Abstract
Remodeling of cortical connectivity is thought to allow initially hippocampus-dependent memories to be expressed independently of the hippocampus at remote time points. Consistent with this, consolidation of a contextual fear memory is associated with dendritic spine growth in neurons of the anterior cingulate cortex (aCC). To directly test whether such cortical structural remodeling is necessary for memory consolidation, we disrupted spine growth in the aCC at different times following contextual fear conditioning in mice. We took advantage of previous studies showing that the transcription factor myocyte enhancer factor 2 (MEF2) negatively regulates spinogenesis both in vitro and in vivo. We found that increasing MEF2-dependent transcription in the aCC during a critical posttraining window (but not at later time points) blocked both the consolidation-associated dendritic spine growth and subsequent memory expression. Together, these data strengthen the causal link between cortical structural remodeling and memory consolidation and, further, identify MEF2 as a key regulator of these processes.
Keywords: structural plasticity, remote memory, viral vector
In experimental animals, damage to the hippocampus disproportionately impacts recently acquired memories, with relative sparing of remote memories (1–8). Such observations have led to the idea that the hippocampus is transiently required for memory expression, with remote memory expression being exclusively dependent on the cortex (9). According to one model (10), posttraining hippocampal activity coordinates reactivation of memory traces in the cortex. This reactivation leads to the remodeling of cortical connections, allowing the memory to eventually be expressed independently of the hippocampus. A recent study in mice (5) provided correlative evidence for posttraining remodeling of neurons in the anterior cingulate cortex (aCC), a subregion of the prefrontal cortex that plays an essential role in remote memory expression (11). Increases in dendritic spine density on layer 2/3 pyramidal aCC neurons were observed 1 mo, but not 1 d, following contextual fear conditioning (5). As layer 2/3 pyramidal neurons send and receive long-range cortical connections (12), such changes may contribute to increased functional connectivity between the aCC and other cortical areas important for remote memory expression (13, 14). However, whether this increase in aCC spine density is necessary for memory consolidation is not known. To test this, it would be necessary to evaluate whether preventing posttraining spinogenesis, specifically in this region, disrupts memory consolidation.
The transcription factor myocyte enhancer factor 2 (MEF2) negatively regulates spinogenesis in an activity-dependent manner and therefore provides a tool to address this question. For example, increasing MEF2 function decreases the number of dendritic spines and excitatory synapses in vitro (15) and blocks increases in spine density normally observed following repeated cocaine administration in rat medium spiny nucleus accumbens neurons in vivo (16). Accordingly, in our experiments, we used a viral vector-based strategy to increase MEF2 function in the aCC at specific times following contextual fear conditioning. We found that increasing MEF2-dependent transcription in layer 2/3 aCC neurons during the first (but not seventh) posttraining week blocks both consolidation-associated structural changes in layer 2/3 aCC neurons and memory consolidation.
Results
Contextual Fear Memory Consolidation Is Associated with Time-Dependent Spine Growth in the aCC.
We first asked whether consolidation of a memory was accompanied by an increase in spine density in aCC pyramidal neurons. We used a contextual fear paradigm in which mice learn to associate a context with an aversive stimulus, such as a mild footshock (SI Materials and Methods). When placed back in the context, mice exhibit a range of species-typical fear reactions, including freezing [the absence of all movement except breathing (1)]. Contextual fear conditioning offers a number of advantages that make it particularly suitable for studying the role of structural plasticity in the aCC in memory consolidation. First, training occurs in a single session (rather than over several days). Second, this training produces a durable memory [that can last as long as a lifetime (17)] and can be quantified using automated procedures (18). Third, lesions of the hippocampus impair expression of recent, but not remote, contextual fear memories (1, 3, 4, 19). Conversely, inactivation of the aCC impairs the expression of remote, but not recent, contextual fear memories (11). In particular, these studies provide direct evidence that the circuits supporting these memories reorganize over time, and that remote memory expression depends, in part, on the aCC (9).
