Abstract
Fibroblasts are important participants in inflammation. Although not leukocytes, their capacity to produce cytokines, chemokines, and other inflammatory factors locally in tissues suggests that they can contribute to inflammatory diseases. For example, fibroblasts in a rheumatoid arthritis (RA) joint are a dominant source of IL-6 and RANKL in the synovium, both of which are therapeutic targets for inflammation and bone erosion. Previously, we found that fibroblasts can be targeted by mAb directed against cadherin-11 (cad-11), a mesenchymal cadherin that fibroblasts selectively express. Targeting cad-11 significantly reduced inflammation as assessed by joint swelling and clinical inflammation scores. However, the mechanism by which anti–cad-11 reduced inflammation was not known. Here, we show that cad-11 engagement induces synovial fibroblasts to secret proinflammatory cytokines including IL-6. Cad-11 engagement strongly synergized with TNF-α and IL-1β in the induction of IL-6. Importantly, cad-11 activated MAP kinases and NF-κB for IL-6 induction. IL-6 levels in ankles of inflamed joints were reduced in cad-11 mutant mice compared to wild-type mice with inflammatory arthritis. Thus, we suggest that cad-11 modulates synovial fibroblasts to evoke inflammatory factors that may contribute to the inflammatory process in RA.
Cadherin-11 (cad-11) is a classical cadherin adhesion molecule that mediates homophilic cell-to-cell adhesion (1–3). The expression pattern of cad-11 is largely restricted to mesenchymal tissues, and thus cad-11 mediates selective association of mesenchymal cells during the development of mouse embryos (4, 5). In humans, mRNA transcripts for cad-11 are detected in placenta, brain, lung, and heart (6, 7). However, high levels of expression are found mainly on osteoblasts and synovial fibroblasts (8).
The synovium in normal joints consists of a lining layer of condensed cells, one to three cells thick, that overlies the loose connective tissue of the synovial sublining (9). Synovial fibroblasts make up the synovial lining and they cocompact with macrophages (10). In rheumatoid arthritis (RA), the synovium is the main site of inflammation and the synovial lining becomes hyperplastic and transforms into a pannus tissue that attaches to, crawls over, invades, and destroys articular cartilage (11, 12). We found that cad-11 is expressed on synovial fibroblasts in the synovium and is required for development of the lining layer of the joint synovium (8, 13–15).
Previously, we reported that cad-11 plays an important role in the pathogenesis of inflammatory arthritis. Mice deficient in cad-11 showed markedly reduced damage to the articular cartilage (15). Because cad-11 is expressed primarily on synovial fibroblasts in the synovium, these findings suggest a key role for synovial fibroblasts in cartilage damage in inflammatory arthritis, consistent with other studies showing that synovial fibroblasts can degrade cartilage (16). Unexpectedly, cad-11–directed therapeutics also markedly reduced synovial inflammation (15). Because cad-11 has not been noted on leukocytes (8, 15), these findings support a role for synovial fibroblasts in inflammatory mechanisms. Although fibroblasts are not typically considered to be inflammatory cells, many studies have revealed their capacity to produce inflammatory factors that may be important in RA such as cytokines, chemokines, growth factors, and lipid mediators (17–19). IL-6 is known to be a key cytokine in RA pathogenesis on the basis of its efficacy in clinical trials (20, 21), and synovial fibroblasts are reported to be the main cells producing IL-6 in the synovium (22, 23).
Here, we show that cad-11–mediated activation of synovial fibroblasts results in their production of proinflammatory factors including IL-6, suggesting that cad-11 may be an important modulator of IL-6 production by synovial fibroblasts. In fact, cad-11 engagement synergized with TNF-α to markedly increase IL-6 production. Furthermore, the levels of IL-6 were decreased in ankles from cad-11–deficient mice compared with wild-type mice with inflammatory arthritis. Together, our findings reveal that cad-11 plays a critical role in evoking synovial fibroblast inflammatory factors that may contribute to RA.
Results
Cad-11 Engagement Induces Cytokine Production by Synovial Fibroblasts.
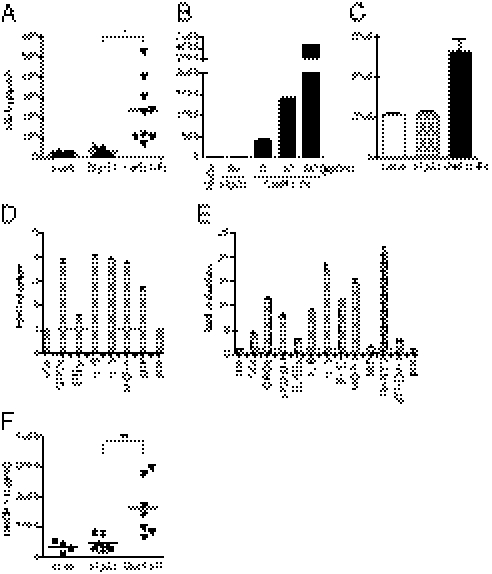
