Abstract
Protease-activated receptor-2 (PAR2), a cell surface receptor for trypsin-like proteases, plays a key role in a number of acute and chronic inflammatory diseases of the joints, lungs, brain, gastrointestinal tract, and vascular systems. Despite considerable effort by the pharmaceutical industry, PAR2 has proven recalcitrant to targeting by small molecule inhibitors, which have been unable to effectively prevent the interaction of the protease-generated tethered ligand with the body of the receptor. Here, we report the development of first-in-class cell-penetrating lipopeptide “pepducin” antagonists of PAR2. The design of the third intracellular (i3) loop pepducins were based on a structural model of a PAR2 dimer and by mutating key pharmacophores in the receptor intracellular loops and analogous pepducins. Individual pharmacophores were identified, which controlled constitutive, agonist, and antagonist activities. This approach culminated in the identification of the P2pal-18S pepducin which completely suppressed trypsin and mast cell tryptase signaling through PAR2 in neutrophils and colon cancer cells. The PAR2 pepducin was highly efficacious in blocking PAR2-dependent inflammatory responses in mouse models. These effects were lost in PAR2-deficient and mast-cell–deficient mice, thereby validating the specificity of the pepducin in vivo. These data provide proof of concept that PAR2 pepducin antagonists may afford effective treatments of potentially debilitating inflammatory diseases and serve as a blueprint for developing highly potent and specific i3-loop–based pepducins for other G protein-coupled receptors (GPCRs).
Keywords: protease-activated receptor-1, protease-activated receptor-4
Protease-activated receptor-2 (PAR2) mediates a number of (patho)physiological pathways involved in acute and chronic inflammation, arthritis, allergic reactions, sepsis, inflammatory pain, as well as cancer cell invasion and metastasis (1–6). PAR2 is a member of the G protein-coupled protease-activated receptor subfamily, which includes the related PAR1, PAR3, and PAR4. Proteases such as trypsin (7), thrombin, and MMP-1 (8, 9) cleave the N-terminal extracellular domain of individual PAR members, thereby unmasking a tethered ligand that binds to the outer surface of the receptor to activate transmembrane signaling to intracellular G proteins. The pleiotropic downstream pathways activated by PAR2 include calcium mobilization, phospholipase C-β–dependent production of inositol phosphates and diacylglycerol, Rho and Rac activation, MAPK signaling, and gene transcription (10). PAR2 itself is strongly up-regulated by diverse inflammatory mediators such as TNF-α, IL-1α, bacterial endotoxin, and autoimmunity-inducing antigens produced during the tissue response to inflammation (11).
As a cell surface sensor of proteases, PAR2 endows the cell with the ability to respond or overrespond to the rapidly changing proteolytic microenvironment that occurs during inflammation. PAR2 is widely expressed in inflammatory cells, stroma, endothelium, and intestinal epithelium (10). PAR2-deficient mice exhibit reduced granulocytic infiltration and tissue damage and suppression of inflammatory cytokines in models of intestinal inflammation, autoimmunity, and encephalomyelitis (11, 12). Reduced cardiac ischemia/reperfusion injury was also observed in PAR2-deficient mice, which correlated with a decline in inflammatory mediators (13). Conversely, overstimulation of PAR2 can lead to severe edema, granulocyte infiltration, increased tissue permeability, tissue damage, and hypotension (1, 12). Agonists of PAR2, including trypsin and the synthetic SLIGRL peptide, also trigger the release of calcitonin and substance P from sensory neurons causing neutrophil infiltration, edema, hyperalgesia, and cancer pain (3, 14). PAR2 has been linked to arthritis as evidenced by significant decreases in joint inflammation in PAR2-deficient mice (15) and up-regulated expression of the receptor in osteoarthritis and rheumatoid arthritis synovial tissues (16).
Tryptase, a major proinflammatory serine protease, can also cleave and activate PAR2 (17). Local or systemic release of high levels of mast cell-derived tryptase can have life-threatening consequences including acute asthma, systemic mastocytosis, and anaphylaxis (18). A specific and effective pharmacological inhibitor of PAR2 therefore has the potential to provide beneficial anti-inflammatory effects and reduce the detrimental activity of mast cells, neutrophils, and other PAR2-expressing leukocytes that contribute to tissue damage. To date, it has been challenging to identify an effective PAR2 antagonist that lacks agonist activity or is efficacious at submillimolar levels (4, 19, 20).
