Abstract
Phantom perception refers to the conscious awareness of a percept in the absence of an external stimulus. On the basis of basic neuroscience on perception and clinical research in phantom pain and phantom sound, we propose a working model for their origin. Sensory deafferentation results in high-frequency, gamma band, synchronized neuronal activity in the sensory cortex. This activity becomes a conscious percept only if it is connected to larger coactivated “(self-)awareness” and “salience” brain networks. Through the involvement of learning mechanisms, the phantom percept becomes associated to distress, which in turn is reflected by a simultaneously coactivated nonspecific distress network consisting of the anterior cingulate cortex, anterior insula, and amygdala. Memory mechanisms play a role in the persistence of the awareness of the phantom percept, as well as in the reinforcement of the associated distress. Thus, different dynamic overlapping brain networks should be considered as targets for the treatment of this disorder.
A fundamental concept in psychology and philosophy of the mind is the notion of perception: The act of interpreting and organizing a sensory stimulus to produce a meaningful experience of the world and of oneself. A stimulus produces an effect on the different sensory receptors, inducing sensation. Further processing of this sensory stimulation generates an internal representation of the outer and inner world called a percept. Since the first days of psychology, two challenging questions have existed: How is sensory information encoded and, in particular, how is this represented information transformed into the individual awareness of a conscious percept (1)? Our understanding of sensory encoding, perception, and consciousness is challenged with a further degree of complexity in the case of phantom perception, the conscious awareness of a percept in the absence of an external stimulus. Deciphering the underlying neural correlates of phantom perception is a scientific endeavor that will aid in understanding the active processes of selecting, organizing, and interpreting information, which ultimately lead to the formation of a conscious percept within the brain.
Although some cases of phantom percepts have been described for the visual, olfactory, and gustatory systems, the vast majority of sensory phantoms are those present in the somatosensory (phantom limb perception/phantom limb pain and neuropathic pain) (2) and auditory (tinnitus) (3) modalities. Upfront we are challenged with the following questions: In the absence of an external sensory stimulus, where and how in the brain is the conscious percept generated? In addition, are the neural substrates underlying the generation of a conscious phantom percept similar for the auditory and somatosensory modalities? If so, can we advance in our understanding and treatment of tinnitus on the basis of what is known for phantom limb and phantom pain perception and vice versa? Here, we address these questions and propose a working model of how phantom perceptions arise from activity in the brain.
Phantom Pain–Phantom Sound Analogy
Activation of nociceptive pathways can trigger brain responses without necessarily causing the feeling of pain, and pain can occur in the absence of activation of nociceptors (4). Neuropathic pain is pain resulting from lesions of the peripheral or central nervous system (5), and phantom limb pain belongs to the group of neuropathic pain syndromes (2). Acute nociceptive pain and neuropathic pain have distinct although overlapping brain activation patterns in the insula, anterior cingulate cortex (ACC), prefrontal cortex, secondary somatosensory cortex, and thalamus (6). After limb amputation almost all people experience a phantom limb (2), whereas 70% suffer from severe phantom pain (2).
In a similar way, stimulus-evoked auditory cortical activation does not necessarily produce conscious auditory perception (7), and auditory perception is possible in the absence of auditory input: More than 80% of people with normal hearing perceive phantom sounds when placed in a soundproof room (8). In addition, deprivation of auditory input can result in an auditory phantom phenomenon called tinnitus. Whereas some people just perceive the phantom sound without being bothered, others suffer severely from their tinnitus (9).
Thus, a clear clinical analogy exists between phantom pain and disabling tinnitus (10, 11): (i) Both symptoms are wholly subjective perceptions and may change in character and quality and (ii) both symptoms occur in the deafferented area. The frequency spectrum of the tinnitus reflects the individual's hearing loss (12), neuropathic pain is felt as coming from the area that was initially innervated by the injured neural structure (2), and phantom pain is perceived in the missing body part (2, 13). The latter has to be differentiated from residual limb (or stump) pain in the still-present body part, adjacent to the amputation or deafferentation line (2). (iii) Both symptoms can be transiently masked and relieved by electrical stimulation of their respective sensory cortex (14) and (iv) similar characteristic symptoms exist in tinnitus and phantom pain (11). For example, a touch stimulus to the skin in patients with neuropathic pain can create a painful sensation (allodynia) and tinnitus patients frequently perceive specific sounds as unpleasant or painful (misophonia). A painful stimulus in neuropathic pain patients often generates an explosive and prolonged reaction to the stimulus (hyperpathia), similar to the hyperacusis seen in some tinnitus patients. Furthermore, a feeling of anxiety and stress responses is often encountered in both phantom pain and tinnitus patients, which can lead to sleep disturbances, concentration problems, fatigue, depression, anxiety disorders, and sometimes even to suicide in both clinical conditions (11).
However, there are also differences between pain and tinnitus. Whereas specific nociceptive pathways lead to physiological nociceptive pain, no analogous physiological tinnitus pathways exist. This observation might account for the fact that, in general, analgesics that are quite efficient for acute physiological body pain are not efficient for the treatment of tinnitus (15). Also, medications such as antiepileptics and antidepressants, which are effective in the treatment of neuropathic pain (15), are generally ineffective for tinnitus (16).
