Abstract
The second critical endpoint in the basalt-H2O system was directly determined by a high-pressure and high-temperature X-ray radiography technique. We found that the second critical endpoint occurs at around 3.4 GPa and 770 °C (corresponding to a depth of approximately 100 km in a subducting slab), which is much shallower than the previously estimated conditions. Our results indicate that the melting temperature of the subducting oceanic crust can no longer be defined beyond this critical condition and that the fluid released from subducting oceanic crust at depths greater than 100 km under volcanic arcs is supercritical fluid rather than aqueous fluid and/or hydrous melts. The position of the second critical endpoint explains why there is a limitation to the slab depth at which adakitic magmas are produced, as well as the origin of across-arc geochemical variations of trace elements in volcanic rocks in subduction zones.
Keywords: water, island arc, silicate melt, synchrotron X-ray, high-pressure research
Water plays an important role in subduction-zone magmatism because it can reduce the melting temperature of rocks in subduction zones and hence can generate magmas (1–7). There is a long-standing debate about whether the fluids released from a subducting slab are aqueous fluid, hydrous silicate melt, supercritical fluid (SCF), or a combination of these (2, 4, 8–12). Whether the subducting slab melts or dehydrates depends on the thermal structure and the phase relation of slab materials under hydrous conditions. Therefore, this long-standing question cannot be answered until the detailed stability fields of these fluids are clarified.
Under high-pressure (P) and high-temperature (T) conditions, it has been shown that the solubility of both water in silicate melt (13–16) and silicate in aqueous fluid (13, 17–25) increases with increasing P. As a result, silicate melt and aqueous fluid in the interior of the Earth are expected to become SCF, and the hydrous solidus of the system can no longer be defined beyond a certain critical condition (26–36). This condition is called the second (or upper) critical endpoint (26) and is the point of intersection between the critical curve and hydrous solidus. Basalt is one of the dominant constituents of a subducting slab and is considered the main carrier of water that triggers the melting of rocks in subduction zones. Therefore, the second critical endpoint in the basalt-H2O system has to be determined in order to understand fully the subduction-zone magmatism.
In some silicic silicate-H2O systems, the location of the second critical endpoint has been reported [e.g., 1.0 GPa, 1,080 °C in the SiO2-H2O system (13); 1.5 GPa, 670 °C in the system NaAlSi3O8-H2O (21); 1.5 GPa, 800 °C in the system KAlSi3O8-H2O (36)]. In mafic systems, however, the determination of the second critical endpoints is not easy because of the difficulty in identifying phases (aqueous fluid versus hydrous silicate melt) in the recovered samples quenched from high-P and high-T conditions (25). The stability fields of fluid phases in mafic systems have to be determined by observing phases directly under high-P and high-T conditions. In the present study, therefore, we determined the second critical endpoint in a basalt-H2O system using a high-P and high-T X-ray radiography technique (37) that enabled us to observe aqueous fluid and hydrous silicate melt directly. In addition to the experiments with X-ray radiography, we also conducted long-duration constant-T quench experiments in order to confirm that the equilibrium was attained in our radiographic observations.
Results
Experimental conditions and results are listed in Table 1. The stability fields of observed minerals (especially amphibole in the run S1176) in the recovered samples are consistent with previous studies (38, 39) within the experimental uncertainties, indicating that our P-T calibrations (30) are reliable. When experimental P is below the second critical endpoint, and both aqueous fluid and hydrous silicate melt are present, round shapes can be observed in the radiographic images because of the differences in their interfacial tension. In this case, the two-phase boundary between these two fluids is enhanced in the radiographic images because of the X-ray refraction contrast. During heating, this two-phase boundary migrates without exception, because the fluid/melt ratio changes with changing T (Movie S1). Therefore, two fluids are easily identified if they coexist under the experimental P-T conditions. Above the second critical endpoint, on the other hand, the two-phase boundary cannot be observed, because only the SCF is a stable fluid phase (Movie S2). Instead, crystals (mostly garnet) are sometimes observed falling uniformly through the entire sample capsule (Movie S3), which is further evidence that only the SCF is a stable fluid phase under the experimental P-T conditions. This is because not all the crystals fall to the bottom of the sample capsule when two fluids coexist; some are sustained in melt. It was difficult to determine the stability fields of minerals from the radiographic observations.
Table 1.
Experimental conditions and results
| Run no.* | P, GPa | TM/TQ, °C | H2O, wt %† | Observed fluid phases in radiography | Quench products |
| S968 | 0.5 | 1,350/1,350 | 59 | F + M (790–1,350)‡ | QM + QF + Vd |
| S969 | 1.8 | 1,320/1,320 | 60 | F + M (950–1,320) | ND |
| S1176 | 2.0 | 1,240/920 | 60 | F + M (820–1,240) | Am + Cpx + QM + QF + Vd |
| QB-1 | 2.0 | 1,200/1,200 | 63 | — | QM + QF + Vd |
| S997 | 2.8 | 1,350/1,350 | 55 | F + M (1,010–1,350) | QM + QF + Vd |
| S998 | 2.8 | 1,350/1,240 | 47 | F + M (790–1,240) | QM + QF + Vd |
| S1195 | 2.8 | 1,240/1,240 | 62 | F + M (850–910) | ND |
| S1194 | 3.0 | 1,240/1,010 | 60 | F + M (1,010–1,125) | QM + QF + Vd |
| S965 | 3.0 | 1,300/1,300 | 62 | F + M (900–1,240) | ND |
| S963 | 3.0 | 1,350/1,350 | 63 | F + M (840–1,040) | ND |
| S996 | 3.0 | 1,300/1,300 | 63 | F + M (880–960) | ND |
| S1083 | 3.0 | 1,350/1,130 | 65 | SCF | QS |
| S1902 | 3.3 | 1,200/1,150 | 62 | F + M (1,150) | QM + QF + Vd |
| S1193 | 3.3 | 1,180/1,180 | 68 | SCF | QS |
| QB-2 | 3.4 | 1,000/1,000 | 57 | — | Gt + QS |
| QB-3 | 3.4 | 1,000/1,000 | 79 | — | Gt + QS |
| QB-5 | 3.4 | 1,150/1,150 | 85 | — | QS |
| S1076 | 3.6 | 1,350/1,130 | 58 | SCF | QS |
| S1077 | 3.6 | 1,350/1,130 | 52 | SCF | QS |
| S1078 | 3.6 | 1,350/1,130 | 45 | SCF | QS |
| S1081 | 3.6 | 1,320/1,130 | 21 | SCF | Gt + QS |
| QB-4 | 3.6 | 1,150/1,150 | 84 | — | QS |
| S966 | 4.0 | 1,190/1,190 | 60 | SCF | QS |
The absolute error in the temperature determination is about ± 50 °C and that of pressure is about ± 0.2 GPa.