We have developed a unique way to inhibit G protein-coupled receptors on the inside surface of the lipid bilayer with cell-penetrating pepducins. Pepducins are derived from the intracellular loops of their target receptor and comprise a lipid tether conjugated to the peptidic portion of the loop (20). These lipidated peptides have the ability to rapidly flip across the membrane and interfere with receptor-G protein signaling in a highly specific manner (20–24). Here, we report the rational design and development of a potent and specific PAR2 antagonist pepducin based on the third intracellular loop, which effectively inhibits protease-PAR2 signaling induced by trypsin and tryptase. In mouse models of acute inflammation, we demonstrate that the PAR2 pepducin provides salutary effects in reducing inflammation and edema, which are lost in PAR2-deficient mice. These data indicate that targeting PAR2 may prove to be beneficial in inflammatory conditions and other diseases that involve trypsin, tryptase, and other protease agonists of PAR2.
Results
Identification of Critical Pharmacophores in the Intracellular i3 Loop of PAR2.
As has been shown with many GPCRs, there is considerable evidence that PARs can interact with each other and form homo- and heterodimers, and pepducins are postulated to mimic these dimeric interactions on the intracellular surface of the lipid bilayer (25). To initiate the design of PAR2 pepducin antagonists, we constructed a molecular model of a PAR2 dimer. The model of the PAR2 monomer was based on the structure of rhodopsin (26), which shares 45% identity with PAR2. Residues 51–397 of PAR2 were substituted for residues 1–346 of bovine rhodopsin. Stereochemically reasonable positions were used for side chains that were later subjected to molecular dynamics and energy minimization. A series of twofold symmetric PAR2 dimer models were then manually constructed with the aim of maximizing the surface area between the adjacent receptors. Among these, we selected the model that was most consistent with available data regarding interactions between the receptor pair and the G protein (SI Materials and Methods).
To provide functional evidence that one PAR2 receptor can interact with an adjacent PAR2 receptor, we constructed a signaling dead PAR2-RQ mutant by transposing residues at Q172 and R173 located in the critical “DRY” TM3 motif (Fig. 1A). The PAR2-RQ mutant has an intact protease cleavage site and tethered ligand but cannot signal to G proteins. A noncleavable PAR2-R36A mutant was also constructed, which retains the ability to fully signal to SLIGRL ligand, but is not able to be proteolyzed and directly activated by trypsin (Fig. S1 A–C). Cells expressing PAR2-R36A or PAR2-RQ alone were unable to migrate toward gradients of trypsin or to activate Gq-PLC-β signaling. However, when the two mutant receptors were cotransfected, chemotactic migration and signaling were restored (Fig. 1B and Fig. S1 B and D), consistent with a mechanism whereby PAR2-RQ can donate its tethered ligand to transactivate PAR2-R36A. To provide direct evidence that PAR2 can form homodimers or oligomers, we showed that myc-tagged PAR2 can stably associate with T7-tagged PAR2 by coimmunoprecipitation (Fig. S2). These data indicate that PAR2 has the ability to associate with itself within a homodimer or oligomeric complex.
Fig. 1.
Constitutive and ligand-dependent activity of PAR2 is regulated by juxtamembrane residues located in the third intracellular (i3) loop. (A) Location of PAR2 mutants and model of the receptor dimer used in this study. The PAR2 model depicting the i3 loop–eighth helix dimer interface was constructed using X-ray structures of rhodopsin as described in Materials and Methods. (B) Migration of HEK cells transiently transfected with PAR2 (wild type, WT), PAR2-R36A, PAR2-RQ, or PAR2-R36A plus PAR2-RQ toward chemotactic gradients of 30 nM trypsin or DMEM media alone for 18 h in a transwell apparatus (8-μm pore), n = 2, repeated three independent times. (C–E) Constitutive and SLIGRL-activated signaling of PAR2 mutants to PLC-β. PLC-β activity was measured by [3H]-InsP formation over 30 min (39) and was typically stimulated four- to fivefold above basal with 30–100 μM SLIGRL. Mean PAR2 mutant surface expression was assessed by FACS and was 1.0 ± 0.3-fold relative to wild type in COS7 cells and 1.00 ± 0.18 in HEK cells (Fig. S1D). Data (n = 2–8) represent the mean ± SD. *P < 0.05 and #P = 0.07.
Interestingly, we also found that wild-type (WT) PAR2 has constitutive activity in the absence of ligand (Fig. 1C). Constitutive signaling has been observed in GPCRs and is often dependent on critical residues located in the C-terminal juxtamembrane region of the i3 loop (27). The PAR2 homodimer model shown in Fig. 1A, predicts that the juxtamembrane i3 loop residues M274, R284, and K287 could potentially interact across the PAR2 dimer interface with the eighth helix region from an adjacent i4 domain. Mutation of M274 to alanine ablated the constitutive signal, whereas mutation of K287 to alanine had no effect (Fig. 1C). Strikingly, mutation of K287 to phenylalanine gave a twofold increase in the constitutive signal. Despite losing its constitutive activity, M274A was able to fully signal to agonist (Fig. 1D). Likewise, K287A and K287F were also able to fully signal to agonist (Fig. 1E). Conversely, the R284S mutant exhibited a loss of constitutive signal and was unable to be activated by even high concentrations of peptide ligand. Similarly, the PAR2ΔH8 mutant, which lacks the eighth helix in the i4 domain, was completely signaling dead to ligand (Fig. 1D). Together, these data indicate that the juxtamembrane residues of the i3 loop of PAR2 play critical roles in both constitutive and ligand-dependent activity.