Cadherins are known for their function in cell-to-cell adhesion in tissues (2), and they can influence β-catenin–determined transcription of the T-cell factor (TCF)/lymphocyte enhancer factor (24, 25). However, cadherins have not previously been known to regulate the expression of inflammatory cytokines and chemokines. In fact, our findings that cad-11–deficient mice or anti–cad-11 mAb therapies significantly reduced inflammation in arthritic mice suggest that cadherin expression might regulate the inflammatory capacity of synovial fibroblasts. In support of this idea, we found that synovial fibroblast cultures at high confluence (where cell-to-cell cad-11 engagement is strong) resulted in more IL-6 secretion than did cultures of the same number of synovial fibroblasts at subconfluent densities (where cell-to-cell cad-11 engagement is limited). Because altering cell density and confluence changes many other factors as well, we developed a cad-11–specific reagent that was designed to engage cad-11 in a manner that mimicked cell-to-cell cadherin engagement. Thus, we generated a human cadherin (hCad)-11-Fc in which the five extracellular cad-11 domains were linked to human IgG1 Fc. Using this recombinant hCad-11-Fc reagent, we asked if direct cad-11 engagement could regulate IL-6 production by synovial fibroblasts. Strikingly, hCad-11-Fc induced IL-6 by primary synovial fibroblasts, compared with isotype control Ig (hIgG1) (Fig. 1A, 234 ± 52 pg/mL by hCad-11-Fc vs. 29 ± 6 pg/mL by hIgG1; n = 9, P = 0.0012). The induction of IL-6 by hCad-11-Fc was regulated at the mRNA level (Fig. S1, peak induction around 2 h stimulation) and showed a clear dose-dependent response (Fig. 1B). Although there were variations of the absolute levels of IL-6 that were produced by primary cells derived from each patient with RA, hCad-11-Fc induced IL-6 (234.0 ± 52.11 pg/mL, n = 9) at the concentration of 5 μg/mL (Fig. 1A). However, when synovial fibroblasts were activated with a higher concentration of hCad-11-Fc at 20 μg/mL, IL-6 secretion was significantly increased up to about 5,000 pg/mL (Fig. 1B). As a control, TNF-α at 1 ng/mL strongly induced IL-6 secretion at the range of 300–3,000 pg/mL in eight different synovial fibroblast lines. hCad-11-Fc also stimulated synovial fibroblasts to induce IL-6 in 3D micromass organ culture, which suggested that cell adherence to the culture dish did not have a significant effect on cytokine production by cad-11 ligation (Fig. 1C). We next performed a cytokine antibody array to identify other proinflammatory factors that cad-11 engagement might stimulate synovial fibroblasts to secrete. IL-6 was up-regulated by hCad-11-Fc stimulation, which was consistent with the results of the single factor ELISA (Fig. 1D). Interestingly, the array revealed that cad-11 also significantly increased secretion of MCP-1 (CCL2), GROα (CXCL1), IL-8, and macrophage migration inhibition factor (MIF) in synovial fibroblasts. For comparison with the factors induced by cad-11 engagement, we used TNF-α that is known to be a major stimulator of synovial fibroblasts. TNF-α activated a broader range of proinflammatory factors, including those that were induced by hCad-11-Fc as well as complement component 5a (C5a), soluble intercellular adhesion molecule 1 (sICAM-1), an IL-1 receptor antagonist (IL-1Ra), IFNγ-induced protein 10 (IP-10, CXCL10), soluble triggering receptor expressed on myeloid cells 1 (sTREM-1), and a regulated upon activation, normal T-cell–expressed and secreted factor (RANTES, CCL5) (Fig. 1E). Interestingly, MIF was uniquely increased by cad-11 stimulation in synovial fibroblasts. To confirm the array data, one of the factors, MCP-1, was quantified by direct ELISA. As shown in Fig. 1F, hCad-11-Fc stimulation significantly induced MCP-1 by synovial fibroblasts (1632 ± 349 pg/mL by hCad-11-Fc vs. 471 ± 89 pg/mL by hIgG1; P = 0.0073, n = 7). These results strongly indicated that cad-11 functions as a signaling receptor to activate synovial fibroblasts to produce proinflammatory factors that may be of significance in synovial inflammation.
Fig. 1.
Cad-11 engagement induces synovial fibroblasts to produce inflammatory factors. (A) Primary synovial fibroblasts were incubated with or without 5 μg/mL hIgG1 or hCad-11-Fc for 2 d in 1% FBS/SF medium. Cell culture supernatants were analyzed for IL-6 by ELISA. The results shown were accumulated from more than five independent experiments. (B) Synovial fibroblasts were cultured with or without 20 μg/mL hIgG1 or the indicated concentrations (μg/mL) of hCad-11-Fc for 2 d in 1% FBS/SF medium, and then the supernatants were analyzed for IL-6 by ELISA. Data shown represent more than three independent experiments. (C) Synovial fibroblasts were stimulated with or without 10 μg/mL hIgG1 or hCad-11-Fc in a 3D micromass organ culture for 24 h. Culture supernatants were analyzed for IL-6 by ELISA. Data shown represent three independent experiments. (D and E) After culturing synovial fibroblasts with 10 μg/mL hIgG1, hCad-11-Fc, or 1 ng/mL TNF-α for 24 h, cell-free supernatants were examined by a cytokine antibody array. Each spot was quantified by a densitometer, and the graph shows fold induction of each molecule in the (D) hCad-11-Fc– or (E) TNF-α–treated group relative to the hIgG1-treated group. The factors shown in D and E were at least more than 1.5-fold induction. (F) Synovial fibroblasts were treated the same way as in A. MCP-1 was measured by ELISA. The results were accumulated from more than four independent experiments. Compared with hIgG1, *P = 0.0012 and **P = 0.0073. pos, positive control.
Specificity of Cad-11 Engagement in Stimulation of IL-6 Expression.