TM, the maximum temperature during the run; TQ, quenched temperature; ND, not determined; QF, fine quench materials from aqueous fluid; QM, glass and/or quench materials from melt; QS, quench materials from supercritical fluid; Am, amphibole; Cpx, clinopyroxene; Gt, garnet; Vd, void; F, aqueous fluid; M, silicate melt; SCF, supercritical fluid.
*S series: radiography, QB series: long-duration constant-T quench experiments.
†Amount of H2O in starting materials. Error: ± 10%.
‡Two fluids were observed during temperature range shown in parentheses.
In experiments up to 3.3 GPa (Fig. 1 A and B and Movie S1), both aqueous fluid and hydrous silicate melt were observed in radiographic images, if the water content of the starting materials was within the solvus between aqueous fluid and silicate melt at the experimental P. On the other hand, these two fluids could not be distinguished in radiographic images (Fig. 1C and Movies S2 and S3) in any of the runs with various water contents above 3.6 GPa. When the samples were quenched under conditions where the two fluids could be observed, void spaces and tiny glass spheres (approximately 10-μm diameter) were observed in the recovered samples (Fig. 1 D–K). These void spaces and glass spheres are considered to be quenched aqueous fluid. Hydrous silicate melts were quenched into glasses at lower P (Fig. 1 E, G, and H), whereas they were quenched into a mixture of quench crystals and glasses at higher P (Fig. 1J). No voids were found in the recovered samples quenched from > 3.6 GPa (Fig. 1 L–P). Instead, quench crystals were distributed throughout the entire sample capsules (Fig. 1 L–P), which is consistent with the fact that the two fluids cannot be distinguished in radiographic images above 3.6 GPa.
Fig. 1.
X-ray radiographic images and photomicrographs of recovered samples. Abbreviations are the same as in Table 1. (A) X-ray radiographic image taken at 0.5 GPa and 1,120 °C (run S968). Both aqueous fluid and silicate melt can be seen. (B) The interface between aqueous fluid and silicate melt in A is indicated by the dotted curve. (C) X-ray radiographic image taken at 3.6 GPa and 1,125 °C (S1077). Only the supercritical fluid phase is observed. The blocky and granular textures in radiographic images (A–C) are instrumental artifacts. (D) Recovered sample quenched from 0.5 GPa and 1,350 °C (S968). A large void space, which was filled with aqueous fluid under the experimental conditions, can be seen. The surface of the QM glass is covered with tiny glass spheres, which are considered to be quenched products from aqueous fluid. (E) Polished surface of D, which is parallel to the X-ray path. Many tiny glass spheres can be seen in the epoxy (dark matrix). (F) Recovered sample quenched from 2.0 GPa and 920 °C (S1176). (G) Polished surface of F. (H) Enlargement of the rectangular area in G. (I) Recovered sample quenched from 2.8 GPa and 1,350 °C (S997). (J) Polished surface of I. (K) Enlargement of QF in the recovered sample S997. (L) Recovered sample quenched from 3.6 GPa and 1,130 °C (S1077). (M) Polished surface of L. (N) Enlargement of QS in the recovered sample S1077. (O) Recovered sample quenched from 3.6 GPa and 1,130 °C (S1081). (P) Enlargement of the rectangular area in O. Note that there is no void space in L, M, N, O, or P, and dense quenched materials are distributed in the entire region in these recovered capsules. The classification of quenched materials into two types (i.e., QF vs. QM) is no longer possible in L–P, indicating that the conditions of these runs are above the second critical endpoint. (E) Secondary electron image. (G, H, J, K, N, and P) Backscattered electron images.
Quench products from long-duration constant-T quench experiments also revealed that aqueous fluid and silicate melt can be distinguished at P less than 3.3 GPa (Table 1). In the samples quenched at 2.0 GPa, void spaces and tiny glass spheres (approximately 10-μm diameter) were observed around spherically shaped globules of a mixture composed of quench crystals and glass. We consider the former void and tiny glass spheres to be quenched from the aqueous fluids, and the mixture globules from melts. In the recovered samples quenched from P values above 3.4 GPa, the two-type discrete quenched materials were no longer recognized; quench crystals were distributed throughout the entire sample capsule. These characteristics are consistent with the textures of the recovered samples quenched after the X-ray radiography. It can be concluded, therefore, that our radiographic studies were done under equilibrium conditions.