Molecular Engineering of PAR2 Antagonist Pepducins.
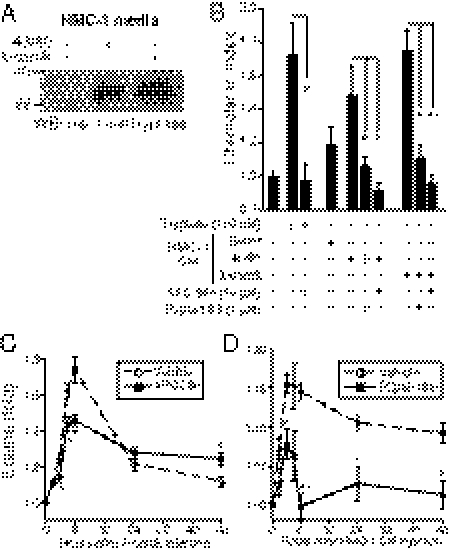
Having identified residues important for the signaling of PAR2, we next synthesized a series of i3 loop-based pepducins with mutations of the critical M274, R284, or K287 pharmacophores (Fig. 2 A and B). An initial screen of PAR2 pepducin agonist and antagonist activity was performed using the PAR2-expressing human colorectal adenocarcinoma cell line SW620. The wild-type full-length i3 loop pepducin, P2pal-21, gave a weak agonist signal and lacked antagonist activity against SLIGRL as assessed by calcium flux (Fig. 2 A and C). Consistent with the gain-of-constitutive activity observed in the PAR2-K287F mutant, the analogous P2pal-21F pepducin (20) gave full agonist activity but no antagonist activity in the SW620 cells (Fig. 2 A and C). Deletion of the first three residues to make P2pal-18F gave a slight decrease in agonist activity, but still was devoid of antagonist activity.
Fig. 2.
Design and screening of agonist and antagonist PAR2 i3-loop pepducins. (A) Agonist and antagonist activity of third intracellular (i3) loop PAR2 pepducins using calcium flux assays with SW620 colon adenocarcinoma cells that endogenously express PAR2. Each column of three calcium flux traces corresponds to the i3 pepducin sequence shown above. The Top row is the agonist activity of 3–4 μM pepducin and the Middle row is the agonist activity of 14–15 μM pepducin. The Bottom row depicts the calcium signal of 100 μM SLIGRL (open arrowheads) following 1-min pretreatment with 6 μM pepducin (closed arrowheads). Final concentration of DMSO vehicle was 0.2%. (B) Model of the WT PAR2 i3 pepducin P2pal-21 bound to the intracellular surface of PAR2. The location of the i3 pepducin was derived by substituting the coordinates of the i3 loop on the intact receptor with the i3 pepducin using the PAR2 dimer model of Fig. 1A. Key pharmacophores M274 (brown), R284 and K287 (yellow), and palmitate (green) are shown. (C) Agonist activity for each pepducin from A is reported as initial velocity of calcium flux at 3–4 μM pepducin (†), or at 14–15 μM pepducin (‡). Antagonist activity of 6 μM pepducin against 100 μM SLIGRL was measured by area under the curve of calcium flux from the Bottom row of A. Experiments were repeated at least two to three times each and gave highly similar results.
The P2pal-18S pepducin, which replaces the critical R284 pharmacophore with serine, was a full antagonist of PAR2 and had no detectable agonist activity in the calcium flux assay (Fig. 2 A and C). Substitution of the C-terminal K287 of P2pal-18S with phenylalanine to make P2pal-18SF restored agonist activity. The replacement of R284 with glutamine to make P2pal-18Q had nearly no agonist activity but had partial antagonist activity. The N-terminally truncated P2pal-14GF had no agonist nor antagonist activity; however, substitution of R284 with glutamine to create P2pal-14GQ gained 53% antagonist activity (Fig. 2 A and C). From this initial calcium flux screen, we chose to further analyze the properties of the P2pal-18S antagonist pepducin for its ability to block other PAR2 functions.
P2pal-18S Is a Specific Antagonist of PAR2 Activity in Neutrophils.
