Abstract
The vascular form of Ehlers-Danlos syndrome (vEDS), a rare disease with grave complications resulting from rupture of major arteries, is caused by mutations of collagen type III [α1 chain of collagen type III (COL3A1)]. The only, recently proven, preventive strategy consists of the reduction of arterial wall stress by β-adrenergic blockers. The heterozygous (HT) Col3a1 knockout mouse has reduced expression of collagen III and recapitulates features of a mild presentation of the disease. The objective of this study was to determine whether changing the balance between synthesis and degradation of collagen by chronic treatment with doxycycline, a nonspecific matrix metalloproteinase (MMP) inhibitor, could prevent the development of vascular pathology in HT mice. After 3 months of treatment with doxycycline or placebo, 9-month-old HT or wild-type (WT) mice were subjected to surgical stressing of the aorta. A 3-fold increase in stress-induced aortic lesions found in untreated HT mice 1 week after intervention (cumulative score 4.5 ± 0.87 versus 1.3 ± 0.34 in WT, p < 0.001) was fully prevented in the doxycycline-treated group (1.1 ± 0.56, p < 0.001). Untreated HT mice showed increased MMP-9 activity in the carotid artery and decreased collagen content in the aorta; however, in doxycycline-treated animals there was normalization to the levels observed in WT mice. Doxycycline treatment inhibits the activity of tissue MMP and attenuates the decrease in the collagen content in aortas of mice haploinsufficient for collagen III, as well as prevents the development of stress-induced vessel pathology. The results suggest that doxycycline merits clinical testing as a treatment for vEDS.
Introduction
The vascular form of Ehlers-Danlos syndrome (vEDS) (Online Mendelian Inheritance in Man, OMIM 130050) is a rare autosomal dominant disorder (incidence 1:100,000) (Germain, 2007) caused by mutations in the α1 chain of type III collagen (COL3A1) (Pope et al., 1975; Smith et al., 1997; Pyeritz, 2000; Germain, 2007). Type III collagen is a homotrimeric fibrillar collagen found abundantly in the wall of arteries, the gastrointestinal tract, the uterus, and other tissues. The quantitative or qualitative deficit of structurally normal collagen III in vEDS and corresponding changes in the wall structure are responsible for major complications observed in afflicted individuals: arterial, bowel, and uterine ruptures. As a result of these dramatic complications, life expectancy in vEDS is shortened to a mean of <50 years (Pepin et al., 2000; Watanabe et al., 2007). Until recently, there was no evidence-based treatment or preventive strategy available (Watanabe and Shimada, 2008). However, in the just-completed first multicenter randomized trial, almost 4 years (47 months) of treatment with celiprolol, a long-acting β1 antagonist with partial β2 agonist properties used for treatment of hypertension, decreased the incidence of arterial ruptures in patients with a clinical diagnosis of vEDS (Ong et al., 2010). Celiprolol was uptitrated every 6 months by steps of 100 mg to a maximum of 400 mg twice daily.
More than 150 mutations in the COL3A1 gene leading to synthesis of an abnormal type III procollagen protein have been identified (The Human Gene Mutation Database, Institute of Medical Genetics, Cardiff University, Cardiff, Wales; http://www.hgmd.cf.ac.uk/ac/index.php). Most of the mutations are single-nucleotide substitutions of the canonical glycine residues in the triple-helical domain of the proα1(III) chain, resulting in a regular quantity of abnormal collagen III; however haploinsufficiency of collagen III (reduced quantity of normal collagen III) also has been reported in patients with vEDS (Schwarze et al., 2001; Khalique et al., 2009).
We have shown that haploinsufficiency for Col3a1 in mice recapitulates mild presentation of vEDS in humans and thus can serve as an experimental model in the search for effective treatments (Cooper et al., 2010).
It has been reported that in the third stage of aneurysm development rapid expansion and increased risk of rupture are associated with accelerated degradation of collagen (Thompson and Baxter, 1999). Doxycycline, a tetracycline antibiotic and broad-spectrum metalloproteinase (MMP) inhibitor, has been successfully tested in pilot clinical trials for the treatment of abdominal aortic aneurysms (Curci et al., 2000; Mosorin et al., 2001; Baxter et al., 2002; Lindeman et al., 2009). Doxycycline, given in a subantimicrobal dose, is also the only MMP inhibitor approved by the U.S. Food and Drug Administration. It is currently used for the treatment of periodontal disease (Wennström et al., 2001) and rosacea (Del Rosso et al., 2007). We hypothesized that MMP inhibition in the mouse experimental model of vEDS would shift the balance between collagen degradation and synthesis in the vascular wall and protect against vascular damage.