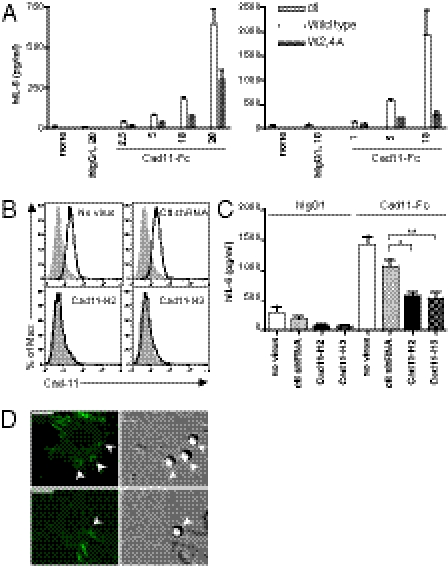
To verify that the action of hCad-11-Fc on synovial fibroblast stimulation is specifically mediated through direct engagement of cad-11 on the cell surface, we performed a series of additional specificity controls. We generated a mutant hCad-11-Fc (W2,4A) that contains two point mutations resulting in amino acid changes from Trp to Ala at position 2 and at position 4 of extracellular domain 1 (EC1) of cad-11. Homophilic binding of cad-11 to cad-11 is known to be mediated by these residues through EC1 interaction from each pair of cadherin molecules binding in trans and is required for forming a strand-swap interaction (26). A mutant form of cadherin EC1 domains (from Trp to Ala at position 2) was known to form an intermediate X-dimer, but not complete mature strand-swap formation, which decreases the binding affinity of cadherin to cadherin (27). Thus, we compared the abilities of wild-type hCad-11-Fc and a mutant hCad-11-Fc to stimulate cad-11 expressing synovial fibroblasts. Importantly, the mutant hCad-11-Fc significantly lost its ability to stimulate IL-6 secretion in both less responsive cells (Fig. 2A, Left) and more responsive cells (Fig. 2A, Right). Additionally, adhesion of synovial fibroblasts to mutant hCad-11-Fc on plates also decreased, which confirmed that the mutant hCad-11-Fc had lower binding affinity to cad-11 on synovial fibroblasts. These results are consistent with the interpretation that the binding of hCad-11-Fc to cell surface cad-11 on synovial fibroblasts is similar to the interactions between natural cell surface cad-11 on adjacent cells. These results further support that the effect of hCad-11-Fc on stimulating synovial fibroblasts to produce IL-6 is mediated by cad-11. Note that synovial fibroblasts are not known to express Fc receptors and that mutant hCad-11-Fc has the same Fc portion and is otherwise the identical molecule to wild-type hCad-11-Fc except for the two amino acids critically involved in homophilic adhesion. In another approach to confirm specificity, we silenced cad-11 expression in synovial fibroblasts by lentivirus containing cad-11 shRNA constructs. Compared with mock or control shRNA-transduced cells, surface cad-11 expression on synovial fibroblasts was almost completely lost when either of the cad-11 shRNA constructs was used (Fig. 2B). Importantly, although lentivirus containing control shRNA had no effect on the ability of hCad-11-Fc to stimulate IL-6 production by synovial fibroblasts, cad-11–silenced cells were markedly impaired in the ability to secrete IL-6 in response to the simulation with hCad-11-Fc (Fig. 2C). Interestingly, the basal levels of IL-6 were also lower in cad-11–silenced synovial fibroblasts than in control groups, suggesting that interactions between endogenous cad-11 may also contribute to constitutive levels of IL-6 expression and that the effect of hCad-11-Fc on IL-6 production was specifically through cad-11 on synovial fibroblasts. Finally, we showed that the hCad-11-Fc binds directly to transmembrane cad-11 expressed on the surface of fibroblasts. When hCad-11-Fc–coated beads were attached to cells, clustering of GFP signals was detected around the beads, whereas control hIgG1-coated beads were not able to induce cadherin clustering on cells (Fig. 2D). Cad-11–mediated adherens junctions were also observed between cells. After incubating cells with hIgG1 or hCad-11-Fc–coated beads, washes removed all of the beads, which indicated that the clustering around the beads was not simply reflected by phagocytosis. Thus, these results suggest that hCad-11-Fc actually binds to cell surface cad-11 and then induces its clustering.
Fig. 2.
Specificity of cad-11–mediated IL-6 production. (A) Synovial fibroblasts were stimulated with wild-type or mutant hCad-11-Fc at various concentrations (μg/mL) in 1% FBS/SF medium. IL-6 secreted into the supernatants was quantified by ELISA. The representative synovial fibroblasts (Left: less responsive cells; Right: more responsive cells) from three independent experiments are shown. (B) After silencing cad-11 by shRNA (cad11-H2 or cad11-H3) in synovial fibroblasts, surface cad-11 was detected by FACS analysis (filled histograms: isotype control; open histograms: cad-11). No virus and control shRNA were used as controls. Data shown represent five independent experiments. (C) Cad-11 shRNA or control shRNA-transduced synovial fibroblasts were stimulated with 5 μg/mL of hIgG1 or hCad-11-Fc in 1% FBS/SF medium for 2 d. Cell-free supernatants were analyzed by ELISA for IL-6. The results shown were accumulated from four independent experiments. When cad11-H2 and cad11-H3 were compared with control shRNA, *P = 0.0018 and **P = 0.0020, respectively. (D) Cad-11–coated beads induce cad-11 clustering on the cell surface. Protein A-precoated polystyrene beads (5.5 μm) were bound with hIgG1 or hCad-11-Fc and added to L cells expressing GFP-cad-11. After fixation, cells were analyzed by confocal microscopy to detect GFP signals clustered around the beads. Digital image correlation was also taken to confirm the locations of beads on the cell surface. Arrowheads indicate the beads on L cells. (Scale bars, 10 μm.)
Cad-11 Activates JNK, ERK, and NF-κB.
