Abstract
Directional drilling and hydraulic-fracturing technologies are dramatically increasing natural-gas extraction. In aquifers overlying the Marcellus and Utica shale formations of northeastern Pennsylvania and upstate New York, we document systematic evidence for methane contamination of drinking water associated with shale-gas extraction. In active gas-extraction areas (one or more gas wells within 1 km), average and maximum methane concentrations in drinking-water wells increased with proximity to the nearest gas well and were 19.2 and 64 mg CH4 L-1 (n = 26), a potential explosion hazard; in contrast, dissolved methane samples in neighboring nonextraction sites (no gas wells within 1 km) within similar geologic formations and hydrogeologic regimes averaged only 1.1 mg L-1 (P < 0.05; n = 34). Average δ13C-CH4 values of dissolved methane in shallow groundwater were significantly less negative for active than for nonactive sites (-37 ± 7‰ and -54 ± 11‰, respectively; P < 0.0001). These δ13C-CH4 data, coupled with the ratios of methane-to-higher-chain hydrocarbons, and δ2H-CH4 values, are consistent with deeper thermogenic methane sources such as the Marcellus and Utica shales at the active sites and matched gas geochemistry from gas wells nearby. In contrast, lower-concentration samples from shallow groundwater at nonactive sites had isotopic signatures reflecting a more biogenic or mixed biogenic/thermogenic methane source. We found no evidence for contamination of drinking-water samples with deep saline brines or fracturing fluids. We conclude that greater stewardship, data, and—possibly—regulation are needed to ensure the sustainable future of shale-gas extraction and to improve public confidence in its use.
Keywords: groundwater, organic-rich shale, isotopes, formation waters, water chemistry
Increases in natural-gas extraction are being driven by rising energy demands, mandates for cleaner burning fuels, and the economics of energy use (1–5). Directional drilling and hydraulic-fracturing technologies are allowing expanded natural-gas extraction from organic-rich shales in the United States and elsewhere (2, 3). Accompanying the benefits of such extraction (6, 7) are public concerns about drinking-water contamination from drilling and hydraulic fracturing that are ubiquitous but lack a strong scientific foundation. In this paper, we evaluate the potential impacts associated with gas-well drilling and fracturing on shallow groundwater systems of the Catskill and Lockhaven formations that overlie the Marcellus Shale in Pennsylvania and the Genesee Group that overlies the Utica Shale in New York (Figs. 1 and 2 and Fig. S1). Our results show evidence for methane contamination of shallow drinking-water systems in at least three areas of the region and suggest important environmental risks accompanying shale-gas exploration worldwide.
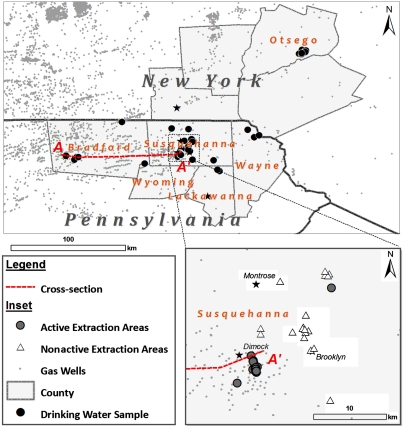
Fig. 1.
Map of drilling operations and well-water sampling locations in Pennsylvania and New York. The star represents the location of Binghamton, New York. (Inset) A close-up in Susquehanna County, Pennsylvania, showing areas of active (closed circles) or nonactive (open triangles) extraction. A drinking-water well is classified as being in an active extraction area if a gas well is within 1 km (see Methods). Note that drilling has already spread to the area around Brooklyn, Pennsylvania, primarily a nonactive location at the time of our sampling (see inset). The stars in the inset represent the towns of Dimock, Brooklyn, and Montrose, Pennsylvania.
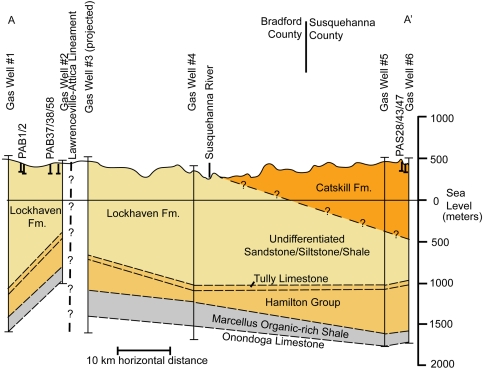
Fig. 2.
Geologic cross-section of Bradford and western Susquehanna Counties created from gas-well log data provided by the Pennsylvania Department of Conservation and Natural Resources. The approximate location of the Lawrenceville-Attica Lineament is taken from Alexander et al. (34). The Ordovician Utica organic-rich shale (not depicted in the figure) underlies the Middle Devonian Marcellus at approximately 3,500 m below the ground surface.