Materials and Methods
See Supplemental Methods for description of quantification of collagen content in skin and description of the colon's biomechanical properties detection.
Subjects.
Heterozygous Col3a1-deficient mice [strain C.129S4(B6)-Col3a1tm1Jae/J] (Liu et al., 1997) were rederived (The Jackson Laboratory, Bar Harbor, ME) and bred in the vivarium of the National Institute on Aging. The Col3a1 genotype was determined by polymerase chain reaction (5′-CTTCTCACCCTTCTTCATCCC-3′, 5′-AGCCTGTTCAAATCGGTACC-3′ and neo 5′-GCTATCAGGACATAGCGTTGG-3′) after weaning, and genotypes were reconfirmed at the end of the experiments. Animals were housed and studied in conformance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, with institutional Animal Care and Use Committee approval. Mice were maintained on ad libitum food (NIH-07 mouse/rat diet; National Institutes of Health, Bethesda, MD) with permanent access to filtered water.
Experimental Protocol.
Two groups of 6-month-old female HT mice were treated for 3 months with doxycycline (compound ID 5281011). Treatment was provided with food containing 200 or 800 mg/kg of doxycycline (Dox Diet pellets; BioServ, Frenchtown, NJ). Because preliminary measured food intake of these mice was averaged at 3.5 g/day and the average body weight of animals was 25 g, the average drug dose for low- and high-dose groups was 25 (Doxy25) or 100 (Doxy100) mg/kg per day, respectively. Untreated WT and HT mice were maintained on a regular diet (NIH-07 mouse/rat diet) and served as controls. After 3 months, under general inhalation anesthesia (2% of isoflurane in oxygen) and aseptic conditions, the abdominal aortas were surgically exposed and stressed by the following technique: the blood flow was stopped by occluding the abdominal aorta against the spinal column with a sterile cotton-tip applicator pressed at the level of the renal arteries. After 30 s a second applicator was pressed at the level of iliac bifurcation and the first applicator was abruptly released, followed by release of the second applicator. As in Weinbrenner et al. (2002), who used a similar procedure for remote preconditioning in rats, the mean arterial pressure had been elevated by approximately 13% immediately after occlusion. The abdominal incision was sutured closed, and mice were returned to home cages. The treatment was continued after the intervention. One week after intervention mice were euthanized by an overdose of isoflurane. Blood was collected, and aortas and segments of colon and skin were harvested.
Tissue Collection.
Blood was collected from the left ventricle. The abdominal aorta was exposed and opened at the bifurcation. A 3% (w/v) agarose solution (SeaPlaque GTG Agarose, low melt; Lonza Inc., Allendale, NJ) diluted in physiological salt solution and colored with Evans blue (Sigma-Aldrich, St. Louis, MO) at 37°C was injected through the left ventricle into the aorta to prevent collapse during tissue processing. The aorta was dissected free from the surrounding connective tissue and pinned onto a wax block before fixation in 10% formalin for 2 days. Cross-sections of the aorta (2 mm in thickness) were placed in 8% agar to create a block with an average of 20 sections of the aorta. The block was stored in 70% ethanol until it was processed and embedded in paraffin (AML Laboratories, Baltimore, MD). Samples of transverse colon were used for the determination of biomechanical properties. Left and right carotid arteries and a piece of the tail and the skin from the back were snap-frozen in liquid nitrogen.
Histological Analysis.
Sections (5 μm) from each block of aortic sections were stained with hematoxylin and eosin and Masson's trichrome. Two veterinary pathologists (T.K.C., H.-J.T.) blinded to genotype independently evaluated the stained sections to count the number of lesions present in aortas and rate the severity of each lesion on a subjective scale of 1 to 4 according to previously reported criteria (Cooper et al., 2010). Because of their mild nature, as well as a high frequency in both sexes and genotypes, grade 1 lesions were excluded from statistical analysis. The sum of the scores of lesions ≥ grade 2 was added for each animal to produce a cumulative score.