Primary Sensory Cortex and Beyond: Conscious Perception
Herman Melville's Captain Ahab following the loss of his leg in a skirmish with the big white whale Moby Dick perceives a phantom leg and phantom pain, and Ludwig von Beethoven, after losing his hearing, perceives tinnitus constantly, resulting in a “wretched life.” Deprivation of sensory input triggers changes in the central nervous system, resulting in phantom percepts.
Both animal (17) and human functional neuroimaging studies (2) demonstrate that phantom limb pain is associated with cortical map plasticity resulting in somatosensory cortex reorganization and that the more pronounced the reorganization is, the more severe the phantom pain (2, 18). Similarly, animal (19) and human data (20) demonstrate that cortical map plasticity in the auditory cortex is associated with tinnitus and that the more pronounced the reorganization is, the more severe the tinnitus is perceived (20). Furthermore, these remapping changes normalize when the pain (21) or the tinnitus improves (22). Topographic development and reorganization in all sensory areas of adult cortex are governed by similar/common mechanisms of synaptic plasticity (23), likely explaining the analogy between phantom pain and tinnitus.
Magnetoencephalography (MEG) studies have demonstrated that nociceptive stimuli induce gamma oscillations in the primary somatosensory cortex (S1) and that they vary with objective stimulus intensity and subjective pain intensity (24). MEG studies have also shown that auditory stimuli elicit gamma band activity in the auditory cortex (25, 26) and that γ-band activity in the sensory cortex correlates with phantom pain (27) and tinnitus (27, 28). Moreover, electroencephalography studies have demonstrated that γ-band activity in the auditory cortex reflects the tinnitus intensity (29), analogous to intensity coding in normal auditory perception (30). The γ-band activity noted in tinnitus patients goes along with decreased α (31) and increased θ activity (27, 32). This coupled θ–γ activity coordinates activity in distributed cortical areas, providing a mechanism for effective communication between these distributed areas (33). The θ–γ coupling has also been shown on intracranial recordings in a patient with tinnitus, which disappears when tinnitus is suppressed by electrical stimulation of the auditory cortex (34). The thalamocortical dysrhythmia model provides an explanation for the emergence and persistence of such a pattern as a consequence of sensory deafferentiation (27).
Are these map plasticity and oscillatory changes in the primary sensory cortices the neural correlate of the conscious phantom percept? More detailed data derived from recordings of the somatosensory system in nonhuman primates indicate that S1 is not sufficient for the generation of a percept and have implicated association cortex and frontal lobe involvement in perception (35), analogous to what has been described for the visual (36) and auditory systems (37). Thus, it has been demonstrated that the activity of S1 neurons covaries with the stimulus strength but not with the animal's perceptual reports. In contrast, the activity of frontal lobe neurons does not covary with the stimulus strength but does so with the animal's perceptual reports (38). Moreover, the transition from sensation to perception gradually builds up across cortical areas, starting at the somatosensory cortex and ending in the premotor areas of the frontal lobe, which might have a hidden sensory function (39). Consistent with this interpretation is the fact that the artificial activation of clusters of S1 neurons is sufficient to drive the full cascade of cognitive events leading to somatosensory perception (40). In addition, very recent studies performed in monkeys are giving hints that what has been described for the somatosensory cortex can be extrapolated to the auditory cortex (41). According to these observations, we could speculate that weak transitions in activity of deafferented sensory cortices trigger abnormal processing across cortical circuits, leading to phantom perceptions. However, phantom perceptions are not necessarily induced only by the triggering signals of sensory cortices. Perceptual systems are formed by interconnected circuits forming distributed systems (42) and therefore phantom perceptions could be generated in any part of the distributed system.
Human imaging studies have given further insight into the neural correlates of conscious perception. For example, brain activity and functional connectivity in patients in a persistent vegetative state, a condition where patients are awake but without awareness and without conscious percepts (43), show that loss of awareness is associated with decreased metabolism in the anterior and posterior cingulate, precuneus, and frontoparietotemporal areas in comparison with that in normal subjects (43). These “awareness areas” anatomically overlap with the brain's default network areas (44), which might also be involved in self-awareness (44, 45). In these persistent vegetative state patients, pain stimuli activate the thalamus and S1, but this primary cortex is functionally disconnected from the secondary somatosensory cortex as well as from the above-mentioned awareness areas (43, 46, 47). Similarly, in these patients activation induced by auditory stimulation is restricted to the primary auditory cortex (A1) bilaterally, without functional connectivity to the inferior parietal cortex, the hippocampus, the anterior cingulate, and the posterior cingulate (43, 48, 49).