The drilling of organic-rich shales, typically of Upper Devonian to Ordovician age, in Pennsylvania, New York, and elsewhere in the Appalachian Basin is spreading rapidly, raising concerns for impacts on water resources (8, 9). In Susquehanna County, Pennsylvania alone, approved gas-well permits in the Marcellus formation increased 27-fold from 2007 to 2009 (10). Concerns for impacts to groundwater resources are based on (i) fluid (water and gas) flow and discharge to shallow aquifers due to the high pressure of the injected fracturing fluids in the gas wells (10); (ii) the toxicity and radioactivity of produced water from a mixture of fracturing fluids and deep saline formation waters that may discharge to the environment (11); (iii) the potential explosion and asphyxiation hazard of natural gas; and (iv) the large number of private wells in rural areas that rely on shallow groundwater for household and agricultural use—up to one million wells in Pennsylvania alone—that are typically unregulated and untested (8, 9, 12). In this study, we analyzed groundwater from 68 private water wells from 36- to 190-m deep in northeast Pennsylvania (Catskill and Lockhaven formations) and upstate New York (Genesee formation) (see Figs. 1 and 2 and SI Text), including measurements of dissolved salts, water isotopes (18O and 2H), and isotopes of dissolved constituents (carbon, boron, and radium). Of the 68 wells, 60 were also analyzed for dissolved-gas concentrations of methane and higher-chain hydrocarbons and for carbon and hydrogen isotope ratios of methane. Although dissolved methane in drinking water is not currently classified as a health hazard for ingestion, it is an asphyxiant in enclosed spaces and an explosion and fire hazard (8). This study seeks to evaluate the potential impact of gas drilling and hydraulic fracturing on shallow groundwater quality by comparing areas that are currently exploited for gas (defined as active—one or more gas wells within 1 km) to those that are not currently associated with gas drilling (nonactive; no gas wells within 1 km), many of which are slated for drilling in the near future.
Results and Discussion
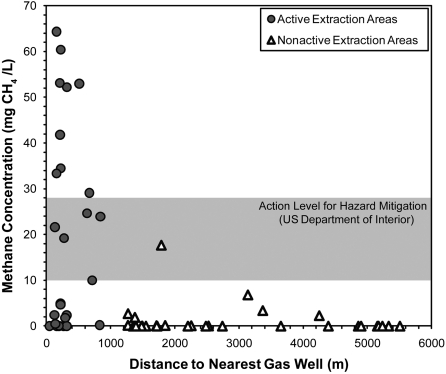
Methane concentrations were detected generally in 51 of 60 drinking-water wells (85%) across the region, regardless of gas industry operations, but concentrations were substantially higher closer to natural-gas wells (Fig. 3). Methane concentrations were 17-times higher on average (19.2 mg CH4 L-1) in shallow wells from active drilling and extraction areas than in wells from nonactive areas (1.1 mg L-1 on average; P < 0.05; Fig. 3 and Table 1). The average methane concentration in shallow groundwater in active drilling areas fell within the defined action level (10–28 mg L-1) for hazard mitigation recommended by the US Office of the Interior (13), and our maximum observed value of 64 mg L-1 is well above this hazard level (Fig. 3). Understanding the origin of this methane, whether it is shallower biogenic or deeper thermogenic gas, is therefore important for identifying the source of contamination in shallow groundwater systems.
Fig. 3.
Methane concentrations (milligrams of CH4 L-1) as a function of distance to the nearest gas well from active (closed circles) and nonactive (open triangles) drilling areas. Note that the distance estimate is an upper limit and does not take into account the direction or extent of horizontal drilling underground, which would decrease the estimated distances to some extraction activities. The precise locations of natural-gas wells were obtained from the Pennsylvania Department of Environmental Protection and Pennsylvania Spatial Data Access databases (ref. 35; accessed Sept. 24, 2010).
Table 1.
Mean values ± standard deviation of methane concentrations (as milligrams of CH4 L-1) and carbon isotope composition in methane in shallow groundwater δ13C-CH4 sorted by aquifers and proximity to gas wells (active vs. nonactive)
| Water source, n | milligrams CH4 L-1 | δ13C-CH4, ‰ |
| Nonactive Catskill, 5 | 1.9 ± 6.3 | −52.5 ± 7.5 |
| Active Catskill, 13 | 26.8 ± 30.3 | −33.5 ± 3.5 |
| Nonactive Genesee, 8 | 1.5 ± 3.0 | −57.5 ± 9.5 |
| Active Genesee, 1 | 0.3 | −34.1 |
| Active Lockhaven, 7 | 50.4 ± 36.1 | −40.7 ± 6.7 |
| Total active wells, 21 | 19.2 | −37 ± 7 |
| Total nonactive wells, 13 | 1.1 | −54 ± 11 |
The variable n refers to the number of samples.