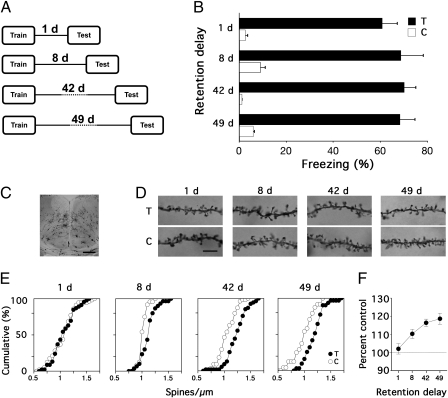
Mice were trained in contextual fear conditioning and tested either 1, 8, 42, or 49 d later (Fig. 1A). Freezing was equivalent at all retention delays, indicating that this training protocol produces robust, durable levels of conditioned fear (Fig. 1B). Consistent with our previous report (5), we observed a time-dependent increase in spine density on apical dendrites of layer 2/3 aCC neurons (Fig. 1 C–F). This increase was limited to layer 2/3 pyramidal neurons [which have long-range cortical connections (12)], as similar changes were not observed in layer 5 aCC neurons [which do not have long-range cortical connections (12)] (Fig. S1). Furthermore, increases in spine density of layer 2/3 aCC pyramidal neurons were most pronounced during the first posttraining week (i.e., day 1–8; P < 0.05, by Fisher's protected least significant difference test) and plateaued thereafter (i.e., days 42–49; P > 0.05) (Fig. 1F). Because memory reactivation is thought to drive structural changes underlying reorganization of cortical networks (20–22), more pronounced increases in spine density shortly following training are consistent with the idea that reactivation strength and frequency decline over time (23). In our previous study, we showed that such changes in spine density are independent of memory recall (5). Therefore, together these data indicate that consolidation of a contextual fear memory is associated with posttraining remodeling of aCC layer 2/3 neurons, and the time course of these structural changes parallels the emergent role of the aCC in the expression of contextual fear memories (11).
Fig. 1.
Time-dependent increases in dendritic spine density in aCC pyramidal neurons following contextual fear conditioning. (A) Experimental design. (B) Conditioned freezing in trained (T) mice was similar at all retention delays, and always greater than in control (C) mice (P < 0.001, by ANOVA). (C) Photomicrograph showing examples of Golgi-impregnated pyramidal neurons in the aCC. (Scale bar, 500 μm.) (D) Golgi-impregnated apical dendritic segments in the aCC in trained and control mice at different retention delays. (Scale bar, 5 μm.) (E) Cumulative frequency of spine density on apical dendrites in layer 2/3 aCC pyramidal neurons. Spine density was greater in trained (closed circles) versus control (open circles) mice 8, 42, and 49 d following training (P < 0.05, by Kolmogorov–Smirnov test). (F) Spine density increased with retention delay in layer 2/3 aCC pyramidal neurons [P < 0.001, by ANOVA (significant delay effect); spine density in trained mice was normalized with respect to controls]. These and subsequent graphs show means ± SEM.
Using a Viral Vector to Acutely Increase MEF2 Function in the aCC.
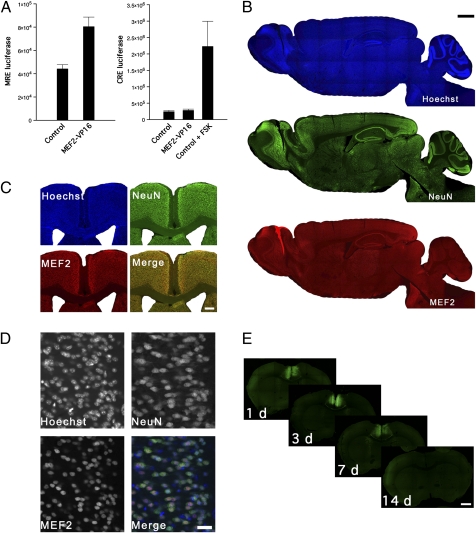
To increase MEF2-dependent transcription, we expressed MEF2-VP16, a version of MEF2 in which the DNA binding and dimerization domains are fused to the transcriptional activation domain of the viral protein VP16 (15). MEF2-VP16 binds MEF2 sites within the promoter region of target genes and leads to their constitutive transcription (15). Accordingly, we verified that this MEF2-VP16 construct specifically increases MEF2-recognition element (MRE)-dependent [but not cAMP-response element (CRE)-dependent] transcription in a luciferase reporter assay in cultured cells (Fig. 2A).
