Abstract
Chronic dopamine replacement therapy in Parkinson's disease (PD) leads to deleterious motor sequelae known as l-DOPA-induced dyskinesia (LID). No known therapeutic can eliminate LID, but preliminary evidence suggests that dl-1-isopropylamino-3-(1-naphthyloxy)-2-propanol [(±)propranolol], a nonselective β-adrenergic receptor (βAR) antagonist, may reduce LID. The present study used the rat unilateral 6-hydroxydopamine model of PD to characterize and localize the efficacy of (±)propranolol as an adjunct to therapy with l-DOPA. We first determined whether (±)propranolol was capable of reducing the development and expression of LID without impairing motor performance ON and OFF l-DOPA. Coincident to this investigation, we used reverse-transcription polymerase chain reaction techniques to analyze the effects of chronic (±)propranolol on markers of striatal activity known to be involved in LID. To determine whether (±)propranolol reduces LID through βAR blockade, we subsequently examined each enantiomer separately because only the (−)enantiomer has significant βAR affinity. We next investigated the effects of a localized striatal βAR blockade on LID by cannulating the region and microinfusing (±)propranolol before systemic l-DOPA injections. Results showed that a dose range of (±)propranolol reduced LID without deleteriously affecting motor activity. Pharmacologically, only (−)propranolol had anti-LID properties indicating βAR-specific effects. Aberrant striatal signaling associated with LID was normalized with (±)propranolol cotreatment, and intrastriatal (±)propranolol was acutely able to reduce LID. This research confirms previous work suggesting that (±)propranolol reduces LID through βAR antagonism and presents novel evidence indicating a potential striatal locus of pharmacological action.
Introduction
For the last half-century, the DA precursor l-DOPA has been the treatment of choice for PD, even though its chronic use induces side effects that can be as debilitating as PD itself (Jankovic, 2008). These side effects, typified by LID, are commonly slow to develop, occurring in less than 10% of patients during the 1st year of l-DOPA therapy but in as many as 90% of patients after 9 years of use (Ahlskog and Muenter, 2001).
The primary mechanism(s) underlying LID are thought to include extraphysiological DA release and aberrant receptor signaling in the striatum, which dysregulates subsequent striatal output (Winkler et al., 2002; Cenci, 2007). After DA depletion, serotonin (5-HT) neurons synthesize DA from exogenous l-DOPA and release it in a pulsatile manner, which is a putative source of LID (Carta et al., 2007). However, recent evidence demonstrates that noradrenergic terminals also synthesize and release l-DOPA-derived DA (Arai et al., 2008). Thus, in recent years, there has been increased interest in the use of adrenergic compounds to prevent or reduce LID (Colosimo and Craus, 2003; Brotchie, 2005). Although most of the research has focused on α-adrenergic compounds (Savola et al., 2003; Rommelfanger and Weinshenker, 2007; Buck et al., 2010), the striatum contains a high density of βARs (Rainbow et al., 1984), which are preserved in PD patients (Waeber et al., 1991). These receptors represent a unique therapeutic target for LID because βAR blockade prevents drug-induced facilitation of DA release in intact and DA-depleted animals, which may blunt downstream signaling abnormalities associated with LID (Reisine et al., 1982; Goshima et al., 1991). In line with these findings (±)propranolol, a nonselective-βAR antagonist, reduces the expression of LID in humans (Carpentier et al., 1996) and in animal models of LID (Gomez-Mancilla and Bedard, 1993; Dekundy et al., 2007; Buck and Ferger, 2010). However, several key pragmatic and mechanistic questions remain regarding the antidyskinetic efficacy of βAR blockade.
Therefore, the following studies were undertaken to determine the cellular and behavioral effects of βAR blockade in l-DOPA-treated hemiparkinsonian rats. The first experiment examined the effects of (±)propranolol on the development of abnormal involuntary movements (AIMs) and on the expression of genes routinely used as markers of pathway-specific striatal DA output: preproenkephalin (PPE), preprodynorphin (PPD), and preprotachykinin (PPT) (Gerfen et al., 1990; Cenci et al., 1998). The second experiment determined whether antidyskinetic doses of (±)propranolol modify l-DOPA efficacy or basal motor activity. By using active and inactive enantiomers, the third experiment examined the βAR-specific effects of (±)propranolol. Lastly, to determine whether the striatum is a pharmacological site of action, striatal microinfusions of (±)propranolol were performed. Results suggest that (±)propranolol dose-dependently suppresses AIMs through a normalization of signaling in the dorsal striatum.
Materials and Methods
Animals.
These studies used male Sprague-Dawley rats (n = 98; Taconic Farms, Hudson, NY), which were 8 weeks old and weighed 225 to 250 g upon arrival. Rats were kept in plastic cages (45 cm long, 23 cm wide, 22 cm high) and given free access to water and food (Rodent Diet 5001; Lab Diet, Brentwood, MO). The colony room was maintained at 22–23°C on a 12-h light/dark cycle with lights on at 7:00 AM. The guidelines of the Institutional Animal Care and Use Committee of Binghamton University and the Guide for the Care and Use of Laboratory Animals were maintained throughout the study (Institute of Laboratory Animal Resources, National Academic Press 1996; NIH publication number 85-23, revised 1996).
Drugs.
Systemic drug administrations were done at a volume of 1 ml/kg, with the injection given intraperitoneally, with the exception of l-DOPA, which was given subcutaneously. 3-(5,6-Dihydrobenzo[b][1]benzazepin-11-yl)-N-methylpropan-1-amine hydrochloride (despiramine hydrochloride; Sigma-Aldrich, St. Louis, MO) was dissolved in dH2O. 21-Cyclopropyl-7-a-[(S)-1-hydroxy-1,2,2-trimethylpropyl]-6,14-endo-ethano-6,7,8,14-tetrahydrooripavine hydrochloride (buprenorphine hydrochloride; Hospira Inc., Lake Forest, IL) was dissolved in dH20 containing 0.9% NaCl (saline). All enantiomers of propranolol hydrochloride (Sigma-Aldrich) were dissolved in 90% saline and 10% dimethyl sulfoxide. l-DOPA methyl ester hydrochloride (Sigma-Aldrich) and 6-hydroxydopamine (6-OHDA) hydrobromide (Sigma-Aldrich) were dissolved in saline with 0.1% ascorbic acid. Doses of 4, 6, and 12 mg/kg l-DOPA were administered during this experiment, but the peripheral decarboxylase inhibitor 2-amino-3-hydroxy-N′-[(2,3,4-trihydroxyphenyl)methyl]propanehydrazide (benserazide; Sigma-Aldrich) was always coadministered with l-DOPA in the same vehicle at a dose of 15 mg/kg. Overall, we believe this to be more beneficial than testing at a single dose of l-DOPA because PD patients differ greatly in l-DOPA dose and dyskinesia severity. In experiment 1, we used a dose of 6 mg/kg l-DOPA to study the development of dyskinesia because animals gradually manifest AIMs of increasing severity for several weeks. We tested 4 and 12 mg/kg l-DOPA in fully primed animals to determine whether propranolol was capable of reducing mild or severe AIMs, respectively.
Surgeries.
One week after arrival, rats were given a unilateral DA lesion to the left medial forebrain bundle (MFB). Before surgery, rats were given injections of desipramine (25 mg/kg) to protect norepinephrine neurons and buprenorphine (0.03 mg/kg) as pre-emptive analgesia. Animals were then anesthetized with 1 to 2% 2-chloro-2-(difluoromethoxy)-1,1,1-trifluoroethane (isoflurane; Baxter Healthcare Corp., Deerfield, IL) mixed with oxygen (2.5 l/min). The following coordinates relative to bregma were used to target the MFB according to Paxinos and Watson (1998): AP, −1.8 mm; ML, + 2.0 mm; DV, −8.6 mm, with the incisor bar 5 mm below the interaural line. A small hole was drilled into the skull, and a 10-μl syringe with a 26-gauge needle (Hamilton Company, Reno, NV) was lowered into the target. 6-OHDA (12 μg in 4 μl) was injected at a constant flow rate of 2 μl/min for 2 min and timed to begin 30 min after desipramine injection. The needle was withdrawn 5 min later.
