Abstract
Previous exposure to amphetamine leads to enhanced locomotor and nucleus accumbens (NAcc) dopamine (DA) responding to the drug as well as enhanced amphetamine self-administration. Here, we investigated the effects of exposure to Δ9-tetrahydrocannibinol (Δ9-THC) on behavioral and biochemical responding to amphetamine. Rats in different groups received five exposure injections of vehicle or one of five doses of Δ9-THC (0.4, 0.75, 1.5, 3.0, and 6.0 mg/kg i.p.) and were tested 2 days and 2 weeks later. Exposure to all but the lowest and highest doses of Δ9-THC enhanced the locomotor response to amphetamine (0.75 mg/kg i.p.), but all failed to enhance NAcc DA overflow in response to the drug. Moreover, exposure to 3.0 mg/kg i.p. Δ9-THC increased forskolin-evoked adenylyl cyclase activity in the NAcc and rats' locomotor response to the direct DA receptor agonist apomorphine (1.0 mg/kg s.c.), suggesting that Δ9-THC sensitized locomotor responding to amphetamine by up-regulating postsynaptic DA receptor signaling in the NAcc. Finally, amphetamine self-administration (200 μg/kg/infusion i.v.) was enhanced in amphetamine (5 × 1.5 mg/kg i.p.)-exposed rats, but not in rats exposed to Δ9-THC (5 × 3.0 mg/kg i.p.). Previous exposure to this dose of Δ9-THC modestly increased apomorphine SA (0.5 mg/kg/infusion i.v.). Thus, unlike amphetamine exposure, exposure to Δ9-THC does not enhance the subsequent NAcc DA response to amphetamine or promote amphetamine self-administration. Although Δ9-THC leads to alterations in postsynaptic DA receptor signaling in the NAcc and these can affect the generation of locomotion, these neuroadaptations do not seem to be linked to the expression of enhanced amphetamine self-administration.
Introduction
The cannabinoid 1 receptor (CB1R), the Δ9-THC binding site (Matsuda et al., 1990), is extensively expressed throughout the mammalian brain, including presynaptic and postsynaptic sites in the NAcc where it has been proposed to contribute to the rewarding effects of psychostimulants and other drugs (Pickel et al., 2006). CB1Rs are located presynaptically at asymmetric excitatory synapses and postsynaptically on GABAergic medium spiny neurons in the NAcc (Pickel et al., 2006; Martin et al., 2008). Although a retrograde inhibitory role for endocannabinoids has been proposed in the ventral tegmental area (VTA) and a number of forebrain sites (Maldonado et al., 2006; Haj-Dahmane and Shen, 2010), evidence from several studies indicates that the reinforcing properties of Δ9-THC are mediated via actions in the NAcc. For example, Δ9-THC has been shown to increase the firing of VTA DA neurons (Gessa et al., 1998) and enhance the overflow of DA in the terminal region of these neurons in the NAcc (Tanda et al., 1997). This effect is blocked by the selective CB1 receptor antagonist SR-141716A [5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-piperidin-1-ylpyrazole-3-carboxamide] (Diana et al., 1998) and thought to involve regulation by Δ9-THC of long-loop GABAergic modulation of midbrain DA neurons (Riegel and Lupica, 2004). Δ9-THC microinjections into the NAcc dose-dependently increase extracellular DA levels in this site, whereas microinjections into the VTA have no effect (Chen et al., 1993).
Behavioral and neurochemical sensitization, phenomena that can persist for weeks to months after repeated exposure to a drug, have been proposed to play a crucial role in drug addiction (Vezina, 2004). For example, intermittent exposure to the psychostimulant amphetamine leads to sensitized locomotion and NAcc DA overflow (Vezina, 1996) in response to the drug and increases work output and self-administration of amphetamine (Vezina et al., 2002) and cocaine (Suto et al., 2004). Although sensitization is the focus of the present experiments, it should be recognized that different effects can be produced by severe binge or continuous drug exposure regimens especially when they are assessed soon after exposure (for discussion, see Vezina et al., 2007). In addition to amphetamine, sensitization has been observed after repeated exposure to a variety of drugs of abuse including morphine, cocaine (Vanderschuren and Kalivas, 2000), and nicotine (Vezina et al., 2007).
Investigations of sensitization of locomotion and stereotypy by Δ9-THC and cannabinoid compounds have yielded mixed results. Some studies have reported that exposure to these compounds fails to enhance their behavioral effects (Arnold et al., 1998; Varvel et al., 2007; Wiley et al., 2008), although others have demonstrated sensitization (Cadoni et al., 2001, 2008; Rubino et al., 2001). Differences in the doses and compounds administered during exposure may account for some of the different findings reported. For example, the dose of Δ9-THC used for exposure by Cadoni et al. (2008) possessed a 5- to 7-fold greater potency than that afforded by the dose of the synthetic cannabinoid agonist CP 55,940 [2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl) cyclohexyl]-5-(2-methyloctan-2-yl)phenol] used for exposure in the study by Arnold et al. (1998). More consistent findings have been obtained in studies assessing the effects of exposure to Δ9-THC on behavioral responding to other drugs. Cross-sensitization has been reported between a wide range of Δ9-THC doses and amphetamine (Gorriti et al., 1999; Lamarque et al., 2001), heroin (Lamarque et al., 2001), and morphine (Cadoni et al., 2001, 2008). Exposure to synthetic CB1R agonists has also been reported to enhance behavioral responding to amphetamine (Muschamp and Siviy, 2002) and morphine (Norwood et al., 2003) but not cocaine (Arnold et al., 1998). The neural mechanisms underlying this cross-sensitization remain unknown. Previous exposure to Δ9-THC has been reported to enhance Δ9-THC- but not morphine-induced DA overflow in the NAcc core (Cadoni et al., 2008). Postsynaptically in the NAcc, the coexpression of DA receptors with CB1Rs by medium spiny neurons together with the fact that both are G protein-coupled and able to modulate overlapping signaling pathways (Howlett, 1995; Glass and Felder, 1997; Martin et al., 2008) introduces the possibility that exposure to Δ9-THC may also enhance behavioral responding to psychostimulants such as amphetamine by altering DA receptor-initiated signaling in this site.
In the current article, rats were exposed to a range of Δ9-THC doses (0.4–6.0 mg/kg i.p.), and their behavioral and biochemical responding to amphetamine was assessed 2 days and 2 weeks after exposure. Our objective was to investigate the presynaptic and postsynaptic mechanisms by which Δ9-THC influences DA neurotransmission in the NAcc and determine whether neuroadaptations in these sites are linked to the behavioral sensitization produced by Δ9-THC exposure.
Materials and Methods
Subjects.
