Abstract
The capsaicin receptor TRPV1 is the principal transduction channel for nociception. Excessive TRPV1 activation causes pathological pain. Ideal pain mangement requires selective inhibition of hyperactive pain-sensing neurons, but sparing normal nociception. We sought to determine whether it is possible to use activity-dependent TRPV1 agonists to identify nerves with excessive TRPV1 activity, as well as exploit the TRPV1 pore to deliver charged anesthetics for neuronal silencing. We synthesized a series of permanently charged capsaicinoids and found that one, cap-ET, efficaciously evoked TRPV1-dependent entry of Ca2+ or the large cationic dye YO-PRO-1 comparably to capsaicin, but far smaller electrical currents. Cap-ET–induced YO-PRO-1 transport required permeation of both the agonist and the dye through the TRPV1 pore and could be enhanced by kinase activation or oxidative covalent modification. Moreover, cap-ET reduced capsaicin-induced currents by a voltage-dependent block of the pore. A low dose of cap-ET elicited entry of permanently charged Na+ channel blockers to effectively suppress Na+ currents in sensory neurons presensitized with oxidative chemicals. These results implicate therapeutic potential of these unique TRPV1 agonists exhibiting activity-dependent ion transport but of minimal pain-producing risks.
Keywords: activity-dependent capsaicinoids, hyperalgesia, ion permeation, selective analgesia
Capsaicin, a small lipophilic molecule from hot chili peppers, acts on sensory neurons by opening the TRPV1 channel. TRPV1 is activated by noxious temperatures (>43 °C) and serves as an integrator for major pain-producing signals (1, 2). Inflammatory hyperalgesia is dramatically reduced in mice lacking TRPV1 (3, 4). Besides being an attractive target for pain management, TRPV1 regulates autonomic function, such as body temperature, blood vessel tone, and release of transmitters from sensory nerves (5–9). TRPV1 activated by capsaicin at a concentration far below the pain-producing threshold triggers neuropeptide release (7) and production of other second messengers (10). In light of the complexity of TRPV1 actions in physiology, one major challenge in developing TRPV1-based pain treatment is to target therapeutic compounds to selectively inhibit hyperactive nociceptive neurons while sparing nerves of normal thresholds for pain detection.
Capsaicin is among the most powerful chemical agonists of TRPV1. Being small and hydrophobic, capsaicin crosses the plasma membrane readily to reach its intracellular ligand-binding site on TRPV1 (11, 12), leading to channel activation and cation permeation. TRPV1 activation rapidly depolarizes nerves to evoke acute pain sensation and allows Ca2+ entry to initiate downstream signaling events such as neuropeptide release or production of other second messengers. Nevertheless, TRPV1 activation facilitates cellular transport of organic cations such as tetraethylammonium (TEA), N-methylglucamine (NMG) (13), and the quaternary ammonium Na+ channel blocker QX-314 (14) or even larger fluorescent organic dyes such as FM1-43 or YO-PRO-1 (15, 16). Therefore, transmembrane cation transport in TRPV1-expressing cells may provide an effective route for agonist-dependent delivery of charged therapeutic molecules. The transport property coupled to TRPV1 activation allows application of capsaicin to load membrane-impermeable QX-314 into primary sensory afferents to suppress thermal nociception (14).
Selective silencing of hyperactive nociceptive neurons in pain management requires use-dependent modulators that preferentially affect aberrantly sensitized TRPV1. To develop such use-dependent TRPV1 modulators, we synthesized chemical derivatives of capsaicin (capsaicinoids) and asked whether they exhibited activity dependence. We found several permanently charged capsaicinoids activating TRPV1 via extracellular application, despite their predicted inability to cross the plasma membrane. Charged capsaicinoids retain substantial ability to induce Ca2+ influx and to transport large cationic molecules, but evoke rather small electric currents. Notably, activation of TRPV1 in intact cells by such cationic capsaicinoids obligates their own entry via TRPV1 pores. This interesting pharmacokinetic property endows these membrane impermeable water-soluble capsaicin derivatives the potential to act as activity-dependent drugs, because these cationic capsaicinoids will preferentially enter cells with elevated TRPV1 activity to perpetuate further increase of membrane permeability to other therapeutic cations. Augmented permeation of large organic cations via TRPV1 sensitized by chemical messengers or other signaling pathways during inflammation or neuropathic pain may enable targeted delivery of cationic anesthetic compounds to selectively tame neurons with hyperactive TRPV1.
Results
Extracellular Application of Charged Capsaicinoids Activated TRPV1.