A sham lesion in the right hemisphere was not performed, which is consistent with current protocols in the field (e.g., Cenci and Lundblad, 2007; Buck and Ferger, 2010). The rationale is that a sham lesion in the right hemisphere would partially deplete DA because of mechanical damage to the MFB.
Rats in experiment 4 had a 15-mm guide cannula (22 gauge, C313/G/SPC; Plastics One Inc., Roanoke, VA) inserted into the dorsal striatum coincident with lesion surgery, using a procedure from Dupre et al. (2008). The following coordinates were used relative to bregma: AP, +0.4 mm; ML, +2.9 mm; DV, −3.6 mm. Cannulae were fixed in place with Jet Denture Repair Acrylic (Lang Dental, Wheeling, IL). The guide cannula was then fitted with a 28-gauge inner stylet (Plastics One) to maintain guide cannula patency.
Abnormal Involuntary Movements Test.
The AIMs test is a metric of dyskinesia, a primary side effect of l-DOPA therapy. Rats were monitored for AIMs using a procedure modified from Lundblad et al. (2002) and described in Eskow et al. (2009). After treatment with l-DOPA, rats were placed in plastic cylinders (22.2 cm diameter, 25.4 cm height; Thermo Fisher Scientific, Waltham, MA) and rated by trained observers blind to the experimental condition for 1 min every 10 min over a 180-min period. Axial AIMs were defined as dystonic twisting of the neck and torso contralateral to the lesioned hemisphere. A limb AIM was operationalized as a rapid, purposeless movement of the forelimb controlled by the lesioned hemisphere. Orolingual AIMs were coded as repetitive mastication or tongue protrusions when the rat's mouth was empty and not in contact with any object. A severity score was assigned to each of these AIMs: 0, not present; 1, present for <50% of the observation period; 2, present for >50% but <100% of the observation period; 3, present for the entire observation period and interrupted by a loud stimulus (a pencil tap on the side of the cylinder); or 4, present for the entire observation period and not interrupted by the stimulus. Scores for axial, limb and orolingual (ALO) were combined to create a single ALO AIMs score for data analysis.
Forepaw Adjusting Steps Test.
The forepaw adjusting steps (FAS) test is a measure of akinesia, a cardinal symptom of PD. Rats with >80% unilateral DA depletion perform poorly on the test with the lesioned side of the body (Chang et al., 1999). l-DOPA reduces this deficit, so the test can be used to determine whether an l-DOPA adjunct is interfering with the relief of PD symptoms provided by l-DOPA (Eskow et al., 2007). To perform the test, an experimenter blind to treatment condition held the rat's hindlimbs and one forelimb, such that the free forelimb was forced to bear the body weight of the rat. Rats were then moved laterally for 90 cm over 10 s across a marked surface while another experimenter counted the number of steps taken in the forehand direction (defined as movement toward the rat's midline) and backhand direction (movement away from the rat's midline). Each FAS test consisted of three backhand and three forehand trials with each limb, for a total of 12 trials per rat. The score for percentage intact stepping was derived by summing the total steps with the lesioned forepaw, dividing by the number of steps with the unlesioned forepaw, and multiplying this number by 100. Lower percentage intact scores indicate greater forelimb akinesia.
Experiment 1: Chronic (±)Propranolol Administration and the Development of Dyskinesia.
Thirty-four rats received unilateral 6-OHDA lesions of the MFB. Two weeks later, DA-lesion severity was assessed using the FAS test. Rats were included in the study if they averaged less than five forehand-adjusting steps with the lesioned forelimb per trial, given that this score is associated with >80% striatal DA depletion (Chang et al., 1999). Thirty-three rats were included in the final analysis and assigned to 3 groups of 11 rats each, with each group having equivalent average disability on the FAS test. These FAS scores, measured off of l-DOPA, were used as a baseline against which scores on l-DOPA would be compared.
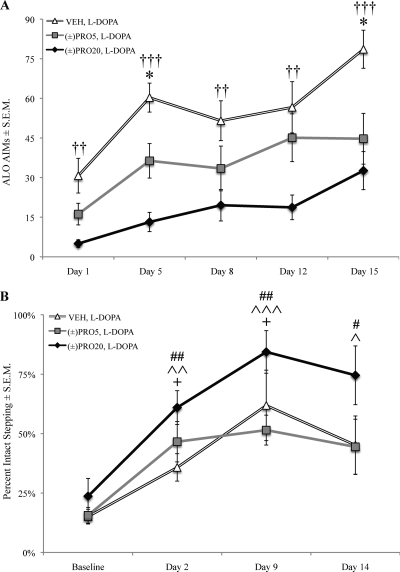
Three weeks after surgery, treatment with (±)propranolol and l-DOPA began. Rats received (±)propranolol (VEH, 5 or 20 mg/kg) 5 min before l-DOPA (6 mg/kg) for 16 consecutive days. AIMs were assessed on days 1, 5, 8, 12, and 15. FAS data were collected 1 h after l-DOPA treatment on days 2, 9, and 14. On day 16, rats were killed by decapitation 2 h after l-DOPA injection. The dorsal striatum was removed for analysis using real-time reverse-transcription polymerase chain reaction (RT-PCR).
Real-Time Reverse Transcription Polymerase Chain Reaction.
Two hours after receiving their last VEH or (±)propranolol injections coincident with l-DOPA, rats in experiment 1 were killed, and the left and right striata were dissected individually and placed in RNAlater (QIAGEN, Valencia, CA) for subsequent analyses of PPE, PPD, and PPT mRNA expression in addition to two housekeeper genes, β-actin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Tissue was processed with RNeasy mini protocol (QIAGEN), as detailed in Barnum et al. (2008). cDNA was amplified with the IQ SYBR Green Supermix kit (Bio-Rad Laboratories, Hercules, CA). A reaction master mix volume of 40 μl was created consisting of 20 μl of SYBR Green, 17.6 μl of RNase-free water, 0.4 μl of cDNA template, and 2 μl of sample. Ten microliters of each master mix was pipetted in triplicate into a 384-well plate (Bio-Rad Laboratories) and analyzed using Bio-Rad CFX1000 Thermal Cycler. Relative gene expression was quantified using the 2−ΔCT method, with expression levels normalized to 100% of ultimate control values. Gene sequences were obtained from GenBank at the National Center for Biotechnology Information, and primer specificity was verified by the Basic Local Alignment Search Tool (http://www.ncbi.nlm.nih.gov/). The primer sequences used were for β-actin (5′-AGCATCACCCCATTTGATGT-3′/5′-GTCGTACCACTGGCATTGTG-3′); GAPDH (5′-GCCATCTCTTGCTCGAAGTC-3′/5′-ATGACTCTACCCACGGCAAG-3′); PPD (5′-GGGTTCGCTGGATTCAAATA-3′/5′-TGTGTGGAGAGGGACACTCA-3′); PPT (5′-AGCCTCAGCAGTTCTTTGGA-3′/5′-CGGACACAGATGGAGATGAA-3′); and PPE (5′-AAAATCTGGGAGACCTGCAA-3′/5′-CATGAAACCGCCATACCTCT-3′).
Experiment 2: Impact of (±)Propranolol on Spontaneous Motor Activity.