Adult male Sprague-Dawley (Harlan, Indianapolis, IN; behavioral characterization, locomotion, microdialysis, and adenylyl cyclase experiments) and Long-Evans rats (Harlan Teklad, Madison, WI; self-administration experiments) weighing 250 to 275 g on arrival were individually housed in a 12-h light/dark reverse-cycle room with food and water available ad libitum. They were afforded a 3- to 5-day acclimation period before the initiation of any procedures. The use of two different strains was not expected to hinder comparisons of the results obtained, because both of these strains have responded similarly when tested in the kind of behavioral and microdialysis experiments reported here (Jolly and Vezina, 1996; Vezina, 1996; Vezina et al., 2002; Panlilio et al., 2007; for additional references, see Vezina, 2004). For the microdialysis experiments, rats were anesthetized with ketamine (100 mg/kg i.p.) and xylazine (6 mg/kg i.p.), placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) with the incisor bar positioned 5.0 mm above the interaural line, and chronically implanted with guide cannulae (20 gauge; Plastics One, Roanoke, VA) aimed bilaterally at the NAcc core (anterior/posterior: +3.6 mm; medial/lateral: ±1.5 mm; dorsal/ventral: −6.5 to −8.5 mm; from bregma and skull). Cannulae were angled at 10°, positioned 5.0 mm above the most ventral aspect of the NAcc, and secured with dental acrylic anchored to stainless-steel skull screws. Guide cannulae were implanted bilaterally to permit one microdialysis test in each hemisphere, one soon and one late after exposure. After surgery, 25-gauge dummy probes (cellulose fiber absent and stainless-steel tubing sealed) were inserted into the guide cannulae flush to the guide tips, and rats were returned to their home cage for a 7- to 10-day recovery period. For the self-administration experiments, rats were anesthetized with ketamine (100 mg/kg i.p.) and xylazine (6 mg/kg i.p.) and surgically implanted with an intravenous catheter as described previously (Vezina et al., 2002). Catheters were made of silastic tubing (Dow Corning, Midland, MI), inserted into the right jugular vein, and positioned to exit slightly caudal to the midscapular region. Catheters were subsequently flushed daily with a sterile 0.9% saline (SAL) solution containing 30 IU/ml heparin and 250 mg/ml ampicillin to promote patency. Self-administration testing began 5 to 7 days later. All procedures were performed according to an approved Institutional Animal Care and Use Committee protocol. All experiments were conducted in accordance with the Declaration of Helsinki and the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
Experimental Design.
Rats in different groups were exposed to doses of Δ9-THC and tested at some time after the last exposure injection in different experiments assessing locomotor responding to amphetamine or the direct DA receptor agonist apomorphine, amphetamine-induced DA overflow in the NAcc, forskolin-evoked adenylyl cyclase activity in the NAcc, and the self-administration of amphetamine and apomorphine. In the self-administration experiments, separate additional rats were exposed to amphetamine to provide comparative data. On the first and last exposures to Δ9-THC, the behavioral response to this drug was quantified in some rats.
Drugs.
S(+)-amphetamine sulfate, R(−)-apomorphine hydrochloride, and Δ9-THC were obtained from Sigma-Aldrich (St. Louis, MO). Amphetamine and apomorphine were dissolved in sterile SAL (0.9% w/v). Δ9-THC was evaporated under nitrogen gas, dissolved in Tween 80, and diluted with 0.9% SAL to the required concentrations. Drug doses refer to the weight of the salt.
Exposure to Δ9-THC and Amphetamine-Induced Locomotion.
Rats in different groups (n = 6/group) were administered five exposure injections of vehicle (1.0 ml/kg i.p.) or one of five doses of Δ9-THC (0.4, 0.75, 1.5, 3.0, and 6.0 mg/kg i.p.), one injection every third day. After each injection, rats were placed in locomotor activity-monitoring chambers, and their locomotion was recorded for 2 h. This regimen of Δ9-THC injections is similar to those described by others with Δ9-THC (e.g., Arnold et al., 1998; Varvel et al., 2007) and is the same as one shown to produce sensitization with amphetamine (Vezina, 1996; Vezina et al., 2002). After the first and fifth injection, the frequency of the following behaviors was recorded by visual observation in four rats from each of the six groups: sniffing, grooming, walking, and motionless. Animals were observed over three 30-min epochs (0–30, 45–75, and 90–120 min) by an experimenter blind to the treatment of each rat. During each of these epochs, each rat was observed for 15 s every 2 min. Soon (2 days) and again later (2 weeks) after the last exposure injection, all rats were tested after an injection of amphetamine (0.75 mg/kg i.p.). On each of these tests, rats were placed in locomotor activity-monitoring chambers, and their locomotion and rearing were recorded for 2 h.
A bank of 12 activity-monitoring chambers was used to measure locomotion. The chambers (22 × 43 × 33 cm) were located in a room lit dimly with red light. Two photocells, positioned 3.5 cm above the floor and spaced evenly along the longitudinal axis, estimated horizontal locomotion. Two-additional photocells, positioned 16.5 cm above the floor and spaced 5 cm from and parallel to the front or back walls, estimated rearing. Separate interruptions of photocell beams were detected and recorded via an electrical interface by a computer located in an adjacent room. Each of a subset of chambers was equipped with a dim light-sensitive camera that permitted observation and filming of rats after a Δ9-THC injection. Monitors and video recorders were situated in an adjacent room.
Exposure to Δ9-THC and Amphetamine-Induced NAcc DA Overflow.
Rats in different groups were administered five exposure injections of vehicle (1.0 ml/kg i.p.) or one of four doses of Δ9-THC (0.75, 1.5, 3.0, and 6.0 mg/kg i.p.), one injection every third day. After each injection, rats were placed in a microdialysis chamber for 2 h. Rats were tested for their NAcc DA response to amphetamine (0.75 mg/kg i.p.) soon (2 days; n = 7–11/group) and again later (2 weeks; n = 5–14/group) after the last Δ9-THC injection. On these tests, microdialysis samples were collected every 20 min 1 h before and 3 h after the challenge injection of amphetamine. To minimize damage to the NAcc, each test was conducted with a microdialysis probe inserted into a separate hemisphere. The night before each test, rats were briefly immobilized with halothane, and a microdialysis probe was lowered into one of the two NAcc guide cannulae. Concentric microdialysis probes were constructed as described previously (Jolly and Vezina, 1996). Rats were placed in the microdialysis testing chambers overnight where they were connected via a steel-spring tether to a liquid swivel and a collection vial positioned outside the chamber. Probes were perfused with a modified Ringer's dialysate (145 mM Na+, 1.2 mM Ca2+, 2.7 mM K+, 1.0 mM Mg2+, and 150 mM Cl−, pH 7.4; 0.3 μl/min overnight and 1.5 μl/min during testing the next day, 18–20 h after probe insertion). After completion of the second test, animals were administered a lethal dose of pentobarbital and transcardially perfused with 10% paraformaldehyde. Probe tips were subsequently verified histologically in 40-μm cresyl violet-stained coronal brain sections. Of the 102 experiments conducted in 51 rats, the results obtained in 15 experiments were dropped because of faulty probe placements.
Dialysate samples (30 μl) were stored frozen before injection of 20-μl aliquots into a high-performance liquid chromatography-electrochemical detection system consisting of a single-piston Gilson, Inc. (Middleton, WI) 302 pump set at 1.1 ml/min, a Gilson diaphragm type pulse dampener, a 10-cm ODS-C18 3-μm column (maintained at 35°C) (Thermo Fisher Scientific, Waltham, MA), an ESA Inc. (Chelmsford, MA) model 5100 Coulochem detector with a conditioning cell (oxidizing at +300 mV) placed before a model 5011 high-sensitivity analytical cell (electrodes set to +50 and −350 mV), and a 0.04 M sodium acetate mobile phase containing 0.3 mM Na2 EDTA, 0.5 mM octyl sodium sulfate, and 3.3% acetonitrile, pH 3.75. Extracellular concentrations of DA, corrected for individual probe recoveries, were estimated from peak areas using EZ Chrom Elite software (Agilent Technologies, Santa Clara, CA). The DA peak eluted at approximately 4.8 min. Probe recoveries were determined in vitro at 20°C after each microdialysis test with known DA standard concentrations and ranged from 6 to 11%.
In vivo microdialysis was performed in eight Plexiglas chambers (38 × 32 × 34 cm) with stainless-steel wire floors that were housed inside light- and sound-attenuating ventilated boxes.
Exposure to Δ9-THC and Apomorphine-Induced Locomotion.