Collagen Detection by Picro-Sirius Red Staining.
To examine collagen content in the vessel wall, 5-μm sections of abdominal aorta were stained with picro-sirius red. Digital images of stained sections were obtained from light microscopy using polarized filters and analyzed using a digital imaging analysis system (MCID; InterFocus Imaging Ltd., Cambridge, UK). The total collagen content in aortic wall as well as collagen content of tunicae adventitia and media separately were calculated as a percentage of the total area of the wall or its respective components (Seeland et al., 2007). Assessment was performed by a single individual (H.-J.T.) blinded to genotype of the animal.
Detection of MMP Activity.
Extracellular matrix proteins were isolated from the right carotid artery, and approximately 5 mg of frozen skin was taken from each animal. Proteins were extracted with 40 μl or 20-fold volume RIPA buffer [50 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1% (v/v) NP-40, 1 mM EDTA, 0.25% Na-deoxycholat, protease inhibitor mix M (Serva, Heidelberg, Germany], overnight at 4°C. Protein content was determined with the BCA method (BCA protein assay kit; Thermo Fisher Scientific, Waltham, MA).
Gelatinase activity was measured as described previously (Briest et al., 2001). Gelatin (0.1% (w/v) (Serva) was added to standard Laemmli acrylamide polymerization mixture. Tissue extract was mixed 1:2 with sample buffer [250 mM Tris-Cl pH 6.8, 10% (w/v) SDS, 20% (v/v) glycerol, 0.005% (w/v) bromphenol blue]. Serum was diluted 1:10 with electrophoresis buffer (2.5 mM Tris, 20 mM glycine, 0.005% SDS) and mixed 1:2 with sample buffer. Twenty microliters were loaded after 10-min incubation at room temperature without boiling. After electrophoresis at 90 V, the gels were soaked in 2.5% (w/v) Triton X-100, incubated 2 to 3 days at 37°C in gelatin digestion buffer [50 mM Tris-Cl, pH 8.0, 8 mM CaCl2, 10 mM ZnSO4, 0.02% (w/v) NaN3], stained in 0.05% Coomassie blue R-250 (Serva) in acetic acid/methanol/water (1:4.5:4.5 by volume), destained in 10% acetic acid and 5% methanol, and scanned for lysis band intensity. The lysis band intensity is proportional to gelatinase activity and was quantified densitometrically by using One-Dimensional Scan software (BD Biosciences Bioimaging, Rockville, MD). The result, a number between 0.07 and 3.75, was normalized to the protein content by dividing the densitometry result with the relative optical density from the BCA protein assay kit result. The result was used for the analysis as the arbitrary unit. For the total MMP activity results of lysis bands of pro-MMP-9, active MMP-9, pro-MMP-2, and active MMP-2 were added. A protein size marker (Page Ruler Plus; Fermentas, St. Leon-Rot, Germany) was used to determine the correct size.
Statistical Analysis.
All data are presented as mean ± S.E.M. The actual number of animals per group might vary in different measurements because of technical reasons; they are presented in the figures. A multiple-sample comparison (analysis of variance and the multiple range test as post hoc test using the criterion of the least significant differences) was applied to test the differences between groups. Statistical significance was accepted at p < 0.05.
Results
Doxycycline Treatment Normalized MMP Activity in Carotid Arteries.
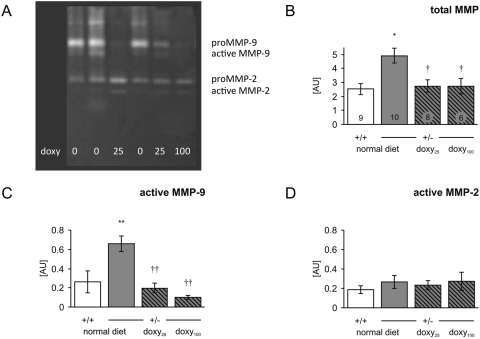
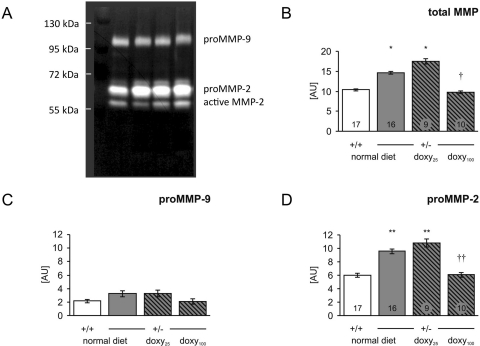
Gelatinases MMP-2 and MMP-9 were detected in extracts from carotid arteries as both an inactive proform and an active form (Fig. 1A). All four bands were summarized as total MMP, which was significantly elevated in untreated HT mice compared with WT, and normalized by doxycycline treatment in HT mice (Fig. 1B). Proforms of both MMPs (data not shown) and active MMP-2 (Fig. 1D) were unchanged in either untreated HT mice or HT mice treated by doxycycline relative to WT. However, there was a significant increase in active MMP-9 in untreated HT mice, which was reduced in a dose-dependent manner by doxycycline (Fig. 1C). The MMP-9 activity in HT mice after doxycycline treatment was statistically not different from WT mice with normal diet.