Fourteen rats received unilateral 6-OHDA lesions in the aforementioned manner. Three weeks later, rats were acclimated to motion chambers 4 times in 1 week for a period of 90 min each, because this length corresponds to the duration of antidyskinetic efficacy of 20 mg/kg (±)propranolol. Rats were kept l-DOPA-naive and tested four times in a within-subjects design. Each test session was separated by a 2- to 3-day washout period, which insured full clearance of the drug given that the half-life of (±)propranolol in rats is approximately 1 h (Bianchetti et al., 1980). Rats were given injections of (±)propranolol (VEH, 5, 20, or 40 mg/kg) and were immediately placed in motion chambers for analysis. The order of treatments was counterbalanced across test days. Testing was done between 10:00 AM and 4:00 PM with each rat placed in the same chamber for all acclimation and testing sessions.
Locomotor activity was assessed in six identical acrylic chambers measuring 41 cm in length and width and 30.5 cm in height (Accuscan Instruments, Columbus, OH). Each chamber was surrounded by infrared photocell arrays synched with a program running Versamax and Versadat software (Accuscan Instruments). The software analyzes patterns of photo beam breaks to measure horizontal and vertical movements. Data were analyzed over a period of 90 min, with data grouped into six blocks of 15 min. We include data on the total distance traveled (in centimeters); the number starts and stops separated by at least 1 s (“movement number”), and the number of beam breaks in the vertical plane (“vertical activity”). These behaviors have been used previously to measure drug-induced locomotor changes in hemiparkinsonian rats (Bishop et al., 2004).
Experiment 3: Enantiomers of (±)Propranolol in the Expression of Established Dyskinesia.
Twenty rats received unilateral 6-OHDA lesions to the MFB. After a 3-week recovery period, all rats were “primed” with daily injections of l-DOPA (12 mg/kg) for 1 week. This process is referred to as priming because subsequent doses of l-DOPA will cause rats to manifest reliably similar levels of dyskinesia in response to a given dose of l-DOPA (Bishop et al., 2009). On day 7 of l-DOPA priming, ALO AIMs were monitored, and rats with >25 ALO AIMs (n = 17) were included in the study.
The following week, rats were given (±)propranolol (VEH, 5 or 20 mg/kg) in a within-subjects design 5 min before l-DOPA (12 mg/kg) and monitored for ALO AIMs. Rats were tested 3 days per week with each test day separated by a 2-day washout period, and the order of treatments was counterbalanced. The individual enantiomers of propranolol (+ and −) were tested in the same manner in weeks 5 and 6, respectively. To address potential changes in dyskinesia levels across treatment, VEH + l-DOPA ALO AIMs of each rat were compared across weeks and did not differ among weeks 4, 5, and 6 (p > 0.05).
Experiment 4: Striatal (±)Propranolol Infusion and Dyskinesia Expression.
Two cohorts each consisting of 15 rats received cannulae in the left dorsal striatum in addition to unilateral 6-OHDA lesion to the MFB. Three weeks later, all rats were primed with l-DOPA (12 mg/kg) for 1 week. On the last day of priming, AIMs were assessed, and only rats with >25 ALO AIMs score were included in the study. Four times during the priming week, rats were wrapped in a small towel for several minutes to habituate them to gentle restraint during microinfusions. Two days after the final priming dose of l-DOPA, the first cohort of rats was given a striatal microinfusion of (±)propranolol (VEH, 1 or 10 μg in 2 μl) at a constant flow rate of 0.5 μl/min over 4 min in a counterbalanced within-subjects manner. Previous research indicated that this flow rate and volume of fluid at the coordinates used would cause the liquid vehicle to permeate only the dorsal striatum (Dupre et al., 2008). For microinfusions, the dummy cannula was removed, and a 16-mm injector was placed inside the 15-mm guide cannula, making the injector extend 1 mm further into the striatum than the guide. The injector was removed 4 min after the flow was stopped, and rats were immediately injected with l-DOPA (4 mg/kg) and assessed for ALO AIMs. The second cohort was tested in the same manner, with the exception that the l-DOPA dose was increased to 12 mg/kg. Rats were removed from the study if they did not become dyskinetic after priming or if the cannula placement was incorrect, leaving 13 rats in the first cohort and 12 in the second.
Tissue Dissection and Cresyl Violet Staining.
After decapitation, the brain was removed, and the striatum was bisected coronally with a razor blade. The posterior striatum was dissected and frozen at −80°C for analysis of DA levels using high-performance liquid chromatography with electrochemical detection (HPLC-ED). The anterior section of the brain (containing the cannula site) was placed in 4% formaldehyde (Thermo Fisher Scientific). Coronal slices (30 μm) showing the cannula placements were sectioned using a microtome (model SM2000R; Leica Microsystems Inc., Bannockburn, IL). Slices were mounted on slides and stained with cresyl violet (FD NeuroTechnologies, Inc., Baltimore, MD). Light microscopy was then used to determine injection sites.
High-Performance Liquid Chromatography.
Reverse-phase HPLC-ED was performed on striatal tissue as outlined by Bishop et al. (2009), based on a protocol for catecholamine analysis by Kilpatrick et al. (1986). The system included an autoinjector (model 542; EMA Services, Chelmsford, MA), a solvent delivery system (model 582; ESA), an external pulse dampener (ESA), and a MD-150 × 3.2 column (150 × 3.2 mm, 3 μm particle size; ESA). Samples were homogenized in 0°C perchloric acid (0.1 M), 1% ethanol, and 0.02% EDTA (Sigma-Aldrich). The homogenates were spun for 45 min at 14,000g with the temperature maintained at 4°C. Aliquots of supernatant were then analyzed for abundance of DA. Samples were separated using a mobile phase composed of 90 mM sodium dihydrogen phosphate, 50 mM citric acid, 50 μM EDTA, 1.7 mM octane sulfonic acid, and 10% acetonitrile, adjusted to pH 3.0 with orthophosphoric acid (Sigma-Aldrich). A coulometric detector configured with three electrodes (Coulochem III; ESA) measured the content of DA. An ESA model 5020 guard cell (+500 mV) was positioned before the autosampler. The analytical cell (ESA model 5011A; first electrode at −100 mV, second electrode at +250 mV) was located immediately after the column. EZChrom Elite software (ESA) recorded and analyzed the electrochemical reaction that occurred on the second analytical electrode. The final oxidation current values were plotted on a standard curve of known concentrations from 10−6 to 10−9 M, and values were adjusted to striatal tissue weights.
Statistical Analysis.
ALO AIMs data for between-subjects comparisons were analyzed using the nonparametric Kruskal-Wallis test. If the omnibus comparison for a given test day was significant, Mann-Whitney contrasts were used to distinguish significant differences between vehicle and treatment groups. For within-subjects designs, the nonparametric Friedman test was used followed by the Wilcoxon-signed rank post hoc. All non-ALO AIMs data were analyzed with standard parametric statistics using between-subjects, repeated measures, and mixed model ANOVAs when appropriate. Fisher's least significant difference contrasts on post hoc comparisons were used throughout the study.
For analysis where the primary comparisons of interest were between-subjects, (experiment 1, ALO AIMs and RT-PCR data) scores with more than 2 S.D.s from the group mean were discarded and replaced with the new group mean; degrees of freedom for comparisons were adjusted accordingly. No data were discarded in other analyses. Statistical analysis was done using SPSS version 18.0 (IBM, Chicago, IL) with alpha set at 0.05.
Results
Experiment 1
(±)Propranolol Dose-Dependently Reduced the Development of ALO AIMs.