Rats in different groups (n = 12/group) were administered five exposure injections of either vehicle or Δ9-THC (3.0 mg/kg i.p.), a dose found to be maximally effective in the locomotor tests with amphetamine. Injections were given once every third day. After each injection, rats were placed in the locomotor activity-monitoring chambers for 2 h. Soon (2 days) and again later (2 weeks) after the last exposure injection, all rats were tested after an injection of apomorphine (1.0 mg/kg s.c.). On each of these tests, rats were placed in locomotor activity-monitoring chambers, and their locomotion was recorded for 1 h.
Exposure to Δ9-THC and Adenylyl Cyclase Activity in the NAcc.
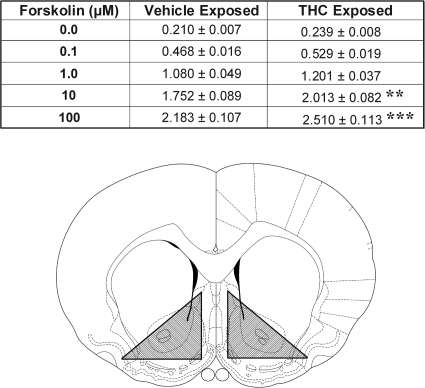
Rats in different groups (n = 6–7/group) were administered five exposure injections of either vehicle or Δ9-THC (3.0 mg/kg i.p.), one injection every third day. Adenylyl cyclase activity was assayed 2 weeks later. On the day of the assay, rats were sacrificed by decapitation, and their brains were removed rapidly and flash-frozen on dry ice. One-millimeter-thick coronal sections were obtained with a brain matrix, and the NAcc was dissected from each hemisphere (Fig. 4). Tissues from each hemisphere were sliced into three, combined, and homogenized by passing them through a 20-gauge syringe needle 10 times in a buffer containing 50 mM HEPES, pH 7.2, 1 mM EDTA, 2 mM EGTA, 10% sucrose, 0.5 μg/ml aprotinin, 100 μM phenylmethylsulfonyl fluoride, 22 μg/ml each of N-tosyl-l-lysine chloromethyl ketone and N-tosyl-l-phenylalanine chloromethyl ketone, and 3.2 μg/ml leupeptin. Lysates were immediately frozen on dry ice and stored at −80°C. To assay adenylyl cyclase activity, samples were rapidly thawed, 5 μl of homogenate was added to 45 μl of a buffer containing 20 mM HEPES, pH 7.2, 10 mM MgCl2, and 1 mM EDTA, and activity was initiated by the addition of forskolin (0, 0.1, 1.0, 10, and 100 μM) in a solution containing 50 mM HEPES, pH 7.2, 10 mM MgCl2, 1 mM EDTA, 0.1 mg/ml bovine serum albumin, 1 mM K2-phosphoenolpyruate, 0.01 mg/ml pyruvate kinase, 100 μM 3-isobutyl-1-methylxanthine, and 32P-labeled ATP. Reactions were measured at 30°C for 10 min and stopped with 2.5% SDS and 50 mM unlabeled ATP. 32P-labeled cAMP was recovered and quantified in nanomoles/minute/milligram of protein to determine adenylyl cyclase activity as described by Salomon et al. (1974). Assays were performed in triplicate.
Fig. 4.
Exposure to Δ9-THC enhances forskolin-evoked adenylyl cyclase activity in the NAcc. Data are shown as group mean (± S.E.M.) adenylyl cyclase activity (nanomoles/minute/milligram of protein) evoked by increasing concentrations of forskolin (0–100 μM) in assays conducted 2 weeks after exposure to Δ9-THC (vehicle or 3.0 mg/kg i.p.). The line drawing (adapted from Paxinos and Watson, 1997) depicts the caudal surface of a coronal section extending +1.2 to +2.2 mm from bregma and illustrates the region corresponding to the NAcc that was dissected bilaterally for the assay (cross-hatched areas). **, p < 0.01; ***, p < 0.001; significantly greater than vehicle as determined by post hoc LSD comparisons after between-within ANOVA (n = 6–7/group).
Exposure to Δ9-THC and the Self-Administration of Amphetamine and Apomorphine.
Rats in different groups (n = 7–12/group) were administered five exposure injections of vehicle, Δ9-THC (3.0 mg/kg i.p.), or amphetamine (1.5 mg/kg i.p.), one injection every third day. After each injection, rats were placed in a self-administration chamber for 2 h. Starting 10 days after the last exposure injection and 5 to 7 days after being surgically implanted with an intravenous catheter, rats were trained on fixed ratio (FR) schedules of reinforcement to self-administer either amphetamine (0.2 mg/kg/infusion) or one of two doses of apomorphine (0.1 or 0.5 mg/kg/infusion) and, after successful training, they were tested on a progressive (PR) schedule in each of six sessions.
Drug self-administration sessions were held daily and lasted up to 4 h. In all cases, reinforced lever presses delivered an infusion of the drug through the intravenous catheter at a rate of 1.6 ml/min in a volume of 0.10 to 0.13 ml/infusion. For 15 s after a reinforced lever press, a stimulus light above the lever was lit and lever presses were recorded but did not lead to further infusions.
During training, an experimenter-delivered intravenous priming infusion was given at the beginning of each session. Rats were then required to self-administer nine infusions in up to 4 h first on a FR1 and then a FR2 schedule. Rats that did not satisfy each criterion within 5 days were excluded from the study. Of the 94 rats tested, 21 were thus excluded. For testing, no priming infusions were given. Under the PR schedule used, the number of lever presses required to obtain each successive drug infusion was determined by round [5 × exp(0.25 × infusion number) − 5] to produce the following sequence: 1, 3, 6, 9, 12, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208, etc. (Richardson and Roberts, 1996). Test sessions were terminated after 4 h or after 1 h elapsed without a drug infusion. The numbers of lever presses and infusions obtained in each session were recorded.
A bank of 15 chambers, each measuring 22 × 22 × 33 cm, was used for self-administration. Each chamber was housed within a sound-attenuating chamber outfitted with an exhaust fan that shielded the animals from extraneous disturbances. A lever (5 cm above the floor) and a stimulus light (13.5 cm above the lever) were positioned on a side wall. Each chamber was equipped with a liquid swivel system comprised of a steel-spring tether, a liquid swivel, and an infusion pump (model A.E.; Razel Scientific, Inc., St. Albans, VT) that allowed free movement of the animal in the chamber and delivery of drug upon depression of the lever. The tether was connected to the animal by screwing its captive collar onto the threaded portion of a custom-designed L-shaped Plastics One cannula (20 gauge). Lever presses and drug infusions were recorded and controlled via an electrical interface by a computer using locally developed software.
Data Analyses.
The 2-h total locomotion and rearing counts obtained after the first and fifth injections of Δ9-THC as well as the frequency of different behaviors observed in three epochs on each of these days were analyzed with between-within ANOVA with dose (6) as the between factor and day (2) as the within factor. The 2-h total locomotion and rearing counts obtained after the amphetamine challenge 2 days and 2 weeks after exposure were analyzed with separate one-way ANOVAs. Total locomotion counts obtained on each test with apomorphine were analyzed with independent samples t tests. DA levels (pg/25 μl) obtained 1 h before and 3 h after the amphetamine challenge on each of the two tests were analyzed with between-within ANOVA with Δ9-THC exposure (5) as the between factor and time (12) as the within factor. Forskolin-evoked adenylyl cyclase activity (nanomoles per minute per milligram of protein) was analyzed with between-within ANOVA with Δ9-THC exposure (2) as the between factor and forskolin concentration (5) as the within factor. Training days to criterion in the self-administration experiments were analyzed with one-way ANOVA (amphetamine self-administration) or independent samples t tests (apomorphine self-administration). The numbers of infusions obtained during PR testing were analyzed with between-within ANOVA with exposure (three for amphetamine and two for apomorphine self-administration) as the between factor and test days (6) as the within factor. All subsequent pairwise comparisons after ANOVA were made using the least square difference (LSD) post hoc test.