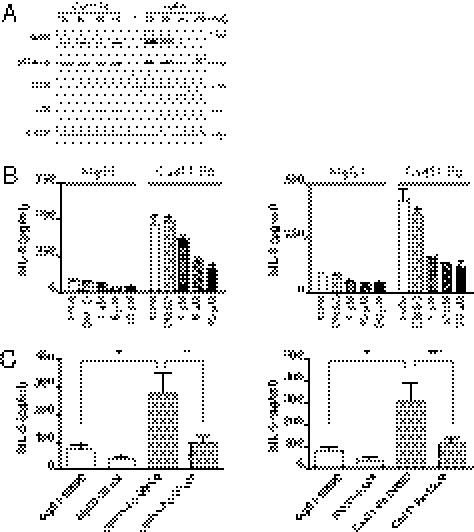
The MAP kinase family and NF-κB are well-known signaling pathways that can result in IL-6 expression (28). Thus, we first tested if the MAP kinases are activated by hCad-11-Fc stimulation in synovial fibroblasts. After stimulating synovial fibroblasts with hCad-11-Fc for various times (15, 30, 60, and 180 min), the levels of phosphorylation of MAP kinase family members were detected by immunoblotting. We found that JNK and ERK1/2 but not p38 were significantly activated by stimulation with hCad-11-Fc (Fig. 3A, Left). We also examined MAP kinase family activation by TNF-α compared with the activation by hCad-11-Fc (Fig. 3A, Right). Interestingly, cad-11 activation resulted in persistent activation of JNK over at least a 180-min time period, whereas the activation of JNK was strong but very transient and back to the basal level at 60 min following stimulation with TNF-α. The kinetics of ERK1/2 activation was similar when cells were stimulated with either hCad-11-Fc or TNF-α (Fig. 3A). The inhibition of JNK by sp600125 and of ERK by U0126 markedly blocked IL-6 secretion following hCad-11-Fc stimulation in a dose-dependent manner (Fig. 3B, sp600125: Left; U0126: Right) and was statistically significant at 10 μM for sp600125 (Fig. 3C, Left) or at 20 μM for U0126 (Fig. 3C, Right). Thus, JNK and ERK1/2 appear to play an important role in IL-6 induction by cad-11 in synovial fibroblasts. Next, because NF-κB is well known to be an important transcription factor for IL-6 expression and is related to RA pathogenesis (29, 30), we also examined whether cad-11 activates NF-κB. Phosphorylation of p65 (p-p65) was increased at 15, 30, and 60 min after hCad-11-Fc stimulation by immunoblotting (Fig. 4A) and was correspondingly increased by ELISA (Fig. 4B). Note that although the level of p-p65 in synovial fibroblasts induced by cad-11 was low, it was reproducible among more than three independent experiments. These results indicate that cad-11 engagement leads to activation of JNK, ERK1/2, and NF-κB, which are involved in IL-6 induction in synovial fibroblasts.
Fig. 3.
Cad-11 engagement strongly activates JNK and ERK in synovial fibroblasts. (A) After synovial fibroblasts were serum-starved overnight and stimulated with or without hCad-11-Fc (20 μg/mL) or TNF-α (10 ng/mL) for the indicated times in 1% FBS/SF medium, total cell lysates were analyzed for pJNK, pERK1/2, or pp38 by immunoblotting. Total p38 and β-actin were used as loading controls. Molecular markers (kDa) are shown. (B) Synovial fibroblasts were pretreated with or without DMSO (0.16%) or inhibitors for JNK (sp600125, Left) or ERK (U0126, Right) at the indicated concentrations for 30 min, and then cells were stimulated with 5 μg/mL hIgG1 or hCad-11-Fc overnight in 1% FBS/SF medium. IL-6 was measured by ELISA. (C) Synovial fibroblasts were treated the same way as in B except for concentrations of inhibitors, 10 μM of sp600125 (Left), and 20 μM of U0126 (Right). Data were accumulated from more than eight independent experiments. When hCad-11-Fc is compared with hIgG1, *P < 0.01. When sp600125 and U0126 were compared with DMSO control in the hCad-11-Fc–stimulated group, **P = 0.0292 and ***P = 0.0349, respectively.
Fig. 4.
Cad-11 stimulates NF-κB activation in synovial fibroblasts. Serum-starved synovial fibroblasts were stimulated with hCad-11-Fc (20 μg/mL) or TNF-α (10 ng/mL) for the indicated times. (A) Total cell lysates were examined for p-p65 and total p65 by immunoblotting (Upper). The band density of p-p65 at each time point was measured by a densitometer. Relative units were calculated by dividing the band density at each time point by the band density at 0 min (Lower). (B) Synovial fibroblasts were stimulated the same way as in A for 15 min and for 30 min. p-p65 was measured by direct ELISA in total cell lysates (Upper). One representative of three independent experiments is shown. The level of p-p65 was confirmed by immunoblotting in the same cell lysates, and p65 was used as a control (Lower). IB, immunoblotting.
Cad-11 Synergizes with TNF-α or IL-1β in Inducing IL-6 Expression.
To model the potential role of cad-11 signaling on IL-6 production in an inflammatory microenvironment, synovial fibroblasts were stimulated with hCad-11-Fc in the presence of TNF-α or IL-1β. The concentrations of TNF-α and IL-1β were titrated down, and 0.1 ng/mL of TNF-α and 0.01 ng/mL of IL-1β were found to suboptimally induce IL-6 in this system. Compared with TNF-α, IL-1β, or hCad-11-Fc alone, hCad-11-Fc significantly increased IL-6 secretion by synovial fibroblasts in the presence of suboptimal concentrations of TNF-α or IL-1β (Fig. 5A). The results suggest that cad-11 engagement on synovial fibroblasts has a synergistic effect with inflammatory cytokines like TNF-α and IL-1β in stimulating IL-6 secretion. Interestingly, compared with stimulation with only one agent, JNK activation was higher when cells were activated with both hCad-11-Fc and TNF-α. In contrast, activation of ERK1/2 was already maximized by stimulation with hCad-11-Fc alone (Fig. 5B). These data imply that the synergistic effect of cad-11 with TNF-α is likely through activation of JNK. The production of IL-6 by these mechanisms may contribute to the initiation or perpetuation of inflammation in inflammatory arthritis.
Fig. 5.
Cad-11 synergizes with TNF-α or IL-1β for IL-6 production. (A) Synovial fibroblasts were stimulated with or without 0.1 ng/mL TNF-α (Left) or 0.01 ng/mL IL-1β (Right) in the combination of 5 μg/mL hIgG1 or hCad-11-Fc for 2 d. Cell culture supernatants were analyzed for IL-6 by ELISA. Data represent more than three independent experiments. (B) Serum-starved synovial fibroblasts were stimulated with or without hCad-11-Fc (20 μg/mL) in the presence or absence of the indicated concentrations of TNF-α for 15 min, and total cell lysates were determined for phosphorylation of ERK1/2 and JNK by immunoblotting.