Taken together, these results indicate that the function of the primary sensory cortices is mainly to generate an appropriate neural discriminatory representation of the sensory input, which does not lead to conscious perception. A stimulus becomes conscious only when functionally connected to a network of frontal and parietal areas. This network, together with the posterior insula (50), is relevant for the integration of sensory experiences in bodily self-consciousness (51, 52). The posterior insula triggers the pain network and the resulting emergence of subjective pain experience (53), possibly because of its involvement in the genesis of our sense of limb ownership and self-awareness (54). Furthermore, a pain (55) or an auditory stimulus (56) delivered near threshold becomes consciously perceived only when the dorsal ACC (dACC) and anterior insula are activated, i.e., when the stimuli are salient (4, 57), meaning behaviorally relevant or functionally significant (58). This concept is consistent with the “global workspace model” of consciousness proposed by Baars (59) and further elaborated by others (36, 60), on the basis of studies of the visual system.
Neuroimaging studies have confirmed the relevance of the coactivation of frontal and parietal areas together with A1 in tinnitus (61, 62). Nonpainful phantom phenomena have been shown to be more closely related to activation of S1 and the posterior parietal cortex, without activation of the secondary somatosensory cortex (63) and without cortical reorganization (64), whereas phantom limb pain is related to activation of the thalamus, the ACC, and the lateral prefrontal cortex (65), similarly to neuropathic pain in general (6), and is associated with plastic changes in S1 (50, 64). The reorganization of A1 in tinnitus (20) and the activation of the ACC and the lateral prefrontal cortex in bothersome tinnitus (9) therefore suggest that distressing tinnitus and phantom pain are more similar than nonbothersome tinnitus and nonpainful phantom phenomena.
Incongruence, Pain, and Tinnitus
Multisensory mechanisms involving crossmodal congruency are involved in bodily self-consciousness (66–68), and crossmodal illusions are observed in most conditions in which there is incongruence among two or three stimuli of different modalities (auditory, visual, somatosensory) (69). Whereas synchronous stimulation of external objects, such as a rubber hand, can lead to the illusion of body ownership (70), asynchronous stimulation in one sensory modality or between the senses can lead to abnormal sensory perception (71, 72). On the basis of this concept it has been proposed that inappropriate cortical representation of proprioception may falsely signal incongruence between motor intention and movement, resulting in pathological pain in the same way that incongruence between vestibular and visual sensation results in motion sickness (73). This sensorimotor conflict can also induce pain in healthy volunteers (72). In this sense, phantom limb pain could hypothetically be related to a temporal incongruence between what is stored in memory (the presence of the limb) and deprivation of sensory input (the absence of the limb). Thus, when a mirror box is used to reinstate the congruence between what was stored in memory and what is seen, the pain can disappear (74).
Incongruence between auditory and visual input can alter auditory perception, as in the well-known McGurk effect (75). Whether multisensory incongruence is involved in tinnitus has not been investigated yet.
Beyond Sensory Percepts: The Affective Dimension
Pain has sensory (discriminative) and affective (the “unpleasantness”) dimensions and can induce an avoidance behavior (76). Likewise, hearing has a sensory and an affective component and can induce avoidance behaviors. Moreover, affective disorders such as anxiety and depression frequently occur together with tinnitus (77). Data based on evoked potentials in humans with implanted electrodes in several brain structures indicate that painful stimuli are processed in parallel in the somatosensory cortex and dACC (78), suggesting that the affective and discriminatory aspects are processed simultaneously and not serially.
MEG studies are aiding toward delineating the network connectivity underlying the affective components of phantom perception. Thus, the presence of distress in tinnitus is related to a network activity, lateralized to the right hemisphere (79). The amount of distress is reflected by functional alterations in a network consisting of the medial temporal lobe (amygdala and hippocampus), parahippocampal areas, insula, and subgenual ACC (sgACC) and dACC (9). This network activation seems to be nonspecific, because pain distress but also distress in dyspnea (80) or social rejection (81) activate the ACC and anterior insula (9).
It is of interest to note that the areas related to the salience network and the distress component of pain and tinnitus overlap with brain areas involved in central control of the autonomic system, which include sgACC, dACC, insula, hypothalamus, and amygdala (82). This overlap supports the idea that the autonomic system is also involved in bringing the phantom percepts to consciousness, flavored by an emotional component, as described in the neurophysiological model for tinnitus (83) and similar pain models (2, 84).
Phantom Percepts and Memory
Almost every amputee experiences a phantom percept (13), but not everybody perceives the phantom as aversive or painful (2, 13). Likewise, not every tinnitus patient experiences tinnitus as aversive or bothersome. In addition, many patients describe their phantom pain as triggered by stressful life events (85), and tinnitus is more common in patients suffering posttraumatic stress disorder (86).