As shown in Fig. 1A, coadministration of (±)propranolol at 5 mg/kg [(±)PRO5] or (±)propranolol at 20 mg/kg [(±)PRO20] along with l-DOPA reduced ALO AIMs compared with l-DOPA alone (VEH). Axial, limb, and orolingual AIMs were examined individually, and it was found that (±)propranolol dose-dependently suppressed all three subtypes (data not shown). Therefore, all further analyses were conducted using a composite ALO AIMs score. Kruskal-Wallis test revealed that this difference was statistically significant on all test days: day 1 (χ2 = 12.74, p < 0.01), day 5 (χ2 = 19.45, p < 0.001), day 8 (χ2 = 7.53, p < 0.05), day 12 (χ2 = 9.40, p < 0.01), and day 15 (χ2 = 12.07, p < 0.01). Mann-Whitney comparisons indicated that, compared with VEH, (±)PRO5 reduced ALO AIMs on days 5 and 15 (p < 0.05), and (±)PRO20 reduced ALO AIMs on all test days (p < 0.05).
Fig. 1.
(±)Propranolol dose-dependently attenuated the development of l-DOPA-induced dyskinesia without interfering with l-DOPA efficacy. Unilaterally lesioned rats (n = 11 per group) were given (±)propranolol (VEH, 5 mg/kg, or 20 mg/kg) 5 min before l-DOPA (6 mg/kg) every day for 16 days in a between-subjects design. ALO AIMs were tested on days 1, 5, 8, 12, and 15. The FAS test was performed at baseline several days before the beginning of l-DOPA treatment and subsequently 1 h after l-DOPA injection on days 2, 9, and 14. Symbols of charts denote treatment group mean ± S.E.M. for ALO AIMs (A) and FAS (B). ALO AIMs were analyzed using the Kruskal-Wallis test with Mann-Whitney post hoc tests. FAS data were analyzed using a one-way repeated measures ANOVA and contrasts comparing treatment days to baseline. AIMs: *, p < 0.05 VEH versus (±)PRO5; ††, p < 0.01, †††, p < 0.001 VEH versus (±)PRO20. FAS: +, p < 0.05, VEH versus baseline; ^, p < 0.05,^^, p < 0.01, ^^^, p < 0.001 (±)PRO5 versus baseline; #,p < 0.05 ##, p < 0.01 (±)PRO20 versus baseline.
(±)Propranolol Did Not Affect the Efficacy of l-DOPA on the FAS Test.
The FAS test was used to determine whether l-DOPA improved stepping in lesioned rats and whether (±)propranolol cotreatment affected stepping (Fig. 1B). A 3 × 4 (treatment by day) mixed model ANOVA was used for analysis. Omnibus ANOVA revealed a main effect of treatment (F2,30 = 4.92, p < 0.05) where (±)PRO20 rats had a higher percentage intact score than (±)PRO5 or VEH rats (p < 0.05). A main effect of day (F3,90 = 17.62, p < 0.001) demonstrated that l-DOPA improved stepping on test days relative to baseline (p < 0.05).
Because there was a main effect of day, a one-way repeated measures ANOVA was run on each treatment group to test for differences between baseline and treatment day stepping. ANOVA revealed an effect of treatment day for VEH (F3,30 = 4.39, p < 0.05), (±)PRO5 (F3,30 = 5.28, p < 0.01), and (±)PRO20 (F3,30 = 9.26, p < 0.001). Post hoc comparisons between baseline and treatment days revealed that l-DOPA administration improved stepping in VEH rats on days 2 and 9 (p < 0.05) and in (±)PRO5 and (±)PRO20 rats on all test days.
(±)Propranolol Modified Gene Expression of LID-Related Striatal Prepropeptides.
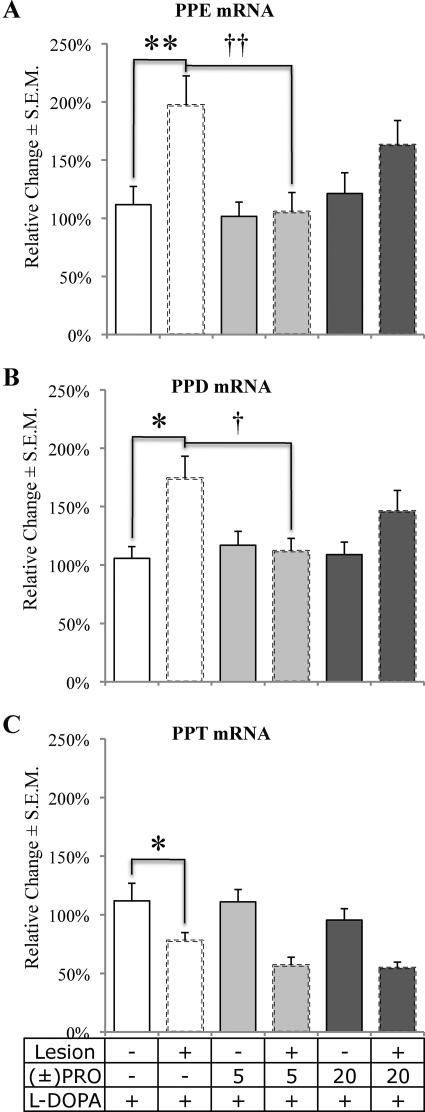
Tissue from the dorsal striatum was analyzed via RT-PCR to examine changes in opioid prepropeptide mRNA for PPE, PPD, and PPT. Expression changes were determined with a 2 × 3 (lesion by treatment) mixed model ANOVA. No significant main effects or interactions were found for the housekeeper genes, β-actin and GAPDH (p > 0.05), thereby allowing for comparison of lesion- and treatment-induced effects. Subsequent opioid mRNA analysis with a 2 × 3 (lesion by treatment) mixed model ANOVA revealed a main effect of lesion, which increased transcription of PPE (F1,26 = 12.97, p < 0.01; Fig. 2A) and PPD (F1,28 = 9.26, p < 0.01; Fig. 2B) while reducing PPT transcription (F1,29 = 46.92, p < 0.001; Fig. 2C). There was also a significant lesion by treatment interaction for PPE (F2,26 = 3.79, p < 0.05) and PPD (F2,28 = 3.72, p < 0.05). Post hoc comparisons of VEH to (±)PRO5 and (±)PRO20 on the lesioned side indicated that transcription of both PPE and PPD on the lesioned side was reduced by (±)PRO5 compared with lesioned striatum treated with VEH (p < 0.05).
Fig. 2.
DA lesion, l-DOPA, and (±)propranolol alter the transcription of PPE, PPD, and PPT mRNA in the striatum. Hemiparkinsonian rats (n = 11 per group) were given (±)propranolol (VEH, 5 or 20 mg/kg) 5 min before l-DOPA (6 mg/kg) every day for 16 days in a between-subjects design. Rats were killed by decapitation 2 h after final l-DOPA injection on day 16, and the dorsal striata from the lesioned and nonlesioned sides were dissected for subsequent RT-PCR analysis. Bars express percentage change in expression compared with control (unlesioned striatum treated with l-DOPA) for PPE (A), PPD (B), and PPT (C) mRNA expression. Effects were determined using a 2 × 3 (lesion by treatment) mixed model ANOVA followed by post hoc tests. *, p < 0.05, **, p < 0.01 VEH+Intact versus VEH+Lesion; †, p < 0.05, ††, p < 0.01 VEH+Lesion versus (±)PRO5+Lesion.
Experiment 2
(±)Propranolol Reduced Motor Activity Only at High Doses.