Cad-11 Expression Influences IL-6 Production in an Inflammatory Arthritis Model in Vivo.
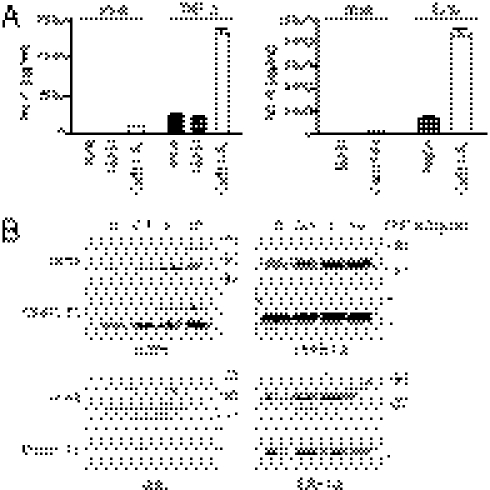
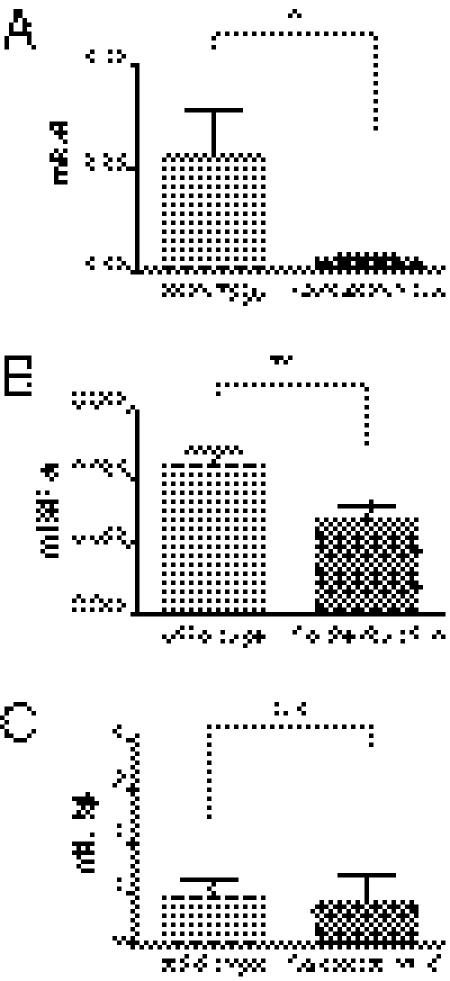
Having found that cad-11 was able to stimulate IL-6 secretion directly and to synergize with TNF-α in enhancing IL-6 secretion in vitro, we next asked if cad-11 modulates IL-6 production in inflammatory arthritis in vivo. Compared with wild-type mice, cad-11–deficient mice had less joint inflammation by measuring ankle thickness and clinical score (Fig. S2 A and B). Interestingly, TNF-α and IL-6 expression were significantly decreased in the ankle joints from cad-11–deficient mice compared with those from wild-type mice (Fig. 6 A and B), whereas differences in IL-1β expression were not statistically significant (Fig. 6C). These results suggest that, as it did in vitro, cad-11 also influenced levels of inflammatory cytokine production in synovium in an inflammatory arthritis model in vivo.
Fig. 6.
Cad-11 regulates IL-6 production in vivo. WT and cad-11–deficient mice (six mice per group) were induced with arthritis by injecting K/BxN serum. Total RNA was isolated from hind ankle joints at day 4. Mouse IL-6 (A), TNF-α (B), and IL-1β (C) were analyzed by quantitative real-time PCR. The expression levels of each cytokine were normalized to GAPDH. When compared the levels of IL-6 and TNF-α in wild-type and cad-11–deficient mice, *P = 0.0472 and **P = 0.0144, respectively. n.s., not significant.
Discussion
Although it has been suggested that fibroblasts are actively involved in chronic inflammatory reactions (31), the molecular mechanisms of their sustained activation and their contribution to inflammatory cascades in tissues remain unclear. Our previous work revealed that cad-11 on synovial fibroblasts plays a role in synovial inflammation (15). However, how cad-11 modulates inflammation is not known. Moreover, the concept that cadherins modulate inflammation is new because they have previously been known as cell adhesion molecules responsible for tissue morphogenesis and architecture (1–3). Here we found that cad-11 directly modulates cytokine production by synovial fibroblasts, particularly IL-6. Cad-11 engagement induced synovial fibroblasts to produce IL-6 and other inflammatory factors. In fact, when we induced inflammatory arthritis by K/BxN serum transfer in mice, the levels of IL-6 were significantly decreased in the ankle joints from cad-11–deficient mice compared with wild-type mice. Elevated levels of IL-6 are found in synovial fluid and serum of patients with RA and can be directly correlated with the extent of joint destruction disease severity (32–34). Moreover, blockade of IL-6 receptor with therapeutic mAb was recently Food and Drug Administration-approved as an effective therapy for RA (20, 21). Several mouse models of inflammatory arthritis support a mechanistic role of IL-6 in pathogenesis (35–38). Given that synovial fibroblasts are the major producers of IL-6 in synovium (22, 23), our in vivo data strongly suggest that IL-6 production in inflammatory arthritis may be modulated by cad-11.
Moreover, there were dramatic synergistic effects of cad-11 with TNF-α or IL-1β on IL-6 induction in synovial fibroblasts, suggesting that cad-11 might set a threshold for fibroblasts responding to activation by inflammatory cytokines. Furthermore, a recent study showed that TNF-α increased cad-11 expression on synovial fibroblasts in vitro and found that the expression level of cad-11 may be higher in inflammatory compared with normal synovium in vivo (39), which may further contribute the synergistic effects of cad-11 with TNF-α on IL-6 production in patients. In addition to increased expression of cad-11 on synovial fibroblasts, activation of JNK was increased when cells were stimulated with cad-11 in the presence of TNF-α (Fig. 5B). Therefore, intrinsic signaling could also participate mechanistically in the synergistic effects of cad-11 with TNF-α.