Results
Previous Exposure to Δ9-THC Enhances Amphetamine-Induced Locomotion.
During exposure, Δ9-THC did not significantly increase locomotion on day 1 but dose-dependently increased it relative to saline on day 5 even though overall activity decreased in all groups on this day relative to day 1 (Table 1). Δ9-THC did not affect rearing overall although a nonsignificant trend was apparent. The ANOVA conducted on these data revealed significant effects of dose (locomotion: F5,30 = 3.08, p < 0.05; rearing: F5,30 = 2.42, p = 0.058) and day (locomotion: F1,30 = 87.55, p < 0.001; rearing: F1,30 = 27.14, p < 0.001). Post hoc LSD tests showed that Δ9-THC-induced locomotion was confined to day 5 (p < 0.05–0.01). More detailed analyses of different behaviors showed a dose-dependent Δ9-THC-induced increase in sniffing and walking on both days (Table 2). Again, although these behaviors declined from days 1 to 5, Δ9-THC continued to increase both relative to saline on day 5 and, paralleling locomotion, this was mostly associated with the 1.5 and 3.0 mg/kg doses of Δ9-THC (see Supplementary Data for ANOVA results). All doses of Δ9-THC decreased grooming on day 1; this effect was less pronounced and restricted to the highest doses on day 5.
TABLE 1.
Group mean (± S.E.M.) 2-h total locomotor and rearing counts after Δ9-THC on days 1 and day 5 of exposure (n = 6/group)
| D9-THC Dose (Intraperitoneal) | Locomotion |
Rearing |
||
|---|---|---|---|---|
| Day 1 | Day 5 | Day 1 | Day 5 | |
| 0 mg/kg | 964.33 ± 106.46 | 467.83 ± 52.45 | 446.83 ± 92.00 | 234.83 ± 49.13 |
| 0.40 mg/kg | 857.83 ± 107.34 | 537.33 ± 62.50 | 349.33 ± 49.50 | 222.50 ± 56.26 |
| 0.75 mg/kg | 954.83 ± 82.38 | 496.33 ± 92.94 | 411.33 ± 75.52 | 260.17 ± 54.10 |
| 1.50 mg/kg | 1095.30 ± 92.39 | 708.83 ± 106.92* | 321.00 ± 72.33 | 245.67 ± 41.07 |
| 3.0 mg/kg | 1246.70 ± 151.42 | 788.17 ± 59.51** | 488.33 ± 75.98 | 370.00 ± 71.34 |
| 6.0 mg/kg | 1179.70 ± 93.44 | 592.67 ± 88.47 | 239.33 ± 40.59 | 116.83 ± 40.58 |
P < 0.05,
P < 0.01, compared with 0 mg/kg.
TABLE 2.
Group mean (± S.E.M.) behavioral counts after Δ9-THC on days 1 and 5 of exposure (n = 4/group)
| Behavior | Δ9-THC Dose |
|||||
|---|---|---|---|---|---|---|
| 0 mg/kg | 0.4 mg/kg | 0.75 mg/kg | 1.5 mg/kg | 3 mg/kg | 6 mg/kg | |
| Day 1 | ||||||
| Epoch 1 (0–30 min) | ||||||
| Sniffing | 24.75 ± 2.50 | 20.75 ± 0.85 | 20.00 ± 1.58 | 22.00 ± 1.96 | 22.25 ± 0.95 | 22.75 ± 4.33 |
| Walking | 10.25 ± 1.03 | 10.00 ± 0.58 | 10.25 ± 0.48 | 11.25 ± 0.25 | 13.00 ± 0.58 | 11.50 ± 1.85 |
| Grooming | 2.00 ± 0.91 | 0.50 ± 0.29 | 1.25 ± 0.48 | 0.75 ± 0.48 | 1.00 ± 0.41 | 0.00 ± 0.00** |
| Motionless | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.25 ± 0.25 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Epoch 2 (45–75 min) | ||||||
| Sniffing | 7.00 ± 1.29 | 16.50 ± 3.93* | 17.25 ± 1.80** | 15.25 ± 3.59* | 20.00 ± 2.42** | 21.75 ± 0.48*** |
| Walking | 2.25 ± 1.11 | 6.50 ± 1.04** | 8.00 ± 0.91*** | 5.25 ± 0.85 | 7.25 ± 1.38** | 9.00 ± 0.71*** |
| Grooming | 6.50 ± 2.10 | 2.50 ± 0.87* | 2.00 ± 0.71* | 1.50 ± 0.87** | 1.75 ± 1.44** | 0.00 ± 0.00*** |
| Motionless | 5.75 ± 2.17 | 2.25 ± 0.87 | 0.25 ± 0.25** | 2.50 ± 1.26 | 0.75 ± 0.48** | 0.00 ± 0.00** |
| Epoch 3 (90–120 min) | ||||||
| Sniffing | 11.25 ± 3.82 | 4.50 ± 1.55 | 15.25 ± 5.76 | 15.50 ± 3.12 | 11.25 ± 3.33 | 12.00 ± 2.86 |
| Walking | 4.75 ± 1.89 | 0.75 ± 0.48 | 3.00 ± 1.58 | 5.50 ± 0.87 | 3.75 ± 1.55 | 4.75 ± 1.32 |
| Grooming | 1.00 ± 0.58 | 0.75 ± 0.48 | 1.75 ± 0.75 | 1.50 ± 0.29 | 0.75 ± 0.48 | 0.50 ± 0.50 |
| Motionless | 7.00 ± 2.48 | 12.50 ± 1.04 | 7.25 ± 0.75 | 3.75 ± 1.44 | 8.00 ± 2.08 | 6.00 ± 1.47 |
| Day 5 | ||||||
| Epoch 1 (0–30 min) | ||||||
| Sniffing | 10.50 ± 1.85 | 15.00 ± 2.80 | 15.05 ± 3.12 | 20.25 ± 0.25** | 18.50 ± 0.87* | 18.25 ± 2.78* |
| Walking | 5.75 ± 1.32 | 6.75 ± 1.65 | 7.75 ± 1.89 | 8.75 ± 1.03 | 8.50 ± 0.65 | 6.00 ± 1.29 |
| Grooming | 3.50 ± 1.19 | 6.25 ± 0.75 | 4.50 ± 1.55 | 2.50 ± 0.29 | 2.50 ± 1.19 | 0.75 ± 0.48 |
| Motionless | 5.25 ± 0.85 | 2.00 ± 1.22** | 4.00 ± 1.00 | 0.75 ± 0.75*** | 1.50 ± 0.50** | 0.50 ± 0.50*** |
| Epoch 2 (45–75 min) | ||||||
| Sniffing | 5.25 ± 1.65 | 8.50 ± 3.28 | 10.50 ± 1.85 | 12.75 ± 0.63* | 14.00 ± 3.08** | 7.25 ± 1.32 |
| Walking | 2.00 ± 0.58 | 2.50 ± 1.04 | 2.50 ± 0.29 | 6.50 ± 1.04** | 6.00 ± 0.71** | 3.50 ± 1.32 |
| Grooming | 3.25 ± 0.75 | 3.25 ± 1.60 | 2.75 ± 1.11 | 2.50 ± 0.96 | 2.25 ± 0.48 | 0.00 ± 0.00** |
| Motionless | 8.00 ± 2.12 | 7.25 ± 2.66 | 6.75 ± 1.03 | 4.00 ± 1.22 | 3.50 ± 1.71 | 3.00 ± 1.22 |
| Epoch 3 (90–120 min) | ||||||
| Sniffing | 7.25 ± 0.63 | 5.00 ± 2.80 | 4.50 ± 1.66 | 7.25 ± 2.69 | 3.50 ± 1.89 | 4.75 ± 2.50 |
| Walking | 2.75 ± 1.03 | 2.25 ± 0.95 | 1.25 ± 0.63 | 3.50 ± 1.04 | 2.00 ± 1.22 | 2.25 ± 1.32 |
| Grooming | 3.25 ± 0.75 | 2.75 ± 1.03 | 1.50 ± 0.65 | 0.75 ± 0.48* | 1.00 ± 0.71* | 0.00 ± 0.00** |
| Motionless | 6.00 ± 1.47 | 8.25 ± 1.80 | 10.50 ± 1.26 | 8.25 ± 2.75 | 11.25 ± 1.55 | 9.25 ± 2.32 |
P < 0.05;
P < 0.01;
P < 0.001 compared with 0 mg/kg.