Fig. 2.
Use of a viral vector to acutely increase MEF2 function in the aCC at specific times after training. (A) MEF2-VP16 robustly and selectively increases MEF2-dependent transcription in cultured cells. Cells were cotransfected with (Left) MRE-luciferase or (Right) CRE-luciferase reporter plasmids and a control (GFP) or MEF2-VP16 plasmid. MEF2-VP16 selectively increased MRE- (P < 0.01, by t test) but not CRE-dependent transcription. Importantly, control data show that forskolin (FSK) increases CRE-mediated transcription (P < 0.001, by t test). Data are expressed as mean ± SEM. (B) Sagittal sections from the adult mouse brain stained for a nuclear marker (Hoechst; Top), a neuron-specific marker (NeuN; Middle), and MEF2 (Bottom), indicating that endogenous MEF2 proteins are highly expressed in neurons throughout the adult brain. (Scale bar, 1 mm.) (C) Coronal sections stained for Hoechst, NeuN, and MEF2 in the aCC. (Scale bar, 200 μm.) (D) High magnification of layer 2/3 pyramidal neurons in the aCC showing that endogenous MEF2 is expressed only in NeuN+ cells. (Scale bar, 50 μm.) (E) Time course of transgene expression following microinfusion of MEF2 vector into the aCC. Robust, bilateral transgene expression was observed in layer 2/3 pyramidal neurons in the aCC up to 1 wk postmicroinfusion, but not thereafter (see also Fig. S2 for quantification of data). (Scale bar, 1 mm.)
To ensure that MEF2 is endogenously expressed in the aCC, we next characterized the expression of MEF2 proteins in the adult mouse brain using an antibody that recognizes the major MEF2 isoforms expressed in the brain (MEF2A, MEF2C, and MEF2D). Consistent with previous data (24), we confirmed that MEF2 is highly expressed in the brain (Fig. 2B), including the aCC (Fig. 2C). Furthermore, MEF2 was colocalized with the neuronal marker NeuN (Fig. 2D), indicating that MEF2 expression is restricted to neurons.
To increase MEF2-dependent transcription only in the aCC at specific times after training, we used a replication-defective HSV vector-based approach (25). We chose HSV because, unlike other vectors, HSV is neurotropic (25) and MEF2 is expressed exclusively in neurons. To visualize infected cells, our HSV coexpressed GFP. Microinfusion of the MEF2 (HSV-MEF2-VP16-GFP) or control (HSV-GFP) vector led to robust transgene expression in ∼5–7% of layer 2/3 pyramidal neurons for up to 1 wk, declining thereafter (Fig. 2E and Fig. S2). Furthermore, up-regulation of MEF2-dependent transcription was not associated with a secondary inflammatory response (e.g., activated microglia; Fig. S3).
Increasing MEF2 Function in the aCC Disrupts Memory Consolidation in a Time-Dependent Manner.
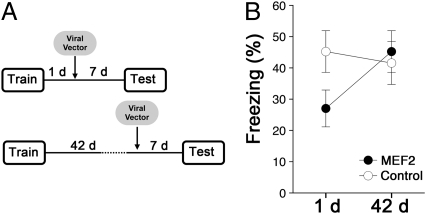
As spine density on apical dendrites of layer 2/3 aCC pyramidal neurons increased during the first, but not the seventh, week following contextual fear conditioning (Fig. 1F), we hypothesized that limiting spine growth shortly following training (but not at later time points) would disrupt memory consolidation. To test this, we trained mice in contextual fear conditioning and microinfused the MEF2 or control vector into the aCC either 1 or 42 d later. Seven days following microinfusion, contextual fear memory was assessed by placing mice back in the context and measuring freezing behavior (Fig. 3A). Only mice with robust, bilateral transgene expression in aCC layer 2/3 pyramidal neurons were included in analyses (for exclusion criteria, see Fig. S4 and SI Materials and Methods). We found that increasing MEF2-dependent transcription in the aCC during the first posttraining week reduced levels of contextual fear. In contrast, increasing MEF2-dependent transcription in the aCC during the seventh posttraining week had no effect on subsequent memory expression. The differential effect of increasing MEF2 function at recent versus remote time points was confirmed by a significant group × delay interaction [F(1,33) = 5.59; P < 0.05] (Fig. 3B). Importantly, spared conditioned freezing in the remote group indicates that increasing MEF2-dependent transcription in the aCC does not impact motor function (e.g., affecting the ability to freeze) or emotion (e.g., changes in basal levels of anxiety) that might nonspecifically reduce conditioned fear. In addition, mice microinfused with the MEF2 vector 1 d after training exhibited reduced freezing when tested 48 d later (Fig. S5), indicating that the memory deficits induced by increasing MEF2-dependent transcription were persistent and did not recover over time. Together, these results are consistent with the idea that increasing MEF2-dependent transcription in the aCC during a critical posttraining window inhibits the spinogenesis that is required for memory consolidation.