The δ13C-CH4 and δ2H-CH4 values and the ratio of methane to higher-chain hydrocarbons (ethane, propane, and butane) can typically be used to differentiate shallower, biologically derived methane from deeper physically derived thermogenic methane (14). Values of δ13C-CH4 less negative than approximately -50‰ are indicative of deeper thermogenic methane, whereas values more negative than -64‰ are strongly indicative of microbial methane (14). Likewise, δ2H-CH4 values more negative than about -175‰, particularly when combined with low δ13C-CH4 values, often represent a purer biogenic methane origin (14).
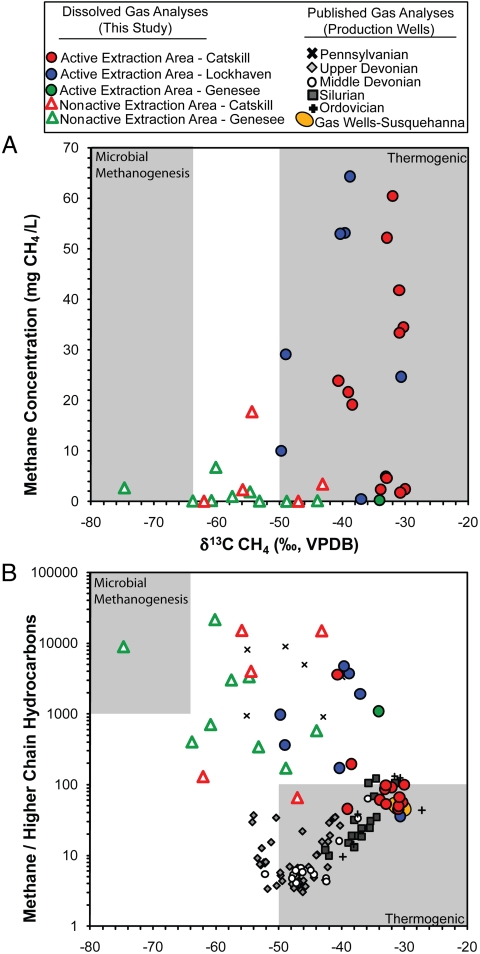
The average δ13C-CH4 value in shallow groundwater in active drilling areas was -37 ± 7‰, consistent with a deeper thermogenic methane source. In contrast, groundwater from nonactive areas in the same aquifers had much lower methane concentrations and significantly lower δ13C-CH4 values (average of -54 ± 11‰; P < 0.0001; Fig. 4 and Table 1). Both our δ13C-CH4 data and δ2H-CH4 data (see Fig. S2) are consistent with a deeper thermogenic methane source at the active sites and a more biogenic or mixed methane source for the lower-concentration samples from nonactive sites (based on the definition of Schoell, ref. 14).
Fig. 4.
(A) Methane concentrations in groundwater versus the carbon isotope values of methane. The nonactive and active data depicted in Fig. 3 are subdivided based on the host aquifer to illustrate that the methane concentrations and δ13C values increase with proximity to natural-gas well drilling regardless of aquifer formation. Gray areas represent the typical range of thermogenic and biogenic methane taken from Osborn and Mcintosh (18). VPDB, Vienna Pee Dee belemnite. (B) Bernard plot (15) of the ratio of methane to higher-chain hydrocarbons versus the δ13C of methane. The smaller symbols in grayscale are from published gas-well samples from gas production across the region (16–18). These data generally plot along a trajectory related to reservoir age and thermal maturity (Upper Devonian through Ordovician; see text for additional details). The gas-well data in the orange ovals are from gas wells in our study area in Susquehanna County, Pennsylvania (data from Pennsylvania Department of Environmental Protection). Gray areas represent typical ranges of thermogenic and biogenic methane (data from Osborn and McIntosh, ref. 18).
Because ethane and propane are generally not coproduced during microbial methanogenesis, the presence of higher-chain hydrocarbons at relatively low methane-to-ethane ratios (less than approximately 100) is often used as another indicator of deeper thermogenic gas (14, 15). Ethane and other higher-chain hydrocarbons were detected in only 3 of 34 drinking-water wells from nonactive drilling sites. In contrast, ethane was detected in 21 of 26 drinking-water wells in active drilling sites. Additionally, propane and butane were detected (> 0.001 mol %) in eight and two well samples, respectively, from active drilling areas but in no wells from nonactive areas.
Further evidence for the difference between methane from water wells near active drilling sites and neighboring nonactive sites is the relationship of methane concentration to δ13C-CH4 values (Fig. 4A) and the ratios of methane to higher-chain hydrocarbons versus δ13C-CH4 (Fig. 4B). Methane concentrations not only increased in proximity to gas wells (Fig. 3), the accompanying δ13C-CH4 values also reflected an increasingly thermogenic methane source (Fig. 4A).