Fig. 1.
MMP activity in the carotid artery of wild-type (+/+) and heterozygous mice (+/−) was analyzed by zymography. A, representative zymogram of extracts from heterozygous mouse carotid artery fed with normal (doxy 0), doxy25, and doxy100 diet. B, all four bands were summarized as total MMP. C and D, active MMP-9 (C) and active MMP-2 (D) were analyzed by densitometry. The result was normalized to the protein content. Data are means ± S.E.M. *, p < 0.05 versus +/+ normal diet. †, p < 0.05 versus +/− normal diet. **, p < 0.001 versus +/+ normal diet. ††, p < 0.001 versus ± normal diet. Numbers of animals in each group are indicated in bars in B.
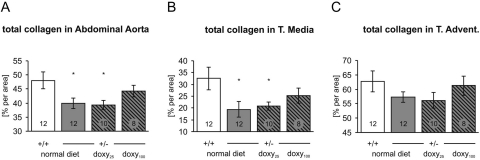
Doxycycline Treatment Attenuates the Reduction of Collagen in the Aorta.
The collagen content was measured by picro-sirius staining in the abdominal aorta, because statistically significant differences in collagen content between WT and HT mice had been detected previously, specifically in that segment of aorta (Cooper et al., 2010). In agreement with our previous findings, there was significantly less total collagen detected in untreated HT mice than in WT mice (Fig. 2A). In the Doxy25 group the collagen content remained significantly reduced compared with WT mice. However, in the Doxy100 group the collagen content increased and became similar to the WT mice. The change in the total collagen content of the aorta was a result of its reduction in the tunica media of untreated and Doxy25-treated mice (Fig. 2B), whereas the collagen content in the tunica adventitia did not differ significantly between groups (Fig. 2C). The differences in collagen content were not accompanied by changes in luminal radius or thickness of the tunica media (Supplemental Fig. 1). The detection of collagen type I in the skin revealed no significant differences between wild-type and untreated heterozygous mice with normal diet. Doxycycline treatment also had no effect on skin collagen (Supplemental Fig. 2).
Fig. 2.
A, heterozygous mice (+/−) had significantly less collagen per unit area than wild-type mice in the abdominal aorta as determined by picro-sirius red staining. This reduction was partially ameliorated in the group given high doses of doxycycline. B and C, the reduction was seen in the tunica media (B) but not in the tunica adventitia (C). Data are means ± S.E.M. *, p < 0.05 versus +/+ normal diet. Numbers of animals in each group are indicated in the bars.
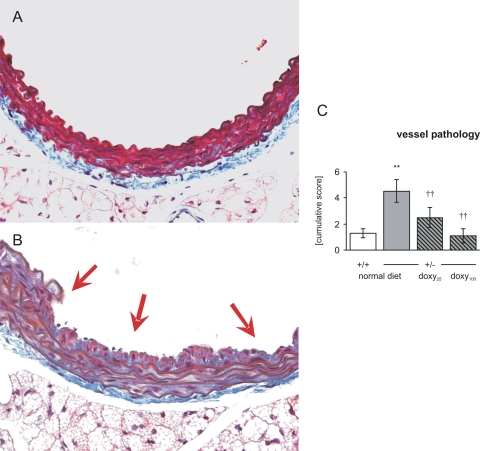
Doxycycline Treatment Prevented Stress-Induced Vessel Pathology.