We first tested the ability of P2pal-18S to antagonize PAR2-dependent activity of human neutrophils. Neutrophils were isolated from the peripheral blood of healthy volunteers and found to express high levels of surface PAR2 and PAR4 and lower apparent levels of PAR1 by flow cytometry (Fig. 3A). Neutrophils robustly migrated toward gradients of the PAR2 agonists trypsin and SLIGRL, which was completely blocked by P2pal-18S with IC50 values of 0.14–0.2 μM (Fig. 3B). Likewise, 0.3 μM P2pal-18S completely blocked chemotactic migration of human neutrophils to 100 nM tryptase (Fig. 3C). P2pal-18S also completely inhibited migration of mouse neutrophils toward 30 nM trypsin (Fig. 3D). This cross-species inhibition was predicted as human PAR2 shares 85% identity with mouse PAR2, and the mouse i3 loop retains all of the critical pharmacophores identified in the human PAR2 i3 loop.
Fig. 3.
The P2pal-18S pepducin is a full antagonist of PAR2-dependent neutrophil chemotaxis. (A) Human neutrophils (n = 4 normal volunteers) were analyzed for surface expression of human PAR1, PAR2, and PAR4 by flow cytometry with PAR-specific antibodies. (B) P2pal-18S inhibits human neutrophil chemotaxis to gradients of trypsin (30 nM) and SLIGRL (10 μM) with IC50 values of 0.14–0.2 μM. Chemotaxis index is the ratio of directed versus random migration over 30 min through a 5-μm pore filter. (C) P2pal-18S completely inhibits human neutrophil chemotaxis to 100 nM tryptase. (D) P2pal-18S completely inhibits mouse neutrophil (n = 6) chemotaxis to 30 nM trypsin. (E) P2pal-18S does not affect human neutrophil chemotaxis to gradients of 100 nM IL-8 (CXCR1/CXCR2), 10 μM TFLLRN (PAR1), or 100 μM AYPGKF (PAR4). n = 4–6, mean ± SEM. *P < 0.05.
Specificity of P2pal-18S for PAR2 was evident as it had no antagonist activity to the closely related PAR1, PAR4, or CXCR1/2 IL-8 receptors in neutrophil chemotaxis assays (Fig. 3E). P2pal-18S did not inhibit PAR1 or PAR4 by calcium flux assays in SW620 cells or in inositol phosphate signaling in COS7 cells, despite providing effective inhibition to the PAR2 ligand SLIGRL (Fig. S3 A and B). The P2pal-14GQ pepducin was also selective for PAR2 and not PAR1, but was not as efficacious in suppressing migration of SW620 cells to SLIGRL compared with P2pal-18S (Fig. S3 A–C). P2pal-18S had no effect on PAR1-dependent platelet aggregation (Fig. S4A). Neither P2pal-18S nor P2pal-14GQ caused membrane disruption or apoptosis as assessed by propidium iodide uptake in SW620 cells with up to 30 μM concentrations of pepducin (Fig. S4B).
We also found that P2pal-18S blocked transactivation of PAR2 homodimers as shown by complete suppression of chemotactic migration of HEK cells coexpressing PAR2-R36A and PAR2-RQ mutants (Fig. S3D). Furthermore, to provide evidence that the P2pal-18S peptide directly interacts with PAR2, we showed that PAR2 had enhanced binding to avidin beads coupled with the PAR2 i3 loop 18S peptide compared with beads alone (Fig. S5). Additionally, we tested whether P2pal-18S inhibited proteolytic cleavage of PAR2 or endocytosis (28–30). P2pal-18S had no effect on trypsin cleavage of PAR2 (Fig. S6) and did not inhibit ligand-dependent endocytosis of PAR2 or PAR1 (Fig. S7). Therefore, the P2pal-18S i3 loop pepducin can inhibit PAR2-dependent calcium signaling, PLC-β inositol phosphate formation, and cell migration, but not proteolytic cleavage or endocytosis.
Efficacy of P2pal-18S in Mouse Models of Inflammatory Paw Edema.
To evaluate the in vivo efficacy and specificity of P2pal-18S, we tested the ability of the pepducin to protect against inflammatory hindlimb paw edema in WT and PAR2-deficient mouse strains (31). Acute inflammatory edema was induced by an intraplantar injection of λ-carrageenan and kaolin, irritants that cause a massive leukocytosis and hyperemic response, which leads to localized swelling (32). We also directly assessed the PAR2-dependent activity of P2pal-18S by quantifying its inhibitory effects against the PAR2-specific agonist SLIGRL when injected into the hind footpad of WT C57BL/6 mice. Acute inflammation induced by λ-carrageenan/kaolin resulted in a nearly twofold increase in paw edema with vehicle-treated WT mice, peaking 8 h after injection (Fig. 4A). The PAR2 agonist peptide, SLIGRL, also induced an increase in edema of WT mice, peaking 4 h after injection (Fig. 4B). Systemic administration of P2pal-18S caused a significant 50% decrease in λ-carrageenan/kaolin–induced edema and an 85% decrease in SLIGRL-induced edema (Fig. 4 A and B). PAR2 deficiency conferred a 50% protective effect relative to WT mice following λ-carrageenan/kaolin injection, which was nearly identical to the protective effect observed in WT mice treated with P2pal-18S. Notably, treatment of PAR2−/− mice with P2pal-18S did not further reduce swelling, confirming that the anti-inflammatory effects of the PAR2 pepducin required the presence of its cognate receptor.