Using a Bernard plot (15) for analysis (Fig. 4B), the enriched δ13C-CH4 (approximately > -50‰) values accompanied by low ratios of methane to higher-chain hydrocarbons (less than approximately 100) in drinking-water wells also suggest that dissolved gas is more thermogenic at active than at nonactive sites (Fig. 4B). For instance, 12 dissolved-gas samples at active drilling sites fell along a regional gas trajectory that increases with reservoir age and thermal maturity of organic matter, with samples from Susquehanna County, Pennsylvania specifically matching natural-gas geochemistry from local gas wells (Fig. 4B, orange oval). These 12 samples and local natural-gas samples are consistent with gas sourced from thermally mature organic matter of Middle Devonian and older depositional ages often found in Marcellus Shale from approximately 2,000 m below the surface in the northern Appalachian Basin (14–19) (Fig. 4B). In contrast, none of the methane samples from nonactive drilling areas fell upon this trajectory (Fig. 4B); eight dissolved-gas samples in Fig. 4B from active drilling areas and all of the values from nonactive areas may instead be interpreted as mixed biogenic/thermogenic gas (18) or, as Laughrey and Baldassare (17) proposed for their Pennsylvanian gas data (Fig. 4B), the early migration of wet thermogenic gases with low-δ13C-CH4 values and high methane-to-higher-chain hydrocarbon ratios. One data point from a nonactive area in New York fell squarely in the parameters of a strictly biogenic source as defined by Schoell (14) (Fig. 4B, upper-left corner).
Carbon isotopes of dissolved inorganic carbon (δ13C-DIC > +10‰) and the positive correlation of δ2H of water and δ2H of methane have been used as strong indicators of microbial methane, further constraining the source of methane in shallow groundwater (depth less than 550 m) (18, 20). Our δ13C-DIC values were fairly negative and show no association with the δ13C-CH4 values (Fig. S3), which is not what would be expected if methanogenesis were occurring locally in the shallow aquifers. Instead, the δ13C-DIC values from the shallow aquifers plot within a narrow range typical for shallow recharge waters, with the dissolution of CO2 produced by respiration as water passes downward through the soil critical zone. Importantly, these values do not indicate extensive microbial methanogenesis or sulfate reduction. The data do suggest gas-phase transport of methane upward to the shallow groundwater zones sampled for this study (< 190 m) and dissolution into shallow recharge waters locally. Additionally, there was no positive correlation between the δ2H values of methane and δ2H of water (Fig. S4), indicating that microbial methane derived in this shallow zone is negligible. Overall, the combined gas and formation-water results indicate that thermogenic gas from thermally mature organic matter of Middle Devonian and older depositional ages is the most likely source of the high methane concentrations observed in the shallow water wells from active extraction sites.
A different potential source of shallow groundwater contamination associated with gas drilling and hydraulic fracturing is the introduction of hypersaline formation brines and/or fracturing fluids. The average depth range of drinking-water wells in northeastern Pennsylvania is from 60 to 90 m (12), making the average vertical separation between drinking-water wells and the Marcellus Shale in our study area between approximately 900 and 1,800 m (Fig. 2). The research area, however, is located in tectonically active areas with mapped faults, earthquakes, and lineament features (Fig. 2 and Fig. S1). The Marcellus formation also contains two major sets of joints (21) that could be conduits for directed pressurized fluid flow. Typical fracturing activities in the Marcellus involve the injection of approximately 13–19 million liters of water per well (22) at pressures of up to 69,000 kPa. The majority of this fracturing water typically stays underground and could in principle displace deep formation water upward into shallow aquifers. Such deep formation waters often have high concentrations of total dissolved solids > 250,000 mg L-1, trace toxic elements, (18), and naturally occurring radioactive materials, with activities as high as 16,000 picocuries per liter (1 pCi L-1 = 0.037 becquerels per liter) for 226Ra compared to a drinking-water standard of 5 pCi L-1 for combined 226Ra and 226Ra (23).