What mechanism turns a phantom percept into an ongoing aversive phenomenon that cannot be extinguished? Behaviorally relevant or functionally significant experiences tend to be well remembered. This observation is reflected by coactivation of the dACC and the anterior insular cortex that form a salience network (57). Functional connectivity studies demonstrate a prolonged enhanced functional coupling in the resting state between amygdala, dACC, anterior insula, and the sympathetic locus coeruleus after psychological stress, resulting in an extended state of hypervigilance that promotes sustained salience and mnemonic processing (87). Thus, the efficient encoding of aversive emotional memories can lead to the formation of a strong, aversive memory trace (88). Pain can induce single-event learning, the memory of which can last for the rest of life. A negative emotional context increases pain perception and this is concomitantly associated with increased neural activity in the anterior insula mediated by the amygdala and parahippocampus (89). In this regard, many people with amputations report phantom limb pain that is similar in both quality and location to pain experienced before the amputation. Moreover, pain experiences before the amputation are powerful predictors and elicitors of phantom limb pain (2). These observations point to the existence of a pain memory system that entrains the chronically persisting phantom pain. The continuous experience of pain produces continuous aversive emotional associations and does not provide an opportunity for extinction of the memory of pain (84). This self-reinforcement process is similar to what has been described in posttraumatic stress disorder, where anxiety and distress are perpetuated by an overactive emotional memory in the amygdala together with a lack of contextual memory in the hippocampus (88).
In the case of tinnitus, enhanced activity of the amygdala is evidenced by c-fos expression in animal models (90, 91), by source-localized electroencephalography (9), by positron emission tomography imaging (92), and by transient tinnitus diminution after suppression of the amygdalo-hippocampal complex by amytal (93). Hippocampal deficits have been documented in animal models of tinnitus and structural imaging in tinnitus patients has demonstrated a decrease in gray matter in the hippocampus (94). Structural deficits have also been observed in the sgACC/nucleus accumbens area (sgACC/Nac) and, on the basis of these findings, it has been postulated that tinnitus is the result of a deficient sensory attentional gating mechanism, originating in the sgACC/Nac and acting on the reticular thalamic nucleus (95). This nucleus accumbens-related inhibitory system is analogous to the nucleus accumbens-based antinociceptive system implicated in pain suppression (96). This sensory gating deficiency is likely mediated via the parahippocampal area, which has a sensory gating function for irrelevant or redundant auditory input (97). The parahippocampal area has been hypothesized to play a central role in memory recollection, sending information from the hippocampus to the association areas, and a dysfunction in this mechanism is posited as an explanation for complex auditory phantom percepts such as auditory hallucinations (98). As the parahippocampal area is involved in tinnitus and tinnitus distress (9), a similar mechanism could be proposed for tinnitus. Moreover, chronic stress is known to reduce neuroplasticity in the hippocampus and to reduce connectivity between hippocampus and sgACC (99). Thus, we suggest that the deficient thalamic gating function emerges as a consequence of an aversive tinnitus memory together with chronic stress and represents an additional factor contributing to the perpetuation of the phantom percept.
In brief, we hypothesize that both tinnitus and phantom pain are perceptual states of continuous learning, where—in the absence of an external input—the phantom percept is reinforced and the connection with aversive emotional associations is continuously updated.
Working Model
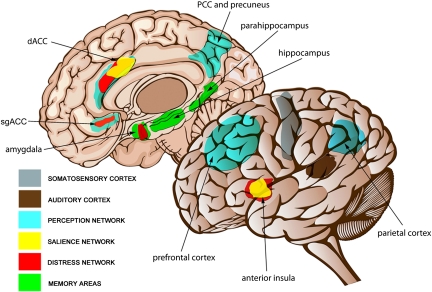
We propose that phantom perception arises as the consequence of multiple parallel overlapping dynamic brain networks (Fig. 1). Thus, any altered activity across these brain networks could generate a phantom perception for any sensory modality. This interpretation casts doubts concerning the sole participation of only one critical circuit in phantom perception. Phantom percepts result from sensory deafferentation and reach awareness only when increased neuronal activity in the primary sensory cortex is connected to a larger coactivated awareness or global workspace brain network, involving frontal and parietal areas. Activity in a salience network consisting of the dACC and anterior insula is required for the percept to reach consciousness. This salience network overlaps with a central autonomic control system and also influences limbic–auditory and –somatosensory interactions that are essential for maintaining the percept into consciousness. These interactions are mediated by the sgACC/Nac and amygdala, modulating the reticular nucleus of the thalamus and thereby potentially further contributing to thalamocortical dysrhythmia. Memory mechanisms play a role in the persistence of the awareness of the salient phantom percept, as well as in the reinforcement of the associated distress. Through the involvement of learning mechanisms, the phantom percept becomes associated to distress, which in turn is reflected by a simultaneously coactivated nonspecific distress network consisting of the parahippocampal area, ACC, anterior insula, and amygdala. Thus, different dynamic and overlapping brain networks should be considered as targets for the treatment of this disorder.
Fig. 1.
Brain networks involved in phantom perception. Sensory deafferentation causes neuroplastic changes resulting in increased activation of the primary sensory cortex: somatosensory cortex (gray) in the case of phantom pain and auditory cortex (brown) in the case of tinnitus. Awareness of the stimulus arises when this activity is connected to a larger coactivated awareness or perceptual network. This perceptual network involves subgenual (sgACC) and dorsal anterior cingulate cortex (dACC) and posterior cingulate cortex (PCC), precuneus, parietal cortex, and frontal cortex (blue). Salience to the phantom percept is reflected by activation of dACC and anterior insula (yellow). As a consequence of a constant learning process, the phantom percept becomes associated to distress, which is reflected by a nonspecific distress network consisting of the anterior cingulate cortex (sgACC and dACC), anterior insula, and amygdala (red). The persistence of the phantom percept is due to memory mechanisms involving the parahippocampal area, amygdala, and hippocampus (green).