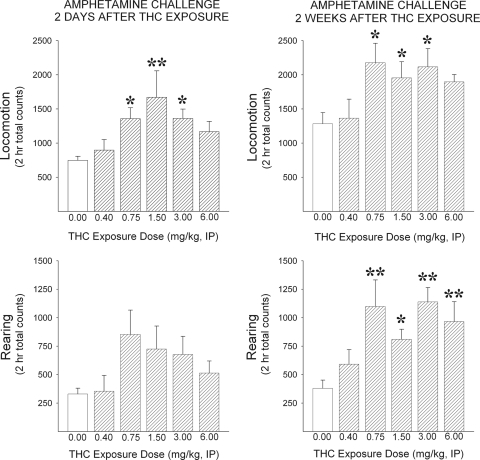
When subsequently tested with amphetamine (Fig. 1), rats previously exposed to Δ9-THC showed a significant dose-dependent increase in locomotion relative to saline-exposed rats both 2 days (F5,30 = 2.80, p < 0.05) and 2 weeks after exposure (F5,30 = 2.70, p < 0.05). Post hoc LSD tests revealed significant increases at the 0.75, 1.5, and 3.0 mg/kg doses of Δ9-THC on both tests (p < 0.05–0.01). Amphetamine did not significantly increase rearing in Δ9-THC relative to saline-exposed rats 2 days after exposure (F5,30 = 1.86, not significant) but did so 2 weeks later (F5,30 = 4.11, p < 0.01). Post hoc LSD tests revealed significant increases at the 0.75, 1.5, 3.0, and 6.0 mg/kg doses of Δ9-THC on the latter test (p < 0.05–0.01).
Fig. 1.
Exposure to Δ9-THC enhances amphetamine-induced locomotion. Data are shown as group mean (+S.E.M.) 2-h total locomotor (top) and rearing (bottom) counts observed after the amphetamine challenge injection (0.75 mg/kg i.p.) in tests conducted 2 days (left) and 2 weeks (right) after exposure to the indicated doses of Δ9-THC. *, p < 0.05; **, p < 0.01; significantly greater than 0 mg/kg as determined by post hoc LSD comparisons after one-way ANOVA (n = 6/group).
Previous Exposure to Δ9-THC Does Not Affect Amphetamine-Induced NAcc DA Overflow.
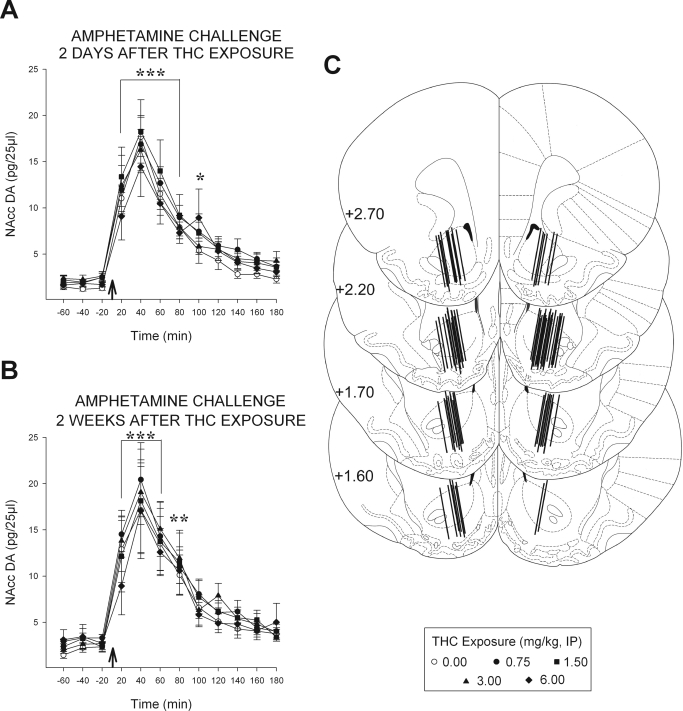
To assess the relationship between NAcc DA overflow and the sensitized locomotor response to amphetamine observed in Δ9-THC-exposed rats, in vivo microdialysis samples were collected in separate groups of animals before and after an amphetamine challenge 2 days and again 2 weeks after exposure (Fig. 2). No evidence was obtained on either test showing an effect of previous exposure to Δ9-THC on amphetamine-evoked NAcc DA overflow. Basal extracellular levels of DA observed in the NAcc before the amphetamine challenges were also unaffected. The ANOVA conducted on the DA levels observed throughout both tests revealed only significant effects of time (2 days: F11,450 = 42.40, p < 0.001; 2 weeks: F11,444 = 18.21, p < 0.001). Post hoc LSD tests indicated significant increases in NAcc DA relative to baseline 20 to 100 min after challenge (p < 0.05–0.001). Thus, amphetamine increased NAcc DA overflow in both tests as expected but did so equally in all groups, indicating no effect of previous exposure to any of the Δ9-THC doses tested. Figure 2C depicts the placements in the NAcc of the microdialysis probes used in the 87 experiments conducted.
Fig. 2.
Exposure to Δ9-THC does not enhance amphetamine-induced NAcc DA overflow. Data are shown as group mean (± S.E.M.). A and B, NAcc DA overflow (pg/25 μl) in the 1 h before and the 3 h after the amphetamine challenge injection (0.75 mg/kg i.p.; arrows) in tests conducted 2 days (A) and 2 weeks (B) after exposure to the indicated doses of Δ9-THC. Amphetamine significantly increased NAcc DA overflow for ∼100 min after injection but no significant differences between exposure groups were detected either before or after the challenge. *, p < 0.05; **, p < 0.01; ***, p < 0.001; significantly greater than preinjection baseline as determined by post hoc LSD comparisons after between-within ANOVA [n = 7–11/group (A); n = 5–14 (B)]. C, line drawings of coronal sections illustrating the location of the active portion of the microdialysis probes in the NAcc of all rats included in the data analyses (adapted from Paxinos and Watson, 1997; numbers to the left indicate millimeters from bregma).
Previous Exposure to Δ9-THC Up-Regulates Postsynaptic DA Receptor Signaling in the NAcc.
Because no relationship was observed between amphetamine-induced NAcc DA overflow and the sensitized locomotor response to amphetamine observed in Δ9-THC-exposed rats, the present experiments assessed whether the latter could have been caused by Δ9-THC-induced alterations in postsynaptic DA receptor signaling in the NAcc.