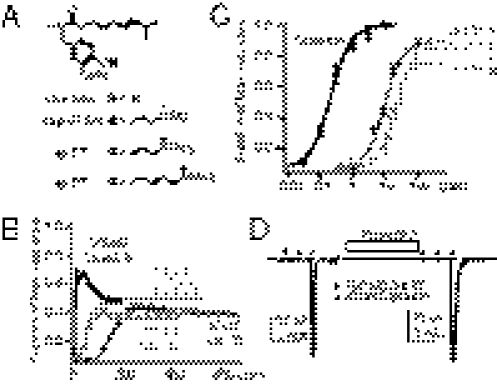
The capsaicinoids shown in Fig. 1 were prepared by alkylation of the potassium phenolate salts of capsaicin, as described in SI Materials and Methods. Three hydrophilic quaternary ammonium capsaicin derivatives were synthesized: capsaicin O-ethyl (trimethylammonium) acetate (cap-ET), capsaicin O-butyl(trimethylammonium) acetate (cap-BT), and capsaicin O-tetraethylammonium acetate (cap-ETEA) (Fig. 1A). All compounds were purified by reverse phase chromatography under conditions that ensured their complete separation from any remaining capsaicin, and all showed satisfactory proton NMR and mass spectral analysis. These three capsaicin derivatives are fully charged cationic quaternary ammonium salts, using the same functionality that renders the cysteine-reactive reagent MTSET membrane impermeable (17, 18). Cap-ET and cap-BT differ in the distance of the trimethylammonium group from the vanilloid ring, whereas cap-ETEA is a more hydrophobic triethylammonium derivative. On the basis of water/octanol partitioning, cap-ET is over 300-fold more hydrophilic than capsaicin (Table S1).
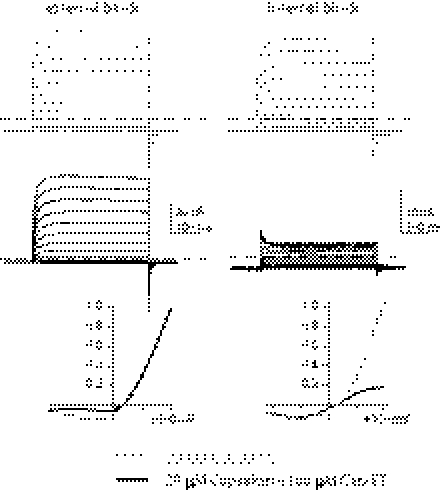
Fig. 1.
Charged capsaicin derivates stimulate TRPV1-dependent calcium influx but fail to induce electrical currents. (A) Three tetraakylammonium capsaicin derivatives bearing permanent positive charges are shown. (B) Representative traces of normalized Ca2+ signals induced by extracelluar application of capsaicin or derived analogs. Signals were normalized to the values from final application of a saturating dose of capsaicin. (C) Dose–response curves of evoked Ca2+ signals of three capsaicin analogs are overlaid for comparison with the parental compound capsaicin. (D) A total of 100 μM cap-ET applied directly to the cytoplamsic side of an inside-out patch from a HEK cell expressing rat TRPV1 induced a barely detectable electric current compared with full activation of TRPV1 by 30 μM capsaicin. The TRPV1 current sensitized by 10 μM PAO, an oxidative chemical that potentiates the receptor, exhibited a recordable but small current activated by 100 μM cap-ET.
When applied extracellularly, capsaicin readily crosses the plasma membrane to reach its intracellular ligand-binding site on TRPV1 to activate the receptor to prompt a large intracellular Ca2+ rise, evidenced by a robust fluorescent signal in ratiometric fura-2 imaging (Fig. 1B). We predicted that extracellular application of permanently charged capsaicinoids would fail to activate TRPV1, because the agonists would fail to access intracellular vanilloid binding sites. Surprisingly, extracellular application of cap-ET, cap-BT, or cap-ETEA still effectively raised intracellular Ca2+ levels (Fig. 1B). The activation is absolutely extracellular Ca2+ dependent, with cap-ET being the most potent and efficacious among the three (Fig. 1C and Figs. S1and S2). Comparable to previous studies, alkylation of capsaicin at the phenolic oxygen reduces the potency of these compounds relative to capsaicin (19): cellular responses evoked by charged TRPV1 agonists exhibited much slower kinetics even when these agonists were applied at several orders of magnitude higher concentration than capsaicin. Direct application of cap-ET to excised inside-out membrane patches from HEK cells, in contrast, opened TRPV1 with no delay by electrophysiological measurements (Fig. 1D and Fig. S3). However, ionic currents evoked by 100 μM cap-ET were much smaller than those by capsaicin (Icap-ET/Icap = 1.1 ± 0.3% at −60 mV, n = 11) even under recording conditions in which no receptor desensitization occurs. The low efficacy of cap-ET to induce currents persisted even for TRPV1 maximally sensitized by phenylarsine oxide (PAO), a cysteine-reacting chemical mimicking cellular oxidative stress (Icap-ET/Icap = 3.0 ± 0.9% at −60 mV, n = 6, after a 5-min PAO sensitization, Fig. 1D) (20). Slow activation kinetics of cationic capsaicin analogs in Ca2+ imaging is, therefore, not a trivial outcome of reduced potency or efficacy of charged capsaicinoids, but more likely a consequence of delayed access of these agonists to their intracellular ligand binding sites. Even though the permanently charged capsaicinoids have dramatically reduced hydrophobicity, which is generally considered to be important for agonist efficacy of TRPV1 ligands (19, 21, 22), their successful activation of TRPV1 predicts sufficient cellular bioavailability, therefore, potential therapeutic usefulness of these compounds.