We evaluated the hydrochemistry of our 68 drinking-water wells and compared these data to historical data of 124 wells in the Catskill and Lockhaven aquifers (24, 25). We used three types of indicators for potential mixing with brines and/or saline fracturing fluids: (i) major inorganic chemicals; (ii) stable isotope signatures of water (δ18O, δ2H); and (iii) isotopes of dissolved constituents (δ13C-DIC, δ11B, and 226Ra). Based on our data (Table 2), we found no evidence for contamination of the shallow wells near active drilling sites from deep brines and/or fracturing fluids. All of the Na+, Cl-, Ca2+, and DIC concentrations in wells from active drilling areas were consistent with the baseline historical data, and none of the shallow wells from active drilling areas had either chloride concentrations > 60 mg L-1 or Na-Ca-Cl compositions that mirrored deeper formation waters (Table 2). Furthermore, the mean isotopic values of δ18O, δ2H, δ13C-DIC, δ11B, and 226Ra in active and nonactive areas were indistinguishable. The 226Ra values were consistent with available historical data (25), and the composition of δ18O and δ2H in the well-water appeared to be of modern meteoric origin for Pennsylvania (26) (Table 2 and Fig. S5). In sum, the geochemical and isotopic features for water we measured in the shallow wells from both active and nonactive areas are consistent with historical data and inconsistent with contamination from mixing Marcellus Shale formation water or saline fracturing fluids (Table 2).
Table 2.
Comparisons of selected major ions and isotopic results in drinking-water wells from this study to data available on the same formations (Catskill and Lockhaven) in previous studies (24, 25) and to underlying brines throughout the Appalachian Basin (18)
| Active | Nonactive | Previous studies (background) | |||||
| Lockhaven formation | Catskill formation | Catskill formation | Genesee group | Lockhaven formation (25) | Catskill formation (24) | Appalachian brines (18, 23) | |
| N = 8 | N = 25 | N = 22 | N = 12 | N = 45 | N = 79 | N = 21 | |
Alkalinity as 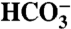 , mg L-1mM , mg L-1mM |
285 ± 36 [4.7 ± 0.6] | 157 ± 56 [2.6 ± 0.9] | 127 ± 53 [2.1 ± 0.9] | 158 ± 56 [2.6 ± 0.9] | 209 ± 77 [3.4 ± 1.3] | 133 ± 61 [2.2 ± 1.0] | 150 ± 171 [2.5 ± 2.8] |
| Sodium, mg L-1 | 87 ± 22 | 23 ± 30 | 17 ± 25 | 29 ± 23 | 100 ± 312 | 21 ± 37 | 33,000 ± 11,000 |
| Chloride, mg L-1 | 25 ± 17 | 11 ± 12 | 17 ± 40 | 9 ± 19 | 132 ± 550 | 13 ± 42 | 92,000 ± 32,000 |
| Calcium, mg L-1 | 22 ± 12 | 31 ± 13 | 27 ± 9 | 26 ± 5 | 49 ± 39 | 29 ± 11 | 16,000 ± 7,000 |
| Boron, μg L-1 | 412 ± 156 | 93 ± 167 | 42 ± 93 | 200 ± 130 | NA | NA | 3,700 ± 3,500 |
| δ11B ‰ | 27 ± 4 | 22 ± 6 | 23 ± 6 | 26 ± 6 | NA | NA | 39 ± 6 |
| 226Ra, pCi L-1 | 0.24 ± 0.2 | 0.16 ± 0.15 | 0.17 ± 0.14 | 0.2 ± 0.15 | 0.56 ± 0.74 | NA | 6,600 ± 5,600 |
| δ2H, ‰, VSMOW | −66 ± 5 | −64 ± 3 | −68 ± 6 | −76 ± 5 | NA | NA | −41 ± 6 |
| δ18O, ‰, VSMOW | −10 ± 1 | −10 ± 0.5 | −11 ± 1 | −12 ± 1 | NA | NA | −5 ± 1 |
Some data for the active Genesee Group and nonactive Lockhaven Formation are not included because of insufficient sample sizes (NA). Values represent means ± 1 standard deviation. NA, not available.
N values for δ11B ‰ analysis are 8, 10, 3, 6, and 5 for active Lockhaven, active Catskill, nonactive Genesee, nonactive Catskill, and brine, respectively. N values for 226Ra are 6, 7, 3, 10, 5, and 13 for active Lockhaven, active Catskill, nonactive Genesee, nonactive Catskill, background Lockhaven, and brine, respectively. δ11B ‰ normalized to National Institute of Standards and Technology Standard Reference Material 951. δ2H and δ18O normalized to Vienna Standard Mean Ocean Water (VSMOW).
There are at least three possible mechanisms for fluid migration into the shallow drinking-water aquifers that could help explain the increased methane concentrations we observed near gas wells (Fig. 3). The first is physical displacement of gas-rich deep solutions from the target formation. Given the lithostatic and hydrostatic pressures for 1–2 km of overlying geological strata, and our results that appear to rule out the rapid movement of deep brines to near the surface, we believe that this mechanism is unlikely. A second mechanism is leaky gas-well casings (e.g., refs. 27 and 28). Such leaks could occur at hundreds of meters underground, with methane passing laterally and vertically through fracture systems. The third mechanism is that the process of hydraulic fracturing generates new fractures or enlarges existing ones above the target shale formation, increasing the connectivity of the fracture system. The reduced pressure following the fracturing activities could release methane in solution, leading to methane exsolving rapidly from solution (29), allowing methane gas to potentially migrate upward through the fracture system.