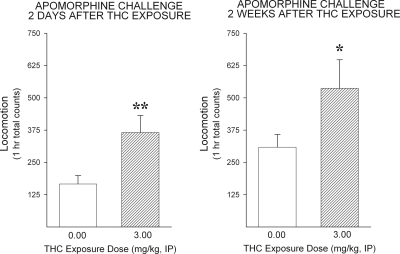
In a first experiment, the effect of exposure to Δ9-THC on locomotor responding to the direct dopamine receptor agonist apomorphine was determined 2 days and again 2 weeks after exposure (Fig. 3). Relative to saline, exposure to 3.0 mg/kg of Δ9-THC, a dose found to be maximally effective in the locomotor tests with amphetamine, significantly increased locomotor responding to apomorphine on both tests (2 days: t22 = 2.71, p < 0.01; 2 weeks: t22 = 1.77, p < 0.05).
Fig. 3.
Exposure to Δ9-THC enhances the locomotor-activating effects of the direct DA receptor agonist apomorphine. Data are shown as group mean (+S.E.M.) 1-h total locomotor counts observed after the apomorphine challenge injection (1.0 mg/kg s.c.) in tests conducted 2 days (left) and 2 weeks (right) after exposure to Δ9-THC (vehicle or 3.0 mg/kg i.p.).*, p < 0.05; **, p < 0.01; significantly greater than vehicle as determined by independent sample t tests (n = 12/group).
To more directly assess the effect of Δ9-THC exposure on postsynaptic DA receptor signaling in the NAcc, tissues from this site were obtained from rats exposed 2 weeks earlier to saline or 3.0 mg/kg of Δ9-THC and used to measure adenylyl cyclase activity evoked by increasing concentrations of forskolin (Fig. 4). As expected, forskolin concentration-dependently increased adenylyl cyclase activity in both groups, but this increase was significantly greater in Δ9-THC relative to saline-exposed rats at the higher concentrations of forskolin. The ANOVA conducted on these data revealed significant effects of exposure (F1,11 = 5.73, p < 0.05) and forskolin concentration (F4,44 = 587.22, p < 0.001) as well as a significant exposure × forskolin concentration interaction (F4,44 = 3.02, p < 0.05). Post hoc LSD tests revealed significantly greater NAcc forskolin-evoked adenylyl cyclase activity in Δ9-THC relative to saline-exposed rats at concentrations of 10 and 100 μM (p < 0.01–0.001). The cross-hatched areas in the line drawing in Fig. 4 illustrate the region of the NAcc that was dissected for the assays.
Previous Exposure to Δ9-THC Does Not Enhance Amphetamine Self-Administration.
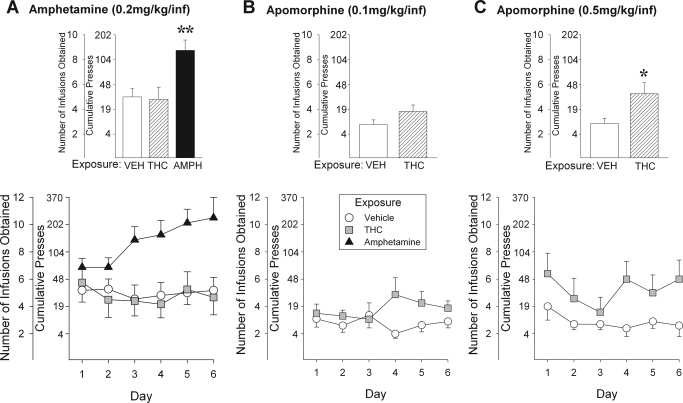
Similar to previous reports (e.g., Vezina et al., 2002), rats in the different exposure groups tested satisfied the amphetamine FR1 and FR2 self-administration training criteria within 2 to 4 days (Table 3). No significant group differences in days to criterion were detected. When subsequently tested on a PR schedule of reinforcement, however, rats previously exposed to amphetamine pressed more and obtained significantly more amphetamine infusions than saline-exposed rats, again consistent with previous reports (Vezina et al., 2002; Vezina, 2004). Increased amphetamine self-administration was not observed in rats previously exposed to Δ9-THC. Those rats showed levels of self-administration indistinguishable from saline-exposed rats (Fig. 5A). The ANOVA conducted on these data revealed a significant effect of exposure (F2,28 = 5.66, p < 0.01) and a significant exposure × day interaction (F10,140 = 1.97, p < 0.05). Post hoc LSD comparisons attributed these effects to an increase in amphetamine infusions obtained by amphetamine compared with saline- or Δ9-THC-exposed rats (p < 0.01).
TABLE 3.
Group mean (± S.E.M.) days to criterion for amphetamine and apomorphine self-administration training
| Exposure | Self-Administered Drug (dose) | Days to Criterion |
|---|---|---|
| Saline (n = 11) | Amphetamine (0.2 mg/kg/infusion) | 3.36 ± 0.54 |
| THC (n = 12) | Amphetamine (0.2 mg/kg/infusion) | 2.42 ± 0.19 |
| Amphetamine (n = 8) | Amphetamine (0.2 mg/kg/infusion) | 3.75 ± 0.41 |
| Saline (n = 11) | Apomorphine (0.1 mg/kg/infusion) | 2.55 ± 0.39 |
| THC (n = 16) | Apomorphine (0.1 mg/kg/infusion) | 2.81 ± 0.40 |
| Saline (n = 7) | Apomorphine (0.5 mg/kg/infusion) | 2.29 ± 0.29 |
| THC (n = 7) | Apomorphine (0.5 mg/kg/infusion) | 2.71 ± 0.36 |
Fig. 5.
Exposure to Δ9-THC does not enhance amphetamine self-administration but does enhance the self-administration of the direct DA receptor agonist apomorphine. Data are shown as mean (± S.E.M.) number of drug infusions obtained. The cumulative number of lever presses required to obtain the infusions is also shown. The bar graphs were derived from the means of the values obtained for each subject on each of the six PR test days. These are shown as group means in the line graphs. PR testing for amphetamine (0.2 mg/kg/infusion; A) or apomorphine (0.1–0.5 mg/kg/infusion; B and C) self-administration was initiated ∼2 weeks after exposure to vehicle (VEH), Δ9-THC (3.0 mg/kg i.p.), or amphetamine (AMPH; 1.5 mg/kg i.p.). *, p < 0.05, significantly greater than vehicle; **, p < 0.01, significantly greater than vehicle and Δ9-THC as determined by post hoc LSD comparisons after between-within ANOVA (n = 7–16/group as outlined in Table 3).
Because exposure to Δ9-THC increased locomotor responding to apomorphine as well as forskolin-induced adenylyl cyclase activity in the NAcc, its effect on apomorphine self-administration was also assessed. As with amphetamine self-administration, Δ9-THC- and saline-exposed rats did not differ in days needed to satisfy the apomorphine FR1 and FR2 self-administration training criteria (Table 3). When subsequently tested on a PR schedule, previous exposure to Δ9-THC did not significantly affect the self-administration of the 0.1 mg/kg/infusion dose of apomorphine but modestly and significantly increased intake of the 0.5 mg/kg/infusion dose (Fig. 5, B and C). The ANOVA conducted on these data revealed only a significant effect of exposure in the latter experiment (F1,12 = 6.08, p < 0.05).
Discussion
In the present experiments, we showed that previous exposure to Δ9-THC enhanced subsequent locomotor responding to amphetamine but failed to enhance NAcc DA overflow in response to the drug. Previous exposure to Δ9-THC also increased rats' locomotor response to apomorphine as well as forskolin-evoked adenylyl cyclase activity in the NAcc. Together, these results suggest that exposure to Δ9-THC sensitized amphetamine-induced locomotor activity by up-regulating postsynaptic DA receptor signaling in the NAcc. Finally, previous exposure to Δ9-THC failed to enhance amphetamine self-administration and only modestly increased apomorphine self-administration. Thus, although Δ9-THC exposure leads to alterations in postsynaptic DA receptor signaling in the NAcc, which can enhance the generation of locomotion by amphetamine and apomorphine, these neuroadaptations do not seem to be sufficient to enhance amphetamine self-administration.
