Abstract
The trace amine-associated receptor 1 (TAAR1), activated by endogenous metabolites of amino acids like the trace amines p-tyramine and β-phenylethylamine, has proven to be an important modulator of the dopaminergic system and is considered a promising target for the treatment of neuropsychiatric disorders. To decipher the brain functions of TAAR1, a selective TAAR1 agonist, RO5166017, was engineered. RO5166017 showed high affinity and potent functional activity at mouse, rat, cynomolgus monkey, and human TAAR1 stably expressed in HEK293 cells as well as high selectivity vs. other targets. In mouse brain slices, RO5166017 inhibited the firing frequency of dopaminergic and serotonergic neurons in regions where Taar1 is expressed (i.e., the ventral tegmental area and dorsal raphe nucleus, respectively). In contrast, RO5166017 did not change the firing frequency of noradrenergic neurons in the locus coeruleus, an area devoid of Taar1 expression. Furthermore, modulation of TAAR1 activity altered the desensitization rate and agonist potency at 5-HT1A receptors in the dorsal raphe, suggesting that TAAR1 modulates not only dopaminergic but also serotonergic neurotransmission. In WT but not Taar1−/− mice, RO5166017 prevented stress-induced hyperthermia and blocked dopamine-dependent hyperlocomotion in cocaine-treated and dopamine transporter knockout mice as well as hyperactivity induced by an NMDA antagonist. These results tie TAAR1 to the control of monoamine-driven behaviors and suggest anxiolytic- and antipsychotic-like properties for agonists such as RO5166017, opening treatment opportunities for psychiatric disorders.
Keywords: drug discovery, serotonin, depression, schizophrenia, anxiety
In 2001, identification of the trace amine-associated receptor 1 (TAAR1) provided evidence for a direct biological effect of so-called trace amines (TAs) (1, 2), a subgroup of biogenic amines previously denoted as false neurotransmitters (3). TAs such as p-tyramine (pTyr), β-phenylethylamine (PEA), octopamine, and tryptamine are metabolites of amino acids with structural similarity to classical biogenic amines. Although they are only found at low concentrations in the brain, TAs have been implicated in a wide range of neuropathological disorders, including schizophrenia, major depression, anxiety states, Parkinson's disease, and attention deficit hyperactivity disorder (3, 4).
TAAR1, a member of the TAAR family (5, 6), is a G protein-coupled receptor (GPCR) that signals through Gs to elevate intracellular cAMP levels in response to TAs (6, 7). In vitro studies have shown a reciprocal regulation between TAAR1 and monoaminergic transporters, particularly the dopamine transporter (DAT) (8, 9). In the mouse brain, Taar1 is expressed throughout the limbic and monoaminergic systems, including the ventral tegmental area (VTA) and dorsal raphe nucleus (DRN) (10). Mice lacking Taar1 (Taar1−/− mice) have no overt phenotype and appear similar to WT littermates in most neurological and behavioral tests (10–12). However, Taar1−/− mice are hypersensitive to the locomotor-stimulating effect of d-amphetamine and show elevated striatal release of dopamine (DA), noradrenaline (NA), and serotonin [5-hydroxytryptamine (5-HT)] after a d-amphetamine challenge (10, 12). Furthermore, the spontaneous firing rate of the VTA DA neurons is augmented in Taar1−/− mice, and only in WT mice does pTyr decrease this firing rate (10). These observations suggest that TAAR1 is a negative modulator of monoaminergic neurotransmission.
Apart from the TAs, TAAR1 is activated by a range of endogenous molecules such as other biogenic amines (2, 5), thyroid hormone-derivative 3-iodothyronamine (T1AM) (13, 14), and catechol-O-methyl transferase products (e.g., 3-methoxytyramine) (2, 15) or by synthetic substances such as amphetamine derivatives and ergolines (2, 12). However, all these ligands have TAAR1-independent effects through other targets, such as the monoaminergic transporters and receptors or the σ-receptors (3, 11, 13, 16). The lack of selective ligands has rendered identification of TAAR1 biological functions challenging. Recently, Bradaia et al. (17) described the first selective TAAR1 antagonist, N-(3-Ethoxy-phenyl)-4-pyrrolidin-1-yl-3-trifluoromethyl-benzamide (EPPTB). Use of EPPTB revealed that TAAR1 tonically activates inwardly rectifying K+ channels in VTA DA neurons to reduce the basal firing activity (17). Importantly, EPPTB increased agonist potency at DA D2 receptors while reducing their desensitization rate, strongly suggesting a functional link between TAAR1 and D2 receptors.
Further elucidation of TAAR1 physiological functions and validation of its promising therapeutic potential require selective agonists. The ideal characteristics of such molecules are (i) high affinity and intrinsic activity (equal or superior to TAs), (ii) high selectivity for TAAR1 (in contrast to TAs), (iii) low metabolic turnover (in contrast to TAs), and (iv) favorable pharmacokinetic properties, such as a good brain penetration, to enable in vivo studies. Here, we report the identification of such a compound, RO5166017, which was used for the study of TAAR1 function in vitro and in vivo.
Results
Identification of RO5166017 and in Vitro Pharmacological Characterization.
There seems to be considerable overlap between the pharmacophore space occupied by TAAR1 ligands and ligands of other biogenic amine receptors (2). Therefore, a medicinal chemistry program seeking to create TAAR1 ligands starting from adrenergic ligands as a source of lead compounds was started. One of the starting compounds was the amino-oxazoline S18616 (18), an α2A-adrenergic receptor agonist, and an iterative series of structural modifications within this chemical class led to the discovery of RO5166017 [(S)-4-[(ethyl-phenyl-amino)-methyl]-4,5-dihydro-oxazol-2-ylamine] (Fig. 1).