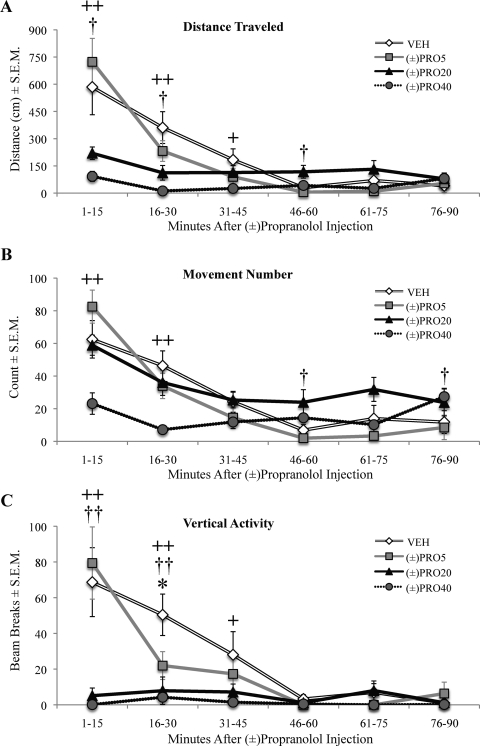
The motor activity of rats given injections of (±)propranolol alone was assayed in motion chambers to determine whether anti-LID efficacy of (±)propranolol is attributable to a general suppression of motor behavior. The four treatment groups were analyzed in six time blocks of 15 min using a 4 × 6 (treatment by time) repeated measures ANOVA. Analysis of total movement (Fig. 3A) revealed a significant effect of treatment (F3,39 = 10.92, p < 0.001), time (F5,65 = 25.52, p < 0.001), and a treatment by time interaction (F15,195 = 6.52, p < 0.001). Compared with VEH, both (±)PRO20 and 40 mg/kg (±)propranolol [(±)PRO40] suppressed total distance traveled. (±)PRO20 suppressed distance traveled for the first 30 min but subsequently increased distance traveled between 46 and 60 min (p < 0.05). (±)PRO40 reduced distance traveled for 45 min (p < 0.05). Movement number (Fig. 3B) was also affected by treatment (F3,39 = 5.91, p < 0.01) and time (F5,65 = 25.76, p < 0.001), and there was an interaction (F15,195 = 5.03, p < 0.001). Collapsing across time, only (±)PRO40 reduced movement number, with significant reductions in the first 30 min (p < 0.05). (±)PRO20 had no overall effect on movement number and actually increased movement at 46 to 60 and 76 to 90 min after injection (p < 0.05). Likewise, a main effect of treatment (F3,39 = 12.75, p < 0.001) and time (F5,65 = 14.15, p < 0.001) and a significant treatment by time interaction (F15,195 = 5.19, p < 0.001) were found for vertical activity. (±)PRO5 reduced vertical activity compared with VEH from 16 to 30 min, but not overall (p > 0.05). Both (±)PRO20 and (±)PRO40 decreased total vertical activity compared with VEH. (±)PRO20 reduced vertical activity compared with VEH for 30 min, whereas (±)PRO40 reduced vertical activity for 45 min (p < 0.05).
Fig. 3.
High doses of (±)propranolol reduce motor activity. Unilaterally lesioned rats (n = 14) were injected with (±)propranolol (VEH, 5, 20, or 40 mg/kg) and immediately placed in motion chambers for analysis of spontaneous motor activity over the next 90 min. Symbols represent mean for 15-min time points ± S.E.M. for distance traveled (A), movement number (B), and vertical activity (C). Data were analyzed using a 4 × 6 (treatment by time) repeated-measures ANOVA and contrasts of vehicle to treatments. *, p < 0.05 VEH versus (±)PRO5; †, p < 0.05, ††, p < 0.01 VEH versus (±)PRO20; +, p < 0.05, ++, p < 0.01 VEH versus (±)PRO40.
Experiment 3
The (−)Propranolol Enantiomer Conveyed Antidyskinetic Properties.
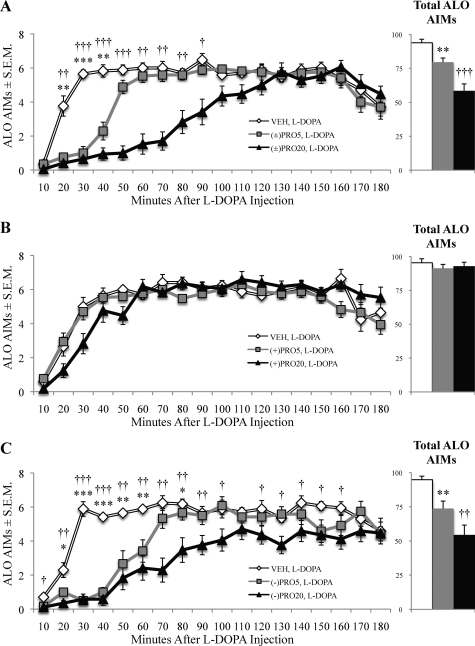
To determine whether the efficacy of (±)propranolol is due to βAR binding, the enantiomers of (±)propranolol were examined individually, given that (−)propranolol has a 100× greater affinity for these receptors than (+)propranolol (Mehvar and Brocks, 2001). Friedman tests showed an ALO AIMs reduction by (±)propranolol (χ2 = 22.91, p < 0.001; Fig. 4A). (+)Propranolol did not reduce ALO AIMs [χ2 = 0.82, p > 0.05; Fig. 4B, (+)PRO], but (−)propranolol was effective [χ2 = 23.06, p < 0.001; Fig. 4C, (−)PRO]. Mann-Whitney post hoc tests demonstrated that anti-LID efficacy began at 20 min after l-DOPA and lasted until 50 min for (±)PRO5 and until 90 min for (±)PRO20 (p < 0.05). (−)PRO5 reduced LID at 20 to 60 and 80 min after l-DOPA, whereas (−)PRO20 reduced LID from time points 20 to 100 and 120 to 160 min (p < 0.05).
Fig. 4.
Propranolol dose-dependently and stereospecifically reduced the expression of ALO AIMs induced by l-DOPA. l-DOPA-primed hemiparkinsonian rats (n = 17) were given injections of racemic (±), levo (−), and dextro (+) propranolol (VEH, 5 or 20 mg/kg) 5 min before l-DOPA (12 mg/kg) in a within-subjects design. ALO AIMs were subsequently monitored. Symbols on main chart represent the treatment group time point mean ALO AIMs ± S.E.M. for (±)propranolol (A), (+)propranolol (B), and (−)propranolol (C). Bars on inlaid figure denote total ALO AIMs ± S.E.M. of treatment groups. Data were analyzed using the Friedman test with Wilcoxon signed-rank post hoc tests. *, p < 0.05, **, p < 0.01, ***, p < 0.001 VEH versus PRO5; †, p < 0.05, ††, p < 0.01, †††, p < 0.001 VEH versus PRO20.
Experiment 4
Dopamine Depletion and Cannula Placements.
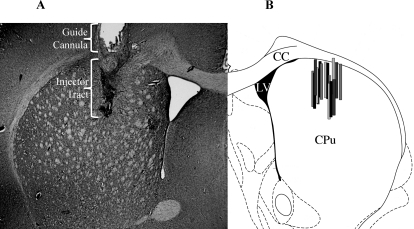
HPLC-ED analysis of posterior striatal tissue revealed that average DA depletion in the lesioned striata compared with intact striata was 97%. Cannula placements were verified using cresyl violet staining (Fig. 5A), and injection sites are represented schematically in Fig. 5B.
Fig. 5.
The cannula placements from rats in experiment 4 were examined post mortem. A, cresyl violet staining of a successful cannula placement in dorsal striatum. Injector tract is darkly stained relative to striatum. B, schematic representation of injector tracts. Modified from a coronal slice at +0.48 mm relative to bregma from Paxinos and Watson (1998). CC, corpus collosum; LV, lateral ventricle; CPu, caudate putamen.