Fig. 3.
Increasing MEF2 function in the aCC following training disrupts consolidation of a contextual fear memory in a time-dependent manner. (A) Experimental design. (B) Reduced freezing levels following MEF2 vector microinfusion 1 d, but not 42 d, after training [P < 0.05, by ANOVA (significant group × delay interaction)].
Increasing MEF2 Function in the aCC Disrupts Spine Growth That Accompanies Memory Consolidation in a Time-Dependent Manner.
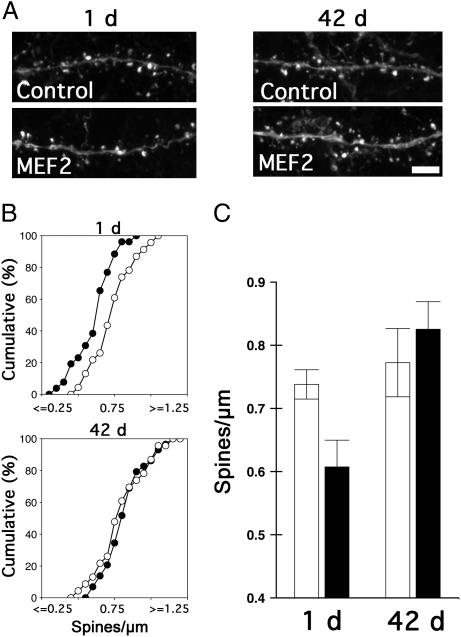
To evaluate whether increasing MEF2-dependent transcription interfered with training-induced spine growth during this critical, early posttraining window, we next quantified spine density in infected aCC pyramidal neurons in mice in the recent and remote groups (Fig. 4 A–C). In the recent group, spine density was reduced in MEF2-infected neurons (relative to the control infected neurons), indicating that increasing MEF2-dependent transcription in aCC neurons during the first posttraining week blocks both memory consolidation and associated increases in spine density. In contrast, spine density was not altered in MEF2-infected neurons in either the remote group (presumably when reactivation levels have declined significantly) or in home-cage control mice (Fig. S6). This indicates that increasing MEF2-dependent transcription does not simply suppress or eliminate spines under basal conditions (15, 16). The dissociable effects of increasing MEF2 function on spine density support the idea that MEF2 regulates reactivation-driven changes in spine density within a defined posttraining window.
Fig. 4.
Increasing MEF2 function in the aCC following training disrupts consolidation-associated changes in spine density in a time-dependent manner. (A) Examples of apical dendritic segments from control and MEF2-infected neurons at different retention delays. (Scale bar, 5 μm.) (B and C) Cumulative frequency plots (B) and group data (C) showing spine density in aCC neurons infected with MEF2 or control vectors either 1 or 42 d following conditioning. Spine density was reduced in MEF2-infected neurons 1 d, but not 42 d, after training (P < 0.01, by Kolmogorov–Smirnov test).