The ability of permanently charged capsaicinoids to access the intracellular ligand-binding site via extracellular application raises the question of how these charged molecules cross biological membranes. Carrying the same positive tetraalkylammonium groups that severely impaired membrane permeability when attached to methanesulfonate (MTS) reagents, these synthetic cationic capsaicinoids cannot cross the lipid bilayer efficiently by passive diffusion. We thus consider two other alternative entry pathways: active transport across the bilayer or permeation through the channel pore.
Capsaicin-Induced Cellular YO-PRO-1 Entry Is a Consequence of YO-PRO-1 Permeation Through the TRPV1 Pore.
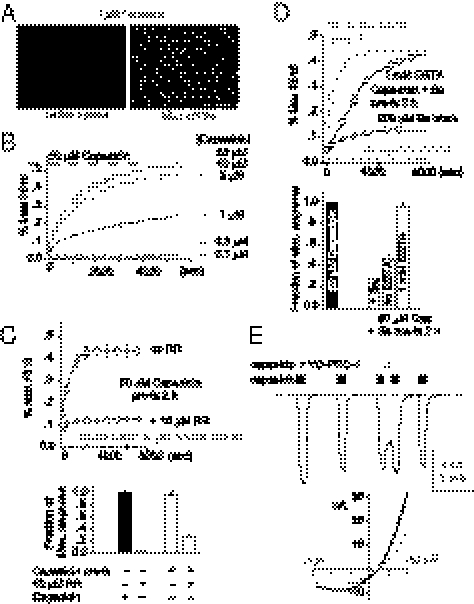
The TRPV1 pore is nonselective enough to allow the passage of structurally unrelated organic cations including TEA and NMG (13). Capsaicin activation of TRPV1 was noted to elicit cellular entry of even larger cationic molecules including the quaternary ammonium Na+ channel blocker QX-314 (14), the biscationic styryl dye FM1-43 (15), and the di-cyanine dye YO-PRO-1 (16). It is conceivable that charged capsaicinoids of comparable molecular weights may enter TRPV1-expressing cells via the same mechanism for transport of other organic cations. We thus used YO-PRO-1 as a reporter to elucidate the general mechanism of TRPV1-agonist dependent organic cation transport. YO-PRO-1 entered cytoplasm of TRPV1-expressing cells upon agonist treatment, whereas control cells without TRPV1 showed little YO-PRO-1 accumulation (Fig. 2 A and B and Movie S1 and Fig. S4). Receptor activation is essential because capsaicin failed to elicit YO-PRO-1 transport in HEK cells expressing rTRPV1 Y511A (Fig. S4). Capsaicin-induced YO-PRO-1 fluorescence developed much slower compared with Ca2+ transients as expected from a larger molecular size of YO-PRO-1.
Fig. 2.
Capsaicin opens the TRPV1 pore to facilitate cellular entry of YO-PRO-1. (A) 1 μM capsaicin induced YO-PRO-1 uptake in rat TRPV1-expressing cells. (B) The fluorescent signals were quantified with plate-reader assays. TRPV1-dependent fluorescent signals, indicating YO-PRO-1 entry, were concentration dependent. (C) Application of the TRPV1 pore blocker ruthenium red (RR, 10 μM) suppressed capsaicin-mediated YO-PRO-1 uptake (Upper, gray trace). To test the time dependence of ruthenium red block, cells were preincubated in capsaicin for 2 h and then the agonist was removed before the subsequent YO-PRO-1 uptake experiments. Ruthenium red (10 μM), when applied concurrently with YO-PRO-1, quickly and effectively blocked the uptake (comparing the two black traces in the chart). (D) Pretreatment with 200 μM Ba2+ also blocked capsaicin-induced YO-PRO-1 uptake (72.1 ± 0.7% block, n = 3 wells). Ba2+ effects could be completely reversed (94.7 ± 3.4% recovery, n = 3 wells) by coapplying 1 mM EGTA, a chelator for this divalent cation, with YO-PRO-1. (E) 5 μM YO-PRO-1 reversibly blocked TRPV1 currents evoked by 10 μM capsaicin in an inside-out patch. The i-V plot shows a weak voltage dependence of YO-PRO-1 block.
We tested whether the TRP channel pore blocker ruthenium red (RR, 10 μM) inhibits YO-PRO-1 transport and found that RR effectively suppressed YO-PRO-1 entry (Fig. 2C). We then pretreated TRPV1-expressing cells with 50 μM capsaicin for 1 h and found that they could efficiently take up YO-PRO-1 even without concurrent presence of extracellular capsaicin afterward (Fig. 2C). In contrast, application of 10 μM RR following pretreatment with capsaicin for 1 h caused an immediate and strong inhibition of YO-PRO-1 transport afterward. An open TRPV1 pore rather than an unrelated transporter activated downstream of the TRPV1 signaling pathway appears to be the major determinant for the cellular transport of large organic cations. This predicts that cells pretreated with both 50 μM capsaicin and TRPV1 blockers should resume YO-PRO-1 transport as soon as the blocker is removed. Ruthenium red, however, has a relative high affinity to TRPV1; RR cannot be effectively washed out to reverse the inhibition. We hence used Ba2+, a divalent ion that is more reversible but still effectively blocks YO-PRO-1 transport, to test whether the reversal of TRPV1 pore block restored permeation of large organic cations. Preincubation of cells in 50 μM capsaicin and 200 μM Ba2+ blocked subsequent YO-PRO-1 transport. Inhibition of transport could be partially reversed by removing Ba2+ from the extracellular solution and almost completely recovered by incubating the cells pretreated with capsaicin and Ba2+ in the chelator EGTA (Fig. 2D). We conclude that Ba2+ functions as a blocker within the TRPV1 pore to hinder YO-PRO-1 transport via the permeation pathway. We also recorded a reversible block of capsaicin-evoked TRPV1 currents by YO-PRO-1, further suggesting a direct interaction of this dye with the pore (Fig. 2E). Agonist-dependent transport of large organic cations in TRPV1-expressing cells is mediated by their permeation through the channel pore, which could be the mechanism used by charged capsaicinoids for cellular entry.
