Abstract
FK506 [tacrolimus; hexadecahydro-5,19-dihydroxy-3-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(2-propenyl)-15,19-epoxy-3H-pyrido[2,1-c][1,4]oxa-azacyclotricosine-1,7,20,21(4H,23H)-tetrone] is used clinically to reduce the incidence of allograft rejection; however, chronic administration leads to endothelial dysfunction and hypertension. We have previously shown that FK506 activates Ca2+/diacylglycerol-dependent conventional protein kinase C (cPKC), which phosphorylates endothelial nitric oxide synthase (eNOS) at one of its inhibitory sites, Thr495. However, which cPKC isoform is responsible for phosphorylating eNOS Thr495 is unknown. The aim of the current study was to determine the cPKC isoform that is activated by FK506, leading to decreased endothelial function. FK506 reduced endothelium-dependent relaxation responses, yet had no effect on endothelium-independent relaxation responses in aortas from control mice. Of the various cPKC isoforms, only the administration of a PKCβII isoform-specific peptide inhibitor restored aortic relaxation responses to that of controls. In aortic endothelial cells, FK506 significantly increased PKCβII activation compared with vehicle-treated controls, and this was prevented by a PKCβII isoform-specific peptide inhibitor. In addition, a PKCβII isoform-specific peptide inhibitor prevented the increase in eNOS Thr495 phosphorylation induced by FK506. Taken together, our results indicate that βII is the cPKC isoform responsible for phosphorylating eNOS at the inhibitory site Thr495 in response to FK506. PKCβII inhibition could prove beneficial in ameliorating the endothelial dysfunction and hypertension in patients treated with FK506.
Introduction
FK506 [tacrolimus; hexadecahydro-5,19-dihydroxy-3-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(2-propenyl)-15,19-epoxy-3H-pyrido[2,1-c][1,4]oxa-azacyclotricosine-1,7,20,21(4H,23H)-tetrone] is a macrolide used clinically for maintenance immunosuppression in organ transplant recipients. Although FK506 greatly reduces the incidence of chronic allograft rejection, it causes hypertension in most patients receiving cardiac, renal, and liver allografts (Miller, 2002; Lindenfeld et al., 2004; Morales and Domínguez-Gil, 2006). Post-transplant hypertension is positively correlated with an increased incidence of allograft failure and is a major limitation in the use of FK506 (Opelz et al., 1998; Mange et al., 2000). We and others have shown previously that a decrease in the production of the potent vasodilator nitric oxide (NO) by endothelial cells contributes to the hypertension caused by FK506 (De Lima et al., 1999; Takeda et al., 1999; Long et al., 2007; Cook et al., 2009). However, the exact molecular mechanisms remain unknown. This knowledge might lead to novel antihypertensive treatments in allograft recipients.
NO can be formed via the enzymatic activity of endothelial NO synthase (eNOS), which converts l-arginine to l-citrulline and NO. The conversion of l-arginine to NO is Ca2+/calmodulin-dependent and is regulated by cofactor availability (i.e., tetrahydrobiopterin), protein-protein interactions, and the phosphorylation status of both stimulatory and inhibitory sites (Fulton et al., 2001). Phosphorylation of Ser1177, Ser633, and Tyr83 stimulates eNOS activity, whereas phosphorylation at Thr495 and Ser116 inhibits eNOS activity (Mount et al., 2007; Fulton et al., 2008). We found that FK506, via inhibition of its intracellular target FK506 binding protein 12 (FKBP12), causes a concentration-dependent increase in eNOS Thr495 phosphorylation, decreases vasodilation, and increases blood pressure (Long et al., 2007; Cook et al., 2009). This is caused by the displacement of FKBP12 from intracellular calcium channels (ryanodine receptors), leading to an intracellular Ca2+ leak that activates the conventional protein kinase C (cPKC) isoforms. Furthermore, administration of Gö6976 [5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propanenitrile], a cPKC inhibitor, prevented the FK506-induced increase in Thr495 phosphorylation and restored NO production and vasodilatory responses (Cook et al., 2009).