Methane migration through the 1- to 2-km-thick geological formations that overlie the Marcellus and Utica shales is less likely as a mechanism for methane contamination than leaky well casings, but might be possible due to both the extensive fracture systems reported for these formations and the many older, uncased wells drilled and abandoned over the last century and a half in Pennsylvania and New York. The hydraulic conductivity in the overlying Catskill and Lockhaven aquifers is controlled by a secondary fracture system (30), with several major faults and lineaments in the research area (Fig. 2 and Fig. S1). Consequently, the high methane concentrations with distinct positive δ13C-CH4 and δ2H-CH4 values in the shallow groundwater from active areas could in principle reflect the transport of a deep methane source associated with gas drilling and hydraulic-fracturing activities. In contrast, the low-level methane migration to the surface groundwater aquifers, as observed in the nonactive areas, is likely a natural phenomenon (e.g., ref. 31). Previous studies have shown that naturally occurring methane in shallow aquifers is typically associated with a relatively strong biogenic signature indicated by depleted δ13C-CH4 and δ2H-CH4 compositions (32) coupled with high ratios of methane to higher-chain hydrocarbons (33), as we observed in Fig. 4B. Several models have been developed to explain the relatively common phenomenon of rapid vertical transport of gases (Rn, CH4, and CO2) from depth to the surface (e.g., ref. 31), including pressure-driven continuous gas-phase flow through dry or water-saturated fractures and density-driven buoyancy of gas microbubbles in aquifers and water-filled fractures (31). More research is needed across this and other regions to determine the mechanism(s) controlling the higher methane concentrations we observed.
Based on our groundwater results and the litigious nature of shale-gas extraction, we believe that long-term, coordinated sampling and monitoring of industry and private homeowners is needed. Compared to other forms of fossil-fuel extraction, hydraulic fracturing is relatively poorly regulated at the federal level. Fracturing wastes are not regulated as a hazardous waste under the Resource Conservation and Recovery Act, fracturing wells are not covered under the Safe Drinking Water Act, and only recently has the Environmental Protection Agency asked fracturing firms to voluntarily report a list of the constituents in the fracturing fluids based on the Emergency Planning and Community Right-to-Know Act. More research is also needed on the mechanism of methane contamination, the potential health consequences of methane, and establishment of baseline methane data in other locations. We believe that systematic and independent data on groundwater quality, including dissolved-gas concentrations and isotopic compositions, should be collected before drilling operations begin in a region, as is already done in some states. Ideally, these data should be made available for public analysis, recognizing the privacy concerns that accompany this issue. Such baseline data would improve environmental safety, scientific knowledge, and public confidence. Similarly, long-term monitoring of groundwater and surface methane emissions during and after extraction would clarify the extent of problems and help identify the mechanisms behind them. Greater stewardship, knowledge, and—possibly—regulation are needed to ensure the sustainable future of shale-gas extraction.
Methods
A total of 68 drinking-water samples were collected in Pennsylvania and New York from bedrock aquifers (Lockhaven, 8; Catskill, 47; and Genesee, 13) that overlie the Marcellus or Utica shale formations (Fig. S1). Wells were purged to remove stagnant water, then monitored for pH, electrical conductance, and temperature until stable values were recorded. Samples were collected “upstream” of any treatment systems, as close to the water well as possible, and preserved in accordance with procedures detailed in SI Methods. Dissolved-gas samples were analyzed at Isotech Laboratories and water chemical and isotope (O, H, B, C, Ra) compositions were measured at Duke University (see SI Methods for analytical details).
Supplementary Material
Acknowledgments.
We thank Rebecca Roter, Peggy Maloof, and many others who allowed us to sample their water wells; Laura Ruhl and Tewodros Rango for coordination and field assistance; Nicolas Cassar for thoughtful suggestions on the research; and Kaiguang Zhao and Rose Merola for help with figures. Jon Karr and the Duke Environmental Isotope Laboratory performed analyses of δ18O, δ2H, and δ13C of groundwater samples. William Chameides, Lincoln Pratson, William Schlesinger, the Jackson Lab, and two anonymous reviewers provided helpful suggestions on the manuscript and research. We gratefully acknowledge financial support from Fred and Alice Stanback to the Nicholas School of the Environment and from the Duke Center on Global Change.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100682108/-/DCSupplemental.
