the untruncated B1/xlmiR16 (Fig. 4D).
Xenopus oocyte immaturity is maintained by critical phosphorylations that render pre-MPF inactive until maturation is induced (20). Our results demonstrate that PKA-regulated microRNA-mediated activation is essential for Myt1 expression (Figs. 4 and 5); Myt1 enables phosphorylation of CDC2, contributing to maintenance of the immature state (8, 9); loss of Myt1 translation leads to maturation (Figs. 5 C–E and Fig. S5 A and B) (9). Oocyte immaturity can be rescued in part by restoring xlmiR16 (Figs. 5 C–E and Fig. S5A, lane 7) or by reexpressing Myt1 (Fig. S5 A, lanes 4 and 8, and B), suggesting that xlmiR16 is necessary for immaturity in part because it maintains the expression of the essential regulator, Myt1 (Figs. 4 and 5 and Fig. S5). Other targets and (undiscovered) microRNA functions likely also contribute to oocyte immaturity. The role of microRNAs in mediating up-regulated expression in the oocyte has implications for related physiological functions in G0 mammalian cells.
Methods
Oocytes.
Human chorionic gonadotropin (hCG)-stimulated oocytes were manipulated as described in SI Methods. A total of 5 nL containing 0.0625 to 0.125 ng DNA plasmid, 0.01–0.1 fmol in vitro-transcribed luciferase reporter mRNAs, 375 fmol siRNA or microRNA, 37.5 ng of antisense or 10 ng of mRNAs were injected per oocyte with blue dextran dye to visualize delivery into the GV. Translation was assayed with a time course ranging from 1 to 6 h and compared at 4 h in hCG-treated oocytes. All experiments were conducted with folliculated immature stage IV-VI oocytes unless specified otherwise.
Plasmids and Reporters.
All plasmids and reporters are described in SI Methods.
Luciferase Assay and Protein and RNA Analyses.
Luciferase assays were performed as described (Promega). Oocytes were manually crushed in passive lysis buffer (Promega), and clarified by centrifugation at 2,000 × g for 5 m. Total oocyte extracts, immunoprecipitations, and RNA analyses are described in SI Methods. The average ratios of luciferase values were calculated with SDs. RNA levels were assessed in each experiment separately (not used to normalize the values graphed) and performed at least three times completely.
Supplementary Material
Acknowledgments
The authors thank Z. Mourelatos, D. Bloch, M. Fritzler, and W. Filipowicz for reagents; P. Vasudevan, K. Tycowski, K. Herbert, A. Alexandrov, and R.-M. Lye for critical comments; and Y. Tong and S. Truesdell for technical and A. Miccinello for editorial assistance. This work was supported by a Cancer Research Institute Investigator Award (to S.V.), Leukemia and Lymphoma Society Special Fellowship 3300-09 (to S.V.), Massachusetts General Hospital start-up funds (S.V.), and National Institutes of Health Grants GM26154 and CA16038 (to J.A.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105401108/-/DCSupplemental.
References
- 1.Carthew RW, Sontheimer EJ. Origins and Mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: MicroRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 3.Coller HA, Sang L, Roberts JM. A new description of cellular quiescence. PLoS Biol. 2006;4:e83. doi: 10.1371/journal.pbio.0040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taieb F, Thibier C, Jessus C. On cyclins, oocytes, and eggs. Mol Reprod Dev. 1997;48:397–411. doi: 10.1002/(SICI)1098-2795(199711)48:3<397::AID-MRD14>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 5.Schorderet-Slatkine S, Schorderet M, Baulieu EE. Cyclic AMP-mediated control of meiosis: Effects of progesterone, cholera toxin, and membrane-active drugs in Xenopus laevis oocytes. Proc Natl Acad Sci USA. 1982;79:850–854. doi: 10.1073/pnas.79.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho-Chung YS, Nesterova MV. Tumor reversion: Protein kinase A isozyme switching. Ann N Y Acad Sci. 2005;1058:76–86. doi: 10.1196/annals.1359.014. [DOI] [PubMed] [Google Scholar]
- 7.Friedman DL. Role of cyclic nucleotides in cell growth and differentiation. Physiol Rev. 1976;56:652–708. doi: 10.1152/physrev.1976.56.4.652. [DOI] [PubMed] [Google Scholar]
- 8.Furuno N, Kawasaki A, Sagata N. Expression of cell-cycle regulators during Xenopus oogenesis. Gene Expr Patterns. 2003;3:165–168. doi: 10.1016/s1567-133x(03)00037-1. [DOI] [PubMed] [Google Scholar]