Fig. 1.
Chemical structure of the selective TAAR1 agonist RO5166017.
RO5166017 exhibited high potency and efficacy at mouse, rat, cynomolgus monkey, and human TAAR1 stably expressed in HEK293 cells (Table 1). Maximal cAMP levels reached by RO5166017 stimulation (maximal efficacy) were in a range similar to that achieved by PEA stimulation (set as 100%) at human, rat, and monkey TAAR1 (81–95%) and somewhat lower at mouse TAAR1 (65%). Electrophysiological recordings in Xenopus oocytes as well as VTA and DRN slices resulted in EC50 and IC50 values for mouse TAAR1 (1.7–8 nM) comparable with those obtained by the cAMP assay in HEK293 cells (3.3 nM).
Table 1.
Binding affinities and EC50/IC50 values of RO5166017 at rodent and primate TAAR1
| Parameter, assay, preparation | Mouse | Rat | Human | Cynomolgus monkey |
| Ki, binding, HEK293* | 1.9 ± 1.2 | 2.7 ± 1.3 | 31 ± 4 | 24 ± 5 |
| EC50, cAMP, HEK293† | 3.3 ± 1.7 (65 ± 15%) | 14 ± 10 (90 ± 17%) | 55 ± 27 (95 ± 8%) | 97 ± 53 (81 ± 1%) |
| EC50, GIRK, Xenopus oocytes‡§ | 8.0 ± 1.2 (72 ± 2%) | n.d. | n.d. | n.d. |
| IC50, patch clamp, VTA slices§ | 1.73 | n.d. | n.d. | n.d. |
| IC50, patch clamp, DRN slices§ | 2.99 | n.d. | n.d. | n.d. |
Values (in nM) represent the mean ± SEM from at least three independent experiments. Data in parentheses represent the maximal efficacy relative to PEA (EC50 and cAMP) or pTyr (EC50 and GIRK). n.d., not determined.
*Radioligand [3H]RO5166017 for mouse and rat TAAR1 and [3H]RO5192022 for human and cynomolgus monkey TAAR1.
†Upstate (Millipore) immunoassay for cAMP.
‡Current mediated by Kir3.1 and Kir3.2 coexpressed with TAAR1.
§Current at −50-mV holding potential.
RO5166017 is highly selective for TAAR1, as evaluated from radioligand binding assays (Cerep) consisting of 123 target proteins (Tables S1 and S2). Whenever the binding of the specific reference labels was significantly inhibited by RO5166017 (10 μM), further determination of the Ki revealed a low affinity (>100-fold weaker vs. mouse TAAR1). The only exceptions were the κ-opioid, adrenergic α2, and imidazoline I1 receptors for which the selectivity ratios (Ki/Ki) were 79-, 64-, and 15-fold, respectively, in favor of mouse TAAR1. Finally, RO5166017 up to 30 μM did not elicit cAMP production from mouse TAAR4, the only other TAAR family member activated by TAs (5).
Electrophysiological Characterization of RO5166017.
The endogenous TAAR1 agonist pTyr inhibits the firing frequency of DA neurons in the VTA, where Taar1 is expressed (10), whereas blockade of TAAR1 with EPPTB strongly increases their firing rate (17). Thus, we examined whether the synthetic TAAR1 agonist RO5166017 influences the firing of DA neurons in a manner similar to pTyr.
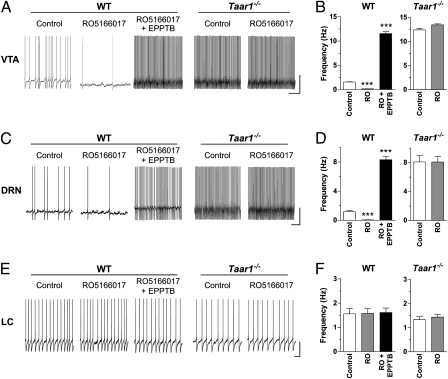
Application of RO5166017 (500 nM) inhibited the firing frequency of DA neurons in the VTA (Fig. 2 A and B) as does pTyr (17). This effect was dose-dependent with an IC50 value of 1.73 nM, which is in agreement with the results obtained in other assays (Table 1). Similar to pTyr, RO5166017 reduced firing frequency by activating a K+-mediated outward current (Figs. S1 and S2 A and B). Application of EPPTB (10 nM) completely reversed the RO5166017-induced effect and even reduced the current beyond the predrug baseline (Fig. S1), which is in line with previous data (17). Importantly, RO5166017 did not change the firing frequency or the membrane current in brain slices from Taar1−/− mice (Fig. 2 A and B and Fig. S1). These results show that RO5166017 activates TAAR1 in DA neurons to lower their firing activity similar to pTyr but with much higher potency.
Fig. 2.
RO5166017 inhibits the firing rate of DA and 5-HT neurons but not that of NA neurons. (A, C, and E) Representative recordings from brain slices of WT and Taar1−/− mice. (Scale bar: VTA, 20 mV/s; DRN and LC, 30 mV/s.) (B, D, and F) Quantification bar graphs (mean ± SEM; n = 5 neurons, recorded from three animals per condition). The firing frequency was assessed before (Control) and during application of RO5166017 (500 nM; RO) alone and in combination with EPPTB (10 nM). In WT mice, RO5166017 decreased firing of DA neurons in the VTA (A and B) and 5-HT neurons in the DRN (C and D), whereas application of EPPTB increased the firing rate above control levels. In brain slices of Taar1−/− mice, the spontaneous firing frequencies of the DA and 5-HT neurons were increased compared with WT, and they were not affected by RO5166017. In the LC (E and F), the spontaneous firing rates of NA neurons in WT and Taar1−/− mice were not significantly different, and RO5166017 had no effect in WT mice. ***P < 0.001 vs. the other two conditions.