Striatal Microinfusion of (±)Propranolol Reduced Established ALO AIMs.
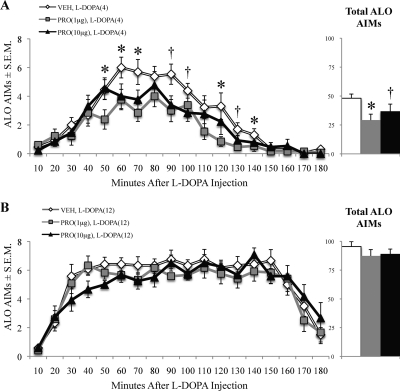
Microinjections targeting the dorsal striatum were used to determine the role of this structure in the antidyskinetic efficacy of (±)propranolol. (±)Propranolol was effective in reducing ALO AIMs induced by 4 mg/kg l-DOPA (χ2 = 7.54, p < 0.05; Fig. 6A), but not those induced by 12 mg/kg l-DOPA (χ2 = 4.67, p > 0.05; Fig. 6B). Post hoc tests run on rats receiving 4 mg/kg indicated that 1 μg of (±)propranolol [(±)PRO1] reduced ALO AIMs from at 50, 60, 70, 120, and 140 min after l-DOPA, whereas 10 μg of (±)propranolol [(±)PRO10] reduced ALO AIMs compared with VEH at the 90-, 100-, and 130-min time points (p < 0.05).
Fig. 6.
Intrastriatal microinfusion of (±)propranolol reduced ALO AIMs induced by low doses of l-DOPA. Unilaterally lesioned rats (n = 12–13) primed with l-DOPA (12 mg/kg) received microinfusions of (±)propranolol (VEH, 1 or 10 μg) into the dorsal striatum. Rats were subsequently injected with l-DOPA and monitored for ALO AIMs. Symbols on graph represent treatment group time point mean of ALO AIMs ± S.E.M. for 4 mg/kg l-DOPA (A) or 12 mg/kg l-DOPA (B). Bars on inlaid figure represent total ALO AIMs ± S.E.M. of treatment groups. Data were analyzed using the Friedman test and Wilcoxon signed-rank post hoc tests. *, p < 0.05 VEH versus (±)PRO1 μg; †, p < 0.05 VEH versus (±)PRO10 μg.
Discussion
Previous preclinical and clinical research has indicated that (±)propranolol reduced the expression of established LID (Carpentier et al., 1996; Buck and Ferger, 2010). In the current investigation, we expand upon these findings, reporting that behaviorally (±)propranolol also reduced the development of LID without impairing motor ability. Pharmacologically, the (−) optical isomer is the only enantiomer that had antidyskinetic efficacy, implicating βAR-specific effects. Neuroanatomically, at least some of therapeutic effects of (±)propranolol occurred in the dorsal striatum. Collectively, these findings suggest that βAR antagonists may be useful l-DOPA adjuncts in PD and may expand the therapeutic window of l-DOPA to allow greater relief of primary PD symptoms while reducing LID.
An ideal l-DOPA adjunct would not only reduce established LID but would also be capable of preventing the development of LID. Therefore, we examined chronic (±)propranolol cotreatment in rats with no prior exposure to l-DOPA. Daily l-DOPA treatment led to a gradual increase in the severity of ALO AIMs, whereas pretreatment with 5 and 20 mg/kg (±)propranolol reduced these dyskinesias by an average of 37 and 70%, respectively, across test days (Fig. 1A). It is noteworthy that both doses of (±)propranolol significantly lowered ALO AIMs on the final test day. Although previous studies have suggested acute LID suppression by (±)propranolol (Dekundy et al., 2007; Buck and Ferger, 2010) and Carpentier et al. (1996) reported long-term antidyskinetic efficacy, this is the first study to suggest a pronounced anti-LID prophylaxis due to chronic βAR blockade.
Coincident with the ALO AIMs investigation, we examined whether (±)propranolol was reducing LID via a general reduction in motor performance, because such a drug would be of limited clinical relevance. Throughout chronic l-DOPA treatment, rats were monitored for motor performance using the FAS test, because DA-lesioned rats exhibit profound stepping deficits that improve with l-DOPA (Chang et al., 1999; Winkler et al., 2002). At baseline, lesioned paw stepping averaged 18% intact paw stepping (Fig. 1B). Although l-DOPA initially improved stepping, it failed to provide a significant improvement on the last day of testing. Meanwhile, pretreatment with (±)PRO20 improved stepping on all test days relative to baseline, with rats performing at more than 70% intact on the last day of testing. Previous studies on the motor effects of (±)propranolol have yielded equivocal results. Dekundy et al. (2007) used the same drug doses as the present investigators and found that (±)propranolol did not affect motor performance in a rotorod test. However, Gomez-Mancilla and Bedard (1993) found that 10 mg/kg (±)propranolol diminished the length of l-DOPA efficacy in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated macaques.
Therefore, to more fully characterize the motor effects of (±)propranolol, we used a second test of motor behavior without l-DOPA. In locomotor chambers, 5 mg/kg had no effect on behavior, whereas 20 mg/kg (±)propranolol reduced some types of motor activity, and 40 mg/kg reduced scores on all metrics of mobility (Fig. 3). A similar study in rats found no significant reduction in motor behavior with either 10 or 20 mg/kg (±)propranolol (Buck and Ferger, 2010). Collectively, these findings indicate that a range of doses of (±)propranolol can reduce LID without suppressing general motor activity or diminishing the anti-PD effects of DA replacement.
Several adrenergic receptor compounds have been investigated for potential anti-LID properties, but most target α-adrenoceptors (Colosimo and Craus, 2003; Brotchie, 2005). Far fewer studies have investigated βAR drugs despite consistent beneficial effects (Carpentier et al., 1996; Dekundy et al., 2007). However, the specificity of these effects for βARs remained untested before the present study. Data from Fig. 4 show that (±) and (−)propranolol reduced ALO AIMs, indicating that anti-LID efficacy is mediated through βAR blockade. Although both enantiomers have membrane effects, including sodium channel blockade (Matthews and Baker, 1982), (−)propranolol has 100× greater affinity for β1/2AR than the (+)enantiomer (Mehvar and Brocks, 2001).
Qualitatively, 20 mg/kg (±)propranolol lowered ALO AIMs for 90 min (Fig. 4A), whereas 20 mg/kg (−)propranolol was effective for almost the entire 180-min rating period (Fig. 4C). (+)Propranolol showed no significant reduction in ALO AIMs, even at 20 mg/kg, but a trend was observed for 50 min after l-DOPA injection (Fig. 4B). This time interval coincided with a reduction in motion chamber activity by 20 mg/kg (±)propranolol (Fig. 3, A and C), which may have accounted for the observed ALO AIMs reduction.
Because (−)propranolol binds to a multitude of receptors, off-target effects are possible but unlikely. For example, (−)propranolol binds to the β3AR but has a 100× lower affinity for this receptor as that for β1/2AR (Mehvar and Brocks, 2001) and β3AR has not been reported in the striatum. (−)Propranolol also has affinity for multiple 5-HT1 receptors, but because it acts as an antagonist (Middlemiss, 1984), the 5-HT binding properties alone would actually be expected to increase dyskinesia (Bishop et al., 2009; Eskow et al., 2009). The results of this study strongly suggest that (±)propranolol reduces LID through β1/2AR antagonism, although future studies should test this theory directly by using antagonists with greater receptor specificity.