One week after surgical intervention, histological evaluation of aortas revealed 3-fold more vessel pathology in untreated HT than in WT mice (p < 0.001; Fig. 3). Doxycycline treatment prevented this increase in a dose-dependent fashion, significantly reducing cumulative score in doxycycline-treated HT groups compared with untreated HT mice (p < 0.001) and bringing the cumulative score to the level of WT mice (Fig. 3). There were no differences in the cumulative scores between WT and HT mice treated with doxycycline.
Fig. 3.
Cumulative lesion score (lesions ≥ grade 2) in each group demonstrates an increase in untreated heterozygotes and a dose-dependent decrease of vessel pathology in the doxycycline-treated groups. A, section of abdominal aorta from a wild-type control animal is shown. Masson's trichrome was used. B, a representative grade 4 lesion (indicated by arrows) with a large defect in the internal elastic lamina and significant subintimal spindle cell proliferation with deposition of collagen is shown. C, data are means (WT = 17, HT = 19, HT-Dox25 = 10, HT-Dox100 = 11) ± S.E.M. **, p < 0.001 versus +/+ normal diet. ††, p < 0.001 versus +/− normal diet.
Colon Biomechanics.
The biomechanical properties of the colon were not changed by doxycycline treatment (data not shown). The stiffness and the maximal holding pressure of the colon from heterozygous animals with and without doxycycline treatment were both reduced in comparison with wild-type mice.
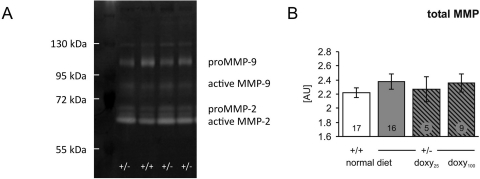
MMP Activity in Blood Serum and Skin.
MMP activity was analyzed in the serum (Fig. 4A). There was a different level of detection of pro-MMPs and active MMPs compared with the carotid arteries extract. There was virtually no detectable proform of MMP-2 in either wild-type or heterozygous mice. Furthermore, there were no significant changes between WT and HT mice, both untreated and treated with doxycycline (Fig. 4B).
Fig. 4.
There was a different pattern in lysis bands in the zymography of serum from heterozygous and wild-type mice. A, a representative zymogram of serum from heterozygous (+/−) and wild-type (+/+) mice is shown. B, all four bands were summarized as total MMP. Numbers of animals in each group are indicated in the bars.
In contrast to the serum, only proforms and no active MMP-9 were detectable in the skin (Fig. 5A). There was remarkably more pro-MMP-2 detectable and some active MMP-2. Similar to the vessel tissue extract, total MMP (Fig. 5B) was significantly elevated in untreated HT mice. This was reduced to the level of WT in the HT group treated with the higher dose of doxycycline. The pattern of total MMP was reflected in the pattern of pro-MMP-2. It was elevated in untreated HT mice and normalized by high-dose doxycycline (Fig. 5D). However, pro-MMP-9 was not affected either by genotype or treatment (Fig. 5C).
Fig. 5.
MMP activities in the skin were elevated in heterozygous mice and normalized to the level of wild-type mice after high-dose doxycycline treatment. A, a representative zymogram of extracts from heterozygote skin is shown. B, all four bands were summarized as total MMP. C and D, proform of MMP-9 (C) and MMP-2 (D) were analyzed by densitometry. The result was normalized to the amount of tissue used (in milligrams). Data are means ± S.E.M. *, p < 0.05 versus +/+ normal diet. †, p < 0.05 versus +/− normal diet. **, p < 0.001 versus +/+ normal diet. ††, p < 0.001 versus +/− normal diet. Numbers of animals in each group are indicated in the bars.
Discussion
Consistent with expectations, we have shown that in the mouse model of collagen III haploinsufficiency chronic MMP inhibition by doxycycline treatment prevented increased MMP activity in the carotid artery and the skin present in untreated HT mice. The normalization of MMP activity was accompanied by partial normalization of collagen content in the tunica media of the abdominal aorta in HT mice. This mild increase in collagen accumulation seems to be sufficient to strengthen the vessel, because the increase in stress-induced vessel pathology in untreated HT mice was prevented by doxycycline treatment. Therefore, on the basis of these results, doxycycline therapy might be considered as a treatment for vEDS, at least of the haploinsufficient type.