Looking Forward
Our understanding of phantom perception has evolved from a “peripheral,” to a “primary sensory cortex,” into a “static network,” reaching a “dynamic multiple parallel overlapping network” problem. Although scientific understanding has advanced in the last decade, much more has yet to be discovered. There are several research directions that promise interesting results in the near future. Application of new structural connectivity techniques such as diffusion tensor imaging and diffusional kurtosis imaging and correlating these to functional connectivity measures would shed light on information flow within and between the parallel networks involved in phantom perception. These connectivity studies could be further evaluated by applying network science methodology (100) and thus could lead to identifying ideal targets and stimulation designs for neuromodulation. Finally, interventional studies using known and new drugs or known and new stimulation designs will enable researchers to prove and refine the proposed working model.
Acknowledgments
We thank Mr. Anselmo Albert Torres for his help with the artwork in Fig. 1. We acknowledge funding from the Tinnitus Research Initiative (D.D.R., B.L, and A.B.E.), the Howard Hughes Medical Institute International Scholar Program (R.R. and A.B.E.), Consejo Nacional de Ciencia y Tecnología and Dirección del Personal Académico de la Universidad Nacional Autónoma de México (R.R.), and University of Buenos Aires, Consejo Nacional de Investigaciones Científicas y Técnicas and Agencia Nacional de Promoción Científica y Tecnológicas, Argentina (A.B.E.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Deco G, Romo R. The role of fluctuations in perception. Trends Neurosci. 2008;31:591–598. doi: 10.1016/j.tins.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Flor H, Nikolajsen L, Staehelin Jensen T. Phantom limb pain: A case of maladaptive CNS plasticity? Nat Rev Neurosci. 2006;7:873–881. doi: 10.1038/nrn1991. [DOI] [PubMed] [Google Scholar]
- 3.Jastreboff PJ. Phantom auditory perception (tinnitus): Mechanisms of generation and perception. Neurosci Res. 1990;8:221–254. doi: 10.1016/0168-0102(90)90031-9. [DOI] [PubMed] [Google Scholar]
- 4.Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: A salience detection system for the body. Prog Neurobiol. 2011;93:111–124. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Merskey R, Bogduk N. Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. Seattle: IASP; 1994. [Google Scholar]
- 6.Moisset X, Bouhassira D. Brain imaging of neuropathic pain. Neuroimage. 2007;37(Suppl 1):S80–S88. doi: 10.1016/j.neuroimage.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 7.Colder BW, Tanenbaum L. Dissociation of fMRI activation and awareness in auditory perception task. Brain Res Cogn Brain Res. 1999;8:177–184. doi: 10.1016/s0926-6410(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 8.Del Bo L, et al. Tinnitus aurium in persons with normal hearing: 55 years later. Otolaryngol Head Neck Surg. 2008;139:391–394. doi: 10.1016/j.otohns.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 9.Vanneste S, et al. The neural correlates of tinnitus-related distress. Neuroimage. 2010;52:470–480. doi: 10.1016/j.neuroimage.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Tonndorf J. The analogy between tinnitus and pain: A suggestion for a physiological basis of chronic tinnitus. Hear Res. 1987;28:271–275. doi: 10.1016/0378-5955(87)90054-2. [DOI] [PubMed] [Google Scholar]
- 11.Møller AR. Similarities between chronic pain and tinnitus. Am J Otol. 1997;18:577–585. [PubMed] [Google Scholar]
- 12.Norena A, Micheyl C, Chéry-Croze S, Collet L. Psychoacoustic characterization of the tinnitus spectrum: Implications for the underlying mechanisms of tinnitus. Audiol Neurootol. 2002;7:358–369. doi: 10.1159/000066156. [DOI] [PubMed] [Google Scholar]
- 13.Ramachandran VS, Hirstein W. The perception of phantom limbs. The D. O. Hebb lecture. Brain. 1998;121:1603–1630. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- 14.De Ridder D, De Mulder G, Menovsky T, Sunaert S, Kovacs S. Electrical stimulation of auditory and somatosensory cortices for treatment of tinnitus and pain. Prog Brain Res. 2007;166:377–388. doi: 10.1016/S0079-6123(07)66036-1. [DOI] [PubMed] [Google Scholar]
- 15.De Ridder D, Moller A. Similarities between treatments of tinnitus and central pain. In: Moller A, Langguth B, De Ridder D, Kleinjung T, editors. Textbook of Tinnitus. New York: Springer; 2011. pp. 753–763. [Google Scholar]
- 16.Elgoyhen AB, Langguth B. Pharmacological approaches to the treatment of tinnitus. Drug Discov Today. 2010;15:300–305. doi: 10.1016/j.drudis.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Kaas JH. Phantoms of the brain. Nature. 1998;391 doi: 10.1038/34782. 331, 333. [DOI] [PubMed] [Google Scholar]