Because Taar1 is also expressed in the DRN (10), we asked whether RO5166017 and pTyr also affect the firing frequency of 5-HT neurons. Both compounds decreased the spike rate of DRN 5-HT neurons in WT mice (Fig. 2 C and D and Fig. S3), with an IC50 value of 2.99 nM for RO5166017 (Table 1). As in the VTA, the inhibitory effects of pTyr and RO5166017 were blocked by EPPTB, which increased the firing frequency over the basal level. The outward current generated by RO5166017 in the DRN was also K+-mediated (Fig. S2 C and D). Finally, neither RO5166017 (Fig. 2 C and D) nor pTyr (Fig. S3) affected the firing rate of 5-HT neurons in Taar1−/− mice, supporting the specificity of their effects in WT mice. Interestingly, the spontaneous spike rate in DRN slices from mutant mice was significantly increased compared with WT, suggesting that, similar to the VTA, TAAR1 in 5-HT neurons is either constitutively active or tonically activated by ambient levels of endogenous agonist(s).
In the locus coeruleus (LC), the absence of detectable Taar1 expression (10) suggests that TAs have no direct effect on NA cells. To test this hypothesis, the electrical activity of NA neurons in this brain region was recorded. We found that neither RO5166017 (Fig. 2 E and F) nor pTyr (Fig. S3) influenced the firing frequency. Furthermore, the spontaneous firing activity did not differ between slices from Taar1−/− and WT mice (Fig. 2 E and F and Fig. S3). This result further shows that the inhibition of neuronal activity by pTyr and RO5166017 observed in the VTA and DRN is specific for Taar1-expressing neurons, and it confirms the absence of functional TAAR1 in the mouse LC.
TAAR1 Interacts with 5-HT1A Autoreceptors in 5-HT Neurons of the DRN.
In DA neurons of the VTA, TAAR1 interacts with D2 autoreceptors to decrease D2 agonist potency and promote D2 desensitization (17). Having observed that TAAR1 influences the neuronal activity of 5-HT neurons, we asked whether TAAR1 in the DRN also interacts with 5-HT1A autoreceptors. This subtype of 5-HT receptors is important for the modulation of mood, cognition, and motor behavior as well as for the response to some antidepressant and antipsychotic drugs (19–21).
Activating TAAR1 with RO5166017 resulted in a twofold increased potency of the 5-HT1A partial agonist ipsapirone, whereas antagonizing TAAR1 activity by EPPTB was associated with a twofold decrease in ipsapirone potency (Fig. 3A). The influence of TAAR1 activity on the 5-HT1A desensitization rate was then examined. Bath application of ipsapirone (10 μM) to DRN slices of WT mice induced an outward current in 5-HT neurons that decreased during long activation (control) (Fig. 3B). Although preincubation of slices with RO5166017 did not significantly change the desensitization, it was clearly prevented by EPPTB. Application of the 5-HT1A receptor antagonist WAY-100135 (1 μM) blocked the current induced by ipsapirone, which in the presence of EPPTB was even reduced below baseline. These observations not only show that TAAR1 and 5-HT1A are functionally coupled but also suggest that a constitutive TAAR1 activity or tonic activation by ambient levels of endogenous agonist(s) is sufficient to drive such an interaction.
Fig. 3.
TAAR1 activity modulates the 5-HT1A receptor pharmacology in 5-HT neurons. (A) Dose–response relationships of the currents induced by the 5-HT1A receptor agonist ipsapirone recorded from DRN 5-HT neurons in control slices and slices preincubated with EPPTB (10 nM) or RO5166017 (19 nM). Current amplitudes were normalized to the maximal current obtained with a saturating concentration of ipsapirone (10 μM). pEC50 values: control = 7.40 ± 0.04 (EC50 = 39 nM); EPPTB = 7.05 ± 0.03 (EC50 = 88 nM); RO5166017 = 7.82 ± 0.04 (EC50 = 15 nM; n = 5). (B) Representative traces of ipsapirone (10 μM)-induced currents in the absence (Control) and presence of EPPTB (10 nM) or RO5166017 (500 nM) followed by application of the 5-HT1A receptor antagonist WAY-100135 (1 μM). EPPTB prevented 5HT1A receptor desensitization and reduced the holding current below the initial baseline (dotted line) when WAY-100135 was added. In contrast, RO5166017 did not significantly reduce 5-HT1A receptor desensitization. (Scale bar: 15 pA per 5 min.) The bar graph quantitatively shows the degree of desensitization rate after continuous ipsapirone application for 15 min [I(t), ratio of current amplitude after 15 min; Imax; peak current amplitude]. Data represent the mean ± SEM (n = 5). ***P < 0.001 vs. control.
RO5166017 Exhibits Anxiolytic-Like Properties.
To estimate whether RO5166017 can be used in vivo, its pharmacokinetic profile was determined in C57BL/6 mice after single i.v. or oral bolus administration (Table S3). RO5166017 showed a moderate volume of distribution at steady state (3.14 L/kg), low binding to plasma proteins (75.9% free fraction), and a high brain to plasma concentration ratio (13:1). Overall, these pharmacokinetic properties are favorable and allow further investigations in vivo.