Cap-ET Crosses the TRPV1 Pore to Reach Cytoplasm for TRPV1 Activation.
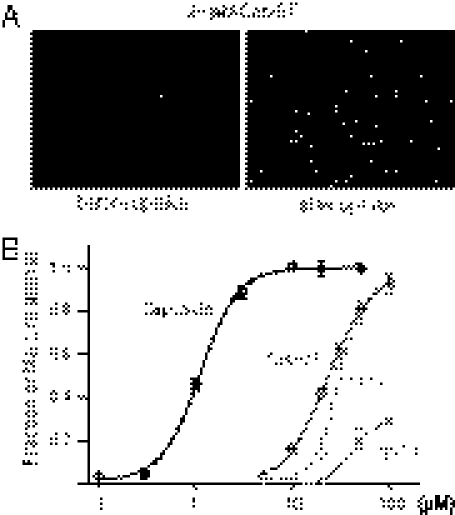
We then asked whether extracellular application of permanently charged capsaicinoids could activate TRPV1 to facilitate YO-PRO-1 transport. All three charged capsaicinoids have considerable efficacies but show even slower kinetics compared with capsaicin (Fig. 3 and Fig. S5). Because cap-ET itself is a cation as large as YO-PRO-1, this charged capsaicinoid may enter cells by crossing the TRPV1 channel pore and then bind to its intracellular vanilloid-binding site to further increase channel opening. We preincubated TRPV1-expressing cells in cap-ET for 2 h to allow its accumulation inside cells, then removed cap-ET and added 5 μM YO-PRO-1 extracellularly. Preincubation of cap-ET enabled TRPV1-expressing cells to transport YO-PRO-1 as effectively and more swiftly: The kinetics of YO-PRO-1 fluorescence in cap-ET pretreated cells were much faster than cells without pretreatment (5.37 ± 0.99-fold decrease of t1/2 to maximal fluorescence, compared with 1.14 ± 0.04-fold in capsaicin treated wells, n = 3 for each group, P = 0.017, Fig. 4A), even though no extracellular cap-ET was given during the phase of the YO-PRO-1 transport experiment. This result implies that cap-ET had been accumulated inside the cells to activate TRPV1 enough to increase the membrane permeability to YO-PRO-1, and consequently the accelerated entry. Given that Ba2+ can effectively inhibit the capsaicin-induced YO-PRO-1 entry, we predicted that coapplication of Ba2+ during the preincubation phase of cap-ET should effectively block permeation of this ligand and the consequent TRPV1 activation, thereby ablating YO-PRO-1 entry in the next step of experiments. Indeed, we observed that cells pretreated with cap-ET in the presence of 200 μM Ba2+ exhibited drastic reduction of YO-PRO-1 transport afterward, whether 1 mM EGTA was provided during the transport experiments or not (Fig. 4B). Coapplication of Ba2+ during preincubation of cap-ET hindered its own transport so much that an insufficient amount of agonist was accumulated inside cells. Taken together, these data indicate that charged capsaicinoids permeate the TRPV1 pore to access the intracellular capsaicin-binding site. Activity-dependent entry of TRPV1 agonists via the channel pore might establish a positive feedback loop to sustain a long-lasting influx of YO-PRO-1 or other therapeutic organic cations, even for an agonist that activates electric currents poorly.
Fig. 3.
Charged capsaicinoids evoke YO-PRO-1 uptake in TRPV1 expressing cells. (A) 20 μM extracellular cap-ET induced YO-PRO-1 uptake in 30 min. (B) Agonist-induced fluorescence at the end of each experiment was normalized to total fluorescence from fixed cells in each well for derivation of dose–response curves of each agonist. Relative efficacy of each agonist was displayed by assuming capsaicin as a full agonist.
Fig. 4.
Block of the TRPV1 permeation pathway inhibits activation by cap-ET but not capsaicin. The Upper panel illustrates that capsaicin can enter the cell by crossing membrane lipid bilayers, whereas cap-ET obligatorily uses the TRPV1 pore as the entry pathway. (A) TRPV1-expressing cells were incubated in 50 μM capsaicin or cap-ET for 2 h. Then the agonist was removed before measuring kinetics of YO-PRO-1 entry. (B) Coincubation of 200 μM Ba2+ with 50 μM cap-ET for 2 h blocked cap-ET–induced YO-PRO-1 uptake nearly completely (black traces in the Left chart). Even incubating the pretreated cells with EGTA could not reverse the block.