The rationale for the only available preventive strategy for vEDS by β1-AR blocker was mainly mechanical, to reduce the arterial wall stress by controlling the rate of increase of pressure over time in the pulse wave (dP/dt) and thus to reduce “wearing and tearing” of arterial wall. The celiprolol, however, used in the successful clinical trial was not a typical β1-AR blocker. It combines β1-AR inhibition with some properties of β2-AR stimulation and was proven beneficial for patients with vEDS without affecting hemodynamic variables (Ong et al., 2010). Heart rate or systolic and diastolic pressure were not decreased, and pulse pressure was even elevated in patients on celiprolol. It was not tested whether celiprolol would be able to reduce a stress response. A possible alternative mechanism of celiprolol's protective effect in vEDS based on stimulation of transforming growth factor β and subsequently stimulation of collagen synthesis has been discussed (Brooke, 2010; Ong et al., 2010). However, this hypothesis has not been proven.
The inhibition of MMPs, the rationale behind this article, targeted the balance of collagen homeostasis on the degradation side. Inhibition of MMPs by the broad-spectrum MMP inhibitor marimastat had been suggested as a potential therapy for vEDS (Sastry, 2002). There have been successful clinical trials testing doxycycline as a MMP inhibitor in patients with abdominal aortic aneurysm (Curci et al., 2000; Mosorin et al., 2001; Baxter et al., 2002; Lindeman et al., 2009). One week of doxycycline treatment effectively suppressed MMP activity in the wall of the aortic aneurysm (Curci et al., 2000); however, its actual clinical effect was not evaluated. In contrast to our results, a reduction of MMP-2 activity associated with doxycycline treatment was reported, whereas we observed a reduction of MMP-9 activity (Fig. 1C). The reason for this discrepancy might lay with the duration of doxycycline treatment, because another study showed a decrease in MMP-9 protein expression after 2 weeks of doxycycline treatment (Lindeman et al., 2009). Longer, 3-month therapy with doxycycline prevented the growth of the abdominal aortic aneurysm as measured in testing 12 and 18 months after treatment (Mosorin et al., 2001). However, at 6-month follow-up testing, no detectable effect was revealed. Side effects of long-term treatment with 150 mg/day doxycycline in people were generally low: 8% incidence of cutaneous photosensitivity, 3% tooth discoloration, and 3% yeast infection (Baxter et al., 2002). Even this low incidence of complications probably could be avoided when doxycycline is used in subantimicrobial doses, which also effectively reduce MMP activity (Brown et al., 2004). Doxycycline is the only U.S. Food and Drug Administration-approved MMP inhibitor for the treatment of periodontal disease and rosacea. Periodontal disease is treated with 20 mg once-daily doxycycline (Periostat). Rosacea is treated with 30 mg of immediate release and 10 mg of delayed release beads of doxycycline (Oracea). Some studies using either 20 mg twice daily (Bikowski, 2003; Thiboutot et al., 2009) or 40 mg once daily (Del Rosso et al., 2007) for the treatment of rosacea reported no adverse events. The comparison of 100 versus 40 mg of doxycycline once daily for the treatment of rosacea revealed significant fewer adverse events in the 40-mg group (Del Rosso et al., 2008). However, doxycycline long-term therapy has been used safely in patients with rosacea and acne vulgaris. Minor side effects are varied [summarized in Valentin et al. (2009)]; serious side effects are rare (Sloan and Scheinfeld, 2008). Furthermore, doxycycline therapy with 100 mg twice daily up to 2 years decreased hemorrhagic risk in brain vascular malformations (Frenzel et al., 2008). Doxycyclin therapy with the same dosage reduced aortic neck dilatation 6 months after endovascular aneurysm repair (Hackmann et al., 2008). Clinical trials with other indications of doxycycline are on the way.