Few studies have assessed the effect of exposure to Δ9-THC on subsequent locomotor responding to amphetamine. In one study that tested rats multiple times with heroin and amphetamine (Lamarque et al., 2001), cross-sensitization was observed only soon after exposure to 20 to 27 injections of Δ9-THC and preferentially in rats showing a higher response to a novel environment (“high responders”). In a second study that tested rats 24 h after exposure to 14 injections of 0.1 or 6.4 mg/kg Δ9-THC (Gorriti et al., 1999), cross-sensitization was observed only in rats exposed to the high dose of Δ9-THC. The present results extend these findings by showing that intermittent exposure to five injections of a range of Δ9-THC doses (0.75–3.00 mg/kg) leads to cross-sensitization of amphetamine-induced locomotion not only soon (2 days) but also long (2 weeks) after exposure. In addition, cross-sensitization to amphetamine-induced rearing was also observed 2 weeks after exposure to the same range of Δ9-THC doses. It is noteworthy that locomotor sensitization was observed from days 1 to 5 of exposure to 1.50 and 3.00 mg/kg Δ9-THC. Because these doses overlap with those found to produce locomotor cross-sensitization to amphetamine, similar neuronal mechanisms may underlie both sensitization to Δ9-THC and cross-sensitization to stimulant drugs. Although a similar dose-response pattern was observed when sniffing, walking, and grooming behaviors were measured during exposure, sensitization of these Δ9-THC-elicited behaviors was not observed from days 1 and 5, indicating a possible response specificity in the sensitization observed during and after exposure to Δ9-THC.
Sensitization of amphetamine-induced locomotion after exposure to Δ9-THC was not accompanied by sensitization of this drug's ability to increase extracellular DA levels in the NAcc. Although consistent with the lack of cross-sensitization to morphine-induced DA overflow in this site after exposure to Δ9-THC (Cadoni et al., 2008), this finding was initially surprising because sensitized NAcc DA overflow is associated with behavioral sensitization to stimulant drugs (Vanderschuren and Kalivas, 2000; Vezina, 2004). A similar failure of Δ9-THC exposure to sensitize amphetamine-induced NAcc DA overflow was also reported in adolescent rats, although in this study locomotor cross-sensitization was not observed (Ellgren et al., 2004). Δ9-THC is known to increase the firing of DA neurons in the VTA (Gessa et al., 1998) and increase DA overflow in the NAcc (Tanda et al., 1997). In addition, the increase in Δ9-THC-induced NAcc DA overflow is enhanced after exposure to the drug (Cadoni et al., 2008). The neuronal mechanisms underlying this plasticity remain unknown but they clearly do not overlap with those underlying stimulant sensitization of NAcc DA overflow and probably do not involve an increase in CB1R density or function because neither has been observed in the NAcc or the VTA after exposure to Δ9-THC (Ellgren et al., 2007). The present findings showing that exposure to Δ9-THC did not enhance amphetamine-induced NAcc DA overflow but did sensitize the locomotor response to apomorphine as well as forskolin-evoked adenylyl cyclase activity in the NAcc suggest that Δ9-THC exposure enhanced the locomotor response to amphetamine by up-regulating postsynaptic DA receptor signaling in this site. Given the lack of effect on amphetamine-induced DA overflow, it is likely that Δ9-THC exposure enhanced stimulant locomotor output by up-regulating DA receptor signaling in NAcc medium spiny neurons projecting to the ventral pallidum rather than to the VTA where they have been proposed to modulate DA neuron activity (Riegel and Lupica, 2004). How DA receptor signaling is up-regulated after Δ9-THC exposure remains unknown. Endogenous cannabinoids acutely block D1 and D2 DA receptor-mediated behaviors (Martin et al., 2008), and it is possible that prolonged intermitted activation of CB1Rs leads to an alteration of this effect. Conversely, because Δ9-THC increases NAcc DA overflow (Tanda et al., 1997) and this would lead to the concurrent stimulation of CB1 and DA receptors on NAcc medium spiny neurons, this may unmask a CB1R-mediated stimulatory effect on DA receptor signaling. Indeed, although both CB1R and D2 DA receptor activation inhibit forskolin-stimulated cAMP accumulation when applied separately, when applied together they reveal a CB1R-mediated augmentation (Glass and Felder, 1997). This effect may intensify with repeated exposure.
Exposure to drugs such as amphetamine sensitizes not only their ability to increase locomotion and NAcc DA overflow but their ability to support self-administration as well. The failure of Δ9-THC to similarly enhance amphetamine-induced NAcc DA overflow and self-administration in the present experiments supports a critical role for sensitized midbrain DA neuron reactivity in the enhanced pursuit of stimulant drugs (Vezina et al., 2002; Vezina, 2004). These results are consistent with others showing no enhancement of cocaine self-administration after Δ9-THC exposure (Panlilio et al., 2007), although increased heroin intake has been reported (Ellgren et al., 2007). The present findings also suggest that the up-regulation of postsynaptic DA receptor signaling observed in the NAcc after Δ9-THC exposure is not interchangeable with enhanced presynaptic DA release because the former modestly increased apomorphine self-administration but was not sufficient to increase the self-administration of amphetamine. Such differences may also underlie known differences in the profile of locomotor behaviors elicited by these two drugs (Ziegler and Szechtman, 1988), and together they may reflect the differential recruitment of additional neurotransmitter systems by amphetamine and apomorphine. Previous exposure to Δ9-THC led to a modest, but significant, increase in apomorphine self-administration, an enhanced locomotor response to this drug, and increased forskolin-evoked adenylyl cyclase activity in the NAcc, findings consistent with those of an earlier report showing enhanced apomorphine intake after 6-hydroxydopamine lesion-induced DA receptor supersensitivity (Roberts, 1989). It is possible that even a modest increase in amphetamine self-administration was not observed after Δ9-THC exposure in the present experiments because the expression of enhanced intake by amphetamine requires a contribution from other neurotransmitter systems that are differentially affected by Δ9-THC. For example, glutamate-DA interactions in the NAcc are known to be critical for the expression of stimulant sensitization (Vanderschuren and Kalivas, 2000; Kim et al., 2001), and they may promote enhanced drug intake in a manner not afforded by functional postsynaptic DA receptor up-regulation alone. Indeed, enhanced apomorphine intake on a PR schedule of reinforcement is much slower and supports lower break points than that observed with amphetamine or cocaine (Richardson and Roberts, 1996; present results). In addition, exposure to either amphetamine or cocaine enhances their ability to subsequently increase glutamate overflow in the NAcc (Vanderschuren and Kalivas, 2000; Kim et al., 2005), an effect required to maintain enhanced amphetamine self-administration (Kim et al., 2005). CB1R activation has been shown to decrease glutamate release in striatal slices (Brown et al., 2003) and this may occlude the self-administration of amphetamine. It remains to be determined whether this effect is altered after repeated Δ9-THC exposure or whether it is associated with other changes including the trafficking of glutamate and possibly other receptors in the NAcc that are known to influence drug self-administration.
Supplementary Material
Acknowledgments
We thank Gretchen M. Arnold and Jennifer D. Austin for expert technical assistance.
This study was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01-DA09397 (to P.V.), T32-DA07255 (to J.J.C.)].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.180208.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- CB1R
- cannabinoid 1 receptor
- ANOVA
- analysis of variance
- DA
- dopamine
- Δ9-THC
- Δ9-tetrahydrocannibinol
- FR
- fixed ratio
- LSD
- least square difference
- NAcc
- nucleus accumbens
- PR
- progressive ratio
- SAL
- saline
- VTA
- ventral tegmental area
- SR-141716A
- 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-piperidin-1-ylpyrazole-3-carboxamide
- CP 55,940
- 2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl) cyclohexyl]-5-(2-methyloctan-2-yl)phenol.