Representative chemical compositions of the phases in the recovered products are listed in Table 2. The composition of the supercritical fluid of the run S1077 is close to that of the starting material, which confirms that our experiments were done under closed-system conditions. It was impossible to determine the composition of aqueous fluid in the experimental runs at lower P, because the size and amount of quenched materials were not sufficient to permit analysis. With increasing P, however, the amount of quenched material from the aqueous fluid increased. Compositions of aqueous fluid could be determined for the runs S997 and S1194, and they are roughly in the range of adakitic dacite on a dry basis (Table 2). Compositions of silicate melts in these runs S997 and S1194 are basaltic on a dry basis, indicating that hydrous basaltic melts can coexist with adakitic dacite under the experimental conditions. Because quenched materials from both the aqueous fluid and SCF are heterogeneously distributed in the epoxy matrix, the difference from 100 wt % does not necessarily represent the water content in the measured phase.
Table 2.
Composition of phases (wt %)
| Run no. | S968 | S1176 | S997 | |||||||
| P, T* | 0.5, 1350 |
2.0, 920 |
2.8, 1350 |
|||||||
| Phase† |
M |
M100‡ |
Am |
Cpx |
M |
M100 |
M |
M100 |
F |
F100 |
| n§ | 10 | — | 7 | 5 | 11 | — | 35 | — | 34 | — |
| SiO2 | 45.40 (0.47) | 50.81 | 44.42 (0.48) | 47.68 (0.97) | 48.50 (0.89) | 57.81 | 36.33 (1.94) | 49.98 | 18.21 (5.45) | 63.49 |
| Al2O3 | 16.19 (0.13) | 18.12 | 15.89 (0.49) | 12.39 (1.63) | 19.10 (0.38) | 22.77 | 12.77 (0.48) | 17.57 | 4.53 (1.40) | 15.80 |
| FeO | 5.03 (0.03) | 5.62 | 9.22 (0.63) | 8.54 (0.66) | 4.42 (0.21) | 5.27 | 7.40 (0.36) | 10.18 | 1.82 (0.55) | 6.35 |
| MgO | 8.29 (0.14) | 9.28 | 14.77 (0.94) | 11.12 (1.64) | 2.90 (0.16) | 3.45 | 6.66 (0.37) | 9.16 | 1.80 (0.56) | 6.27 |
| CaO | 12.41 (0.18) | 13.89 | 10.88 (0.37) | 19.68 (1.50) | 8.10 (0.35) | 9.65 | 8.32 (0.78) | 11.44 | 1.78 (1.39) | 6.21 |
| Na2O | 1.96 (0.08) | 2.19 | 2.00 (0.11) | 0.44 (0.25) | 0.80 (0.14) | 0.95 | 1.16 (0.49) | 1.59 | 0.48 (0.21) | 1.67 |
| K2O | 0.08 (0.01) | 0.09 | 0.07 (0.01) | 0.01 (0.01) | 0.08 (0.02) | 0.10 | 0.06 (0.02) | 0.08 | 0.06 (0.02) | 0.21 |
| Total | 89.36 (0.56) | 100.00 | 97.25 (0.40) | 99.86 (1.42) | 83.90 (1.29) | 100.00 | 72.70 (2.32) | 100.00 | 28.68 (8.19) | 100.00 |
| Run no. | S1194 | S1077 | S1081 | ||||||
| P, T | 3.0, 1010 |
3.6, 1130 |
3.6, 1130 |
||||||
| Phase |
M |
M100 |
F |
F100 |
SCF |
SCF100 |
Gt |
SCF |
SCF100 |
| n | 19 | — | 34 | — | 11 | — | 9 | 15 | — |
| SiO2 | 35.62 (1.91) | 48.78 | 16.04 (3.19) | 65.23 | 33.37 (1.65) | 51.31 | 40.70 (0.46) | 40.59 (1.92) | 55.90 |
| Al2O3 | 12.49 (0.74) | 17.10 | 3.92 (0.79) | 15.94 | 10.62 (0.54) | 16.33 | 22.78 (0.19) | 10.87 (0.49) | 14.97 |
| FeO | 6.10 (0.37) | 8.36 | 1.11 (0.35) | 4.51 | 6.51 (0.56) | 10.01 | 14.93 (0.49) | 5.78 (0.27) | 7.96 |
| MgO | 6.64 (0.47) | 9.09 | 1.36 (0.49) | 5.53 | 5.35 (0.64) | 8.23 | 13.05 (0.46) | 4.64 (0.31) | 6.39 |
| CaO | 10.02 (0.78) | 13.73 | 1.27 (0.30) | 5.17 | 7.43 (1.43) | 11.42 | 8.97 (0.36) | 9.00 (0.89) | 12.39 |
| Na2O | 2.05 (0.46) | 2.81 | 0.82 (0.18) | 3.34 | 1.61 (0.28) | 2.47 | 0.02 (0.01) | 1.37 (0.26) | 1.89 |
| K2O | 0.09 (0.02) | 0.13 | 0.07 (0.02) | 0.28 | 0.15 (0.03) | 0.23 | 0.00 (0.00) | 0.36 (0.07) | 0.50 |
| Total | 73.01 (3.61) | 100.00 | 24.59 (4.44) | 100.00 | 65.04 (2.24) | 100.00 | 100.45 (0.75) | 72.61 (2.64) | 100.00 |
Numbers in parentheses represent one standard deviation.
*Pressure in GPa, quenched temperature in °C.
†Abbreviations are the same as in Table 1.
‡Normalized to 100 wt %.
§Number of analyses.
Discussion
Second Critical Endpoint in Basalt-H2O System.