The β1/2ARs are widely distributed throughout the central nervous system but are found in particularly high concentrations in the striatum (Rainbow et al., 1984). Ample evidence exists showing that βAR activity modulates DA release in the striatum, suggesting a presynaptic mechanism for (±)propranolol in LID (Reisine et al., 1982; Goshima et al., 1991). Recent research has suggested an additional postsynaptic mechanism; antagonism of β1AR was shown to reduce downstream phosphorylation of dopamine- and cyclic-AMP-regulated phosphoprotein of 32 kDa (DARPP-32) at Thr34 (Hara et al., 2010), which is clinically important because high levels of pThr34-DARPP-32 are implicated in the expression of LID (Santini et al., 2007; Bateup et al., 2010).
The striatum was hypothesized to be the anatomical locus of the therapeutic action of (±)propranolol, given the evidence that LID is caused by aberrant striatal DA signaling (Winkler et al., 2002; Cenci, 2007) and that (±)propranolol directly effects striatal DA signaling (Reisine et al., 1982; Goshima et al., 1991). Microinjection of (±)propranolol (1 or 10 μg) into the dorsal striatum reduced ALO AIMs when l-DOPA was given at a therapeutic dose (4 mg/kg; Fig. 6A) but not when given at a higher dose (12 mg/kg; Fig. 6B). The reduction in ALO AIMs did not occur until 50 min after 4 mg/kg l-DOPA injection, which corresponded to peak dyskinesia, so it is possible that intrastriatal (±)propranolol is only capable of blunting maximal DA spikes. Striatal (±)propranolol microinjection had no significant effect on ALO AIMs with 12 mg/kg l-DOPA, even though systemic (±)propranolol did. It is unlikely that increasing the dose of (±)propranolol would have provided efficacy with 12 mg/kg l-DOPA because we did not observe a dose response between 1 and 10 μg with 4 mg/kg l-DOPA. Thus, results in Fig. 6 simultaneously confirm that the dorsal striatum is a key site of anti-LID action for (±)propranolol and suggest the existence of additional loci of action, such as the ventral striatum, globus pallidus, and substantia nigra pars reticulata (Rainbow et al., 1984).
Regardless of the site(s) of anti-LID action of (±)propranolol, the drug seems to be affecting striatal signaling since gene expression changes were observed in striatal mRNA following (±)propranolol adjunct therapy (Fig. 2). PPE mRNA expression, an index of D2-mediated indirect pathway activity (Gerfen et al., 1990), was increased by l-DOPA in the lesioned hemisphere relative to the intact hemisphere (Fig. 2A). It is noteworthy that pretreatment with (±)PRO5 prevented this increase. It is surprising that we did not observe a significant change in PPE or PPD expression among (±)PRO20-treated rats on the lesioned side compared with l-DOPA alone. The expression of PPD and PPT (Fig. 2, B and C) was also measured because they are markers of D1-mediated direct pathway activity (Gerfen et al., 1990). In corroboration with previous research, l-DOPA was found to preferentially increase PPD expression in the lesioned striata (Cenci et al., 1998). Figure 2B highlights the novel finding that this increase was blocked with (±)PRO5 pretreatment. We confirm previous reports of a lesion-induced decrease in PPT mRNA (Cenci et al., 1998); however, treatment group did not effect PPT expression.
The use of (±)propranolol may be warranted clinically as it is currently U.S. Food and Drug Administration-approved for a variety of conditions, including essential tremor, but potential side effects should be considered. For example, PD is associated with a reduction in sympathetic nervous system activity, and patients often display basal hypotension and bradycardia (Oka et al., 2007). It is feasible that (±)propranolol might exacerbate these symptoms. However, Carpentier et al. (1996) administered (±)propranolol to PD patients for 5 to 6 weeks and did not observe any side effects that precluded patients from taking the drug. Systolic blood pressure and heart rate were unaffected by the maximal dose tested (60 mg/day), but this dose was able to reduce LID scores by an average of 40%.
The present data suggest that there is a dose range of (±)propranolol, which will reduce dyskinesia without impairing motor activity or exacerbating PD symptoms. Mechanistically, we provide evidence that the dorsal striatum is one site of action for (±)propranolol in the reduction of LID. We show that aberrant striatal signaling associated with dyskinesia is normalized with doses of (±)propranolol that have antidyskinetic efficacy. When combined with previous data in humans and in animal models, the present research provides convergent preclinical evidence for the efficacy of βAR blockade for the treatment of LID.
Acknowledgments
We thank Dr. Terrence Deak for providing expertise with RT-PCR research design and analysis. Thomas Button and Jess George analyzed HPLC-ED data. Margaret Surrena, Melanie Salamon, Evan Feinberg, Adam Goldenberg, Hannah Mitchell, Yuchen Liu, and Sando O. Dickinson helped with behavioral testing. Molly M. Deak, Hollin Buck, Cara Hueston, and Nancy Monteith assisted with RT-PCR analysis. Drs. Lisa M. Savage and Ryan P. Vetreno aided with histology.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS059600]; and the Center for Development and Behavioral Neuroscience at Binghamton University.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.179416.
- DA
- dopamine
- PD
- Parkinson's disease
- l-DOPA
- l-3,4-dihydroxyphenylalanine
- LID
- l-DOPA-induced dyskinesia
- 5-HT
- serotonin
- βAR
- β-adrenergic receptor
- AIMs
- abnormal involuntary movements
- PPD
- preprodynorphin
- PPE
- preproenkephalin
- PPT
- preprotachykinin
- 6-OHDA
- 6-hydroxydopamine
- MFB
- medial forebrain bundle
- ALO
- axial limb and orolingual
- FAS
- forepaw adjusting steps
- VEH
- vehicle
- RT-PCR
- reverse transcription-polymerase chain reaction
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- HPLC-ED
- high-performance liquid chromatography with electrochemical detection
- PRO(1μg)
- 1 μg (±)propranolol
- (±)PRO5
- 5 mg/kg (±)propranolol
- (±)PRO20
- 20 mg/kg (±)propranolol
- (±)PRO40
- 40 mg/kg (±)propranolol
- PRO(10μg)
- 10 μg (±)propranolol
- DARPP-32
- dopamine- and cAMP-regulated phosphoprotein of 32 kDa.
Authorship Contributions
Participated in research design: Lindenbach, Ostock, Eskow Jaunarajs, Dupre, Barnum, Bhide, and Bishop.
Conducted experiments: Lindenbach, Ostock, Eskow Jaunarajs, Dupre, Barnum, and Bhide.
Contributed new reagents or analytic tools: Bishop.
Performed data analysis: Lindenbach and Bishop.
Wrote or contributed to the writing of the manuscript: Lindenbach and Bishop.
Provided funding: Bishop.