Authorship Contributions
Participated in research design: Lorrain and Vezina.
Conducted experiments: Lorrain, Beeler, Tang, and Vezina.
Performed data analysis: Cortright and Vezina.
Wrote or contributed to the writing of the manuscript: Cortright and Vezina.
Other: Vezina acquired funding for the research.
References
- Arnold JC, Topple AN, Hunt GE, McGregor IS. (1998) Effects of pre-exposure and co-administration of the cannabinoid receptor agonist CP 55,940 on behavioral sensitization to cocaine. Eur J Pharmacol 354:9–16 [DOI] [PubMed] [Google Scholar]
- Brown TM, Brotchie JM, Fitzjohn SM. (2003) Cannabinoids decrease corticostriatal synaptic transmission via an effect on glutamate uptake. J Neurosci 23:11073–11077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoni C, Pisanu A, Solinas M, Acquas E, Di Chiara G. (2001) Behavioral sensitization after repeated exposure to Δ9-tetrahydrocannabinol and cross-sensitization with morphine. Psychopharmacology 158:259–266 [DOI] [PubMed] [Google Scholar]
- Cadoni C, Valentini V, Di Chiara G. (2008) Behavioral sensitization to Δ9-tetrahydrocannabinol and cross-sensitization with morphine: differential changes in accumbal shell and core dopamine transmission. J Neurochem 106:1586–1593 [DOI] [PubMed] [Google Scholar]
- Chen J, Marmur R, Pulles A, Paredes W, Gardner EL. (1993) Ventral tegmental microinjection of Δ9-tetrahydrocannabinol enhances ventral tegmental somatodendritic dopamine levels but not forebrain dopamine levels: evidence for local neural action by marijuana's psychoactive ingredient. Brain Res 621:65–70 [DOI] [PubMed] [Google Scholar]
- Diana M, Melis M, Gessa GL. (1998) Increase in meso-prefrontal dopaminergic activity after stimulation of CB1 receptors by cannabinoids. Eur J Neurosci 10:2825–2830 [DOI] [PubMed] [Google Scholar]
- Ellgren M, Hurd YL, Franck J. (2004) Amphetamine effects on dopamine levels and behavior following cannabinoid exposure during adolescence. Eur J Pharmacol 497:205–213 [DOI] [PubMed] [Google Scholar]
- Ellgren M, Spano SM, Hurd YL. (2007) Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology 32:607–615 [DOI] [PubMed] [Google Scholar]
- Gessa GL, Melis M, Muntoni AL, Diana M. (1998) Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol 341:39–44 [DOI] [PubMed] [Google Scholar]
- Glass M, Felder CC. (1997) Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci 17:5327–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorriti MA, Rodríguez de Fonseca F, Navarro M, Palomo T. (1999) Chronic (−)-Δ9-tetrahydrocannabinol treatment induces sensitization to the psychomotor effects of amphetamine in rats. Eur J Pharmacol 365:133–142 [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY. (2010) Regulation of plasticity of glutamate synapses by endocannabinoids and the cyclic-AMP/protein kinase A pathway in midbrain dopamine neurons. J Physiol 588:2589–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC. (1995) Pharmacology of cannabinoid receptors. Annu Rev Pharmacol Toxicol 35:607–634 [DOI] [PubMed] [Google Scholar]
- Jolly D, Vezina P. (1996) In vivo microdialysis in the rat: low cost and low labor construction of a small diameter, removable, concentric-style microdialysis probe system. J Neurosci Methods 68:259–267 [DOI] [PubMed] [Google Scholar]
- Kim JH, Austin JD, Tanabe L, Creekmore E, Vezina P. (2005) Activation of group II mGlu receptors blocks the enhanced drug taking induced by previous exposure to amphetamine. Eur J Neurosci 21:295–300 [DOI] [PubMed] [Google Scholar]
- Kim JH, Perugini M, Austin JD, Vezina P. (2001) Previous exposure to amphetamine enhances the subsequent locomotor response to a D1 dopamine receptor agonist when glutamate reuptake is inhibited. J Neurosci 21:RC133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarque S, Taghzouti K, Simon H. (2001) Chronic treatment with Δ(9)-tetrahydrocannabinol enhances the locomotor response to amphetamine and heroin. Implications for vulnerability to drug addiction. Neuropharmacology 41:118–129 [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. (2006) Involvement of the endocannabinoid system in drug addiction. Trends Neurosci 29:225–232 [DOI] [PubMed] [Google Scholar]
- Martín AB, Fernandez-Espejo E, Ferrer B, Gorriti MA, Bilbao A, Navarro M, Rodriguez de Fonseca F, Moratalla R. (2008) Expression and function of CB1 receptor in rat striatum: localization and effects on D1 and D2 dopamine receptor-mediated motor behaviors. Neuropsychopharmacology 33:1667–1679 [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564 [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Siviy SM. (2002) Behavioral sensitization to amphetamine follows chronic administration of the CB1 agonist WIN 55,212-2 in Lewis rats. Pharmacol Biochem Behav 73:835–842 [DOI] [PubMed] [Google Scholar]
- Norwood CS, Cornish JL, Mallet PE, McGregor IS. (2003) Pre-exposure to the cannabinoid receptor agonist CP 55940 enhances morphine behavioral sensitization and alters morphine self-administration in Lewis rats. Eur J Pharmacol 465:105–114 [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Solinas M, Matthews SA, Goldberg SR. (2007) Previous exposure to THC alters the reinforcing efficacy and anxiety-related effects of cocaine in rats. Neuropsychopharmacology 32:646–657 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1997) The Rat Brain in Stereotaxic Coordinates, Compact 3rd ed., Academic Press, San Diego [Google Scholar]
- Pickel VM, Chan J, Kearn CS, Mackie K. (2006) Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. J Comp Neurol 495:299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11 [DOI] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR. (2004) Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci 24:11070–11078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC. (1989) Breaking points on a progressive ratio schedule reinforced by intravenous apomorphine increase daily following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav 32:43–47 [DOI] [PubMed] [Google Scholar]
- Rubino T, Viganò D, Massi P, Parolaro D. (2001) The psychoactive ingredient of marijuana induces behavioral sensitization. Eur J Neurosci 14:884–886 [DOI] [PubMed] [Google Scholar]
- Salomon Y, Londos C, Rodbell M. (1974) A highly sensitive adenylate cyclase assay. Anal Biochem 58:541–548 [DOI] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P. (2004) Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology 29:2149–2159 [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. (1997) Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common μ1 opioid receptor mechanism. Science 276:2048–2050 [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology 151:99–120 [DOI] [PubMed] [Google Scholar]
- Varvel SA, Martin BR, Lichtman AH. (2007) Lack of behavioral sensitization after repeated exposure to THC in mice and comparison to methamphetamine. Psychopharmacology 193:511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. (1996) D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. J Neurosci 16:2411–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. (2004) Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev 27:827–839 [DOI] [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. (2002) Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci 22:4654–4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, McGehee DS, Green WN. (2007) Exposure to nicotine and sensitization of nicotine-induced behaviors. Prog Neuropsychopharmacol Biol Psychiatry 31:1625–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Evans RL, Grainger DB, Nicholson KL. (2008) Age-dependent differences in sensitivity and sensitization to cannabinoids and “club drugs” in male adolescent and adult rats. Addict Biol 13:277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler M, Szechtman H. (1988) Differences in the behavioral profile of circling under amphetamine and apomorphine in rats with unilateral lesions of the substantia nigra. Behav Neurosci 102:276–288, 327 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.