Our direct observations by X-ray radiography and careful examination of the quenched samples indicate that the second critical endpoint in the basalt-H2O system should occur at 3.4 ± 0.2 GPa (Fig. 2). Because the second critical endpoint occurs on the hydrous solidus, the T of the second critical endpoint is estimated to be at around 770 ± 50 °C, based on previous studies (38, 39). From the runs S996 and S1083 at 3.0 GPa and also runs S1902 and S1193 at 3.3 GPa (Table 1), the water content of the aqueous fluid on the solvus between the aqueous fluid and the silicate melt at P between 3.0 and 3.3 GPa must be between 62 and 68 wt % H2O. Therefore, the critical water content at the second critical endpoint is considered to be less than and probably close to 65 ± 3 wt % H2O. Our results are consistent with most of the previous studies: None of the previous experimental studies have shown any direct evidence that the aqueous fluid and hydrous silicate melt could coexist in the basalt-H2O system above 3 GPa.
Fig. 2.
Phase diagrams in the vicinity of the second critical endpoint in the basalt-H2O system. (A) P-T diagram showing the experimental results and phase relations in the basalt-H2O system. Open bars represent the P-T conditions at which the two fluid phases (aqueous fluid and silicate melt) were observed, whereas filled bars represent the P-T conditions at which the two fluids were not observed in the radiographic images. These results indicate that the P of the second critical endpoint can be bracketed between 3.3 and 3.6 GPa. The amount of H2O in the starting materials of data plotted here is 62 ± 6 wt % (Table 1). Solid curve is the hydrous solidus in the basalt-H2O system (38). The transitional T from filled bars to open bars does not necessarily coincide with the hydrous solidus. Because the spherical melt appears upon heating when the experimental condition reaches well above the solidus and hence the degree of melting becomes quite high, it follows that all the open bars in A are located above the hydrous solidus. The dotted line and the star represent the estimated position of the critical curve and second critical endpoint (3.4 ± 0.2 GPa) in the basalt-H2O system. (B) Isobaric phase diagram below the second critical endpoint. S: silicate solid, M: silicate melt, F: aqueous fluid. Note that the stability field of S + M + F (red field) exists on the lower-T side of the stability field of M + F (blue field), because the basalt-H2O system is the multicomponent system. This stability field of S + M + F extends toward the water-poor side. The P-T path in experiments below the second critical endpoint should encounter the stability field of S + M + F, if only a small amount of water is present in the system. The miscibility gap therefore cannot be overlooked, even when the water content in the starting material is less than the critical water content at the experimental P. (See ref. 35 for a detailed discussion.) The experimental P of data plotted here ranges from 2.8 to 3.3 GPa. (C) Isobaric phase diagram above the second critical endpoint. The meaning of open and filled bars in B and C is the same as in A. Filled squares in C represent the P-T conditions at which only the SCF is stable fluid phase observed in the long-duration constant-T quench experiments. The experimental P of data plotted here ranges from 3.4 to 3.6 GPa. (D) P - T - XH2O phase diagram showing the position of the second critical endpoint (star) in the basalt-H2O system. The second critical endpoint occurs at around 3.4 ± 0.2 GPa and 770 ± 50 °C, where the hydrous solidus (Solidus) intersects the critical curve (dotted curve). Note that the solidus of the hydrous basalt is no longer defined above the second critical endpoint, and the aqueous fluid changes continuously to the silicate melt with T. Details of these phase diagrams have yet to be determined in future works.
Kessel et al. (40) reported that the second critical endpoint in a K-free basalt-H2O system occurs at around 5.5 GPa, which is 2.1 GPa higher than the result determined in the present study. Kessel et al. (40) determined the solubility of silicate components in H2O with changing T under constant P. The solubility of silicate components changes continuously with T when the experimental P is above the second critical endpoint, whereas the solubility changes discontinuously with T when the experimental P is below the second critical endpoint. However, as can be seen from the flat of the solubility curve in Fig. 2C (XH2O ∼ 30 to 80), the change in solubility is drastic in the case when the experimental P is above the second critical endpoint. It is, therefore, difficult to determine which type of phase diagram (i.e., Fig. 2 B versus C) is valid under the experimental P by determining the solubility only. Using their indirect method (40), which involved some uncertainty in determining the solubility of silicates, it is considered that Kessel et al. (40) were unable to determine precisely the P of the second critical endpoint.
Depths of the Second Critical Endpoints and Their Bearing on the Subduction-Zone Magmatism.
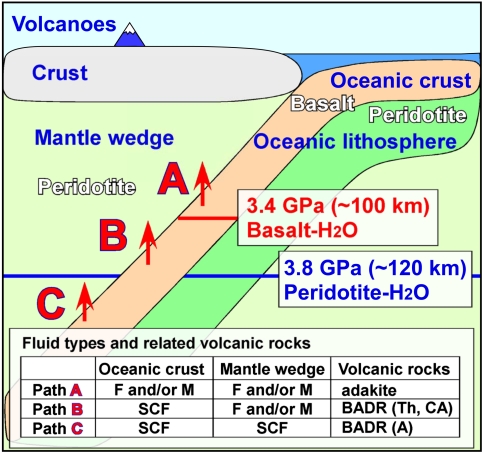
In most of the Earth’s present “normal-type” subduction zones, basaltic magmas are considered to be generated by the melting of mantle wedge peridotite by the addition of aqueous fluid released from the subducting slab (1, 3–7). The mean depth below subduction-zone volcanoes to the top of the deep seismic zone (Wadati–Benioff zone) has been given as 124 ± 38 km (ref. 41) or 108 ± 14 km (ref. 5). Recent studies have shown that this depth ranges from 65–130 km (ref. 42) or 72–173 km, with a global average of 105 km (ref. 43). Based on our experimental results, the position of the second critical endpoint in the subducting slab should be located at around 100-km depth, if the dominant fluid component is H2O (Fig. 3). Therefore, our experimental results strongly indicate that the slab-derived fluid under most subduction-zone volcanoes is SCF rather than a separate aqueous fluid, hydrous melt, or both of them. Only in the case when dehydration reactions of hydrous minerals in subducting slabs occur at depths shallower than 100 km may aqueous fluid and/or hydrous melt be released from the slabs, depending on P-T conditions.