On the basis of the presented data, it is difficult to distinguish whether increased total MMP and active MMP-9 in the carotid arteries of heterozygous mice was a result of increased extracellular matrix turnover in heterozygous mice or a response to stressing of the vascular system by physically manipulating the aorta. The former hypothesis is more probable, because of an elevated urine concentration of carboxyl-terminal peptide of collagen I in heterozygous males (Cooper et al., 2010). Increase of total MMP activity in the skin shown in our experiment indicates that changes in collagen turnover are observed not only in the vascular system, but outside of it as well. However, to our knowledge there have been no studies evaluating MMP activity in the serum of patients with vEDS. Nevertheless, elevated MMP-9 activity was found in abdominal aortic aneurysms among the general population (Thompson et al., 1995; Yamashita et al., 2001). This activity was localized to infiltrating adventitial macrophages (Thompson et al., 1995). The absence of active MMP-9 in the skin in wild-type and heterozygous mice (Fig. 5) might be explained by the absence of inflammation and macrophage infiltration. MMP-2 is unique in its ability to degrade both elastin and fibrillar collagen (Kadoglou and Liapis, 2004). Mesenchymal cells (including vascular smooth muscle cells) constitutively express MMP-2, but other cell types such as macrophages and fibroblasts produce small amounts of it. The main cellular sources of MMP-2 are located in the media and adventitia of the aortic wall, facilitating the destruction of elastin and collagen fibers and the disorganization of the aortic wall structure in abdominal aortic aneurysm (Kadoglou and Liapis, 2004). The failure to observe increased MMP-2 activity in our model (Fig. 1C) may be because the actual vascular lesions were not sufficiently severe. The activity was rarely elevated in vivo in abdominal aortic aneurysm in patients (Thompson et al., 1995). It was also increased in vitro in vascular smooth muscle cells isolated from abdominal aortic aneurysm (Crowther et al., 2000).
In our experiment, doxycycline prevented an increase in MMP activity in the vascular wall, as was seen for MMP-9 in carotid artery. These results are compatible with the reported reduction of MMP-9 activity in the serum of patients with abdominal aortic aneurysm treated with doxycycline (Baxter et al., 2002). Moreover, the activity of MMP-9 was reduced only in patients whose MMP-9 levels were elevated before the start of doxycycline treatment. It is noteworthy that the reported lack of doxycycline effect on normal MMP levels was in concordance with our observation on skin MMP activity. The normal levels of MMP activity in the skin of HT mice were not affected by doxycycline treatment, and thus accumulation of the collagen in the skin was not induced. Furthermore, doxycycline treatment did not alleviate the weakness of heterozygous mouse colon as determined by biomechanical measurements. The outcomes of this measurement support the hypothesis that the effects of doxycycline might be tissue- or age-specific in preventing pathology. For instance, the biomechanical properties of the abdominal aorta were not different between HT and WT mice at 9 months, but they did differ at 21 months of age (Cooper et al., 2010).
In our mouse model the reduction of MMP activity in the vessel was accompanied by reduced MMP concentration in the skin, whereas MMP concentration in serum was not affected. This suggests a possibility to monitor the progression of potential doxycycline treatment of patients with vEDS by skin biopsy, if MMP activity would not be elevated in the serum of patients with vEDS.
Doxycycline prevents the excess of stress-induced vessel pathology in mice haploinsufficient for Col3a1 by inhibiting activity of tissue MMP-9, thus reducing the degradation of the elastic matrix. The results suggest that doxycycline merits clinical testing as a possible treatment for vEDS.
Limitation.
The efficacy of proposed doxycycline therapy was tested in the haploinsufficient mouse model. Therefore, it definitely can be proposed as a potential treatment for only a small subset of patients with vEDS with haploinsufficiency for COL3A1. Whether this treatment can be successful in the rest of patients with vEDS will be tested in an appropriate genetic mouse model yet to be developed. However, doxycycline treatment was successful in another mouse model with a mutation in a protein of the extracellular matrix causing aortic ruptures: a mouse model of Marfan syndrome based on a mutation in fibrilin-1 (Fbn1C1039/+) (Chung et al., 2008; Xiong et al., 2008; Yang et al., 2010).
Supplementary Material
Acknowledgments
We thank Chris Morell (National Institute on Aging) for the statistical calculation of the vessel pathology and Tia Turner and Shannon Marshall (National Institute on Aging) for technical assistance.
This work was supported by the Intramural Research Program of the National Institutes of Health National Institute on Aging. W.B. was partially supported by the research grants of Gerhard Schuler, Department of Cardiology, Heart Center, Leipzig, Germany.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.177782.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- vEDS
- vascular form of Ehlers-Danlos syndrome
- AR
- adrenergic receptor
- BCA
- bicinchoninic acid
- COL3A1
- α1 chain of collagen type III
- HT
- heterozygous
- MMP
- matrix metalloproteinase
- WT
- wild type
- Doxy25 (doxy25)
- doxycycline dose of 25 mg/kg
- Doxy100 (doxy100)
- doxycycline dose of 100 mg/kg
- AU
- arbitrary units.