The FK506-FKBP12 complex also binds and inhibits the Ca2+-dependent phosphatase calcineurin, which may also affect eNOS phosphorylation and endothelial function. However, we found that the acute effects of FK506 on eNOS were completely independent of calcineurin inhibition (Cook et al., 2009). Thus, it still remains to be elucidated which of the four cPKC isoforms (α, βI, βII, and γ) is responsible for the phosphorylation of eNOS at Thr495 in response to FK506. These four PKC isoforms are Ca2+/diacylglycerol-dependent, and all control an array of functions in normal tissue. With respect to cPKC and eNOS phosphorylation, a previous study showed that PKCα activates eNOS by increasing the phosphorylation of eNOS at the stimulatory site Ser1177, leading to increased NO production and increased arterial blood flow (Partovian et al., 2005). In obese, diabetic rats cPKC is activated by the de novo synthesis of diacylglycerol from glucose (Naruse et al., 2006). Once activated in endothelial cells, PKCβI and PKCβII were shown to inhibit Akt activation by insulin and vascular endothelial growth factor, inhibit Akt-dependent eNOS regulation by insulin, and lead to endothelial dysfunction in obese diabetic rats (Naruse et al., 2006). The observed endothelial dysfunction and decrease in NO production was reversed with the PKCβ inhibitor ruboxistaurin. These studies suggest that the PKCβ isoforms, but not PKCα, may be responsible for phosphorylating eNOS Thr495. Therefore, we hypothesized that FK506 activates PKCβII, which phosphorylates eNOS Thr495, leading to endothelial dysfunction.
Materials and Methods
Animals.
Male C57BL/6 mice aged 10 to 18 weeks were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice were kept on a 12:12 light/dark cycle and fed standard chow ad libitum. All procedures were approved by the Texas A&M Health Science Center/Scott and White Memorial Hospital Institutional Animal Care and Use Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Peptide Inhibitors.
PKC isoform-specific peptide inhibitors (Mochly-Rosen, 1995; Souroujon and Mochly-Rosen, 1998) for PKCβI (KIBI31-1), PKCβII (KIBII31-1), PKCγ (KIG31-1), a pan cPKC inhibitor (KIC1-1), and their carrier control (C1) were provided in kind by KAI Pharmaceuticals (San Francisco, CA). The development and functions of these PKC isoform-specific peptide inhibitors have been described previously (Ron et al., 1995; Stebbins and Mochly-Rosen, 2001).
Vascular Reactivity.
Vascular reactivity was measured as described previously (Long et al., 2007; Cook et al., 2009). Indomethacin (10 μM, 40 min) was present in all experiments to inhibit cyclooxygenase to examine NO-mediated vasorelaxation. Endothelium-intact aortas were incubated in the presence of PKCβI, PKCβII, PKCγ, or pan cPKC peptide inhibitors (10 μM, 20 min each). FK506 or vehicle was then added to each bath (10 μM, 20 min). Phenylephrine was added (1 μM) to obtain an EC70 contraction, and upon reaching a plateau, measurements of relaxation responses to the endothelium-dependent dilator acetylcholine or the endothelium-independent dilator sodium nitroprusside were performed.
Cell Culture.
Rat aortic endothelial cells (RAECs) were obtained from Cell Applications (San Diego, CA). RAECs were cotreated with the respective PKC isoform-specific peptide inhibitors (10 μM, 20 min) plus FK506 (10 μM, 20 min) and incubated in 95% O2/5% CO2. All cells were treated at passage six.
Immunoblotting.
RAECs treated with PKC isoform-specific peptide inhibitors and/or FK506 were lysed in 200 μl of cell lysis buffer (Cell Signaling Technology, Danvers, MA) containing phenylmethylsulfonyl fluoride (Sigma-Aldrich, St. Louis, MO) for 5 min on ice. Cells were scraped and centrifuged at 14,000 rpm for 10 min at 4°C. Supernatant was then collected, and protein estimation was performed using the Bradford assay with bovine serum albumin (Sigma-Aldrich) as the protein standard. Cellular homogenates (55 μg of total protein) were separated by electrophoresis on a NuPage 4 to 12% Bis-Tris gel (Invitrogen, Carlsbad, CA) and then transferred onto a 0.45-μm pure nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). Western blot analysis was performed with the following antibodies: peNOS Thr495 1:1000 (Millipore Corporation, Billerica, MA), eNOS 1:2500 (BD Biosciences, Franklin Lakes, NJ), pPKCβII Thr641 1:1000 (Cell Signaling Technology), and β-actin 1:5000 (Sigma-Aldrich). Secondary antibodies consisted of anti-mouse and anti-rabbit IgGs conjugated to either Alexa-Fluor 680 or IR800DYE (LI-COR Biosciences, Lincoln, NE). Imaging and densitometry were performed using a LI-COR Biosciences Odyssey imager.