References
- Ahlskog JE, Muenter MD. (2001) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 16:448–458 [DOI] [PubMed] [Google Scholar]
- Arai A, Tomiyama M, Kannari K, Kimura T, Suzuki C, Watanabe M, Kawarabayashi T, Shen H, Shoji M. (2008) Reuptake of L-DOPA-derived extracellular DA in the striatum of a rodent model of Parkinson's disease via norepinephrine transporter. Synapse 62:632–635 [DOI] [PubMed] [Google Scholar]
- Barnum CJ, Eskow KL, Dupre K, Blandino P, Jr., Deak T, Bishop C. (2008) Exogenous corticosterone reduces L-DOPA-induced dyskinesia in the hemi-parkinsonian rat: role for interleukin-1beta. Neuroscience 156:30–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup HS, Santini E, Shen W, Birnbaum S, Valjent E, Surmeier DJ, Fisone G, Nestler EJ, Greengard P. (2010) Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci USA 107:14845–14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchetti G, Elghozi JL, Gomeni R, Meyer P, Morselli PL. (1980) Kinetics of distribution of di-propranolol in various organs and discrete brain areas of the rat. J Pharmacol Exp Ther 214:682–687 [PubMed] [Google Scholar]
- Bishop C, Krolewski DM, Eskow KL, Barnum CJ, Dupre KB, Deak T, Walker PD. (2009) Contribution of the striatum to the effects of 5-HT1A receptor stimulation in L-DOPA-treated hemiparkinsonian rats. J Neurosci Res 87:1645–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C, Tessmer JL, Ullrich T, Rice KC, Walker PD. (2004) Serotonin 5-HT2A receptors underlie increased motor behaviors induced in dopamine-depleted rats by intrastriatal 5-HT2A/2C agonism. J Pharmacol Exp Ther 310:687–694 [DOI] [PubMed] [Google Scholar]
- Brotchie JM. (2005) Nondopaminergic mechanisms in levodopa-induced dyskinesia. Mov Disord 20:919–931 [DOI] [PubMed] [Google Scholar]
- Buck K, Voehringer P, Ferger B. (2010) The α2 adrenoceptor antagonist idazoxan alleviates L-DOPA-induced dyskinesia by reduction of striatal dopamine levels: an in vivo microdialysis study in 6-hydroxydopamine-lesioned rats. J Neurochem 112:444–452 [DOI] [PubMed] [Google Scholar]
- Buck K, Ferger B. (2010) The selective α1 adrenoceptor antagonist HEAT reduces L-DOPA-induced dyskinesia in a rat model of Parkinson's disease. Synapse 64:117–126 [DOI] [PubMed] [Google Scholar]
- Carpentier AF, Bonnet AM, Vidailhet M, Agid Y. (1996) Improvement of levodopa-induced dyskinesia by propranolol in Parkinson's disease. Neurology 46:1548–1551 [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Björklund A. (2007) Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain 130:1819–1833 [DOI] [PubMed] [Google Scholar]
- Cenci MA. (2007) Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci 30:236–243 [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Björklund A. (1998) L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci 10:2694–2706 [PubMed] [Google Scholar]
- Cenci MA, Lundblad M. (2007) Ratings of L-DOPA-induced dyskinesia in the unilateral 6-OHDA lesion model of Parkinson's disease in rats and mice. Curr Protoc Neurosci Chapter 9:Unit 9.25 [DOI] [PubMed] [Google Scholar]
- Chang JW, Wachtel SR, Young D, Kang UJ. (1999) Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson's disease: studies on medial forebrain bundle and striatal lesions. Neuroscience 88:617–628 [DOI] [PubMed] [Google Scholar]
- Colosimo C, Craus A. (2003) Noradrenergic drugs for levodopa-induced dyskinesia. Clin Neuropharmacol 26:299–305 [DOI] [PubMed] [Google Scholar]
- Dekundy A, Lundblad M, Danysz W, Cenci MA. (2007) Modulation of L-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav Brain Res 179:76–89 [DOI] [PubMed] [Google Scholar]
- Dupre KB, Eskow KL, Barnum CJ, Bishop C. (2008) Striatal 5-HT1A receptor stimulation reduces D1 receptor-induced dyskinesia and improves movement in the hemiparkinsonian rat. Neuropharmacology 55:1321–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskow KL, Dupre KB, Barnum CJ, Dickinson SO, Park JY, Bishop C. (2009) The role of the dorsal raphe nucleus in the development, expression, and treatment of L-dopa-induced dyskinesia in hemiparkinsonian rats. Synapse 63:610–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskow KL, Gupta V, Alam S, Park JY, Bishop C. (2007) The partial 5-HT(1A) agonist buspirone reduces the expression and development of l-DOPA-induced dyskinesia in rats and improves l-DOPA efficacy. Pharmacol Biochem Behav 87:306–314 [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250:1429–1432 [DOI] [PubMed] [Google Scholar]
- Gomez-Mancilla B, Bédard PJ. (1993) Effect of nondopaminergic drugs on L-dopa-induced dyskinesias in MPTP-treated monkeys. Clin Neuropharmacol 16:418–427 [DOI] [PubMed] [Google Scholar]
- Goshima Y, Misu Y, Arai N, Misugi K. (1991) Nanomolar L-dopa facilitates release of dopamine via presynaptic β-adrenoceptors: comparative studies on the actions in striatal slices from control and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated C57 black mice, an animal model for Parkinson's disease. Jpn J Pharmacol 55:93–100 [DOI] [PubMed] [Google Scholar]
- Hara M, Fukui R, Hieda E, Kuroiwa M, Bateup HS, Kano T, Greengard P, Nishi A. (2010) Role of adrenoceptors in the regulation of dopamine/DARPP-32 signaling in neostriatal neurons. J Neurochem 113:1046–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J. (2008) Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79:368–376 [DOI] [PubMed] [Google Scholar]
- Kilpatrick IC, Jones MW, Phillipson OT. (1986) A semiautomated analysis method for catecholamines, indoleamines, and some prominent metabolites in microdissected regions of the nervous system: an isocratic HPLC technique employing coulometric detection and minimal sample preparation. J Neurochem 46:1865–1876 [DOI] [PubMed] [Google Scholar]
- Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. (2002) Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson's disease. Eur J Neurosci 15:120–132 [DOI] [PubMed] [Google Scholar]
- Matthews JC, Baker JK. (1982) Effects of propranolol and a number of its analogues on sodium channels. Biochem Pharmacol 31:1681–1685 [DOI] [PubMed] [Google Scholar]
- Mehvar R, Brocks DR. (2001) Stereospecific pharmacokinetics and pharmacodynamics of β-adrenergic blockers in humans. J Pharm Pharm Sci 4:185–200 [PubMed] [Google Scholar]
- Middlemiss DN. (1984) Stereoselective blockade at [3H]5-HT binding sites and at the 5-HT autoreceptor by propranolol. Eur J Pharmacol 101:289–293 [DOI] [PubMed] [Google Scholar]
- Oka H, Morita M, Onouchi K, Yoshioka M, Mochio S, Inoue K. (2007) Cardiovascular autonomic dysfunction in dementia with Lewy bodies and Parkinson's disease. J Neurol Sci 254:72–77 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson W. (1998) The Rat Brain in Stereotaxic Coordinates, Academic Press, San Diego, CA [Google Scholar]
- Rainbow TC, Parsons B, Wolfe BB. (1984) Quantitative autoradiography of β1- and β2-adrenergic receptors in rat brain. Proc Natl Acad Sci USA 81:1585–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisine TD, Chesselet MF, Lubetzki C, Chéramy A, Glowinski J. (1982) A role for striatal beta-adrenergic receptors in the regulation of dopamine release. Brain Res 241:123–130 [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Weinshenker D. (2007) Norepinephrine: The redheaded stepchild of Parkinson's disease. Biochem Pharmacol 74:177–190 [DOI] [PubMed] [Google Scholar]
- Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA, Hervé D, Greengard P, Fisone G. (2007) Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci 27:6995–7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savola JM, Hill M, Engstrom M, Merivuori H, Wurster S, McGuire SG, Fox SH, Crossman AR, Brotchie JM. (2003) Fipamezole (JP-1730) is a potent α2 adrenergic receptor antagonist that reduces levodopa-induced dyskinesia in the MPTP-lesioned primate model of Parkinson's disease. Mov Disord 18:872–883 [DOI] [PubMed] [Google Scholar]
- Waeber C, Rigo M, Chinaglia G, Probst A, Palacios JM. (1991) β-Adrenergic receptor subtypes in the basal ganglia of patients with Huntington's chorea and Parkinson's disease. Synapse 8:270–280 [DOI] [PubMed] [Google Scholar]
- Winkler C, Kirik D, Björklund A, Cenci MA. (2002) L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of Parkinson's disease: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis 10:165–186 [DOI] [PubMed] [Google Scholar]