Fig. 3.
Schematic illustration showing the types of fluids and related volcanic rocks in subduction zones. Not to scale. When dehydration occurs like path A, both aqueous fluid and silicate melt could exist in both the subducting slab and mantle wedge, because path A is shallower than the second critical endpoint in both basalt-H2O (approximately 100 km) and peridotite-H2O (approximately 120 km) (ref. 35) systems. In this case, adakitic magmas could be produced by the slab melting. The depth of path B is between the second critical endpoint in the basalt-H2O system and that in the peridotite-H2O system. Therefore, the slab-derived fluid is SCF, whereas both aqueous fluid and silicate melt could exist in the mantle wedge. In path C, only SCF is stable in both the subducting slab and the mantle wedge, because path C is deeper than the second critical endpoint in both basalt-H2O and peridotite-H2O systems. In paths B and C, normal types of island-arc volcanic rocks of basalt, andesite, dacite, and rhyolite (BADR) could be generated. The primitive basalt on the volcanic front in normal-type island-arc magmatism (path B) could become tholeiitic (Th) or calc-alkaline (CA) as a result of the possible removal of alkaline-rich aqueous fluid from magma (35). On the other hand, the primitive magma on the back-arc side (path C) should remain alkaline-rich (BADR, A) because the extraction of the alkaline elements by the fluid-melt immiscibility is impossible beyond the second critical endpoint (35). Abbreviations for F, M, and SCF are the same as in Table 1.
It is often suggested that the fluid released from the subducting slab plays an important role in the transport of trace elements from the slab and in the generation of the geochemical characteristics of magmas in subduction zones (5, 44). In the fore-arc region, where the P is less than the second critical endpoint, the fluid mobile elements (FMEs) (i.e., aqueous fluid/mineral partition coefficient, Dfluid/mineral > 1, and/or fluid/melt partition coefficient, Dfluid/melt > 1), such as B, Sr, and Pb (ref. 44) are preferentially extracted from the slab to the mantle wedge. With increasing P, however, not only the FMEs but also the fluid immobile elements (FIEs), such as Nb, Th, and REE, enter into the SCF (45).
It has been reported that the ratio of FME to FIE in volcanic rocks decreases continuously from the volcanic front to the back-arc side (46, 47). These across-arc geochemical variations have often been interpreted as a sign that the amount of fluid released from a slab decreases continuously with increasing depth of the Wadati–Benioff zone, assuming that the trace element composition of the fluid remains constant with slab depth (46). However, the composition of slab-derived fluids cannot be constant with P (45). Our experimental results suggest that these across-arc geochemical variations are direct evidence that the fluids released from the subducting slab change from an aqueous fluid to SCF with increasing depth under volcanic arcs.
Concerning subduction zones where young and hot oceanic crust is subducted, it has been argued that the melting of subducting oceanic crust itself, rather than mantle wedge peridotite, could happen and generate adakitic magmas (48–50). One of the interesting features of adakites is that they are mainly situated where the Wadati–Benioff zone is located at 70–90 km beneath the volcanic arc (51, 52). It has been suggested (52) that this depth limitation of 90 km is related to the stability limit of amphibole in the subducting oceanic crust. However, serpentine in the oceanic lithosphere could also carry water to the depths of 150–200 km (ref. 53) and hence could be the source of water that might trigger the melting of oceanic crust at depths greater than 90 km.
Our results lead to an alternative explanation as to why adakitic magmatism occurrences are generally situated where the depth of the Wadati–Benioff zone is shallower than 90 km. According to our phase diagrams (Fig. 2C), it would be easier to generate hydrous melt (i.e., adakites) below 3.4 GPa because a relatively high T is required to produce melt (i.e., silicate-rich SCF2 in Fig. 2C) above 3.4 GPa. Above 3.4 GPa, water-rich SCF (SCF1 in Fig. 2C) would be released successively from the slab to the mantle wedge before the slab T becomes high enough. In this case, this low-T, water-rich SCF (SCF1) could become the source of water that generates “normal” basaltic magmas by the hydrous melting of mantle wedge peridotite, instead of forming adakites.
Our experimental results lead to the conclusion that the depth limit of 90 km for the formation of adakitic magmatism is controlled by the position of the second critical endpoint in the basalt-H2O system, rather than the availability of water in the slab. For some subduction zones, it has been reported that magmatism changes from an adakite type to a normal subduction-zone type with increasing depth of the Wadati–Benioff zone (51, 54). This change in the major-element chemistry of volcanic rocks is considered further proof that the slab melting is limited to the fore-arc side, whereas SCF as a result of slab dehydration causes normal-type magmatism in most of the subduction zone.
Materials and Methods
Experiments were carried out using an X-ray radiography technique (37) together with a Kawai-type double-stage multianvil high-P apparatus (SPEED-1500) installed at SPring-8 (55). The composition of basalt used as a starting material was close to the average for mid-ocean-ridge basalt glasses (56) (SiO2: 50.4 wt %, Al2O3: 16.8 wt %, FeO∗: 10.9 wt %, MgO: 7.9 wt %, CaO: 12.0 wt %, Na2O: 1.9 wt %, and K2O: 0.1 wt %). Water was added as hydroxides and deionized water. The sample container was an AuPd tube with a cylindrical single-crystal diamond placed on each end. Because diamonds are transparent to X-rays, the real-time images of the samples under high-P and high-T conditions could be directly observed using an X-ray camera.