Fig. 4.
The PAR2 antagonist pepducin P2pal-18S significantly reduces mouse paw edema and inflammation in wild-type but not PAR2-deficient mice. (A) λ-carrageenan/kaolin or (B) the PAR2-specific agonist SLIGRL was administered by intraplantar injection to the left hindpaw of C57BL/6 wild-type or PAR2−/− mice that were treated with s.c. injection of 10 mg/kg of P2pal-18S or vehicle. Paw area was measured and reported as fold increase relative to baseline paw area. (C) Histology of representative H&E-stained footpads 7 h after λ-carrageenan/kaolin injection and quantification of the infiltrating inflammatory cells at 40× magnification. Data (n = 4–6 per group) mean ± SEM. *P < 0.05 and **P < 0.005.
Histologic analysis of the inflamed footpads harvested 7 h post λ-carrageenan/kaolin injection revealed that P2pal-18S provided significant 60% protection (P < 0.005) against the leukocytic infiltrates in the dermis of the footpads, which was identical to the protection observed in PAR2−/− relative to WT mice (Fig. 4C and Fig. S8A). The λ-carrageenan/kaolin challenge caused a twofold increase in myeloperoxidase activity in WT mice, which was blocked by P2pal-18S (Fig. S8B). Together, these data demonstrate that the PAR2 pepducin P2pal-18S affords significant protection against acute leukocytic inflammation and edema and these protective effects are dependent on the presence of PAR2.
P2pal-18S Protects Against Mast Cell Tryptase-Induced Inflammation.
Previous studies have established that mast cell tryptase cleaves and activates PAR2 signaling in human endothelium and keratinocytes and in mouse models of arthritis (4, 17, 33). To determine whether mast cells and mast cell tryptase were contributing to the observed PAR2-dependent effects in the mouse models of paw inflammation, we stimulated mast cells with the degranulating agent 48/80, or λ-carrageenan/kaolin and collected conditioned media. As shown in Fig. 5A, the stimulated mast cells secreted tryptase, which was then used as a chemoattractant source in neutrophil chemotaxis assays. The conditioned media from the stimulated mast cells gave comparable chemotactic migration as 100 nM tryptase (Fig. 5B). Treatment of human neutrophils with tryptase inhibitor, APC-366, or the PAR2 pepducin P2pal-18S, completely inhibited chemotactic migration toward the tryptase-containing mast cell media (Fig. 5B). To provide in vivo evidence that mast cells and mast cell tryptase were activating PAR2 in the paw edema model, we depleted mice of mast cells by pretreatment with compound 48/80 (34). A decrease in λ-carrageenan/kaolin-induced edema in the 48/80-depleted animals was observed, compared with nontreated controls (Fig. S9A). Similarly, mice treated with tryptase inhibitor APC-366, gave a significant 40% protection in λ-carrageenan/kaolin–induced paw edema (Fig. 5C). An intraplantar injection of the tryptase-containing mast cell media resulted in a similar peak increase in paw edema (Fig. 5D), as induced by the selective PAR2 agonist SLIGRL (Fig. 4B). Systemic treatment with P2pal-18S gave a 50% decrease in peak development of edema at 4 h and afforded complete protection at 8 h and thereafter (Fig. 5D). To provide further support for the notion that mast cell-derived tryptase mediates the observed PAR2-dependent inflammatory responses, we challenged mast cell-deficient mice with λ-carrageenan/kaolin and observed that these mice had an identical reduced paw edema response as P2pal-18S–treated littermate control mice that have intact mast cells (Fig. S9B). Furthermore, the observed paw edema in the mast cell-deficient mice could not be further reduced by treatment with P2pal-18S (Fig. S9B). Together, these data suggest that the P2pal-18S pepducin provides significant protection against inflammatory edema triggered by mast cell-derived tryptase.
Fig. 5.
P2pal-18S significantly attenuates mast cell tryptase-dependent neutrophil migration and paw edema in mice. (A) Human mast cells (HMC-1) were treated with 2 mg/mL of mast cell degranulating agent 48/80 or 2% λ-carrageenan/4% kaolin. Additionally, conditioned media (CM) was harvested at 24 h and a Western blot of mast cell tryptase shows release of tryptase. (B) P2pal-18S inhibits human neutrophil chemotaxis (n = 6) to mast cell media. Human neutrophils were incubated with 1 μM P2pal-18S, or 10 μM mast cell tryptase inhibitor APC-366 and allowed to migrate 30 min toward CM from mast cells. (C) C57BL/6 mice (n = 5) were pretreated with the tryptase inhibitor APC-366 (5 mg/kg, s.c.) or vehicle (20% DMSO) and then challenged with intraplantar injection of λ-carrageenan/kaolin. (D) Mast cell-conditioned media (30 μL of λ-carrageenan/kaolin–stimulated media) was injected into the hindpaws of C57BL/6 mice treated with 10 mg/kg P2pal-18S or vehicle (n = 5). Data represent mean ± SEM. #P = 0.07, *P < 0.05, and **P < 0.005.