Monoamines are involved in the processing of anxious states. As TAAR1 modulates their activity, we examined if TAAR1 activation by RO5166017 influences anxious states. The stress-induced hyperthermia (SIH) paradigm, a model that reflects the activation of the autonomic nervous system by measuring body temperature (Tb) in response to mild stress, was used. This model is considered robust and reproducible, with good clinical predictive validity (22, 23). In the SIH paradigm, the measure for anxiety is the increase in temperature (dT) over a 15-min time window in response to the mild stress of measuring rectal temperature.
RO5166017 given orally dose-dependently prevented the SIH in Naval Medical Research Institute (NMRI) mice (Fig. 4A), a standard, easy to handle, outbred mouse line that enables reliable rectal temperature reading. Although ineffective at low concentrations, RO5166017 significantly reversed SIH at doses 0.1 and 0.3 mg/kg (Fig. 4A) without decreasing basal Tb (T1) (Fig. S4). Mice treated with a higher dose (1 mg/kg) experienced a decrease in basal Tb by ∼1 °C compared with those with vehicle only (Fig. S4A). Importantly, RO5166017 (0.1 mg/kg) significantly reversed the SIH in WT but not Taar1−/− C57BL/6 mice (Fig. 4B) without alteration of basal Tb (Fig. S4A). These data suggest that RO5166017 exhibits TAAR1-mediated anxiolytic-like properties at doses 0.1–0.3 mg/kg.
Fig. 4.
RO5166017 reverses stress-induced hyperthermia (SIH). (A) In NMRI mice, RO5166017 significantly reversed the SIH (dT) at doses 0.1 and 0.3 mg/kg without affecting basal Tb (Fig. S4). At 1 mg/kg, the decrease of dT was attributed to a nonspecific effect on Tb (Fig. S4A). The diagram combines the data of two experiments. ***P < 0.001, **P < 0.01 vs. Veh (n = 8–16 per group). (B) RO5166017 (0.1 mg/kg) failed to decrease dT in Taar1−/− C57BL/6 mice in contrast to their WT littermates. In both genotypes, basal Tb was not affected (Fig. S4A). **P < 0.01 vs. WT/Veh (n = 8–10 per group). Numbers on the x axes are oral doses of RO5166017 in mg/kg. Veh, vehicle. All data represent the mean ± SEM.
RO5166017 Inhibits Psychostimulant- and Genetically-Induced Hyperlocomotion.
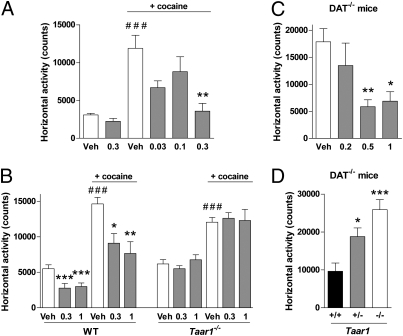
Taar1−/− mice are hypersensitive to the effects of amphetamine, with enhanced locomotor activity (LMA) and increased striatal release of DA, 5-HT, and NA compared with WT (10, 12), suggesting that TAAR1 is an important modulator of the monoaminergic systems. Because most psychostimulant drugs interact with monoaminergic neurons to elevate extracellular monoamine concentration (24), we investigated how the TAAR1 agonist RO5166017 alters their effects in vivo. For this, the effects of RO5166017 were examined on psychostimulant-induced hyperlocomotion. Mice injected with cocaine, a nonselective competitive inhibitor of monoamine transporters, displayed elevated LMA compared with vehicle-treated mice (Fig. 5A and Fig. S5A). RO5166017 given orally prevented this effect in a dose-dependent manner, similar to the atypical antipsychotic olanzapine (Fig. S5B). RO5166017 alone had little or no effect on LMA. In Taar1−/− mice submitted to the same paradigm, cocaine elevated LMA to a similar extent as in WT mice, but only in WT mice, RO5166017 prevented the cocaine-induced hyperlocomotion (Fig. 5B). Interestingly, RO5166017 also inhibited stereotypies induced by cocaine in WT mice (Fig. S6A) similar to olanzapine (Fig. S6B), and this effect was lost in Taar1−/− mice (Fig. S6C). This suggests that, in mice, RO5166017 blocks the psychostimulant effects of cocaine through TAAR1.
Fig. 5.
RO5166017 blocks hyperlocomotion induced by cocaine or lack of DAT. (A) RO5166017 administered orally dose-dependently reduced cocaine (15 mg/kg i.p.) -induced hyperlocomotion. ###P < 0.001 vs. saline/Veh; **P < 0.01 vs. cocaine/Veh. Cocaine/RO5166017 (0.3 mg/kg) does not differ from groups without cocaine (n = 8 per group). (B) RO5166017 failed to antagonize cocaine (20 mg/kg i.p.) -induced hyperlocomotion in Taar1−/− mice but not in WT littermates. ###P < 0.001 vs. saline groups; ***P < 0.001, **P < 0.01, and *P < 0.05 vs. Veh (n = 10 per group). (C) RO5166017 given i.p. dose-dependently reduced hyperlocomotion in spontaneously hyperactive dopamine transporter knockout (DAT−/−) mice. **P < 0.01, *P < 0.05 vs. Veh (n = 7–8 per group). (D) RO5166017 (0.5 mg/kg i.p.) failed to inhibit hyperlocomotion in DAT−/− mice deficient for Taar1 (−/−) and had reduced effects in DAT−/−/Taar1+/− mice (+/−). +/+, Taar1 WT. ***P < 0.001, *P < 0.05 vs. (+/+) (n = 12–22 per group). Numbers on the x axes are oral doses of RO5166017 in mg/kg. Veh, vehicle. Data represent the mean ± SEM.