The T was monitored with a W5%Re-W26%Re thermocouple. Because the high-T junction of the thermocouple was far from the sample chamber for the radiography experiments, a correction was made to estimate the real sample T from the thermocouple readings. The T values reported here are real (or estimated) sample T values that can be directly compared to the other studies on phase relations. No correction for the effect of P on the thermocouple electromotive force was applied. Detailed experimental methods and P-T calibrations have been reported elsewhere (30, 35).
The T was increased or decreased under a constant load. The heating and cooling rates were about 5 °C/s, which is comparable to those adopted in the studies on the observation of critical behavior between aqueous fluid and silicate melt using a hydrothermal diamond-anvil cell (27, 28, 32, 36). The X-ray camera was on at all times during the experiment, and images were recorded from the beginning of the heating (i.e., room T) until quenching. In the experiments at P values below the second critical endpoint, the experimental P-T path encountered the stability field of aqueous fluid + silicate melt with increasing T under a constant P. In this case, one fluid phase (either aqueous fluid or silicate melt) formed spheres in the other phase because of the differences in the interfacial tension between them. A round shape is therefore expected to be observed in the radiographic images. On the other hand, in experiments beyond the second critical endpoint, a round shape should not be observed, because SCF is the only homogeneous fluid phase existing at high T. The P of the second critical endpoint can therefore be determined by bracketing the upper and the lower P limit where the round shape is present or absent in the radiographic images. It has been reported that no visible change in the size of the silicate melt sphere in an aqueous fluid matrix was observed in the radiographic images while the T was kept at 1,180 °C for 45 min (35). This indicates that the modal abundance of phases does not change with time during radiographic observation, suggesting that chemical equilibrium was attained.
After observation with the X-ray camera, the samples were quenched under the desired P-T conditions so that the X-ray radiographic images could be compared with the quenched textures. Phases in the recovered samples were identified with a JEOL JXA-8900 electron microprobe at the Cornell Center for Materials Research, Cornell University. Chemical composition was analyzed by wavelength dispersive spectrometers with a JEOL JXA-8800R electron microprobe at the Earthquake Research Institute, University of Tokyo. The data were reduced using the correction procedure of Bence and Albee (57). An acceleration voltage of 15 kV and a beam current of 12 nA were employed. Counting time was 10 s for all elements. A focused electron beam was employed for analysis of minerals. A 10- to 30-μm diameter electron beam was used to obtain the composition of quenched materials from the silicate melt and aqueous fluid.
Because radiographic studies were done within a relatively short experimental duration, such as tens of minutes (30, 35), the equilibrium was checked by conducting a series of long-duration constant-T quench experiments to see if we could reproduce quench textures of the aqueous fluids, hydrous melts, and SCFs, which were observed in the experimental products quenched after the radiography observations. Experiments were carried out using a Kawai-type multianvil high-P apparatus installed at the Earthquake Research Institute, University of Tokyo (58). In these long-duration constant-T quench experiments, only one diamond end cap was used with an AuPd sample capsule, and the high-T junction of the thermocouple was placed immediately adjacent to the AuPd sample capsule. The thermocouple reading was therefore exactly the same as the sample T. The duration of the long-duration constant-T quench experiments was 6 h. The recovered samples were polished and examined with a JEOL JXA-8800R electron microprobe and a JEOL JSM-5600LV scanning electron microscope at the Earthquake Research Institute.
Supplementary Material
Acknowledgments.
We thank K. Funakoshi, T. Fujii, A. Yasuda, S. Nakada, Y. Orihashi, T. Iizuka, S. Urakawa, Y. Nishihara, E. Takahashi, T. Okada, A. Nozawa, Y. Yoshimura, G. H. Gudfinnsson, M. Frank, H. Watson, S. Keshav, C. Hadidiacos, J. Sinnott, J. Hunt, and W. A. Bassett for helpful discussions and technical support. This study was partly supported by the Research Fellowships of the Japan Society for the Promotion of Science (JSPS) for Young Scientists, the JSPS postdoctoral fellowships for research abroad, and grants from a joint research program at the Institute for Study of the Earth’s Interior, Okayama University, the Nissan Science Foundation, and the Japanese Ministry of Education, Science, Sports, and Culture. The synchrotron radiation experiments were performed at the BL04B1 in the SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute. Reviews by three anonymous referees significantly improved the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010968108/-/DCSupplemental.