- 9.Nakajo N, et al. Absence of Wee1 ensures the meiotic cell cycle in Xenopus oocytes. Genes Dev. 2000;14:328–338. [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Doench JG, Petersen CP, Sharp PA. siRNAs can function as miRNAs. Genes Dev. 2003;17:438–442. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skålhegg BS, Taskén K. Specificity in the cAMP/PKA signaling pathway. differential expression, regulation, and subcellular localization of subunits of PKA. Front Biosci. 1997;2:d331–d342. doi: 10.2741/a195. [DOI] [PubMed] [Google Scholar]
- 13.Faure S, et al. Control of G2/M transition in Xenopus by a member of the p21-activated kinase (PAK) family: A link between protein kinase A and PAK signaling pathways? J Biol Chem. 1999;274:3573–3579. doi: 10.1074/jbc.274.6.3573. [DOI] [PubMed] [Google Scholar]
- 14.Armisen J, Gilchrist MJ, Wilczynska A, Standart N, Miska EA. Abundant and dynamically expressed miRNAs, piRNAs, and other small RNAs in the vertebrate Xenopus tropicalis. Genome Res. 2009;19:1766–1775. doi: 10.1101/gr.093054.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Didiano D, Hobert O. Molecular architecture of a miRNA-regulated 3′ UTR. RNA. 2008;14:1297–1317. doi: 10.1261/rna.1082708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nahvi A, Shoemaker CJ, Green R. An expanded seed sequence definition accounts for full regulation of the hid 3′ UTR by bantam miRNA. RNA. 2009;15:814–822. doi: 10.1261/rna.1565109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi WY, Giraldez AJ, Schier AF. Target protectors reveal dampening and balancing of Nodal agonist and antagonist by miR-430. Science. 2007;318:271–274. doi: 10.1126/science.1147535. [DOI] [PubMed] [Google Scholar]
- 19.Nakahara K, et al. Targets of microRNA regulation in the Drosophila oocyte proteome. Proc Natl Acad Sci USA. 2005;102:12023–12028. doi: 10.1073/pnas.0500053102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motlík J, Kubelka M. Cell-cycle aspects of growth and maturation of mammalian oocytes. Mol Reprod Dev. 1990;27:366–375. doi: 10.1002/mrd.1080270411. [DOI] [PubMed] [Google Scholar]
- 21.Kovo M, et al. An active protein kinase A (PKA) is involved in meiotic arrest of rat growing oocytes. Reproduction. 2006;132:33–43. doi: 10.1530/rep.1.00824. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, et al. A role for the P-body component GW182 in microRNA function. Nat Cell Biol. 2005;7:1261–1266. doi: 10.1038/ncb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma J, et al. MicroRNA activity is suppressed in mouse oocytes. Curr Biol. 2010;20:265–270. doi: 10.1016/j.cub.2009.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Z, et al. GW182 is critical for the stability of GW bodies expressed during the cell cycle and cell proliferation. J Cell Sci. 2004;117:5567–5578. doi: 10.1242/jcs.01477. [DOI] [PubMed] [Google Scholar]
- 25.Till S, et al. A conserved motif in Argonaute-interacting proteins mediates functional interactions through the Argonaute PIWI domain. Nat Struct Mol Biol. 2007;14:897–903. doi: 10.1038/nsmb1302. [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki S, Tomari Y. Argonaute-mediated translational repression (and activation) Fly (Austin) 2009;3:204–206. [PubMed] [Google Scholar]
- 27.Say E, et al. A functional requirement for PAK1 binding to the KH(2) domain of the fragile X protein-related FXR1. Mol Cell. 2010;38:236–249. doi: 10.1016/j.molcel.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Moore MJ, Proudfoot NJ. Pre-mRNA processing reaches back to transcription and ahead to translation. Cell. 2009;136:688–700. doi: 10.1016/j.cell.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Weinmann L, et al. Importin 8 is a gene silencing factor that targets argonaute proteins to distinct mRNAs. Cell. 2009;136:496–507. doi: 10.1016/j.cell.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Lau NC, Ohsumi T, Borowsky M, Kingston RE, Blower MD. Systematic and single cell analysis of Xenopus Piwi-interacting RNAs and Xiwi. EMBO J. 2009;28:2945–2958. doi: 10.1038/emboj.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.