Results
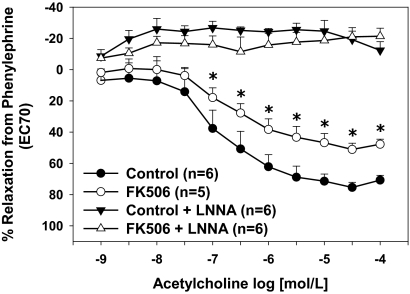
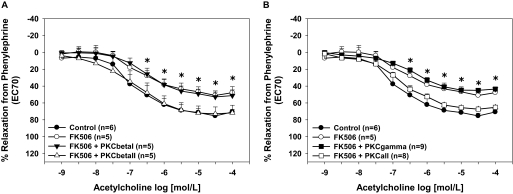
Maximal relaxation responses to acetylcholine were significantly decreased in control aortas treated with FK506 compared with vehicle-treated control aortas (p < 0.05; Fig. 1), which supports our previous findings (Long et al., 2007; Cook et al., 2009). NOS inhibition completely blocked aortic relaxation responses in both groups (Fig. 1). Specific inhibition of PKCβII restored acetylcholine-induced relaxation responses of FK506-treated aortas to that of controls (p < 0.05; Fig. 2A), whereas inhibition of PKCβI had no effect on acetylcholine-induced relaxation responses. Specific inhibition of PKCγ had no effect on acetylcholine-induced relaxation responses in FK506-treated aortas (Fig. 2B). Inhibition of all cPKC isoforms restored endothelium-dependent relaxation responses in FK506-treated aortas (Fig. 2B). Control aortas treated with the various PKC isoform-specific inhibitors alone exhibited no differences in acetylcholine-induced relaxation responses compared with vehicle-treated controls (data not shown).
Fig. 1.
FK506 causes endothelial dysfunction. FK506 decreased NO-mediated relaxation responses in aortas from control mice. All relaxation responses were inhibited completely by the eNOS inhibitor LNNA. *, p < 0.05. The numbers of aortas are given in parentheses.
Fig. 2.
Inhibition of cPKCβII restored endothelial function. A, the FK506-induced decrease in aortic endothelium-dependent relaxation responses was prevented by the cPKCβII isoform-specific peptide inhibitor, whereas the cPKCβI isoform-specific peptide inhibitor had no effect. B, the cPKCγ isoform-specific peptide inhibitor had no effect on the FK506-induced decrease in aortic endothelium-dependent relaxation responses, whereas inhibition of all cPKC isoforms increased relaxation responses. *, p < 0.05. The numbers of aortas are given in parentheses.
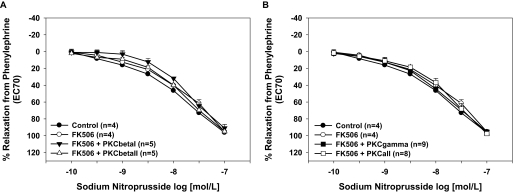
Endothelium-independent relaxation responses to sodium nitroprusside were not different between FK506-treated aortas and vehicle-treated aortas or after treatment with PKCβI or PKCβII isoform-specific peptide inhibitors (p > 0.05; Fig. 3A). Specific inhibition of PKCγ or inhibition of all cPKC isoforms also had no effect on endothelium-independent relaxation responses (Fig. 3B). Control aortas treated with the various PKC isoform-specific inhibitors alone exhibited no significant differences in sodium nitroprusside-induced relaxation responses compared with vehicle-treated controls (data not shown). These data suggest that PKCβII mediates the endothelial dysfunction caused by FK506.
Fig. 3.
Inhibition of cPKCβII had no significant effect on smooth muscle function. A, neither FK506 nor treatment with the cPKCβI or cPKCβII isoform-specific peptide inhibitors affected aortic endothelium-independent relaxation responses. B, the cPKCγ isoform-specific peptide inhibitor or inhibition of all cPKC isoforms had no effect on relaxation responses to the endothelium-dependent dilator sodium nitroprusside. *, p < 0.05. The numbers of aortas are given in parentheses.