The mutant mouse line that lacks the Slc6a3 gene encoding the DAT (DAT−/− mice) represents a model of persistent hyperdopaminergia and related behavioral abnormalities used to evaluate compounds on endophenotypes of DA-related psychiatric disorders (24). As previously shown (25, 26), saline-treated DAT−/− mice are spontaneously hyperactive in a novel environment. Treatment with RO5166017 i.p. dose-dependently suppressed hyperlocomotion in DAT−/− mice (Fig. 5C) similar to classical (haloperidol) and atypical (olanzapine and clozapine) antipsychotics (27). This finding further indicates that at least this effect of TAAR1 activation on DA-related function is independent of DAT. In WT mice, the reduction of LMA after introduction in the novel environment was reduced at 0.5 mg/kg but not at the other doses tested (Fig. S5C). Importantly, the effect of RO5166017 (0.5 mg/kg, i.p.) was reduced in DAT−/−/Taar1+/− mice and was completely absent in double mutant DAT−/−/Taar1−/− mice (Fig. 5D). Thus, the inhibitory action of RO5166017 on DA-dependent hyperactivity, at least at this dose, is fully mediated by TAAR1.
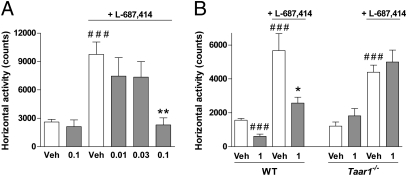
Finally, preclinical and clinical evidence suggest that hypofunction of glutamatergic NMDA receptors plays an important role in the pathophysiology of schizophrenia (28). Hyperlocomotion induced by (3R,4R)-3-amino-1-hydroxy-4-methyl-2-pyrrolidinone (L-687,414), a well-characterized selective and brain-penetrating glycine site NMDA receptor antagonist, has been recently described as a method to identify antipsychotic-like action of drugs (29) in relation to the glutamatergic hypoactivity theory of schizophrenia. In this paradigm, RO5166017 dose-dependently reversed hyperlocomotion induced by L-687,414 (Fig. 6A and Fig. S5D). This effect was similar to known antipsychotic drugs (29), including olanzapine (Fig. S5E), and it was not seen in Taar1−/− mice (Fig. 6B). These observations further show that selective TAAR1 activation by RO5166017 produces antipsychotic-like effects.
Fig. 6.
RO5166017 dose-dependently blocks L-687,414–induced hyperlocomotion. (A) In NMRI mice, RO5166017 administered orally dose-dependently blocked hyperlocomotion triggered by L-687,414 (50 mg/kg s.c.). ###P < 0.001 vs. saline/Veh; **P < 0.01 vs. L-687,414/Veh. L-687,414/RO5166017 (0.1 mg/kg) does not differ from groups without L-687,414 (n = 7–8 per group). (B) In Taar1−/− C57BL/6 mice but not in WT littermates, RO5166017 failed to antagonize hyperlocomotion triggered by L-687,414 (75 mg/kg s.c.). ###P < 0.001 vs. saline/Veh; *P < 0.05 vs. L-687,414/Veh (n = 6–8 per group). Numbers on the x axes are oral doses of RO5166017 in mg/kg. Veh, vehicle. Data represent the mean ± SEM.
Discussion
To date, all studies of TAAR1 function have relied on nonselective agonists such as TAs or thyronamine derivatives (7). Selective and potent TAAR1 compounds were not available to elucidate the physiological role of the receptor and verify the promising therapeutic hypotheses. Here, we report on RO5166017, a TAAR1 agonist with high affinity, efficacy, and selectivity as well as favorable pharmacokinetic properties. RO5166017 was used in vitro and in vivo to show that selective stimulation of TAAR1 affects firing activity of DA and 5-HT neurons and triggers anxiolytic- and antipsychotic-like effects in mice.
RO5166017 Is a High-Affinity, Potent, Selective, and Bioavailable TAAR1 Agonist.
RO5166017 exhibits high binding affinity for TAAR1 and high potency to stimulate cAMP production, particularly at rodent TAAR1. Compared with pTyr, RO5166017 exhibited 200-fold higher affinity (Ki = 1.9 vs. 404 nM) and potency to activate cAMP production (EC50 = 3.3 vs. 545 nM) at mouse TAAR1 (17), whereas T1AM and its derivative o-phenyl-3-iodotyramine show EC50 values of 112 and 35 nM, respectively (30). In Xenopus oocytes coexpressing mouse TAAR1 with human Kir3 channels, RO5166017 evoked an inward current similar to pTyr but with a 21-fold lower IC50 (8 vs. 167 nM) (17). Finally, RO5166017 also reduced the firing frequency of VTA DA neurons by activating an outward K+ current similar to pTyr but with 179-fold higher potency (1.7 vs. 305 nM) (17). Such electrophysiological effects are presumably of postsynaptic nature, although presynaptic action cannot be excluded.
In contrast to the TAAR1 agonists currently available, RO5166017 is selective against a large panel of targets, including the monoaminergic receptors and transporters. An exception is the imidazoline receptor I1, against which the selectivity ratio is only 15-fold. However, I1 imidazoline binding sites are unlikely to mediate the effects of RO5166017. RO5166017 did not affect NA neurons in the LC where harmane, a putative endogenous imidazoline binding sites ligand, increases firing activity (31). In contrast, it reduced the firing of DA neurons in the VTA, where no I1 imidazoline binding sites were seen (31). Importantly, RO5166017 had no effect in Taar1−/− mice, both in electrophysiological and behavioral studies.