Discussion
With time, the importance of the hippocampus in memory expression diminishes as a more permanent trace is established in the cortex. This time-dependent reorganization process is thought to depend on the remodeling of cortical connections allowing the memory to be expressed independently of the hippocampus. Here we tested whether inhibiting spine growth specifically in the aCC at different times following training disrupted memory consolidation. To inhibit spine growth, we used MEF2, a transcription factor that has been shown to negatively regulate spine growth both in vitro and in vivo (15, 16). We found that increasing MEF2-dependent transcription in aCC neurons immediately following training (but not thereafter) blocked both memory consolidation and associated increases in spine density. Together, these data strengthen the causal link between structural remodeling in the aCC and memory consolidation and, further, identify MEF2 as a key regulator of the molecular machinery mediating these changes.
Our finding that increasing MEF2-dependent transcription inhibits spine growth normally associated with consolidation of a fear memory is consistent with reports showing that several MEF2 target genes encode proteins that weaken excitatory synaptic transmission. For instance, genome-wide analysis of developing neurons (26) identified MEF2 target genes that (i) decrease surface AMPAR expression [arc, JNK, SynGAP (27–31)], (ii) uncouple mGluR5 from its effector targets in the postsynaptic density and reduce the amplitude of AMPAR- and NMDAR-mediated excitatory postsynaptic currents [Homer1a (32–34)], (iii) internalize N-cadherin, a synaptic adhesion molecule implicated in synaptic transmission [PCDH (protocadherin) family members, including protocadherins 9, 10, and 17 (35)], and (iv) decrease neuronal excitability [voltage-gated K+ channels (including kcna1 and kcna4) (36–38)]. Therefore, increasing MEF2-dependent transcription may orchestrate a coordinated increase in expression of genes that suppress excitatory synapse function.
In our experiments, increasing MEF2 function just in the aCC was sufficient to interfere with the formation of enduring contextual fear memories. Previous studies suggest that the aCC plays an increasingly important role in the expression of contextual fear (as well as other, initially hippocampus-dependent) memories over time (6, 9, 39–41). For example, the aCC is activated following remote, but not recent, contextual fear memory recall (11). Also, inactivating the aCC disrupts the expression of remote, but not recent, contextual fear memory (11). Although structural remodeling likely occurs in other cortical regions, these data suggest that the aCC is an essential node within a broader network supporting expression of remote memory. Such time-dependent changes in dendritic spine density in aCC neurons may reflect consolidation-associated modification of functional connections between the aCC and other cortical (13, 14) and subcortical [e.g., amygdala (42)] regions.
Memory reactivation is thought to drive structural changes necessary for the consolidation of memories in cortical networks (20–22). Consistent with this model, interfering with memory reactivation disrupts memory consolidation (20, 21). Similarly, here we have shown that interfering with spine growth in the week following training (but not at a later time point) disrupted memory consolidation. The time-limited nature of these effects is consistent with observations that (i) the most pronounced increases in spine density occur during the first posttraining week, and (ii) memory reactivation strength and frequency decline over time (23).
Materials and Methods
Quantification of Spine Density in aCC Pyramidal Neurons Following Contextual Fear Conditioning.
Mice were trained in contextual fear conditioning and then tested 1 (trained, n = 6; control, n = 6), 8 (trained, n = 5; control, n = 4), 42 (trained, n = 11; control, n = 11), or 49 (trained, n = 6; control, n = 5) d later (for a detailed description of apparatus and behavioral procedures, see SI Materials and Methods). Following testing, their brains were removed and prepared for Golgi-Cox staining. Golgi-impregnated neurons in layer 2/3 and layer 5 of the aCC were first identified using a light microscope. For each neuron, spine density was quantified along five randomly selected segments on secondary and tertiary branches of apical dendrites. Segments were at least 20 μm in length. Only protuberances with a clear connection of the head of the spine to the shaft of the dendrite were counted as spines (see SI Materials and Methods for further details).
Immunohistochemistry.
To characterize endogenous MEF2 expression in the adult mouse brain, naïve mice were perfused transcardially with PBS and paraformaldehyde. Sections were cut and then incubated with primary antibodies against MEF2 and neuron-specific nuclear protein (NeuN). Alexa-488 goat anti-mouse and Alexa-568 goat anti-rabbit were used as secondary antibodies (for detailed procedures, see SI Materials and Methods).