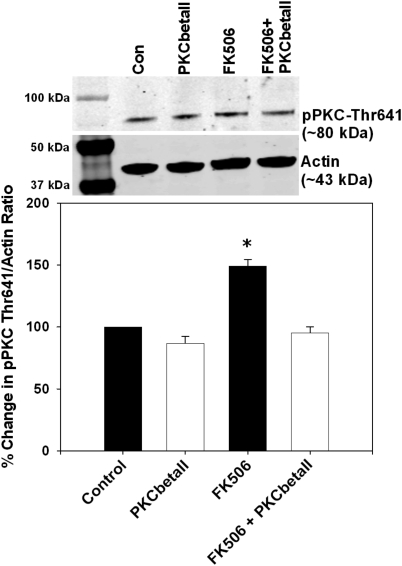
To determine whether FK506 activates PKCβII we treated RAECs with FK506 and measured pPKC Thr641, a specific marker of PKCβII activation (Keranen et al., 1995). We found that RAECs treated with FK506 had significantly increased levels of PKC Thr641 phosphorylation compared with controls (p < 0.05; Fig. 4). Furthermore, selective inhibition of PKCβII prevented the FK506-induced increase in PKC Thr641 phosphorylation while having no significant effects in controls (Fig. 4).
Fig. 4.
FK506 increased endothelial cPKCβII activation, which was prevented by the cPKCβII isoform-specific peptide inhibitor. FK506 treatment significantly increased phosphorylation of cPKCβII at Thr641, and this was prevented by the cPKCβII isoform-specific peptide inhibitor. Top, representative immunoblots. Con, control. Bottom, densitometry for the percentage change in the ratio of pPKC Thr641 to actin. *, p < 0.05. n = 4 independent experiments.
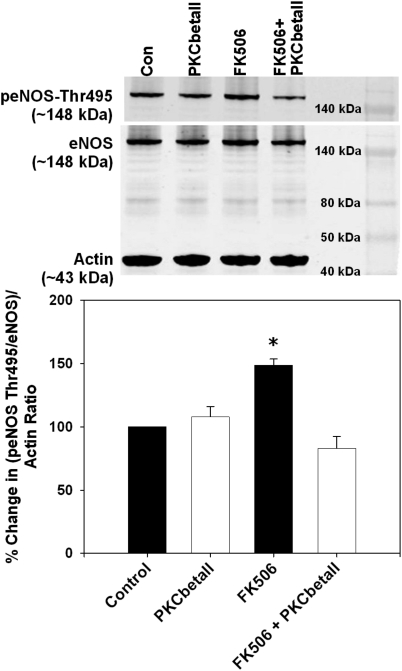
Because cPKC is known to phosphorylate eNOS Thr495, we analyzed whether the FK506-induced PKCβII activation led to increased phosphorylation of eNOS at Thr495, which decreases eNOS activity. We found that RAECs treated with FK506 exhibited significantly increased the levels of eNOS Thr495 phosphorylation compared with vehicle-treated control RAECs (p < 0.05; Fig. 5). Next, we determined whether selective inhibition of the PKCβII isoform would prevent eNOS Thr495 phosphorylation. Selective PKCβII inhibition in the presence of FK506 restored eNOS Thr495 phosphorylation levels to that of vehicle-treated RAECs while having no significant effects on controls (Fig. 5).
Fig. 5.
FK506 increased endothelial eNOS Thr495 phosphorylation, which was prevented by the cPKCβII isoform-specific peptide inhibitor. FK506 treatment significantly increased phosphorylation of eNOS at Thr495, and this was prevented by the cPKCβII isoform-specific peptide inhibitor. Top, representative immunoblots. Con, control. Bottom, densitometry for the percentage change in the ratio of peNOS Thr495/eNOS to actin. *, p < 0.05. n = 4 independent experiments.