- 18.Birbaumer N, et al. Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. J Neurosci. 1997;17:5503–5508. doi: 10.1523/JNEUROSCI.17-14-05503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Mühlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci USA. 1998;95:10340–10343. doi: 10.1073/pnas.95.17.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacIver K, Lloyd DM, Kelly S, Roberts N, Nurmikko T. Phantom limb pain, cortical reorganization and the therapeutic effect of mental imagery. Brain. 2008;131:2181–2191. doi: 10.1093/brain/awn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engineer ND, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buonomano DV, Merzenich MM. Cortical plasticity: From synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 24.Gross J, Schnitzler A, Timmermann L, Ploner M. Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biol. 2007;5:e133. doi: 10.1371/journal.pbio.0050133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Brazier Award-winning article, 2001. Clin Neurophysiol. 2001;112:565–582. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- 26.Joliot M, Ribary U, Llinás R. Human oscillatory brain activity near 40 Hz coexists with cognitive temporal binding. Proc Natl Acad Sci USA. 1994;91:11748–11751. doi: 10.1073/pnas.91.24.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisz N, et al. The neural code of auditory phantom perception. J Neurosci. 2007;27:1479–1484. doi: 10.1523/JNEUROSCI.3711-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Loo E, et al. Tinnitus intensity dependent gamma oscillations of the contralateral auditory cortex. PLoS ONE. 2009;4:e7396. doi: 10.1371/journal.pone.0007396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schadow J, et al. Stimulus intensity affects early sensory processing: Sound intensity modulates auditory evoked gamma-band activity in human EEG. Int J Psychophysiol. 2007;65:152–161. doi: 10.1016/j.ijpsycho.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Lorenz I, Müller N, Schlee W, Hartmann T, Weisz N. Loss of alpha power is related to increased gamma synchronization-A marker of reduced inhibition in tinnitus? Neurosci Lett. 2009;453:225–228. doi: 10.1016/j.neulet.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 32.Weisz N, Dohrmann K, Elbert T. The relevance of spontaneous activity for the coding of the tinnitus sensation. Prog Brain Res. 2007;166:61–70. doi: 10.1016/S0079-6123(07)66006-3. [DOI] [PubMed] [Google Scholar]
- 33.Canolty RT, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Ridder D, et al. Theta-gamma dysrhythmia and auditory phantom perception. J Neurosurg. 2011;114:912–921. doi: 10.3171/2010.11.JNS10335. [DOI] [PubMed] [Google Scholar]
- 35.Romo R, Salinas E. Flutter discrimination: Neural codes, perception, memory and decision making. Nat Rev Neurosci. 2003;4:203–218. doi: 10.1038/nrn1058. [DOI] [PubMed] [Google Scholar]
- 36.Crick F, Koch C. Are we aware of neural activity in primary visual cortex? Nature. 1995;375:121–123. doi: 10.1038/375121a0. [DOI] [PubMed] [Google Scholar]
- 37.Lemus L, Hernández A, Romo R. Neural encoding of auditory discrimination in ventral premotor cortex. Proc Natl Acad Sci USA. 2009;106:14640–14645. doi: 10.1073/pnas.0907505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Lafuente V, Romo R. Neuronal correlates of subjective sensory experience. Nat Neurosci. 2005;8:1698–1703. doi: 10.1038/nn1587. [DOI] [PubMed] [Google Scholar]
- 39.de Lafuente V, Romo R. Neural correlate of subjective sensory experience gradually builds up across cortical areas. Proc Natl Acad Sci USA. 2006;103:14266–14271. doi: 10.1073/pnas.0605826103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romo R, Hernández A, Zainos A, Salinas E. Somatosensory discrimination based on cortical microstimulation. Nature. 1998;392:387–390. doi: 10.1038/32891. [DOI] [PubMed] [Google Scholar]
- 41.Lemus L, Hernández A, Romo R. Neural codes for perceptual discrimination of acoustic flutter in the primate auditory cortex. Proc Natl Acad Sci USA. 2009;106:9471–9476. doi: 10.1073/pnas.0904066106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mountcastle VB. An organizing principle for cerebral function: The unit module and the distributed system. In: Edelman G, Mountcastle VB, editors. The Mindful Brain: Cortical Organization and Group Selective Theory of Higher Brain Function. Cambridge, MA: MIT Press; 1982. pp. 7–50. [Google Scholar]
- 43.Laureys S. Eyes open, brain shut. Sci Am. 2007;296:84–89. doi: 10.1038/scientificamerican0507-84. [DOI] [PubMed] [Google Scholar]
- 44.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 45.Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boly M, et al. Perception of pain in the minimally conscious state with PET activation: An observational study. Lancet Neurol. 2008;7:1013–1020. doi: 10.1016/S1474-4422(08)70219-9. [DOI] [PubMed] [Google Scholar]