Finally, a critical advantage of RO5166017 over TAs and other TAAR1 ligands comes from its pharmacokinetic properties. TAs, due to rapid degradation by monoamine oxidases (7), have a very short half-life in the order of 30 s in the brain and 5 min in plasma (32, 33). In contrast, RO5166017 is stable, with a half-life of several hours in plasma. Furthermore, it shows good bioavailability and high brain penetration, enabling peripheral administration for in vivo studies, including oral dosing.
TAAR1 Modulates 5-HT Activity.
Previous studies indicated that TAAR1 modulates dopaminergic activity, presumably through functional interaction with DAT and the D2 receptor (8–10, 12, 17). We now show that both RO5166017 and pTyr reduce the firing frequency of 5-HT neurons in the DRN, strongly suggesting that TAAR1 also modulates the serotonergic system. In the VTA, TAAR1 alters neuronal firing through activation of G protein-coupled inwardly-rectifying potassium (GIRK) channels (17), and the mechanism is likely to be similar in the DRN 5-HT neurons. In addition, it was observed that TAAR1 influences the functioning of 5-HT1A autoreceptors in the DRN. Their desensitization upon ipsapirone application is TAAR1-dependent, as coapplication of EPPTB prevents 5-HT1A desensitization. This observation is reminiscent of the VTA, where D2 desensitization after quinpirole application was abolished by the TAAR1 antagonist (17). Promotion of monoamine autoreceptor desensitization, thus, emerges as an important function of TAAR1. This may occur either by direct interaction or by favoring interactions with the GPCR desensitization machinery.
Finally, agonist potency at 5-HT1A increased with TAAR1 activation, whereas it decreased with TAAR1 blockade. Interestingly, this situation is reversed compared with the D2 receptors, at which agonist potency is promoted by TAAR1 blockade (17). Thus, although TAAR1 activation decreases firing activity in both DA and 5-HT neurons, it modulates the pharmacology of monoamine autoreceptors according to the cellular environment. This is likely to have critical impacts on the functioning of the dopaminergic and serotonergic systems. Interestingly, 5-HT1A autoreceptors in the DRN show greater sensitivity to 5-HT compared with postsynaptic 5-HT1A heteroreceptors located in the corticolimbic structures (19, 21). It is tempting to hypothesize that TAAR1 contributes to this difference of sensitivity, which is important in the management of psychiatric diseases like schizophrenia, anxiety, and depression (21).
The 5-HT1A autoreceptor is considered a therapeutic target for some neuropsychiatric disorders (20, 21, 34). It is believed to delay the therapeutic action of antidepressants that increase 5-HT levels, and only after the progressive desensitization of 5-HT1A can therapeutic effects occur (20, 34). Because TAAR1 seems necessary for the desensitization of 5-HT1A autoreceptors, adequate activation of TAAR1 may be critical for the therapeutic action of antidepressants. Depression is indeed associated with insufficiency of brain TAs, notably PEA (35), and coadministration of PEA or its precursor phenylalanine with the MAOI selegiline improves mood in patients with major depression, including those that are treatment-resistant (35, 36). Thus, association of specific TAAR1 agonists such as RO5166017 to classical antidepressant treatments might accelerate and improve therapeutic efficacy.
Selective Activation of TAAR1 Shows Anxiolytic- and Antipsychotic-Like Actions.
A variety of molecules activate TAAR1 in vitro (2, 14), but their administration to animals results in an array of responses from which TAAR1-mediated effects are challenging to distinguish. PEA produces amphetamine-like hyperactivity, although not in DAT−/− mice (26). T1AM decreases Tb dramatically to produce torpor-like states (14), but WT and Taar1−/− mice are affected equally, suggesting TAAR1-independent mechanisms (13). Thus, RO5166017 was designed to examine in vivo the effects of TAAR1 selective activation. In the mouse, dosing of RO5166017 alone did not trigger any obvious behavioral or physiological manifestations. RO5166017 did not produce hyperlocomotion like PEA but rather, an occasional and minor decrease of LMA. At high dose, it reduced Tb by ∼1 °C in the SIH test but clearly did not cause torpor episodes like T1AM. Instead, we found that RO5166017 displays psychoactive properties in mice. Selective TAAR1 activation reduced anxiety in the SIH paradigm and fully prevented psychostimulant-induced and persistent hyperdopaminergia-related hyperactivity similar to the antipsychotic drug olanzapine (27). Importantly, these effects were not observed in Taar1−/− mice, showing that the anxiolytic- and antipsychotic-like properties of RO5166017 result from selective activation of TAAR1. Furthermore, the fact that RO5166017 suppressed hyperactivity of mice lacking DAT strongly suggests that DAT is not required for this effect of TAAR1 on DA-related functions. Potent activity of RO5166017 in pharmacological or genetic models of hyperdopaminergia indicates potential activity of TAAR1 agonists in conditions that may result from abnormally enhanced dopaminergic transmission such as schizophrenia.
Conclusion
TAAR1 is a promising therapeutic target for the treatment of neuropsychiatric disorders, but the diversity and polypharmacology of the agonists available have rendered dissection of its physiological functions challenging. Here, we report on RO5166017, a synthetic selective and in vivo active TAAR1 agonist. In vitro, RO5166017 inhibited the firing frequency of VTA DA and DRN 5-HT neurons. Furthermore, modulation of TAAR1 activity altered the pharmacology of DRN 5-HT1A receptors, demonstrating that TAAR1 modulates not only DA but also 5-HT neurotransmission. In vivo, although silent by itself, RO5166017 prevented psychostimulant-induced hyperlocomotion, inhibited novelty-driven hyperactivity of DAT−/− mice, and prevented SIH in WT but not Taar1−/− mice. These results link TAAR1 to the control of monoaminergic-driven behaviors and underline the antipsychotic and anxiolytic potential of TAAR1 agonists.