Luciferase Reporter Assays.
To verify that our MEF2-VP16 construct specifically increases MRE-dependent (but not CRE-dependent) transcription, we used a luciferase reporter assay in Neuro2A cells. Cells were transiently transfected with either MEF2-VP16 or control (GFP) constructs and an MRE- or CRE-luciferase reporter plasmid. Luciferase expression was assessed 48 h later (for detailed procedures, see SI Materials and Methods).
HSV Vectors.
To locally increase MEF2 function in the aCC at different times following fear conditioning, we used replication-defective HSV. The MEF2-VP16 construct was subcloned into an HSV amplicon backbone (pHSV-prPUC). Transgene expression was regulated by the constitutive promoter for the HSV immediate-early gene IE 4/5. This vector also expressed GFP to allow infected neurons to be visualized. A control vector similarly expressed GFP alone. Microinfusion of the MEF2 (HSV-MEF2-VP16-GFP) or control (HSV-GFP) vector produced robust transgene expression in layer 2/3 aCC pyramidal neurons (for more details, see SI Materials and Methods).
Examination of the Effects of Increasing MEF2 Function on Consolidation of a Contextual Fear Memory.
Mice were trained in contextual fear conditioning. Either 1 or 42 d later, mice were microinfused with the control (1 d, n = 11; 42 d, n = 14) or MEF2 (1 d, n = 11; 42 d, n = 17) vector into the aCC (for detailed behavioral and surgical procedures, see SI Materials and Methods). One week later, mice were placed back in the context and freezing was measured. Following testing, brains were removed and spine density of infected neurons was quantified in layer 2/3 aCC neurons using native GFP fluorescence. For each neuron, spine density was quantified along five randomly selected segments on secondary and tertiary branches of apical dendrites (segments > 20 μm in length) (for detailed procedures, see SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank Kaori Takehara-Nishiuchi for comments on this manuscript and Rachael Neve for providing HSV constructs. This work was supported by grants from the Canadian Institutes of Health Research (MOP-77561 and MOP-74650 to P.W.F. and S.A.J., respectively). G.V. received support from the University of Tor Vergata, Rome. L.R. and C.J.C. received support from The Hospital for Sick Children, and P.J.R. received support from the Ontario Ministry of Research and Innovation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016275108/-/DCSupplemental.
References
- 1.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 2.Clark RE, Broadbent NJ, Zola SM, Squire LR. Anterograde amnesia and temporally graded retrograde amnesia for a nonspatial memory task after lesions of hippocampus and subiculum. J Neurosci. 2002;22:4663–4669. doi: 10.1523/JNEUROSCI.22-11-04663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: Within-subjects examination. J Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 5.Restivo L, Vetere G, Bontempi B, Ammassari-Teule M. The formation of recent and remote memory is associated with time-dependent formation of dendritic spines in the hippocampus and anterior cingulate cortex. J Neurosci. 2009;29:8206–8214. doi: 10.1523/JNEUROSCI.0966-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- 7.Takehara K, Kawahara S, Kirino Y. Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci. 2003;23:9897–9905. doi: 10.1523/JNEUROSCI.23-30-09897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- 9.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 10.Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: A neurobiological perspective. Curr Opin Neurobiol. 1995;5:169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 11.Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- 12.González-Burgos G, Barrionuevo G, Lewis DA. Horizontal synaptic connections in monkey prefrontal cortex: An in vitro electrophysiological study. Cereb Cortex. 2000;10:82–92. doi: 10.1093/cercor/10.1.82. [DOI] [PubMed] [Google Scholar]
- 13.Takashima A, et al. Shift from hippocampal to neocortical centered retrieval network with consolidation. J Neurosci. 2009;29:10087–10093. doi: 10.1523/JNEUROSCI.0799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sacco T, Sacchetti B. Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science. 2010;329:649–656. doi: 10.1126/science.1183165. [DOI] [PubMed] [Google Scholar]