Transport of cap-ET via the channel pore suggests that this charged capsaicinoid should also reduce TRPV1 current by a pore blocking mechanism, in addition to its gating effect. Direct application of cap-ET alone to the cytoplasmic surface of an excised membrane patch induced rather small TRPV1 currents that exhibited a voltage-dependent pore block. To more accurately measure the extent of pore block by cap-ET, we used capsaicin to activate the receptor (Fig. 5). A saturating concentration of capsaicin robustly activated TRPV1 currents even in the presence of cap-ET, supporting the notion that capsaicin does interact with higher affinity to the vanilloid-binding site than cap-ET. Coapplied cap-ET exerted a substantial pore block with strong voltage dependence, whether cap-ET was administered to the extracellular or the intracellular side. Given that cytoplasmic cap-ET only suppressed outward capsaicin-evoked TRPV1 currents, the inability of internal cap-ET to effectively activate TRPV1 currents in the negative voltage range must result from its low intrinsic efficacy. The voltage-dependent pore block by extracellular cap-ET may further reduce any inward TRPV1 currents. The residual ionic currents at extreme membrane potentials suggested that cap-ET block does not completely occlude the pore. These observations are mostly consistent with the idea that cap-ET, albeit a TRPV1 blocker, can still permeate an open TRPV1 pore, provided that there is sufficient driving force.
Fig. 5.
Cap-ET blocks TRPV1 pore in a voltage-dependent manner. Traces from capsaicin-induced currents in the absence (gray) or the presence (black) of cap-ET were displayed to demonstrate cap-ET block of capsaicin-induced TRPV1 currents. A total of 100 μM cap-ET, when coapplied with 30 μM capsaicin, exhibited a strong voltage-dependent block from either outside (Left) or inside (Right). Cap-ET block was incomplete in either case. I–V curves were generated by normalizing the current amplitude at each voltage to the maximal TRPV1 current activated by 30 μM capsaicin at +100 mV.
TRPV1 Sensitization Augments cap-ET–Induced Cation Transport.
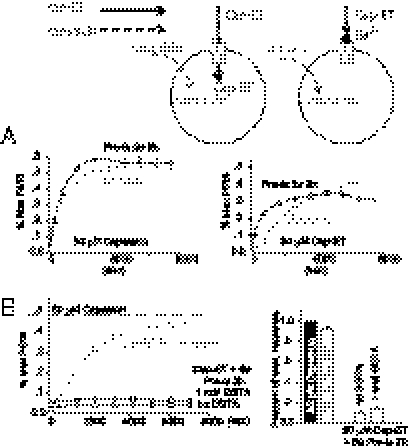
In many disease conditions, particularly the inflammatory states, TRPV1 becomes hypersensitized and triggers excessive pain. An agonist with the potential to stimulate activity-dependent therapeutic molecule transport will be most useful if it also exhibits a parallel increase of potency or efficacy for sensitized versus normal TRPV1. Protein kinase C activation (23–26) and oxidative modification (20, 27, 28) are major biochemical pathways that enhance TRPV1 sensitivity to chemical agonists. PDBu and PAO, chemical activators stimulating PKC or mimicking cellular oxidation, respectively, could elicit TRPV1-dependent intracellular Ca2+ rise (20, 29). They also dramatically shifted dose–response curves of agonist-evoked YO-PRO-1 entry leftward (Fig. 6 A and B and Fig. S6).
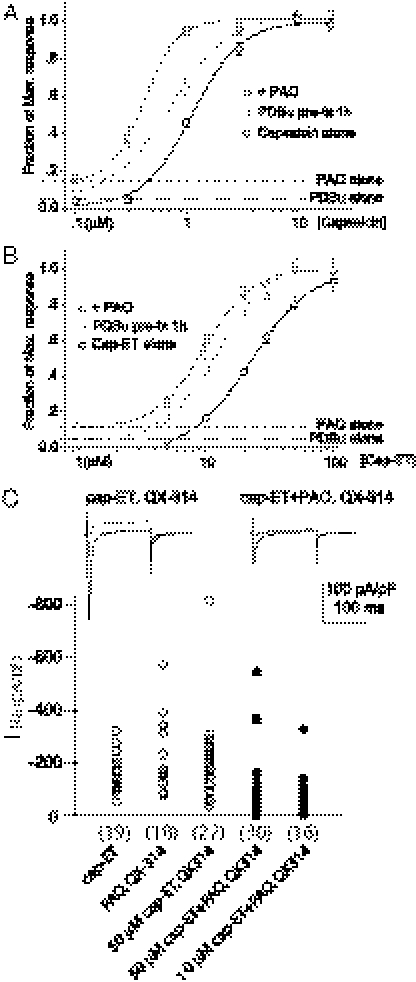
Fig. 6.