References
- 1.McBirney AR. Compositional variations in Cenozoic calc-alkaline suites of Central America; Proceedings of the Andesite Conference; Prtland, OR: Oregon Department of Geology and Mineral Industries; 1969. pp. 185–189. [Google Scholar]
- 2.Wyllie PJ, Sekine T. The formation of mantle phlogopite in subduction zone hybridization. Contrib Mineral Petrol. 1982;79:375–380. [Google Scholar]
- 3.Kushiro I. On the lateral variations in chemical composition and volume of Quaternary volcanic rocks across Japanese arcs. J Volcanol Geotherm Res. 1983;18:435–447. [Google Scholar]
- 4.Sakuyama M, Nesbitt RW. Geochemistry of the Quaternary volcanic rocks of the northeast Japan arc. J Volcan Geotherm Res. 1986;29:413–450. [Google Scholar]
- 5.Tatsumi Y, Eggins S. Subduction Zone Magmatism. Boston: Blackwell Science; 1986. [Google Scholar]
- 6.Mibe K, Fujii T, Yasuda A. Control of the location of the volcanic front by aqueous fluid connectivity in the mantle wedge. Nature. 1999;401:259–262. [Google Scholar]
- 7.Hattori KH, Guillot S. Volcanic fronts form as a consequence of serpentine dehydration in the forarc mantle wedge. Geology. 2003;31:525–528. [Google Scholar]
- 8.Elliott T, Plank T, Zindler A, White W, Bourdon B. Element transport from slab to volcanic front at the Mariana arc. J Geophys Res. 1997;102:14991–15019. [Google Scholar]
- 9.Class C, Miller DM, Goldstein SL, Langmuir CH. Distinguishing melt and fluid subduction components in Umnak Volcanics, Aleutian Arc. Geochem Geophys Geosyst. 2000;1(6):1004. 10.1029/1999GC000010. [Google Scholar]
- 10.Prouteau G, Scaillet B, Pichavant M, Maury R. Evidence for mantle metasomatism by hydrous silicic melts derived from subducted oceanic crust. Nature. 2001;410:197–200. doi: 10.1038/35065583. [DOI] [PubMed] [Google Scholar]
- 11.Grove TL, Parman SW, Bowring SA, Price RC, Baker MB. The role of an H2O-rich fluid component in the generation of primitive basaltic andesites and andesites from Mt. Shasta region, N California. Contrib Mineral Petrol. 2002;142:375–396. [Google Scholar]
- 12.Wysoczanski RJ, et al. Volatile contents of Kermadec Arc—Havre Trough pillow glasses: Fingerprinting slab-derived aqueous fluids in the mantle sources of arc and back-arc lavas. J Volcan Geotherm Res. 2006;152:51–73. [Google Scholar]
- 13.Kennedy GC, Wasserburg GJ, Heard HC, Newton RC. The upper three phase region in the system SiO2-H2O. Am J Sci. 1962;260:501–521. [Google Scholar]
- 14.Burnham CW, Jahns RH. A method for determining the solubility of water in silicate melts. Am J Sci. 1962;260:721–745. [Google Scholar]
- 15.Hamilton DL, Burnham CW, Osborn EF. The solubility of water and effects of oxygen fugacity and water content on crystallization in mafic magmas. J Petrol. 1964;5:21–39. [Google Scholar]
- 16.Eggler DH, Burnham CW. Solution of H2O in diopside melts: A thermodynamic model. Contrib Mineral Petrol. 1984;85:58–66. [Google Scholar]
- 17.Anderson GM, Burnham CW. The solubility of quartz in supercritical water. Am J Sci. 1965;263:494–511. [Google Scholar]
- 18.Nakamura Y, Kushiro I. Composition of the gas phase in Mg2SiO4-SiO2-H2O at 15 kbar. Year Book Carnegie Inst Washington. 1974;73:255–258. [Google Scholar]
- 19.Schneider ME, Eggler DH. Fluids in equilibrium with peridotite minerals: Implications for mantle metasomatism. Geochim Cosmochim Acta. 1986;50:711–724. [Google Scholar]
- 20.Ryabchikov ID. Geochemical Evolution of Earth’s Mantle. Moscow: Nauka; 1988. p. 37. (in Russian) [Google Scholar]
- 21.Paillat O, Elphick SC, Brown WL. The solubility of water in NaAlSi3O8 melts: A re-examination of Ab-H2O phase relationships and critical behaviour at high pressures. Contrib Mineral Petrol. 1992;112:490–500. [Google Scholar]
- 22.Ryabchikov ID. Fluid transport of ore metals in ultramafic mantle rocks; Proceedings of the Eighth Quadrennial International Association on the Genesis of Ore Deposits (IAGOD) Symposium; Stuttgart: Schweizerbartsche Ferlagbuchhandlung; 1993. pp. 425–433. [Google Scholar]
- 23.Manning CE. The solubility of quartz in H2O in the lower crust and upper mantle. Geochim Cosmochim Acta. 1994;58:4831–4839. [Google Scholar]
- 24.Stalder R, Ulmer P, Thompson AB, Gunther D. High pressure fluids in the system MgO-SiO2-H2O under upper mantle conditions. Contrib Mineral Petrol. 2001;140:607–618. [Google Scholar]
- 25.Mibe K, Fujii T, Yasuda A. Composition of aqueous fluid coexisting with mantle minerals at high pressure and its bearing on the differentiation of the Earth’s mantle. Geochim Cosmochim Acta. 2002;66:2273–2285. [Google Scholar]
- 26.Niggli P. Die leichtfluchtigen Bestandteile im Magma. Leipzig: BG Teubner; 1920. [Google Scholar]
- 27.Shen A, Keppler H. Direct observation of complete miscibility in the albite-H2O system. Nature. 1997;385:710–712. [Google Scholar]
- 28.Bureau H, Keppler H. Complete miscibility between silicate melts and hydrous fluids in the upper mantle: Experimental evidence and geochemical implications. Earth Planet Sci Lett. 1999;165:187–196. [Google Scholar]
- 29.Wyllie PJ, Ryabchikov ID. Volatile components, magmas, and critical fluids in upwelling mantle. J Petrol. 2000;41:1195–1206. [Google Scholar]