Materials and Methods
Details on animals, membrane preparation, radioligand binding, cAMP assay, pharmacokinetic measurements, stress-induced hyperthermia, and statistical analysis are provided in SI Materials and Methods. Electrophysiological recordings were made as in ref. 17 and are detailed in SI Materials and Methods.
Compounds.
All compounds were purchased from Sigma except for cocaine, d-amphetamine, olanzapine, L-687,414, EPPTB (RO5212773), RO5166017, [3H]RO5166017, and [3H]RO5192022 ([3H](S)-4-(2,4-Difluoro-phenyl)-4,5-dihydro-oxazol-2-ylamine), which were synthesized at Roche.
Measurement of Locomotor Activity and Stereotypies.
For psychostimulant studies, recordings were as reported (10). LMA was measured as the number of horizontal beam breaks (horizontal activity) cumulated over 30 min. Stereotypy time was assessed as the total time that stereotypic behaviors (repetitive beam breaks with intervals less than 1 s) were monitored over 30 min. For cocaine studies, C57BL/6 mice (n = 8 per group) were treated per os (p.o.) with vehicle (H2O + 0.3% tween 80) or RO5166017 (0.03–3 mg/kg in vehicle), placed into the activity monitor chamber for 30 min (habituation period), injected i.p. with saline (0.9% NaCl + 0.3% tween 80) or cocaine (15 mg/kg in saline), and returned to the recording chamber for immediate monitoring of behavior (recording period). The same paradigm was used in Taar1−/− mice (n = 10 per group) with RO5166017 (0.3–1 mg/kg p.o.) and cocaine (20 mg/kg i.p.) using a repeated measures design with at least 10 d between two sessions. For L-687,414 studies, NMRI mice (n = 8 per group) were dosed p.o. with vehicle or RO5166017 (0.01–1 mg/kg in vehicle) 15 min before receiving saline or L-687,414 (50 mg/kg in saline) s.c. The habituation period was 15 min. For Taar1−/− mice, animals (n = 8 per group) were dosed with RO5166017 (0.3 mg/kg p.o.), were placed into the recording chamber for 45 min, received saline or L-687,414 (75 mg/kg) s.c., and were returned to the recording chamber for 15 min (thus, 60-min habituation) before LMA was recorded.
For DAT−/−, WT, and double mutant (DAT−/−/Taar1−/−) mice, LMA was measured as reported (26). DAT−/− mice were placed into activity monitor chambers for 30 min to fully manifest their novelty-driven hyperactivity; then, they were treated with saline or RO5166017 (0.2–1 mg/kg i.p.), and horizontal activity was monitored for 90 min. Nonhabituated WT mice were treated before placement into the monitoring chambers, and LMA was recorded for 90 min.
Supplementary Material
Acknowledgments
We thank D. Buchy, M.-T. Miss, and M. Kapps for technical assistance; M. Howard for valuable input on the manuscript; and S. Lazic for help with statistics. R.R.G. and M.G.C. acknowledge Lundbeck A/G and Lundbeck USA for generously sharing Taar1−/− mice, which were used in this work to generate DAT−/−/Taar1−/− mice. R.R.G. is supported by Roche and Compagnia di San Paolo Fondazione (Turin, Italy). We acknowledge the continued support of Roche and Swiss Science Foundation Grant 3100A0-117816 (to B.B.).
Footnotes
Conflict of interest statement: F.G.R., J.-L.M., R.M., S.D., K.G.Z., R.N., C.A.M., V.M., S.C., L.O., G.T., B.P., J.G.W., and M.C.H. are F. Hoffmann-La Roche employees. R.R.G. is supported in part by research grants from F. Hoffmann-La Roche Ltd. (Basel, Switzerland) and Compagnia di San Paolo Fondazione (Turin, Italy). A.B. is employed by Neuroservice. M.G.C. has received funds for Sponsored Research Agreements unrelated to this work from Forest Laboratories, NeuroSearch, and Lundbeck USA as well as consulting fees from Merck and F. Hoffmann-La Roche Ltd. An unrestricted gift to Duke University was provided by Lundbeck USA to support Neuroscience research in the laboratory of M.G.C.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103029108/-/DCSupplemental.