- 15.Flavell SW, et al. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- 16.Pulipparacharuvil S, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gale GD, et al. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anagnostaras SG, Josselyn SA, Frankland PW, Silva AJ. Computer-assisted behavioral assessment of Pavlovian fear conditioning in mice. Learn Mem. 2000;7:58–72. doi: 10.1101/lm.7.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 20.Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 22.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 23.Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: Effects of behavioral state, experience, and EEG dynamics. J Neurosci. 1999;19:4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neely MD, et al. Localization of myocyte enhancer factor 2 in the rodent forebrain: Regionally-specific cytoplasmic expression of MEF2A. Brain Res. 2009;1274:55–65. doi: 10.1016/j.brainres.2009.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fink DJ, DeLuca NA, Goins WF, Glorioso JC. Gene transfer to neurons using herpes simplex virus-based vectors. Annu Rev Neurosci. 1996;19:265–287. doi: 10.1146/annurev.ne.19.030196.001405. [DOI] [PubMed] [Google Scholar]
- 26.Flavell SW, et al. Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron. 2008;60:1022–1038. doi: 10.1016/j.neuron.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shepherd JD, et al. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chowdhury S, et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rumbaugh G, Adams JP, Kim JH, Huganir RL. SynGAP regulates synaptic strength and mitogen-activated protein kinases in cultured neurons. Proc Natl Acad Sci USA. 2006;103:4344–4351. doi: 10.1073/pnas.0600084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas GM, Lin DT, Nuriya M, Huganir RL. Rapid and bi-directional regulation of AMPA receptor phosphorylation and trafficking by JNK. EMBO J. 2008;27:361–372. doi: 10.1038/sj.emboj.7601969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu Y, et al. Rap2-JNK removes synaptic AMPA receptors during depotentiation. Neuron. 2005;46:905–916. doi: 10.1016/j.neuron.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 32.Sala C, et al. Inhibition of dendritic spine morphogenesis and synaptic transmission by activity-inducible protein Homer1a. J Neurosci. 2003;23:6327–6337. doi: 10.1523/JNEUROSCI.23-15-06327.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tu JC, et al. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 34.Xiao B, Tu JC, Worley PF. Homer: A link between neural activity and glutamate receptor function. Curr Opin Neurobiol. 2000;10:370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 35.Yasuda S, et al. Activity-induced protocadherin arcadlin regulates dendritic spine number by triggering N-cadherin endocytosis via TAO2β and p38 MAP kinases. Neuron. 2007;56:456–471. doi: 10.1016/j.neuron.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brew HM, et al. Seizures and reduced life span in mice lacking the potassium channel subunit Kv1.2, but hypoexcitability and enlarged Kv1 currents in auditory neurons. J Neurophysiol. 2007;98:1501–1525. doi: 10.1152/jn.00640.2006. [DOI] [PubMed] [Google Scholar]
- 37.Kopp-Scheinpflug C, Fuchs K, Lippe WR, Tempel BL, Rübsamen R. Decreased temporal precision of auditory signaling in Kcna1-null mice: An electrophysiological study in vivo. J Neurosci. 2003;23:9199–9207. doi: 10.1523/JNEUROSCI.23-27-09199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luján R, et al. Immunohistochemical localization of the voltage-gated potassium channel subunit Kv1.4 in the central nervous system of the adult rat. J Chem Neuroanat. 2003;26:209–224. doi: 10.1016/j.jchemneu.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Miller CA, et al. Cortical DNA methylation maintains remote memory. Nat Neurosci. 2010;13:664–666. doi: 10.1038/nn.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teixeira CM, Pomedli SR, Maei HR, Kee N, Frankland PW. Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J Neurosci. 2006;26:7555–7564. doi: 10.1523/JNEUROSCI.1068-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding HK, Teixeira CM, Frankland PW. Inactivation of the anterior cingulate cortex blocks expression of remote, but not recent, conditioned taste aversion memory. Learn Mem. 2008;15:290–293. doi: 10.1101/lm.905008. [DOI] [PubMed] [Google Scholar]
- 42.Malin EL, Ibrahim DY, Tu JW, McGaugh JL. Involvement of the rostral anterior cingulate cortex in consolidation of inhibitory avoidance memory: Interaction with the basolateral amygdala. Neurobiol Learn Mem. 2007;87:295–302. doi: 10.1016/j.nlm.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.