- 30.Mibe K, et al. Determination of the second critical end point in silicate-H2O systems using high-pressure and high-temperature X-ray radiography. Geochim Cosmochim Acta. 2004;68:5189–5195. [Google Scholar]
- 31.Kawamoto T, et al. Mg/Si ratios of aqueous fluids coexisting with forsterite and enstatite based on the phase relations in the Mg2SiO4-SiO2-H2O system. Am Mineral. 2004;89:1433–1437. [Google Scholar]
- 32.Kawamoto T. Hydrous phases and water transport in the subducting slab. Rev Mineral Geochem. 2006;62:273–289. [Google Scholar]
- 33.Hermann J, Spandler C, Hack A, Korsatov AV. Aqueous fluids and hydrous melts in high-pressure and ultra-high pressure rocks: Implications for element transfer in subduction zones. Lithos. 2006;92:399–417. [Google Scholar]
- 34.Hack AC, Thompson AB, Aerts M. Liebscher A, Heinrich CA, editors. Phase relations involving hydrous silicate melts, aqueous fluids, and minerals. Rev Mineral Geochem. 2007;65:129–185. (in Fluid-fluid interactions) [Google Scholar]
- 35.Mibe K, et al. Second critical endpoint in the peridotite-H2O system. J Geophys Res. 2007;112:B03201. 10.1029/2005JB004125. [Google Scholar]
- 36.Mibe K, Chou I-M, Bassett WA. In situ Raman spectroscopic investigation of the structure of subduction-zone fluids. J Geophys Res. 2008;113:B04208. 10.1029/2007JB005179. [Google Scholar]
- 37.Kanzaki M, et al. A new technique to measure the viscosity and density of silicate melts at high pressure. In: Manghnani MH, Syono Y, editors. High-Pressure Research in Mineral Physics. Washington, DC: Am Geophys Union; 1987. pp. 195–200. [Google Scholar]
- 38.Lambert IB, Wyllie PJ. Melting of gabbro (quartz eclogite) with excess water to 35 kilobars, with geological applications. J Geol. 1972;80:693–708. [Google Scholar]
- 39.Green TH. Anatexis of mafic crust and high pressure crystallization of andesite. In: Thorpe RS, editor. Andesites: Orogrenic Andesites and Related Rocks. New York: Wiley; 1982. pp. 465–487. [Google Scholar]
- 40.Kessel R, Ulmer P, Pettke T, Schmidt MW, Thompson AB. The water-basalt system at 4 to 6 GPa: Phase relations and second critical endpoint in a K-free eclogite at 700 to 1400 °C. Earth Planet Sci Lett. 2005;237:873–892. [Google Scholar]
- 41.Gill JB. Orogenic Andesite and Plate Tectonics. Berlin: Springer; 1981. [Google Scholar]
- 42.England P, Engdahl R, Thatcher W. Systematic variation in the depths of slabs beneath arc volcanoes. Geophys J Int. 2004;156:377–408. [Google Scholar]
- 43.Syracuse EM, Abers GA. Global compilation of variations in slab depth beneath arc volcanoes and implications. Geochem Geophys Geosyst. 2006;7:Q05017. 10.1029/2005GC001045. [Google Scholar]
- 44.Keppler H. Constraints from partitioning experiments on the composition of subduction-zone fluids. Nature. 1996;380:237–240. [Google Scholar]
- 45.Kessel R, Schmidt MW, Ulmer P, Pettke T. Trace element signature of subduction-zone fluids, melts and supercritical liquids at 120–180 km depth. Nature. 2005;437:724–727. doi: 10.1038/nature03971. [DOI] [PubMed] [Google Scholar]
- 46.Ishikawa T, Nakamura E. Origin of the slab component in arc lavas from across-arc variation of B and Pb isotopes. Nature. 1994;370:205–208. [Google Scholar]
- 47.Ryan JG, Morris J, Tera F, Leeman WP, Tsvetkov A. Cross-arc geochemical variations in the Kurile Arc as a function of slab depth. Science. 1995;270:625–627. [Google Scholar]
- 48.Kay RW. Aleutian magnesian andesites: Melts from subducted Pacific Ocean crust. J Volcanol Geotherm Res. 1978;4:117–132. [Google Scholar]
- 49.Defant MJ, Drummond MS. Derivation of some modern arc magmas by melting of young subducted lithosphere. Nature. 1990;347:662–665. [Google Scholar]
- 50.Rapp RP, Watson EB. Dehydration melting of metabasalt at 8–32 kbar: Implications for continental growth and crust-mantle recycling. J Petrol. 1995;36(4):891–931. [Google Scholar]
- 51.Defant MJ, Drummond MS. Mount St. Helens: Potential example of the partial melting of the subducted lithosphere in a volcanic arc. Geology. 1993;21:547–550. [Google Scholar]
- 52.Martin H. Adakitic magmas: Modern analogues of Archaean granitoids. Lithos. 1999;46:411–429. [Google Scholar]
- 53.Ulmer P, Trommsdorff V. Serpentine stability to mantle depths and subduction-related magmatism. Science. 1995;268:858–861. doi: 10.1126/science.268.5212.858. [DOI] [PubMed] [Google Scholar]
- 54.Samsonov AV, et al. The relationship between adakitic, calc-alkaline volcanic rocks and TTGs: Implications for the tectonic setting of the Karelian greenstone belts, Baltic Shield. Lithos. 2005;79:83–106. [Google Scholar]
- 55.Utsumi W, et al. High-pressure science with a multi-anvil apparatus at SPring-8. J Phys Condens Matter. 2002;14:10497. 10.1088/0953-8984/14/44/322. [Google Scholar]
- 56.Hofmann AW. Chemical differentiation of the Earth: The relationship between mantle. continental crust, and oceanic crust. Earth Planet Sci Lett. 1988;90:297–314. [Google Scholar]
- 57.Bence AE, Albee AL. Empirical correction factors for the electron microanalysis of silicates and oxides. J Geol. 1968;76:382–403. [Google Scholar]
- 58.Mibe K, Yoshino T, Ono S, Yasuda A, Fujii T. Connectivity of aqueous fluid in eclogite and its implications for fluid migration in the Earth’s interior. J Geophys Res. 2003;108(B6):2295. 10.1029/2002JB001960. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.