Discussion
Hypertension and endothelial dysfunction are major limiting factors in maintenance immunosuppression with the macrolide FK506 (Miller, 2002; Lindenfeld et al., 2004). We have shown previously that administration of FK506 leads to an intracellular Ca2+ leak that activates cPKC (Long et al., 2007). PKC is known to phosphorylate eNOS at Thr495, which attenuates eNOS activity and NO generation (Fleming et al., 2001; Michell et al., 2001; Lenasi et al., 2003). It is still unknown, however, which cPKC isoform is responsible for phosphorylating eNOS Thr495 in response to FK506. By using cPKC isoform-specific peptide inhibitors we demonstrate that PKCβII is the isoform activated by FK506, leading to eNOS Thr495 phosphorylation and endothelial dysfunction.
The generation of NO depends on the enzymatic activity of eNOS, which is regulated by a variety of post-transcriptional mechanisms including protein-protein interactions, subcellular localization, and phosphorylation of serine, threonine, and tyrosine residues (Fulton et al., 2001). To maintain normal vascular function NO must be tightly regulated at the post-transcriptional level to avoid the cellular toxicity of excess NO while still maintaining vascular tone. One mechanism by which NO synthesis is controlled is by the phosphorylation status of both stimulatory and inhibitory sites. To generate NO efficiently eNOS must be phosphorylated at its stimulatory site as well as dephosphorylated at its inhibitory sites (Fulton et al., 2001; Michell et al., 2001). PKC activation is known to decrease NO production, which may be mediated by its direct effects on eNOS phosphorylation sites (Fleming et al., 2001; Matsubara et al., 2003).
Fleming et al. (2001) reported that PKC directly phosphorylates eNOS at Thr495 because the PKC inhibitor Ro 31-8220 [3-[3-[2,5-dihydro-4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-1H-pyrrol-3-yl]-1H-indol-1-yl]propyl carbamimidothioic acid ester mesylate] or down-regulation of PKC via phorbol 12-myristate 13-acetate was able to prevent eNOS Thr495 phosphorylation. Because PKC-mediated phosphorylation of eNOS Thr495 exerts negative vascular effects by reducing NO production, it is important to know which PKC isoform is responsible, which may lead to the development of therapies for diseases characterized by reduced NO and endothelial dysfunction. We have shown that FK506 exerts direct, detrimental vascular effects because ex vivo treatment of mouse aortas with FK506 significantly increases PKC activation and eNOS Thr495 phosphorylation and decreases endothelium-dependent relaxation responses (Long et al., 2007; Cook et al., 2009). These effects were prevented by the cPKC inhibitor Gö6976, implicating a cPKC isoform. Another inhibitory phosphorylation site on eNOS that may be phosphorylated by a PKC isoform is Ser116. Kou et al. (2002) showed that the PKC inhibitor calphostin could reduce eNOS Ser116 phosphorylation; however, others have shown that PKC cannot phosphorylate eNOS Ser116 because it is followed by a proline (Fujii et al., 2004; Zhu et al., 2005). Therefore, although FK506 may be having an indirect effect on Ser116, it is not related directly to the PKC-mediated phosphorylation of eNOS Thr495 induced by FK506 (Fig. 6).
Fig. 6.
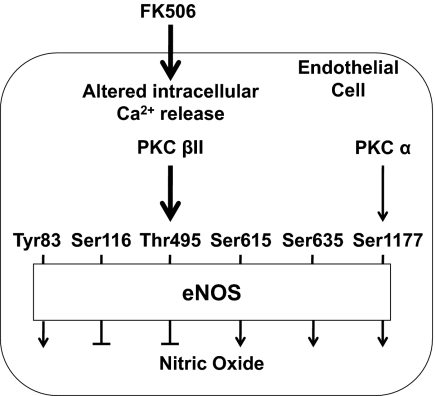
Proposed mechanisms by which cPKC isoforms affect eNOS phosphorylation. FK506 induces an endothelial cell intracellular calcium release, which activates cPKC isoforms, leading to changes in eNOS phosphorylation and NO production. Predominantly, PKCβII activation leads to eNOS Thr495 phosphorylation, which negatively affects NO production, whereas PKCα activation may lead to Ser1177 phosphorylation, which positively affects NO production.