References
- 1.Pacala S, Socolow R. Stabilization wedges: Solving the climate problem for the next 50 years with current technologies. Science. 2004;305:968–972. doi: 10.1126/science.1100103. [DOI] [PubMed] [Google Scholar]
- 2.Tour JM, Kittrell C, Colvin VL. Green carbon as a bridge to renewable energy. Nature Mater. 2010;9:871–874. doi: 10.1038/nmat2887. [DOI] [PubMed] [Google Scholar]
- 3.Kerr RA. Natural gas from shale bursts onto the scene. Science. 2010;328:1624–1626. doi: 10.1126/science.328.5986.1624. [DOI] [PubMed] [Google Scholar]
- 4.Raupach MR, et al. Global and regional drivers of accelerating CO2 emissions. Proc Natl Acad Sci USA. 2007;104:10288–10293. doi: 10.1073/pnas.0700609104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Energy Information Administration. Annual Energy Outlook 2010 with Projections to 2035. Washington, DC: US Energy Information Administration; 2010. DOE/EIA-0383; http://www.eia.doe.gov/oiaf/aeo/pdf/0383(2010).pdf. [Google Scholar]
- 6.US Environmental Protection Agency. Washington, DC: US Environmental Protection Agency; 2011. Hydraulic Fracturing. http://water.epa.gov/type/groundwater/uic/class2/hydraulicfracturing/ [Google Scholar]
- 7.Kargbo DM, Wilhelm RG, Campbell DJ. Natural gas plays in the Marcellus shale: Challenges and potential opportunities. Environ Sci Technol. 2010;44:5679–5684. doi: 10.1021/es903811p. [DOI] [PubMed] [Google Scholar]
- 8.Revesz KM, Breen KJ, Baldassare AJ, Burruss RC. Carbon and hydrogen isotopic evidence for the origin of combustible gases in water supply wells in north-central Pennsylvania. Appl Geochem. 2010;25:1845–1859. [Google Scholar]
- 9.Zoback M, Kitasei S, Copithorne B. Worldwatch Institute Briefing Paper 1. Washington, DC: Worldwatch Inst; Addressing the environmental risks from shale gas development. http://blogs.worldwatch.org/revolt/wp-content/uploads/2010/07/Environmental-Risks-Paper-July-2010-FOR-PRINT.pdf. [Google Scholar]
- 10.Pennsylvania Department of Environmental Protection, Bureau of Oil and Gas Management. Harrisburg, PA: Pennsylvania Dept of Environmental Protection, Bureau of Oil and Gas Management; 2010. 2009 Year End Workload Report. http://www.dep.state.pa.us/dep/deputate/minres/oilgas/2009%20Year%20End%20Report-WEBSITE.pdf. [Google Scholar]
- 11.Colborn T, Kwiatkowski C, Schultz K, Bachran M. Natural gas operations from a public health perspective. Hum Ecol Risk Assess. 2010 in press. [Google Scholar]
- 12.Pennsylvania Department of Environmental Protection. Harrisburg, PA: Pennsylvania Dept of Environmental Protection; 2011. Private Water Wells in Pennsylvania. http://www.dep.state.pa.us/dep/deputate/watermgt/wc/Subjects/SrceProt/well/ [Google Scholar]
- 13.Eltschlager KK, Hawkins JW, Ehler WC, Baldassare F. Technical Measures for the Investigation and Mitigation of Fugitive Methane Hazards in Areas of Coal Mining. Pittsburgh: US Dept of the Interior, Office of Surface Mining Reclamation and Enforcement; 2001. [Google Scholar]
- 14.Schoell M. The hydrogen and carbon isotopic composition of methane from natural gases of various origins. Geochim Cosmochim Acta. 1980;44:649–661. [Google Scholar]
- 15.Bernard BB. College Station, TX: Texas A&M Univ; 1978. Light hydrocarbons in marine sediments. PhD Dissertation. [Google Scholar]
- 16.Jenden PD, Drazan DJ, Kaplan IR. Mixing of thermogenic natural gases in northern Appalachian Basin. Am Assoc Pet Geol Bull. 1993;77:980–998. [Google Scholar]
- 17.Laughrey CD, Baldassare FJ. Geochemistry and origin of some natural gases in the Plateau Province Central Appalachian Basin, Pennsylvania and Ohio. Am Assoc Pet Geol Bull. 1998;82:317–335. [Google Scholar]
- 18.Osborn SG, McIntosh JC. Chemical and isotopic tracers of the contribution of microbial gas in Devonian organic-rich shales and reservoir sandstones, northern Appalachian Basin. Appl Geochem. 2010;25:456–471. [Google Scholar]
- 19.Repetski JE, Ryder RT, Harper JA, Trippi MH. Thermal maturity patterns in the Ordovician and Devonian of Pennsylvania using conodont color alteration index (CAI) and vitrinite reflectance (%Ro) Northeastern Geology Environmental Sciences. 2006;28:266–294. [Google Scholar]