Cad-11 induced several proinflammatory cytokines in addition to IL-6, including IL-8, MIF, and MCP-1. All of these factors have been shown to be relevant to joint inflammation in RA. IL-8 and MCP-1 are key chemotactic factors for macrophages that play an important role in inflammatory arthritis. IL-8 also recruits neutrophils and promotes angiogenesis (40). Notably, MIF was strongly induced by hCad-11-Fc, but not by TNF-α, and has been implicated in cell migration, invasion, and inflammation. MIF-deficient mice had decreased inflammation in the collagen induced arthritis model (41, 42). Thus, these factors may also participate in cad-11–mediated joint inflammation. Taken together, cad-11 may regulate the intrinsic activation of synovial fibroblasts to influence the expression of proinflammatory factors, which may contribute to inflammation in RA.
It was also relevant to understand the mechanisms by which cad-11 modulates cytokine production in synovial fibroblasts. Cadherin signaling pathways that modulate cytokine production have not been previously described. In this study, we found that cad-11 engagement activated the MAP kinases, JNK, and ERK1/2, but not p38 in synovial fibroblasts. Furthermore, evidence for classical NF-κB activation was detected by phosphorylation of p65 following cad-11 stimulation. Both MAP kinases and NF-κB have been shown to induce IL-6 in several cell types. Human IL-6 promoter contains glucocorticoid response element, multiple response element, cAMP response element, CCAAT response element binding protein, activator protein 1, and NF-κB binding sites. Each specific inhibitor for JNK or ERK significantly blocked IL-6 production, implying that JNK and ERK are responsible for IL-6 induction by cad-11 stimulation. AP-1 and other transcriptional factors may be regulated by JNK and ERK to induce IL-6, respectively. The known intracellular binding partners of cadherin are the catenins that include p120 ctn, β-catenin, and α-catenin. p120ctn regulates small GTPases, Rac1, CDC42, and RhoA that have been shown to activate MAP kinase family members and NF-κB (43). A study showed that depletion of p120ctn increased skin inflammation by activating RhoA, ROCK, and the NF-κB pathway. A chemical inhibitor for RhoA kinase, Y-27632, decreased NF-κB activation in keratinocytes (44). However, Y-27632 did not block IL-6 production by cad-11 in synovial fibroblasts. In contrast, the Rac1 inhibitor, NSC-23672, reduced IL-6 expression by cad-11. It was suggested that Rac1 mediated STAT3 activation through autocrine IL-6 induction. IL-6 mRNA was higher in the cells transfected with a constitutively active form of Rac1, and STAT 3 activity in the cells was blocked by anti–IL-6 neutralizing antibody (45). Therefore, the p120ctn and Rac1 pathway might also contribute to cad-11 signaling to induce IL-6 in synovial fibroblasts. β-Catenin binds to a distal region of the cad-11 intracellular domain. TCF/β-catenin is a well-known transcription factor that is activated by canonical Wnt signaling. A study showed that IL-6 was induced at the level of transcription in wnt-5α–transfected synovial fibroblasts (46). Thus, it is also possible that β-catenin contributes to IL-6 production in response to cad-11. Another possibility is that cad-11 may associate with other signaling receptors on the cell surface in certain conditions. Accumulating evidence suggests that crosstalk between cadherins and growth factor receptor tyrosine kinases (RTKs) occurs (47). Interestingly, a recent report found that cad-11 can associate with the FGFR-1 on ventral spinal cord explants from mouse embryos to enhance neurite outgrowth (48). Thus, it is possible that crosstalk between cad-11 and RTKs may be also involved in cytokine production by synovial fibroblasts.
Although much remains to be learned regarding the potential mechanisms by which cad-11 on synovial fibroblasts might modulate fibroblast activation and contribute to tissue inflammation, our studies reveal the existence of this pathway and clearly implicate MAP kinases and NF-κB as important participants. Because cad-11 has also been reported to be up-regulated on fibroblasts in other inflamed tissues such as skin (49) and lung (39), it is likely that cad-11 plays a similar role in fibroblast-mediated inflammation in these and other tissues as well.
Materials and Methods
Reagents.
Human cad-11 extracellular domain (nucleotides 1–1,827) was cloned into pFUSE-human IgG1-Fc1 vector (InvivoGen) to generate hCad-11-Fc. A mutant hCad-11-Fc (W2,4A) was generated by site-directed mutagenesis (Stratagene). WT and a mutant hCad-11-Fc constructs were stably expressed in 293T cells. hCad-11-Fc in supernatants was purified by protein A column (Bio X Cell). hIgG1 was purchased from Sigma-Aldrich. Recombinant human TNF-α and IL-1β were purchased from R&D Systems. Sp600125 was from Calbiochem. U0126 (9903) was from Cell Signaling Technology, Inc.
Synovial Fibroblast Culture and Stimulation.
Human primary synovial fibroblasts were derived from synovial tissues of patients with RA and cultured as described previously (8). Cultures of synovial fibroblasts between passage 4 and passage 9 were used for our experiments. Synovial fibroblasts were grown in synovial fibroblast medium [SF medium; DMEM supplemented with 10% FBS (HyClone), 2 mM l-glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin sulfate, 10 μg/mL gentamycin, 55 μM 2-mercaptoethanol, and essential and nonessential amino acids (Gibco BRL)] at 37 °C under 10% CO2. For stimulation, cells were released from tissue culture flasks using 0.02% (wt/vol) irradiated and L-1-tosylamido-2-phenylethyl chloromethyl ketone-treated trypsin (Worthington Biochemical Corp.) in Hepes-buffered saline (HBS) containing 2 mM CaCl2 (HBS− Ca2+) for 5 min at 37 °C to minimize cadherin proteolysis. For stimulation, synovial fibroblasts were resuspended in 1% FBS/SF medium and then seeded in flat-bottomed 96-well plates at 15,000 cells/200 μl/well, unless otherwise indicated. For 3D micromass organ culture, cells (1 × 106/mL) were resuspended with matrigel (#354234; BD Biosciences) and dropped (25 μl) onto poly-2-hydroxyethyl-methacrylate (poly-HEMA; Aldrich Chemical Co.)-coated culture dishes. Poly-HEMA coating prevented attachment of the micromass to the culture dish plastic. Gelation was allowed for 1 h at 37 °C and then culture medium was added (14, 15).