Additional molecules have been shown to affect cPKC activity and eNOS Thr495 phosphorylation. β-Amyloid peptide, which plays a role in the development of Alzheimer's disease, has been shown to alter intracellular Ca2+ homeostasis, which activates cPKC (Gentile et al., 2004). A β-amyloid peptide-induced Ca2+ leak from endothelial intracellular stores leads to an increase in cPKC-mediated eNOS Thr495 phosphorylation and decreased endothelium-dependent relaxation responses in mouse aortas. Similar to our previous studies, these effects were blocked by the cPKC inhibitor Gö6976. Hyperhomocystinemia (HHcy) is also associated with endothelial dysfunction and decreased NO production. The endothelial dysfunction observed with HHcy has been shown to be mediated by the phosphorylation of eNOS Thr495 via cPKC (Jiang et al., 2005). Jiang et al. (2005) showed that the administration of GFX [2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl) maleimide], a cPKC-selective inhibitor, could restore relaxation responses in vivo as well as ex vivo and that NO production was normalized in CBS(−/−) (a mouse model of HHcy) or Hcy-treated mice. We cannot rule out the possible effects of FK506 on homocysteine levels in vivo because serum homocysteine levels are increased in allograft recipients compared with controls. However, the levels are typically lower in FK506-treated recipients compared with cyclosporine-treated recipients. Thus, it is possible that in vivo this could be contributing to the increased eNOS Thr495 phosphorylation after FK506. However, an in vitro study showed that FK506 treatment of renal proximal tubule epithelial cells had no effect on homocysteine production (Ignatescu et al., 2002). This finding would fit with our ex vivo and in vitro results, which support a mechanism in which cPKC activation by the removal of FKBP12/12.6 from ryanodine receptors causes the increase in eNOS Thr495 phosphorylation. Finally, the Ca2+ channel antagonist amlodipine has been shown to increase NO generation and increase relaxation responses through the inhibition of cPKC (Lenasi et al., 2003). These studies suggest that a cPKC isoform is responsible for phosphorylating eNOS Thr495.
PKCβII activation, as evidenced by increased PKCβII Thr641 phosphorylation (Keranen et al., 1995), was increased after administration of FK506, confirming that PKCβII is activated by FK506. Inhibition of PKCβII by a selective peptide inhibitor blocked the activation of PKCβII and prevented the FK506-induced increase in eNOS Thr495 phosphorylation and decrease in endothelium-dependent relaxation responses. In addition to increasing eNOS Thr495 phosphorylation, FK506-mediated activation of PKCβII probably leads to increased reactive oxygen species produced by NADPH oxidase. Both FK506 and PKCβII have been shown to lead to NADPH oxidase activation, which would increase the oxygen radical-induced decrease in NO bioavailability and result in oxidative stress and endothelial dysfunction (Dekker et al., 2000; Paolocci et al., 2001; Kitada et al., 2003; Khanna and Pieper, 2007; Pandey and Fulton, 2008). Further studies are needed to confirm the role of PKCβII in NADPH oxidase activation and whether antioxidant therapy would be effective in restoring endothelial function in FK506-treated patients.
This study identifies βII of the cPKC family as the isoform activated by the immunosuppressive drug FK506. We have shown that, once activated, PKCβII phosphorylates eNOS at its inhibitory site Thr495, leading to a decrease in endothelial function that could contribute to the hypertension observed with chronic FK506 administration. PKCβII inhibition with a selective peptide inhibitor could prove beneficial in rectifying the endothelial dysfunction and hypertension induced by chronic administration of FK506 as well as the endothelial dysfunction induced by β-amyloid peptide and HHcy.
Acknowledgments
We thank KAI Pharmaceuticals for providing the PKC isoform-specific peptide inhibitors.
This work was supported by the National Institutes of Health National Heart, Lung and Blood Institute [Grant HL084299].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.178095.
- FK506
- hexadecahydro-5,19-dihydroxy-3-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(2-propenyl)-15,19-epoxy-3H-pyrido[2,1-c][1,4]oxa-azacyclotricosine-1,7,20,21(4H,23H)-tetrone
- FKBP12
- FK506 binding protein 12
- PKC
- protein kinase C
- cPKC
- conventional PKC
- NO
- nitric oxide
- eNOS
- endothelial NO synthase
- RAEC
- rat aortic endothelial cell
- HHcy
- hyperhomocystinemia
- Gö6976
- 5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propanenitrile
- Ro 31-8220
- 3-[3-[2,5-dihydro-4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-1H-pyrrol-3-yl]-1H-indol-1-yl]propyl carbamimidothioic acid ester mesylate
- GFX
- 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl) maleimide
- LNNA
- N5-[imino(nitroamino)methyl]-l-ornithine.