PKC activation and receptor oxidation enhanced cap-ET–evoked cation entry. (A and B) Treatment of TRPV1-expressing cells with 1 μM PDBu or 10 μM PAO increased the potency of capsaicin or cap-ET for YO-PRO-1 uptake. (C) Cap-ET evoked substantial QX-314 entry in PAO sensitized neurons (filled circles) to reduce the current density of voltage-gated Na+ channels compared with control groups (open circles) that showed no suppression (number of recordings indicated in parentheses, P < 0.001, Mann–Whitney test). Representative traces of Na+ currents at −70, −10, and +20 mV from the medians of the uninhibited and inhibited groups are shown at the Top of panel C.
We therefore tested whether cap-ET could present a prototype of TRPV1 agonists with therapeutic potential due to their preferential targeting of pathologically sensitized sensory nerves during inflammation or oxidative injuries. We recorded an effective reduction of voltage-gated Na+ currents by brief extracellular application of QX-314 in PAO-sensitized neurons pretreated with 10 or 50 μM cap-ET, whereas Na+ current densities from neurons treated with either PAO or cap-ET alone were comparable to cells not exposed to QX-314 (Fig. 6C). Taken together, TRPV1 sensitization could allow one to exploit this ion channel as the permeation pathway to deliver membrane impermeable local anesthetics preferentially into hyperactive nociceptors to suppress their electrical activity.
Discussion
The identification of TRPV1 has facilitated the development of novel analgesics (30, 31). However, most lead compounds developed for treating pain are TRPV1 antagonists that suppress channel activity without discriminating between normal and hyperactive TRPV1. Alternatively, a permeation pathway for large organic ions coupled to ligand-gated channel activity, similar to that of ionotropic purinergic P2X receptors (32–34), represents an attractive conduit to introduce charged therapeutics into cytoplasms of select neuronal populations. For example, the mustard oil receptor TRPA1 responds to allyl-isothiocyanate to mediate YO-PRO-1 transport (35, 36). Moreover, anionic transmitters ATP or GABA can pass pores of pannexin or bestrophin, respectively, underlying nonvesicular release of autocrine or paracrine factors in synapses (37–41). Analogously, the remarkable permeability of the TRPV1 pore presents a novel route for delivery of membrane impermeable local anesthetics selectively into pain sensing neurons (14).
Regardless of approach, pain alleviation strategies exploiting TRPV1 pharmacology still face two major issues: specificity and side effects. Both are related to the electrical excitation of sensory nerves downstream of TRPV1 activation. Indiscriminate suppression of all TRPV1 by receptor antagonists or TRPV1 blockers cripples its protective role in alarming imminent or existing tissue damage. It is therefore advantageous to search for TRPV1 modulators that display activity-dependent efficacy. The strategy of coadministering capsaicin or strong TRPV1 agonists with membrane impermeable QX-314 to deliver local anesthetics into nerve terminals to reduce pain cannot bypass initial electric excitation-induced pain before sufficient accumulation of this Na+ channel blocker to suppress action potentials; one cannot ease pain without eliciting it first. Besides high specificity and selectivity, an ideal TRPV1 agonist used for stimulating pore-mediated transport of therapeutic organic cations should also retain the ability to activate chemical signals downstream of receptors, but cause little electrical excitation.
In this study, we demonstrate the possibility of designing TRPV1 drugs with these properties. Simple chemical modification of the highly effective TRPV1 ligand capsaicin yields charged membrane-impermeable analogs. TRPV1 channels challenged with charged capsaicin derivatives exhibit a dramatic reduction of electrical current but preserve the ability to evoke Ca2+ transients and to transport large cationic molecules. Most importantly, the efficacies of some charged analogs could approach that of capsaicin for Ca2+ signaling and cation transport. The special pharmacological property of these cationic capsaicin derivatives is a consequence of their permeation through TRPV1: the receptor ligand goes through the ion channel pore to directly access its own binding site. An activity-dependent permeation pathway can potentially permit sustained entry of externally applied charged capsaicinoids and Na+ channel blockers preferentially into hyperactive neurons without causing excessive electrical excitation.
Although an open TRPV1 pore is reported to be wide enough to accommodate large cations (13, 15, 16), the relative contribution of permeation through the TRPV1 pore as a cellular transport pathway of large cationic molecules has not been fully determined. Our electrophysiological data provided direct evidence that YO-PRO-1 and cap-ET do interact with the TRPV1 ion permeation pathway. Two pore blockers, RR and Ba2+, could also effectively suppress capsaicin-mediated YO-PRO-1 uptake. Both the rapid onset of RR block on capsaicin pretreated cells and the reversal of Ba2+ inhibition by its removal or EGTA chelation suggest that the TRPV1 pore itself is the major entry pathway for YO-PRO-1 into the cell.
Cap-ET and related analogs have limited permeability across the lipid bilayer. Even if these ligands may enter cells via TRPV1 pores, one might question how these hydrophilic agonists could enter cells initially. Given that relative permeability of small metal ions for activated TRPV1 is dependent on modes of channel activation (2, 42), chemical modulators might activate specific modes of TRPV1 opening and cause a rapid change of relative permeability of large organic cations (16). Such dynamic permeability change could not have happened before agonist application, that is, for hydrophilic cap-ET at starting time points of our YO-PRO-1 transport experiments. It is more likely that initial cap-ET entry was due to a sufficient basal channel activity of TRPV1 at room temperature (43–45).