Mice and Real-Time PCR.
Control and cad-11–deficient mice (B6:129) were maintained at Taconic. Males aged 10 wk were used for these studies. K/BxN serum (150 μL) was injected i.p. into mice at day 0 and day 2. Ankle thickness and clinical score were measured on days 0, 2, and 4. Two hind ankles were collected at day 4, and total RNA was isolated from the tissues by using a RNeasy Mini Kit (Qiagen) and converted to cDNA (QuantiTect Reverse Transcription kit, Qiagen) according to the manufacturer's instructions. Quantitative RT-PCR (Agilent Technologies) was performed to analyze the levels of mIL-6, mTNF-α, and mIL-1β (the probes were from SABiosciences).
Cytokine Array.
The profile of inflammatory cytokines secreted by synovial fibroblasts was analyzed by using a human Cytokine Antibody Array kit (#ARY005; R&D Systems). Cells (100,000 cells/500 μL/well in 24-well plates) were stimulated with 10 μg/mL hIgG1, hCad-11-Fc, or 1 ng/mL TNF-α for 24 h, and cell culture supernatants were collected and analyzed according to the manufacturer's instruction.
Statistical Analysis.
Statistical analysis was performed using the Student's t test. Values of P < 0.05 were considered to be significant. All error bars in figures represent SEM.
Supplementary Material
Acknowledgments
We thank Gerald F. M. Watts for hCad-11-Fc preparation. This work was supported by Grants P01 AI 065858 and R01 AR 48114 from the National Institutes of Health (to M.B.B.); an award from the American College of Rheumatology Research and Education Foundation (to M.B.B.); a postdoctoral fellowship from the Arthritis Foundation (to S.K.C. and K.T.); the Abbott Scholar Award in Rheumatology Research and American College of Rheumatology Research and Education Foundation (to E.H.N.); and a Howard Hughes Gilliam Fellowship for Advanced Study (to L.L.).
Footnotes
Conflict of interest statement: M.B.B. has equity options and receives consulting fees from Synovex Corp., a company pursuing rheumatoid arthritis therapies.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019437108/-/DCSupplemental.
References
- 1.Takeichi M. Cadherins: A molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- 2.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 3.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann I, Balling R. Cloning and expression analysis of a novel mesodermally expressed cadherin. Dev Biol. 1995;169:337–346. doi: 10.1006/dbio.1995.1148. [DOI] [PubMed] [Google Scholar]
- 5.Kimura Y, et al. Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Dev Biol. 1995;169:347–358. doi: 10.1006/dbio.1995.1149. [DOI] [PubMed] [Google Scholar]
- 6.Shibata T, Ochiai A, Gotoh M, Machinami R, Hirohashi S. Simultaneous expression of cadherin-11 in signet-ring cell carcinoma and stromal cells of diffuse-type gastric cancer. Cancer Lett. 1996;99:147–153. doi: 10.1016/0304-3835(95)04047-1. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi J, et al. Expression and function of the splice variant of the human cadherin-11 gene in subordination to intact cadherin-11. J Bone Miner Res. 1999;14:764–775. doi: 10.1359/jbmr.1999.14.5.764. [DOI] [PubMed] [Google Scholar]
- 8.Valencia X, et al. Cadherin-11 provides specific cellular adhesion between fibroblast-like synoviocytes. J Exp Med. 2004;200:1673–1679. doi: 10.1084/jem.20041545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards JC. Fibroblast biology. Development and differentiation of synovial fibroblasts in arthritis. Arthritis Res. 2000;2:344–347. doi: 10.1186/ar110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helder AE, Feltkamp-Vroom TM, Nienhuis RL. Electron and light microscopical observations and serological findings in rheumatoid arthritis. Ann Rheum Dis. 1973;32:515–523. doi: 10.1136/ard.32.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson B, Pettipher ER. The synovial lining cell: Biology and pathobiology. Semin Arthritis Rheum. 1985;15:1–32. doi: 10.1016/0049-0172(85)90007-1. [DOI] [PubMed] [Google Scholar]
- 12.Zvaifler NJ, Boyle D, Firestein GS. Early synovitis: Synoviocytes and mononuclear cells. Semin Arthritis Rheum. 1994;23(6, Suppl 2):11–16. doi: 10.1016/0049-0172(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 13.Chang SK, Gu Z, Brenner MB. Fibroblast-like synoviocytes in inflammatory arthritis pathology: The emerging role of cadherin-11. Immunol Rev. 2010;233:256–266. doi: 10.1111/j.0105-2896.2009.00854.x. [DOI] [PubMed] [Google Scholar]
- 14.Kiener HP, Lee DM, Agarwal SK, Brenner MB. Cadherin-11 induces rheumatoid arthritis fibroblast-like synoviocytes to form lining layers in vitro. Am J Pathol. 2006;168:1486–1499. doi: 10.2353/ajpath.2006.050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DM, et al. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315:1006–1010. doi: 10.1126/science.1137306. [DOI] [PubMed] [Google Scholar]
- 16.Geiler T, Kriegsmann J, Keyszer GM, Gay RE, Gay S. A new model for rheumatoid arthritis generated by engraftment of rheumatoid synovial tissue and normal human cartilage into SCID mice. Arthritis Rheum. 1994;37:1664–1671. doi: 10.1002/art.1780371116. [DOI] [PubMed] [Google Scholar]
- 17.Noss EH, Brenner MB. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev. 2008;223:252–270. doi: 10.1111/j.1600-065X.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 18.Mor A, Abramson SB, Pillinger MH. The fibroblast-like synovial cell in rheumatoid arthritis: A key player in inflammation and joint destruction. Clin Immunol. 2005;115:118–128. doi: 10.1016/j.clim.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 19.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 20.Mima T, Nishimoto N. Clinical value of blocking IL-6 receptor. Curr Opin Rheumatol. 2009;21:224–230. doi: 10.1097/BOR.0b013e3283295fec. [DOI] [PubMed] [Google Scholar]