Discussion
In this paper, we report the development of first-in-class lipopeptide pepducin antagonists of PAR2. Pepducins are an emerging new technology to target recalcitrant transmembrane receptors such as PAR2. These highly stable lipidated peptides are targeted to the intracellular surface of their cognate GPCR and stabilize the receptor in either an active or inactive conformation, resulting in modulation of signal transduction (20, 21, 24). Pepducins typically comprise two components: a short peptide sequence, derived from an i1–i4 intracellular loop of the target GPCR, and an acyl-chain fatty acid (e.g., palmitate) or other lipid conjugated to the peptide. The rational design of the i3 loop agonist and antagonist pepducins was based on a structural model of a PAR2 dimer and by manipulating key residues in the receptor loops and analogous pepducins. We identified individual pharmacophores that controlled constitutive, agonist, and antagonist activites. The most potent pepducin antagonist, P2pal-18S, fully ablated PAR2 signaling but did not inhibit the closely related PAR1 or PAR4 receptors or other tested GPCRs.
The PAR2 pepducin antagonist had significant in vivo efficacy in suppressing leukocytic infiltration and edema induced by λ-carrageenan/kaolin or a PAR2-selective agonist in mouse paw inflammation models. The anti-inflammatory effect of the P2pal-18S pepducin was lost in PAR2-deficient mice, demonstrating that the pepducin was highly specific for PAR2 in vivo. Moreover, the anti-inflammatory effect observed in the PAR2-deficient mice relative to wild type was nearly identical to that observed in wild-type mice treated with P2pal-18S. Together, these data indicate that P2pal-18S affords effective pharmacologic blockade of PAR2 in models of acute inflammation and that these effects require the presence of PAR2.
Many studies have implicated PAR2 as playing critical roles in a wide range of diseases including asthma (2), arthritis (16), hyperalgesia (3), neurogenic and cancer pain (14), and cancer invasion (5). We provided several lines of evidence that the inflammatory response observed in the mouse footpad model was largely dependent on mast cells and mast cell-derived tryptase, an important agonist of PAR2-driven inflammation. We found that the PAR2 pepducin could completely suppress tryptase signaling through PAR2. Moreover, mast cell-deficient mice had an identical reduced paw edema response as P2pal-18S treated littermate controls which had intact mast cells. Furthermore, the paw edema in the mast cell-deficient mice could not be further reduced by treatment with P2pal-18S, providing further support for the notion that mast cell-derived tryptase mediates the PAR2-dependent inflammatory responses in this acute inflammation model. It is possible that the PAR2 pepducin may inhibit signaling induced by other PAR2 protease agonists present in the inflammatory milieu in addition to tryptase. Indeed, PAR2 has been shown to be activated by other proteases, including the TF-FXa-FVIIa complex and membrane-type serine protease 1 (MT-SP1) matriptase (35).
Several studies indicate that it may not always be advantageous to inhibit PAR2 (21, 36, 37). For example, PAR2 agonists can provide physiologic protective responses against bronchoconstriction in rat models (36), and PAR2 has been shown to be essential for the late protective effects of PAR1 agonism in mouse models of cecal ligation and puncture sepsis (21). Additionally, PAR2 has been observed to be beneficial in the mechanism of clearance of Pseudomonas aeruginosa pathogens from infected lungs using PAR2−/− mice (37). In this model, PAR2−/− mice had severe defects in their neutrophil and macrophage phagocytic efficiency, suggesting that PAR2 may also confer protective effects in the host response to bacterial pathogens (37).
Two other groups have disclosed PAR2 antagonists on the basis of the tethered peptide ligand (4, 38). A PAR2 small molecule inhibitor, ENMD-1068, was tested in a model of joint inflammation (4). ENMD-1068 requires millimolar concentrations to observe its protective effects in vitro and considerably higher doses in vivo. The peptide antagonist K-14585 was shown to inhibit PAR2-dependent IL-8 production, NF-κB phosphorylation, and p38 signaling (19). However, the K-14585 compound has partial agonist activity (19, 38), as also observed with the wild-type PAR2 pepducin P2pal-21 (20). In this regard, we discovered that wild-type PAR2 has constitutive activity, indicating that certain extracellular or intracellular PAR2 ligands might stablize the latent on state. The realization that constitutive activity could be ablated or enhanced by mutation of critical i3 loop pharmacophores in the intact receptor led us to rationally design PAR2 pepducin antagonists that lost residual agonist activity. The ability to design pepducin antagonists against difficult GPCR targets such as PAR2 is a valuable research and therapeutic approach, which will aid in the delineation of complex mechanisms of GPCR signaling and pathophysiology and may lead to novel pharmacological agents in a potentially wide range of diseases.