Authorship Contributions
Participated in research design: Chiasson and Mitchell.
Conducted experiments: Chiasson, Quinn, Young, and Mitchell.
Performed data analysis: Chiasson, Quinn, and Mitchell.
Wrote or contributed to the writing of the manuscript: Chiasson, Quinn, and Mitchell.
Other: Mitchell acquired funding for the research.
References
- Cook LG, Chiasson VL, Long C, Wu GY, Mitchell BM. (2009) Tacrolimus reduces nitric oxide synthase function by binding to FKBP rather than by its calcineurin effect. Kidney Int 75:719–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker LV, Leitges M, Altschuler G, Mistry N, McDermott A, Roes J, Segal AW. (2000) Protein kinase C-β contributes to NADPH oxidase activation in neutrophils. Biochem J 347:285–289 [PMC free article] [PubMed] [Google Scholar]
- De Lima JJ, Xue H, Coburn L, Andoh TF, McCarron DA, Bennett WM, Roullet JB. (1999) Effects of FK506 in rat and human resistance arteries. Kidney Int 55:1518–1527 [DOI] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. (2001) Phosphorylation of Thr495 regulates Ca2+/camodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88:E68–E75 [DOI] [PubMed] [Google Scholar]
- Fujii K, Zhu G, Liu Y, Hallam J, Chen L, Herrero J, Shaw S. (2004) Kinase peptide specificity: improved determination and relevance to protein phosphorylation. Proc Natl Acad Sci USA 101:13744–13749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton D, Gratton JP, Sessa WC. (2001) Post-translational control of endothelial nitric oxide synthase: why isn't calcium/calmodulin enough? J Pharmacol Exp Ther 299:818–824 [PubMed] [Google Scholar]
- Fulton D, Ruan L, Sood SG, Li C, Zhang Q, Venema RC. (2008) Agonist-stimulated endothelial nitric oxide synthase activation and vascular relaxation. Role of eNOS phosphorylation at Tyr83. Circ Res 102:497–504 [DOI] [PubMed] [Google Scholar]
- Gentile MT, Vecchione C, Maffei A, Aretini A, Marino G, Poulet R, Capobianco L, Selvetella G, Lembo G. (2004) Mechanisms of soluble β-amyloid impairment of endothelial function. J Biol Chem 279:48135–48142 [DOI] [PubMed] [Google Scholar]
- Ignatescu MC, Kletzmayr J, Födinger M, Bieglmayer C, Hörl WH, Sunder-Plassmann G. (2002) Influence of mycophenolic acid and tacrolimus on homocysteine metabolism. Kidney Int 61:1894–1898 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Jiang X, Yang F, Tan H, Liao D, Bryan RM, Jr, Randhawa JK, Rumbaut RE, Durante W, Schafer AI, Yang X, et al. (2005) Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscl Thromb Vasc Biol 25:2515–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keranen LM, Dutil EM, Newton AC. (1995) Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr Biol 5:1394–1403 [DOI] [PubMed] [Google Scholar]
- Khanna AK, Pieper GM. (2007) NADPH oxidase subunits (NOX-1, p22phox, Rac-1) and tacrolimus-induced nephrotoxicity in a rat renal transplant model. Nephrol Dial Transplant 22:376–385 [DOI] [PubMed] [Google Scholar]
- Kitada M, Koya D, Sugimoto T, Isono M, Araki S, Kashiwagi A, Haneda M. (2003) Translocation of glomerular p47phox and p67phox by protein kinase C-β activation is required for oxidative stress in diabetic nephropathy. Diabetes 52:2603–2614 [DOI] [PubMed] [Google Scholar]
- Kou R, Greif D, Michel T. (2002) Dephosphorylation of endothelial nitric oxide synthase by vascular endothelial growth factor. Implications for the vascular responses to cyclosporine A. J Biol Chem 277:29669–29673 [DOI] [PubMed] [Google Scholar]
- Lenasi H, Kohlstedt K, Fichtlscherer B, Mülsch A, Busse R, Fleming I. (2003) Amlodipine activates the endothelial nitric oxide synthase by altering phosphorylation on Ser1177 and Thr495. Cardiovasc Res 59:844–853 [DOI] [PubMed] [Google Scholar]