Although crystallographic data are not currently available to depict how organic cations permeate the TRPV1 pore, large ions may pass the channel using a similar principle as adopted by the more selective K+ channels. That is, the entire permeation pathway may simultaneously accommodate multiple cations, which create local electrostatic interactions among different or the same species of cations (46, 47). Charged capsaicinoids possess aliphatic tails that might mediate hydrophobic interaction with the nonpolar residues lining the pore also. Multiple ion bindings in the channel pore and hydrophobic interaction of aliphatic tails of charged capsaicinoids with other regions of the pore may collectively contribute to the permeation of cap-ET through TRPV1 channels. Further analysis will be required to delegate the contribution of each of these factors in the permeation of a charged capsaicinoid through TRPV1.
The charged capsaicinoids we synthesized are all partial agonists. The relative reduction in efficacy, however, is assay dependent. The remarkable permeability of TRPV1 to Ca2+ and receptor reserve may well be sufficient for a low efficacy agonist like cap-ET to elicit substantial Ca2+ rise to induce an effector response. The observation of a markedly reduced efficacy of cap-ET in electrophysiological studies compared with that in YO-PRO-1 transport experiments was, however, somewhat unanticipated. It is worth noting that the ability of TRPV1 ligands to cause electrical excitation depends critically on the Na+ influx per second. In contrast, Ca2+ entry and YO-PRO-1 transport reflect the capacity of TRPV1 channels to serve as hydrophilic permeation pathways to allow gradual accumulation of cations inside cells over a long period. The relatively inefficient coupling to electrical currents suggests an intriguing possibility to apply a chemical agonist with a pharmacological profile similar to cap-ET to manage pain. In contrast with capsaicin that causes substantial initial irritation, cap-ET will have reduced risk to evoke pain. Despite the fact that cap-ET might still accumulate over a prolonged period to cause indiscriminate increase of membrane permeability even in nonsensitized nociceptors, our neuronal data suggested that cap-ET can distinguish sensitized versus nonsensitized neurons if applied within an appropriate therapeutic window. Charged capsaicinoids can also trigger Ca2+-dependent desensitization, which may also blunt TRPV1’s ability to translate pain-producing stimuli into electric excitation. Moreover, extracellular cap-ET can block the inward current mediated by a strong pain-producing chemical like capsaicin. Unlike partial agonists generally exhibiting compromise in all downstream signaling pathways coupled to receptors, these charged synthetic ligands reveal an alternative and unconventional avenue for agonist design. Their activity dependence for cellular entry and differential alteration of agonistic efficacy highlights the possibility to further engineer existing TRPV1 ligands into ideal analgesics that can reduce pain arising from electrical activity without impairing signaling critical for other TRPV1 functions.
Materials and Methods
Ca2+ Imaging.
HEK293 cells were loaded with 5 μM Fura-2 AM and 0.01% pluronic acid in Ca2+ imaging buffer for 3 h. The imaging solution contained 8.5 mM Hepes, 140 mM NaCl, 3.4 mM KCl, 1.7 mM MgCl2, and 1 mM CaCl2, pH to 7.4 with NaOH. A 2× solution containing different concentrations of capsaicin and capsaicin analogs was pipetted into individual wells for agonist delivery. The images were acquired every 2 s for capsaicin and every 5 s for synthetic analogs. The plots displayed mean ± SEM values of F340/F380 (150-ms exposure time for each wavelength) ratios from fields containing 180–400 cells.
YO-PRO-1 Imaging.
Cells were plated in 96-well plates to near confluence. Images were acquired at a rate of 1 frame per 15 s. The fields were illuminated for 2 s for each frame. The fluorescent intensity was measured at the emission wavelength of 510/20 nm. A 2× concentration of agonists and 10 μM YO-PRO-1 were mixed and added at equal volume to a bath solution with the same electrolyte composition except omitting CaCl2.
Plate-Reader Assays.
TRPV1 cell lines were passed into 96-well plates, grown to confluence, and assayed in a physiological Ringer solution. A total of 50 μL of solution as used for YO-PRO-1 imaging with agonists of various concentrations was added to each well. Fluorescence intensity (485-nm excitation, 516-nm emission) was monitored by Multidetection Microplate Reader (BioTek). For normalization, we fixed cells by 5% PFA at the end of experiments and counted total fluorescence from each well after adding digitonin solution (Assay Designs) with 5 μM YO-PRO-1.
Supplementary Material
Acknowledgments
The authors thank Drs. Robert Oswald, Linda Nowak, Robert Gilmour, and Benjamin R. Myers for critical comments on the manuscript. This work was supported by a Scientist Development Grant from the American Heart Association (to H.-h.C.) and Cornell University college funds.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018550108/-/DCSupplemental.