- 21.Mircic M, Kavanaugh A. The clinical efficacy of tocilizumab in rheumatoid arthritis. Drugs Today (Barc) 2009;45:189–197. doi: 10.1358/dot.2009.45.3.1343794. [DOI] [PubMed] [Google Scholar]
- 22.Guerne PA, Zuraw BL, Vaughan JH, Carson DA, Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989;83:585–592. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bucala R, Ritchlin C, Winchester R, Cerami A. Constitutive production of inflammatory and mitogenic cytokines by rheumatoid synovial fibroblasts. J Exp Med. 1991;173:569–574. doi: 10.1084/jem.173.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 25.Gottardi CJ, Gumbiner BM. Adhesion signaling: How beta-catenin interacts with its partners. Curr Biol. 2001;11:R792–R794. doi: 10.1016/s0960-9822(01)00473-0. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro L, Weis WI. Structure and biochemistry of cadherins and catenins. Cold Spring Harb Perspect Biol. 2009;1:a003053. doi: 10.1101/cshperspect.a003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison OJ, et al. Two-step adhesive binding by classical cadherins. Nat Struct Mol Biol. 2010;17:348–357. doi: 10.1038/nsmb.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 29.Miyazawa K, Mori A, Yamamoto K, Okudaira H. Constitutive transcription of the human interleukin-6 gene by rheumatoid synoviocytes: Spontaneous activation of NF-kappaB and CBF1. Am J Pathol. 1998;152:793–803. [PMC free article] [PubMed] [Google Scholar]
- 30.Miagkov AV, et al. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci USA. 1998;95:13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckley CD, et al. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22:199–204. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
- 32.Usón J, et al. Soluble interleukin 6 (IL-6) receptor and IL-6 levels in serum and synovial fluid of patients with different arthropathies. J Rheumatol. 1997;24:2069–2075. [PubMed] [Google Scholar]
- 33.Madhok R, Crilly A, Watson J, Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: Correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993;52:232–234. doi: 10.1136/ard.52.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsumoto T, Tsurumoto T, Shindo H. Interleukin-6 levels in synovial fluids of patients with rheumatoid arthritis correlated with the infiltration of inflammatory cells in synovial membrane. Rheumatol Int. 2006;26:1096–1100. doi: 10.1007/s00296-006-0143-2. [DOI] [PubMed] [Google Scholar]
- 35.Nowell MA, et al. Soluble IL-6 receptor governs IL-6 activity in experimental arthritis: Blockade of arthritis severity by soluble glycoprotein 130. J Immunol. 2003;171:3202–3209. doi: 10.4049/jimmunol.171.6.3202. [DOI] [PubMed] [Google Scholar]
- 36.Alonzi T, et al. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187:461–468. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohshima S, et al. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci USA. 1998;95:8222–8226. doi: 10.1073/pnas.95.14.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atsumi T, et al. A point mutation of Tyr-759 in interleukin 6 family cytokine receptor subunit gp130 causes autoimmune arthritis. J Exp Med. 2002;196:979–990. doi: 10.1084/jem.20020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vandooren B, et al. Tumor necrosis factor alpha drives cadherin 11 expression in rheumatoid inflammation. Arthritis Rheum. 2008;58:3051–3062. doi: 10.1002/art.23886. [DOI] [PubMed] [Google Scholar]
- 40.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:289–295. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 41.Mikulowska A, Metz CN, Bucala R, Holmdahl R. Macrophage migration inhibitory factor is involved in the pathogenesis of collagen type II-induced arthritis in mice. J Immunol. 1997;158:5514–5517. [PubMed] [Google Scholar]
- 42.Ichiyama H, et al. Inhibition of joint inflammation and destruction induced by anti-type II collagen antibody/lipopolysaccharide (LPS)-induced arthritis in mice due to deletion of macrophage migration inhibitory factor (MIF) Cytokine. 2004;26:187–194. doi: 10.1016/j.cyto.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Perona R, et al. Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 1997;11:463–475. doi: 10.1101/gad.11.4.463. [DOI] [PubMed] [Google Scholar]
- 44.Perez-Moreno M, et al. p120-catenin mediates inflammatory responses in the skin. Cell. 2006;124:631–644. doi: 10.1016/j.cell.2005.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faruqi TR, Gomez D, Bustelo XR, Bar-Sagi D, Reich NC. Rac1 mediates STAT3 activation by autocrine IL-6. Proc Natl Acad Sci USA. 2001;98:9014–9019. doi: 10.1073/pnas.161281298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sen M, et al. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci USA. 2000;97:2791–2796. doi: 10.1073/pnas.050574297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andl CD, Rustgi AK. No one-way street: Cross-talk between e-cadherin and receptor tyrosine kinase (RTK) signaling: A mechanism to regulate RTK activity. Cancer Biol Ther. 2005;4:28–31. doi: 10.4161/cbt.4.1.1431. [DOI] [PubMed] [Google Scholar]
- 48.Boscher C, Mège RM. Cadherin-11 interacts with the FGF receptor and induces neurite outgrowth through associated downstream signalling. Cell Signal. 2008;20:1061–1072. doi: 10.1016/j.cellsig.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Whitfield ML, et al. Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc Natl Acad Sci USA. 2003;100:12319–12324. doi: 10.1073/pnas.1635114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.