- 47.Laureys S, et al. Cortical processing of noxious somatosensory stimuli in the persistent vegetative state. Neuroimage. 2002;17:732–741. [PubMed] [Google Scholar]
- 48.Boly M, et al. Auditory processing in severely brain injured patients: Differences between the minimally conscious state and the persistent vegetative state. Arch Neurol. 2004;61:233–238. doi: 10.1001/archneur.61.2.233. [DOI] [PubMed] [Google Scholar]
- 49.Laureys S, et al. Auditory processing in the vegetative state. Brain. 2000;123:1589–1601. doi: 10.1093/brain/123.8.1589. [DOI] [PubMed] [Google Scholar]
- 50.Flor H, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 51.Blanke O, Morgenthaler FD, Brugger P, Overney LS. Preliminary evidence for a fronto-parietal dysfunction in able-bodied participants with a desire for limb amputation. J Neuropsychol. 2009;3:181–200. doi: 10.1348/174866408X318653. [DOI] [PubMed] [Google Scholar]
- 52.Tsakiris M. My body in the brain: A neurocognitive model of body-ownership. Neuropsychologia. 2010;48:703–712. doi: 10.1016/j.neuropsychologia.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 53.Isnard J, Magnin M, Jung J, Mauguière F, Garcia-Larrea L. Does the insula tell our brain that we are in pain? Pain. 2011;152:946–951. doi: 10.1016/j.pain.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 54.Baier B, Karnath HO. Tight link between our sense of limb ownership and self-awareness of actions. Stroke. 2008;39:486–488. doi: 10.1161/STROKEAHA.107.495606. [DOI] [PubMed] [Google Scholar]
- 55.Boly M, et al. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA. 2007;104:12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadaghiani S, Hesselmann G, Kleinschmidt A. Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. J Neurosci. 2009;29:13410–13417. doi: 10.1523/JNEUROSCI.2592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fecteau JH, Munoz DP. Salience, relevance, and firing: A priority map for target selection. Trends Cogn Sci. 2006;10:382–390. doi: 10.1016/j.tics.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 59.Baars BJ. How does a serial, integrated and very limited stream of consciousness emerge from a nervous system that is mostly unconscious, distributed, parallel and of enormous capacity? Ciba Found Symp. 1993;174:282–290. doi: 10.1002/9780470514412.ch14. discussion 291–303. [DOI] [PubMed] [Google Scholar]
- 60.Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: A testable taxonomy. Trends Cogn Sci. 2006;10:204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Lockwood AH, et al. The functional anatomy of gaze-evoked tinnitus and sustained lateral gaze. Neurology. 2001;56:472–480. doi: 10.1212/wnl.56.4.472. [DOI] [PubMed] [Google Scholar]
- 62.Schlee W, Hartmann T, Langguth B, Weisz N. Abnormal resting-state cortical coupling in chronic tinnitus. BMC Neurosci. 2009;10:1–11. doi: 10.1186/1471-2202-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flor H, et al. A neural substrate for nonpainful phantom limb phenomena. Neuroreport. 2000;11:1407–1411. doi: 10.1097/00001756-200005150-00011. [DOI] [PubMed] [Google Scholar]
- 64.Grüsser SM, et al. The relationship of perceptual phenomena and cortical reorganization in upper extremity amputees. Neuroscience. 2001;102:263–272. doi: 10.1016/s0306-4522(00)00491-7. [DOI] [PubMed] [Google Scholar]
- 65.Willoch F, et al. Phantom limb pain in the human brain: Unraveling neural circuitries of phantom limb sensations using positron emission tomography. Ann Neurol. 2000;48:842–849. [PubMed] [Google Scholar]
- 66.Blanke O, Ortigue S, Landis T, Seeck M. Stimulating illusory own-body perceptions. Nature. 2002;419:269–270. doi: 10.1038/419269a. [DOI] [PubMed] [Google Scholar]
- 67.De Ridder D, Van Laere K, Dupont P, Menovsky T, Van de Heyning P. Visualizing out-of-body experience in the brain. N Engl J Med. 2007;357:1829–1833. doi: 10.1056/NEJMoa070010. [DOI] [PubMed] [Google Scholar]
- 68.Aspell JE, Lenggenhager B, Blanke O. Keeping in touch with one's self: Multisensory mechanisms of self-consciousness. PLoS ONE. 2009;4:e6488. doi: 10.1371/journal.pone.0006488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wozny DR, Beierholm UR, Shams L. Human trimodal perception follows optimal statistical inference. J Vis. 2008;8 doi: 10.1167/8.3.24. 24.1–11. [DOI] [PubMed] [Google Scholar]
- 70.Armel KC, Ramachandran VS. Projecting sensations to external objects: Evidence from skin conductance response. Proc Biol Sci. 2003;270:1499–1506. doi: 10.1098/rspb.2003.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramachandran V, Blakeslee S. Phantoms in the Brain. New York: Harper Collins; 1998. [Google Scholar]
- 72.McCabe CS, Haigh RC, Halligan PW, Blake DR. Simulating sensory-motor incongruence in healthy volunteers: Implications for a cortical model of pain. Rheumatology (Oxford) 2005;44:509–516. doi: 10.1093/rheumatology/keh529. [DOI] [PubMed] [Google Scholar]