Materials and Methods
Detailed methods, including PAR2 structural modeling, reagents, cell culture, PAR surface expression analysis, transwell migration, mouse paw edema models, neutrophil chemotaxis, and statistical analyses, appear in SI Materials and Methods. All animal experiments were performed in accordance with the National Institutes of Health guidelines and approved by Tufts University Institutional Animal Care and Use Committee. Neutrophils were obtained from the peripheral blood of healthy volunteers using informed consent procedures approved by the institutional review board of Tufts Medical Center. Statistical significance was defined as *P < 0.05 or **P < 0.005.
Supplementary Material
Acknowledgments
We thank Dr. J. H. Butterfield for providing the HMC-1 cells; Dr. Martin Beinborn for the COS7 cells; the Rosenblatt laboratory for the HEK cells; George Koukos for technical assistance; and Katie O'Callaghan, Anika Agarwal, Caitlin Foley, and Nga Nguyen for their expert advice. This work was funded in part by Grants R01 HL-57905, R01 HL-64701, and R01 CA-122992 from the National Institutes of Health (to A.K.) and R01 CA-104406 (to L.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017091108/-/DCSupplemental.
References
- 1.Vergnolle N, Hollenberg MD, Sharkey KA, Wallace JL. Characterization of the inflammatory response to proteinase-activated receptor-2 (PAR2)-activating peptides in the rat paw. Br J Pharmacol. 1999;127:1083–1090. doi: 10.1038/sj.bjp.0702634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidlin F, et al. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169:5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- 3.Vergnolle N, et al. Proteinase-activated receptor-2 and hyperalgesia: A novel pain pathway. Nat Med. 2001;7:821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- 4.Kelso EB, et al. Therapeutic promise of proteinase-activated receptor-2 antagonism in joint inflammation. J Pharmacol Exp Ther. 2006;316:1017–1024. doi: 10.1124/jpet.105.093807. [DOI] [PubMed] [Google Scholar]
- 5.Shi X, Gangadharan B, Brass LF, Ruf W, Mueller BM. Protease-activated receptors (PAR1 and PAR2) contribute to tumor cell motility and metastasis. Mol Cancer Res. 2004;2:395–402. [PubMed] [Google Scholar]
- 6.Kaneider NC, Agarwal A, Leger AJ, Kuliopulos A. Reversing systemic inflammatory response syndrome with chemokine receptor pepducins. Nat Med. 2005;11:661–665. doi: 10.1038/nm1245. [DOI] [PubMed] [Google Scholar]
- 7.Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci USA. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boire A, et al. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Trivedi V, et al. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell. 2009;137:332–343. doi: 10.1016/j.cell.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ossovskaya VS, Bunnett NW. Protease-activated receptors: Contribution to physiology and disease. Physiol Rev. 2004;84:579–621. doi: 10.1152/physrev.00028.2003. [DOI] [PubMed] [Google Scholar]
- 11.Noorbakhsh F, et al. Proteinase-activated receptor 2 modulates neuroinflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. J Exp Med. 2006;203:425–435. doi: 10.1084/jem.20052148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cenac N, et al. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am J Pathol. 2002;161:1903–1915. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoniak S, et al. Protease-activated receptor 2 deficiency reduces cardiac ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2010;30:2136–2142. doi: 10.1161/ATVBAHA.110.213280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam DK, Schmidt BL. Serine proteases and protease-activated receptor 2-dependent allodynia: A novel cancer pain pathway. Pain. 2010;149:263–272. doi: 10.1016/j.pain.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrell WR, et al. Essential role for proteinase-activated receptor-2 in arthritis. J Clin Invest. 2003;111:35–41. doi: 10.1172/JCI16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrell WR, Kelso EB, Lockhart JC, Plevin R, McInnes IB. Protease-activated receptor 2: A novel pathogenic pathway in a murine model of osteoarthritis. Ann Rheum Dis. 2010;69:2051–2054. doi: 10.1136/ard.2010.130336. [DOI] [PubMed] [Google Scholar]