- Lindenfeld J, Miller GG, Shakar SF, Zolty R, Lowes BD, Wolfel EE, Mestroni L, Page RL, 2nd, Kobashigawa J. (2004) Drug therapy in the heart transplant recipient: part II: immunosuppressive drugs. Circulation 110:3858–3865 [DOI] [PubMed] [Google Scholar]
- Long C, Cook LG, Wu GY, Mitchell BM. (2007) Removal of FKBP12/12.6 from endothelial ryanodine receptors leads to an intracellular calcium leak and endothelial dysfunction. Arterioscler Thromb Vasc Biol 27:1580–1586 [DOI] [PubMed] [Google Scholar]
- Mange KC, Cizman B, Joffe M, Feldman HI. (2000) Arterial hypertension and renal allograft survival. JAMA 283:633–638 [DOI] [PubMed] [Google Scholar]
- Matsubara M, Hayashi N, Jing T, Titani K. (2003) Regulation of endothelial nitric oxide synthase by protein kinase C. J Biochem 133:773–781 [DOI] [PubMed] [Google Scholar]
- Michell BJ, Chen ZP, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. (2001) Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem 276:17625–17628 [DOI] [PubMed] [Google Scholar]
- Miller LW. (2002) Cardiovascular toxicities of immunosuppressive agents. Am J Transplant 2:807–818 [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D. (1995) Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science 268:247–251 [DOI] [PubMed] [Google Scholar]
- Morales JM, Domínguez-Gil B. (2006) Impact of tacrolimus and mycophenolate mofetil combination on cardiovascular risk profile after kidney transplantation. J Am Soc Nephrol 17:S296–S303 [DOI] [PubMed] [Google Scholar]
- Mount PF, Kemp BE, Power DA. (2007) Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 42:271–279 [DOI] [PubMed] [Google Scholar]
- Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, Jacobs JR, Clermont AC, Ueki K, Ohshiro Y, et al. (2006) Activation of vascular protein kinase C-β inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes 55:691–698 [DOI] [PubMed] [Google Scholar]
- Opelz G, Wujciak T, Ritz E. (1998) Association of chronic kidney graft failure with recipient blood pressure. Collaborative Transplant Study. Kidney Int 53:217–222 [DOI] [PubMed] [Google Scholar]
- Pandey D, Fulton D. (2008) Regulation of NADPH oxidase 5 activity by PKC and MAPK pathways. FASEB J 22:758.30 [Google Scholar]
- Paolocci N, Biondi R, Bettini M, Lee CI, Berlowitz CO, Rossi R, Xia Y, Ambrosio G, L'Abbate A, Kass DA, et al. (2001) Oxygen radical-mediated reduction in basal and agonist-evoked NO release in isolated rat heart. J Mol Cell Cardiol 33:671–679 [DOI] [PubMed] [Google Scholar]
- Partovian C, Zhuang Z, Moodie K, Lin M, Ouchi N, Sessa WC, Walsh K, Simons M. (2005) PKCα activates eNOS and increases arterial blood flow in vivo. Circ Res 97:482–487 [DOI] [PubMed] [Google Scholar]
- Ron D, Luo J, Mochly-Rosen D. (1995) C2 region-derived peptides inhibit translocation and function of β protein kinase C in vivo. J Biol Chem 270:24180–24187 [DOI] [PubMed] [Google Scholar]
- Souroujon MC, Mochly-Rosen D. (1998) Peptide modulators of protein-protein interactions in intracellular signaling. Nat Biotechnol 16:919–924 [DOI] [PubMed] [Google Scholar]
- Stebbins EG, Mochly-Rosen D. (2001) Binding specificity for RACK1 resides in the V5 region of βII protein kinase C. J Biol Chem 276:29644–29650 [DOI] [PubMed] [Google Scholar]
- Takeda Y, Miyamori I, Furukawa K, Inaba S, Mabuchi H. (1999) Mechanisms of FK 506-induced hypertension in the rat. Hypertension 33:130–136 [DOI] [PubMed] [Google Scholar]
- Zhu G, Fujii K, Belkina N, Liu Y, James M, Herrero J, Shaw S. (2005) Exceptional disfavor for proline at the P+1 position among AGC and CAMK kinases establishes reciprocal specificity between them and the proline-directed kinases. J Biol Chem 280:10743–10748 [DOI] [PubMed] [Google Scholar]