- 73.Harris AJ. Cortical origin of pathological pain. Lancet. 1999;354:1464–1466. doi: 10.1016/S0140-6736(99)05003-5. [DOI] [PubMed] [Google Scholar]
- 74.Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci. 1996;263:377–386. doi: 10.1098/rspb.1996.0058. [DOI] [PubMed] [Google Scholar]
- 75.McGurk H, MacDonald J. Hearing lips and seeing voices. Nature. 1976;264:746–748. doi: 10.1038/264746a0. [DOI] [PubMed] [Google Scholar]
- 76.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 77.Sullivan MD, et al. Disabling tinnitus. Association with affective disorder. Gen Hosp Psychiatry. 1988;10:285–291. doi: 10.1016/0163-8343(88)90037-0. [DOI] [PubMed] [Google Scholar]
- 78.Frot M, Mauguière F, Magnin M, Garcia-Larrea L. Parallel processing of nociceptive A-delta inputs in SII and midcingulate cortex in humans. J Neurosci. 2008;28:944–952. doi: 10.1523/JNEUROSCI.2934-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schlee W, Weisz N, Bertrand O, Hartmann T, Elbert T. Using auditory steady state responses to outline the functional connectivity in the tinnitus brain. PLoS ONE. 2008;3:e3720. doi: 10.1371/journal.pone.0003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.von Leupoldt A, et al. Dyspnea and pain share emotion-related brain network. Neuroimage. 2009;48:200–206. doi: 10.1016/j.neuroimage.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 81.Masten CL, et al. Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: A functional neuroimaging investigation in humans. J Physiol. 2000;523:259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jastreboff PJ. Tinnitus retraining therapy. Prog Brain Res. 2007;166:415–423. doi: 10.1016/S0079-6123(07)66040-3. [DOI] [PubMed] [Google Scholar]
- 84.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jensen TS, Krebs B, Nielsen J, Rasmussen P. Immediate and long-term phantom limb pain in amputees: Incidence, clinical characteristics and relationship to pre-amputation limb pain. Pain. 1985;21:267–278. doi: 10.1016/0304-3959(85)90090-9. [DOI] [PubMed] [Google Scholar]
- 86.Hinton DE, Chhean D, Pich V, Hofmann SG, Barlow DH. Tinnitus among Cambodian refugees: Relationship to PTSD severity. J Trauma Stress. 2006;19:541–546. doi: 10.1002/jts.20138. [DOI] [PubMed] [Google Scholar]
- 87.van Marle HJ, Hermans EJ, Qin S, Fernández G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage. 2010;53:348–354. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- 88.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 89.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Flexible cerebral connectivity patterns subserve contextual modulations of pain. Cereb Cortex. 2011;21:719–726. doi: 10.1093/cercor/bhq146. [DOI] [PubMed] [Google Scholar]
- 90.Mahlke C, Wallhäusser-Franke E. Evidence for tinnitus-related plasticity in the auditory and limbic system, demonstrated by arg3.1 and c-fos immunocytochemistry. Hear Res. 2004;195:17–34. doi: 10.1016/j.heares.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 91.Wallhäusser-Franke E, et al. Expression of c-fos in auditory and non-auditory brain regions of the gerbil after manipulations that induce tinnitus. Exp Brain Res. 2003;153:649–654. doi: 10.1007/s00221-003-1614-2. [DOI] [PubMed] [Google Scholar]
- 92.Mirz F, Gjedde A, Sødkilde-Jrgensen H, Pedersen CB. Functional brain imaging of tinnitus-like perception induced by aversive auditory stimuli. Neuroreport. 2000;11:633–637. doi: 10.1097/00001756-200002280-00039. [DOI] [PubMed] [Google Scholar]
- 93.De Ridder D, et al. Amygdalohippocampal involvement in tinnitus and auditory memory. Acta Otolaryngol Suppl. 2006;556:50–53. doi: 10.1080/03655230600895580. [DOI] [PubMed] [Google Scholar]
- 94.Landgrebe M, et al. Structural brain changes in tinnitus: Grey matter decrease in auditory and non-auditory brain areas. Neuroimage. 2009;46:213–218. doi: 10.1016/j.neuroimage.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 95.Rauschecker JP, Leaver AM, Mühlau M. Tuning out the noise: Limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–826. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gear RW, Aley KO, Levine JD. Pain-induced analgesia mediated by mesolimbic reward circuits. J Neurosci. 1999;19:7175–7181. doi: 10.1523/JNEUROSCI.19-16-07175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boutros NN, et al. Sensory gating in the human hippocampal and rhinal regions: Regional differences. Hippocampus. 2008;18:310–316. doi: 10.1002/hipo.20388. [DOI] [PubMed] [Google Scholar]
- 98.Diederen KM, et al. Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. Am J Psychiatry. 2010;167:427–435. doi: 10.1176/appi.ajp.2009.09040456. [DOI] [PubMed] [Google Scholar]
- 99.Spedding M, Jay T, Costa e Silva J, Perret L. A pathophysiological paradigm for the therapy of psychiatric disease. Nat Rev Drug Discov. 2005;4:467–476. doi: 10.1038/nrd1753. [DOI] [PubMed] [Google Scholar]
- 100.Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]