References
- 1.Borowsky B, et al. Trace amines: Identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunzow JR, et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol. 2001;60:1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- 3.Burchett SA, Hicks TP. The mysterious trace amines: Protean neuromodulators of synaptic transmission in mammalian brain. Prog Neurobiol. 2006;79:223–246. doi: 10.1016/j.pneurobio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Berry MD. The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Rev Recent Clin Trials. 2007;2:3–19. doi: 10.2174/157488707779318107. [DOI] [PubMed] [Google Scholar]
- 5.Lindemann L, et al. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics. 2005;85:372–385. doi: 10.1016/j.ygeno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Lindemann L, Hoener MC. A renaissance in trace amines inspired by a novel GPCR family. Trends Pharmacol Sci. 2005;26:274–281. doi: 10.1016/j.tips.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Grandy DK. Trace amine-associated receptor 1-Family archetype or iconoclast? Pharmacol Ther. 2007;116:355–390. doi: 10.1016/j.pharmthera.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller GM. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J Neurochem. 2011;116:164–176. doi: 10.1111/j.1471-4159.2010.07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie Z, Miller GM. Trace amine-associated receptor 1 is a modulator of the dopamine transporter. J Pharmacol Exp Ther. 2007;321:128–136. doi: 10.1124/jpet.106.117382. [DOI] [PubMed] [Google Scholar]
- 10.Lindemann L, et al. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther. 2008;324:948–956. doi: 10.1124/jpet.107.132647. [DOI] [PubMed] [Google Scholar]
- 11.Ledonne A, et al. Trace amines depress D(2)-autoreceptor-mediated responses on midbrain dopaminergic cells. Br J Pharmacol. 2010;160:1509–1520. doi: 10.1111/j.1476-5381.2010.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolinsky TD, et al. The Trace Amine 1 receptor knockout mouse: An animal model with relevance to schizophrenia. Genes Brain Behav. 2007;6:628–639. doi: 10.1111/j.1601-183X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 13.Panas HN, et al. Normal thermoregulatory responses to 3-iodothyronamine, trace amines and amphetamine-like psychostimulants in trace amine associated receptor 1 knockout mice. J Neurosci Res. 2010;88:1962–1969. doi: 10.1002/jnr.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scanlan TS, et al. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med. 2004;10:638–642. doi: 10.1038/nm1051. [DOI] [PubMed] [Google Scholar]
- 15.Sotnikova TD, et al. The dopamine metabolite 3-methoxytyramine is a neuromodulator. PLoS One. 2010;5:e13452. doi: 10.1371/journal.pone.0013452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fontanilla D, et al. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradaia A, et al. The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc Natl Acad Sci USA. 2009;106:20081–20086. doi: 10.1073/pnas.0906522106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millan MJ, et al. S18616, a highly potent, spiroimidazoline agonist at alpha(2)-adrenoceptors: I. Receptor profile, antinociceptive and hypothermic actions in comparison with dexmedetomidine and clonidine. J Pharmacol Exp Ther. 2000;295:1192–1205. [PubMed] [Google Scholar]
- 19.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 20.Gardier AM, Malagié I, Trillat AC, Jacquot C, Artigas F. Role of 5-HT1A autoreceptors in the mechanism of action of serotoninergic antidepressant drugs: Recent findings from in vivo microdialysis studies. Fundam Clin Pharmacol. 1996;10:16–27. doi: 10.1111/j.1472-8206.1996.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 21.Millan MJ. Improving the treatment of schizophrenia: Focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295:853–861. [PubMed] [Google Scholar]
- 22.Adriaan Bouwknecht J, Olivier B, Paylor RE. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: A review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev. 2007;31:41–59. doi: 10.1016/j.neubiorev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Van der Heyden JA, Zethof TJ, Olivier B. Stress-induced hyperthermia in singly housed mice. Physiol Behav. 1997;62:463–470. doi: 10.1016/s0031-9384(97)00157-1. [DOI] [PubMed] [Google Scholar]
- 24.Gainetdinov RR, Caron MG. Monoamine transporters: From genes to behavior. Annu Rev Pharmacol Toxicol. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- 25.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 26.Sotnikova TD, et al. Dopamine transporter-dependent and -independent actions of trace amine beta-phenylethylamine. J Neurochem. 2004;91:362–373. doi: 10.1111/j.1471-4159.2004.02721.x. [DOI] [PubMed] [Google Scholar]
- 27.Boulay D, Bergis O, Avenet P, Griebel G. The glycine transporter-1 inhibitor SSR103800 displays a selective and specific antipsychotic-like profile in normal and transgenic mice. Neuropsychopharmacology. 2010;35:416–427. doi: 10.1038/npp.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Javitt DC. Glutamate and schizophrenia: Phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- 29.Alberati D, Moreau JL, Mory R, Pinard E, Wettstein JG. Pharmacological evaluation of a novel assay for detecting glycine transporter 1 inhibitors and their antipsychotic potential. Pharmacol Biochem Behav. 2010;97:185–191. doi: 10.1016/j.pbb.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Hart ME, et al. Trace amine-associated receptor agonists: Synthesis and evaluation of thyronamines and related analogues. J Med Chem. 2006;49:1101–1112. doi: 10.1021/jm0505718. [DOI] [PubMed] [Google Scholar]
- 31.Smith KL, Jessop DS, Finn DP. Modulation of stress by imidazoline binding sites: Implications for psychiatric disorders. Stress. 2009;12:97–114. doi: 10.1080/10253890802302908. [DOI] [PubMed] [Google Scholar]
- 32.Durden DA, Philips SR. Kinetic measurements of the turnover rates of phenylethylamine and tryptamine in vivo in the rat brain. J Neurochem. 1980;34:1725–1732. doi: 10.1111/j.1471-4159.1980.tb11267.x. [DOI] [PubMed] [Google Scholar]
- 33.Shannon HE, Cone EJ, Yousefnejad D. Physiologic effects and plasma kinetics of beta-phenylethylamine and its N-methyl homolog in the dog. J Pharmacol Exp Ther. 1982;223:190–196. [PubMed] [Google Scholar]
- 34.Blier P, Piñeyro G, el Mansari M, Bergeron R, de Montigny C. Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Ann N Y Acad Sci. 1998;861:204–216. doi: 10.1111/j.1749-6632.1998.tb10192.x. [DOI] [PubMed] [Google Scholar]
- 35.Sabelli HC, Javaid JI. Phenylethylamine modulation of affect: Therapeutic and diagnostic implications. J Neuropsychiatry Clin Neurosci. 1995;7:6–14. doi: 10.1176/jnp.7.1.6. [DOI] [PubMed] [Google Scholar]
- 36.Sabelli H, Fink P, Fawcett J, Tom C. Sustained antidepressant effect of PEA replacement. J Neuropsychiatry Clin Neurosci. 1996;8:168–171. doi: 10.1176/jnp.8.2.168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.