References
- 1.Caterina MJ, et al. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 2.Tominaga M, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 3.Caterina MJ, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 4.Davis JB, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 5.Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 6.Gamse R, Molnar A, Lembeck F. Substance P release from spinal cord slices by capsaicin. Life Sci. 1979;25:629–636. doi: 10.1016/0024-3205(79)90558-7. [DOI] [PubMed] [Google Scholar]
- 7.Zygmunt PM, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 8.Jancsó-Gábor A, Szolcsányi J, Jancsó N. Stimulation and desensitization of the hypothalamic heat-sensitive structures by capsaicin in rats. J Physiol. 1970;208:449–459. doi: 10.1113/jphysiol.1970.sp009130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gavva NR, et al. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci. 2007;27:3366–3374. doi: 10.1523/JNEUROSCI.4833-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang D, et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010;12:130–141. doi: 10.1016/j.cmet.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- 12.Jung J, et al. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellwig N, et al. TRPV1 acts as proton channel to induce acidification in nociceptive neurons. J Biol Chem. 2004;279:34553–34561. doi: 10.1074/jbc.M402966200. [DOI] [PubMed] [Google Scholar]
- 14.Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–610. doi: 10.1038/nature06191. [DOI] [PubMed] [Google Scholar]
- 15.Meyers JR, et al. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung MK, Güler AD, Caterina MJ. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci. 2008;11:555–564. doi: 10.1038/nn.2102. [DOI] [PubMed] [Google Scholar]
- 17.Akabas MH, Stauffer DA, Xu M, Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992;258:307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- 18.Stauffer DA, Karlin A. Electrostatic potential of the acetylcholine binding sites in the nicotinic receptor probed by reactions of binding-site cysteines with charged methanethiosulfonates. Biochemistry. 1994;33:6840–6849. doi: 10.1021/bi00188a013. [DOI] [PubMed] [Google Scholar]
- 19.Walpole CS, et al. Analogues of capsaicin with agonist activity as novel analgesic agents; structure-activity studies. 1. The aromatic “A-region”. J Med Chem. 1993;36:2362–2372. doi: 10.1021/jm00068a014. [DOI] [PubMed] [Google Scholar]
- 20.Chuang HH, Lin S. Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proc Natl Acad Sci USA. 2009;106:20097–20102. doi: 10.1073/pnas.0902675106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walpole CS, et al. Analogues of capsaicin with agonist activity as novel analgesic agents; structure-activity studies. 2. The amide bond “B-region”. J Med Chem. 1993;36:2373–2380. doi: 10.1021/jm00068a015. [DOI] [PubMed] [Google Scholar]
- 22.Walpole CS, et al. Analogues of capsaicin with agonist activity as novel analgesic agents; structure-activity studies. 3. The hydrophobic side-chain “C-region”. J Med Chem. 1993;36:2381–2389. doi: 10.1021/jm00068a016. [DOI] [PubMed] [Google Scholar]
- 23.Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci USA. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- 25.Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- 26.Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida T, et al. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- 28.Salazar H, et al. A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat Neurosci. 2008;11:255–261. doi: 10.1038/nn2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhave G, et al. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci USA. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- 31.Vay L, Gu C, McNaughton PA. Current perspectives on the modulation of thermo-TRP channels: New advances and therapeutic implications. Expert Rev Clin Pharmacol. 2010;3:687–704. doi: 10.1586/ecp.10.41. [DOI] [PubMed] [Google Scholar]
- 32.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 33.Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- 34.Khakh BS, Bao XR, Labarca C, Lester HA. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nat Neurosci. 1999;2:322–330. doi: 10.1038/7233. [DOI] [PubMed] [Google Scholar]
- 35.Banke TG, Chaplan SR, Wickenden AD. Dynamic changes in the TRPA1 selectivity filter lead to progressive but reversible pore dilation. Am J Physiol Cell Physiol. 2010;298:C1457–C1468. doi: 10.1152/ajpcell.00489.2009. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, et al. Pore dilation occurs in TRPA1 but not in TRPM8 channels. Mol Pain. 2009;5:3. doi: 10.1186/1744-8069-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schenk U, et al. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 38.Kronlage M, et al. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal. 2010;3:ra55. doi: 10.1126/scisignal.2000588. [DOI] [PubMed] [Google Scholar]
- 39.Chekeni FB, et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacVicar BA, Thompson RJ. Non-junction functions of pannexin-1 channels. Trends Neurosci. 2010;33:93–102. doi: 10.1016/j.tins.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Lee S, et al. Channel-mediated tonic GABA release from glia. Science. 2010;330:790–796. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- 42.Samways DS, Khakh BS, Egan TM. Tunable calcium current through TRPV1 receptor channels. J Biol Chem. 2008;283:31274–31278. doi: 10.1074/jbc.C800131200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voets T, et al. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 44.Ahern GP, Premkumar LS. Voltage-dependent priming of rat vanilloid receptor: Effects of agonist and protein kinase C activation. J Physiol. 2002;545:441–451. doi: 10.1113/jphysiol.2002.029561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matta JA, Ahern GP. Voltage is a partial activator of rat thermosensitive TRP channels. J Physiol. 2007;585:469–482. doi: 10.1113/jphysiol.2007.144287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doyle DA, et al. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 47.Valiyaveetil FI, Leonetti M, Muir TW, Mackinnon R. Ion selectivity in a semisynthetic K+ channel locked in the conductive conformation. Science. 2006;314:1004–1007. doi: 10.1126/science.1133415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.