- 20.Martini AM, et al. Genetic and temporal relations between formation waters and biogenic methane: Upper Devonian Antrim Shale, Michigan Basin, USA. Geochim Cosmochim Acta. 1998;62:1699–1720. [Google Scholar]
- 21.Engelder T, Lash GG, Uzcategui RS. Joint sets that enhance production from Middle and Upper Devonian gas shales of the Appalachian Basin. Am Assoc Pet Geol Bull. 2009;93:857–889. [Google Scholar]
- 22.Pennsylvania Department of Environmental Protection. Harrisburg, PA: Pennsylvania Dept of Environmental Protection; 2011. Marcellus Shale, http://www.dep.state.pa.us/dep/deputate/minres/oilgas/new_forms/marcellus/marcellus.htm. [Google Scholar]
- 23.New York State Department of Health, Bureau of Environmental Radiation Protection. Troy, NY: New York State Dept of Health; 2009. Comments, July 21, 2009, Supplemental Generic Environmental Statement on the Oil and Gas Regulatory Program Well Permit Issuance for Horizontal Drilling and Hydraulic-Fracturing to Develop the Marcellus Shale and other Low Permeability Gas Reservoirs; http://www.riverkeeper.org/wp-content/uploads/2010/01/Riverkeeper-DSGEIS-Comments-Appendix-3-NYSDOH-Environmental-Radiation-Memo.pdf. [Google Scholar]
- 24.Taylor LE. Harrisburg, PA: Pennsylvania Dept of Environmental Resources-Office of Parks and Forestry—Bureau of Topographic and Geologic Survey; 1984. Groundwater Resources of the Upper Susquehanna River Basin, Pennsylvania: Water Resources Report 58; p. 139. [Google Scholar]
- 25.Williams JH, Taylor L, Low D. Harrisburg, PA: Commonwealth of Pennsylvania Dept of Conservation and Natural Resources; 1998. Hydrogeology and Groundwater Quality of the Glaciated Valleys of Bradford, Tioga, and Potter Counties, Pennsylvania: Water Resources Report 68; p. 89. [Google Scholar]
- 26.Kendall C, Coplan TB. Distribution of oxygen-18 and deuterium in river waters across the United States. Hydrol Processes. 2001;15:1363–1393. [Google Scholar]
- 27.Van Stempvoort D, Maathuis H, Jaworski E, Mayer B, Rich K. Oxidation of fugitive methane in groundwater linked to bacterial sulfate reduction. Ground Water. 2005;43:187–199. doi: 10.1111/j.1745-6584.2005.0005.x. [DOI] [PubMed] [Google Scholar]
- 28.Taylor SW, Sherwood Lollar B, Wassenaar LI. Bacteriogenic ethane in near-surface aquifers: Implications for leaking hydrocarbon well bores. Environ Sci Technol. 2000;34:4727–4732. [Google Scholar]
- 29.Cramer B, Schlomer S, Poelchau HS. Vol. 196. London: Geological Society Special Publications; 2002. Uplift-related hydrocarbon accumulations: the release of natural gas from groundwater; pp. 447–455. [Google Scholar]
- 30.Geyer AR, Wilshusen JP. Harrisburg, PA: Dept of Environmental Resources, Office of Resources Management; 1982. Engineering characteristics of the rocks of Pennsylvania; environmental geology supplement to the state geologic map, 1982 Pennsylvania Geological Survey. [Google Scholar]
- 31.Etiope G, Martinelli G. Migration of carrier and trace gases in the geosphere: An overview. Phys Earth Planet Inter. 2002;129:185–204. [Google Scholar]
- 32.Aravena R, Wassenaar LI. Dissolved organic carbon and methane in a regional confined aquifer, southern Ontario, Canada: Carbon isotope evidence for associated subsurface sources. Appl Geochem. 1993;8:483–493. [Google Scholar]
- 33.Coleman DD, Liu C, Riley KM. Microbial methane in the shallow Paleozoic sediments and glacial deposits of the Illinois, USA. Chem Geol. 1988;71:23–40. [Google Scholar]
- 34.Alexander SS, Cakir R, Doden AG, Gold DP, Root SI. Middletown, PA: Pennsylvania Dept of Conservation and Natural Resources; 2005. Basement depth and related geospatial database for Pennsylvania: Pennsylvania Geological Survey, 4th ser., Open-File General Geology Report 05-01.0. http://www.dcnr.state.pa.us/topogeo/openfile. [Google Scholar]
- 35.Pennsylvania Spatial Data Access (PASDA) Harrisburg, PA: Pennsylvania Dept of Environmental Protection; Online mapping, data access wizard, oil and gas locations. http://www.pasda.psu.edu/uci/SearchResults.aspx?searchType=mapservice&condition=OR&entry=PASDA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.