- 17.Molino M, et al. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- 18.Caughey GH. Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev. 2007;217:141–154. doi: 10.1111/j.1600-065X.2007.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goh FG, Ng PY, Nilsson M, Kanke T, Plevin R. Dual effect of the novel peptide antagonist K-14585 on proteinase-activated receptor-2-mediated signalling. Br J Pharmacol. 2009;158:1695–1704. doi: 10.1111/j.1476-5381.2009.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc Natl Acad Sci USA. 2002;99:643–648. doi: 10.1073/pnas.022460899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneider NC, et al. ‘Role reversal’ for the receptor PAR1 in sepsis-induced vascular damage. Nat Immunol. 2007;8:1303–1312. doi: 10.1038/ni1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Covic L, Tchernychev B, Jacques S, Kuliopulos A. In: Handbook of Cell-Penetrating Peptides. Langel U, editor. Boca Raton: Taylor and Francis; 2007. pp. 245–257. [Google Scholar]
- 23.Wielders SJ, Bennaghmouch A, Reutelingsperger CP, Bevers EM, Lindhout T. Anticoagulant and antithrombotic properties of intracellular protease-activated receptor antagonists. J Thromb Haemost. 2007;5:571–576. doi: 10.1111/j.1538-7836.2007.02364.x. [DOI] [PubMed] [Google Scholar]
- 24.Tressel SL, et al. Pharmacology, biodistribution, and efficacy of GPCR-based pepducins in disease models. Methods Mol Biol. 2011;683:259–275. doi: 10.1007/978-1-60761-919-2_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leger AJ, et al. Blocking the protease-activated receptor 1-4 heterodimer in platelet-mediated thrombosis. Circulation. 2006;113:1244–1254. doi: 10.1161/CIRCULATIONAHA.105.587758. [DOI] [PubMed] [Google Scholar]
- 26.Teller DC, Okada T, Behnke CA, Palczewski K, Stenkamp RE. Advances in determination of a high-resolution three-dimensional structure of rhodopsin, a model of G-protein-coupled receptors (GPCRs) Biochemistry. 2001;40:7761–7772. doi: 10.1021/bi0155091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kjelsberg MA, Cotecchia S, Ostrowski J, Caron MG, Lefkowitz RJ. Constitutive activation of the alpha 1B-adrenergic receptor by all amino acid substitutions at a single site. Evidence for a region which constrains receptor activation. J Biol Chem. 1992;267:1430–1433. [PubMed] [Google Scholar]
- 28.DeFea KA, et al. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roosterman D, Schmidlin F, Bunnett NW. Rab5a and rab11a mediate agonist-induced trafficking of protease-activated receptor 2. Am J Physiol Cell Physiol. 2003;284:C1319–C1329. doi: 10.1152/ajpcell.00540.2002. [DOI] [PubMed] [Google Scholar]
- 30.Stalheim L, et al. Multiple independent functions of arrestins in the regulation of protease-activated receptor-2 signaling and trafficking. Mol Pharmacol. 2005;67:78–87. doi: 10.1124/mol.104.006072. [DOI] [PubMed] [Google Scholar]
- 31.Damiano BP, et al. Cardiovascular responses mediated by protease-activated receptor-2 (PAR-2) and thrombin receptor (PAR-1) are distinguished in mice deficient in PAR-2 or PAR-1. J Pharmacol Exp Ther. 1999;288:671–678. [PubMed] [Google Scholar]
- 32.Day SM, Lockhart JC, Ferrell WR, McLean JS. Divergent roles of nitrergic and prostanoid pathways in chronic joint inflammation. Ann Rheum Dis. 2004;63:1564–1570. doi: 10.1136/ard.2003.017269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer HS, et al. Protease-activated receptor 2 mediates the proinflammatory effects of synovial mast cells. Arthritis Rheum. 2007;56:3532–3540. doi: 10.1002/art.22936. [DOI] [PubMed] [Google Scholar]
- 34.Carvalho M, Benjamim C, Santos F, Ferreira S, Cunha F. Effect of mast cells depletion on the failure of neutrophil migration during sepsis. Eur J Pharmacol. 2005;525:161–169. doi: 10.1016/j.ejphar.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi T, et al. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem. 2000;275:26333–26342. doi: 10.1074/jbc.M002941200. [DOI] [PubMed] [Google Scholar]
- 36.Cocks TM, et al. A protective role for protease-activated receptors in the airways. Nature. 1999;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- 37.Moraes TJ, et al. Role of PAR2 in murine pulmonary pseudomonal infection. Am J Physiol Lung Cell Mol Physiol. 2008;294:L368–L377. doi: 10.1152/ajplung.00036.2007. [DOI] [PubMed] [Google Scholar]
- 38.Kanke T, et al. Novel antagonists for proteinase-activated receptor 2: Inhibition of cellular and vascular responses in vitro and in vivo. Br J Pharmacol. 2009;158:361–371. doi: 10.1111/j.1476-5381.2009.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swift S, et al. A novel protease-activated receptor-1 interactor, Bicaudal D1, regulates G protein signaling and internalization. J Biol Chem. 2010;285:11402–11410. doi: 10.1074/jbc.M110.105403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.