Authorship Contributions
Participated in research design: Briest, McDonnell, and Talan.
Conducted experiments: Briest, Cooper, Tae, and Krawczyk.
Performed data analysis: Briest, Cooper, Tae, and Krawcyk.
Wrote or contributed to the writing of the manuscript: Briest, Cooper, Tae, and Talan.
References
- Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett JW, Jr, Kent KC, Upchurch GR, Jr, Chaikof EL, Mills JL, Fleckten B, et al. (2002) Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (phase II) multicenter study. J Vasc Surg 36:1–12 [DOI] [PubMed] [Google Scholar]
- Bikowski JB. (2003) Subantimicrobial dose doxycycline for acne and rosacea. Skinmed 2:234–245 [DOI] [PubMed] [Google Scholar]
- Briest W, Hölzl A, Rassler B, Deten A, Leicht M, Baba HA, Zimmer HG. (2001) Cardiac remodeling after long term norepinephrine treatment in rats. Cardiovasc Res 52:265–273 [DOI] [PubMed] [Google Scholar]
- Brooke BS. (2010) Celiprolol therapy for vascular Ehlers-Danlos syndrome. Lancet 376:1443–1444 [DOI] [PubMed] [Google Scholar]
- Brown DL, Desai KK, Vakili BA, Nouneh C, Lee HM, Golub LM. (2004) Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler Thromb Vasc Biol 24:733–738 [DOI] [PubMed] [Google Scholar]
- Chung AW, Yang HH, Radomski MW, van Breemen C. (2008) Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ Res 102:e73–e85 [DOI] [PubMed] [Google Scholar]
- Cooper TK, Zhong Q, Krawczyk M, Tae HJ, Müller GA, Schubert R, Myers LA, Dietz HC, Talan MI, Briest W. (2010) The haploinsufficient Col3a1 mouse is a model for vascular Ehlers-Danlos syndrome. Vet Pathol 47:1028–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther M, Goodall S, Jones JL, Bell PR, Thompson MM. (2000) Increased matrix metalloproteinase 2 expression in vascular smooth muscle cells cultured from abdominal aortic aneurysms. J Vasc Surg 32:575–583 [DOI] [PubMed] [Google Scholar]
- Curci JA, Mao D, Bohner DG, Allen BT, Rubin BG, Reilly JM, Sicard GA, Thompson RW. (2000) Preoperative treatment with doxycycline reduces aortic wall expression and activation of matrix metalloproteinases in patients with abdominal aortic aneurysms. J Vasc Surg 31:325–342 [DOI] [PubMed] [Google Scholar]
- Del Rosso JQ, Schlessinger J, Werschler P. (2008) Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J Drugs Dermatol 7:573–576 [PubMed] [Google Scholar]
- Del Rosso JQ, Webster GF, Jackson M, Rendon M, Rich P, Torok H, Bradshaw M. (2007) Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol 56:791–802 [DOI] [PubMed] [Google Scholar]
- Frenzel T, Lee CZ, Kim H, Quinnine NJ, Hashimoto T, Lawton MT, Guglielmo BJ, McCulloch CE, Young WL. (2008) Feasibility of minocycline and doxycycline use as potential vasculostatic therapy for brain vascular malformations: pilot study of adverse events and tolerance. Cerebrovasc Dis 25:157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain DP. (2007) Ehlers-Danlos syndrome type IV. Orphanet J Rare Dis 2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackmann AE, Rubin BG, Sanchez LA, Geraghty PA, Thompson RW, Curci JA. (2008) A randomized, placebo-controlled trial of doxycycline after endoluminal aneurysm repair. J Vasc Surg 48:519–526; discussion 526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoglou NP, Liapis CD. (2004) Matrix metalloproteinases: contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms. Curr Med Res Opin 20:419–432 [DOI] [PubMed] [Google Scholar]
- Khalique Z, Lyons OT, Clough RE, Bell RE, Reidy JF, Schwarze U, Byers PH, Taylor PR. (2009) Successful endovascular repair of acute type B aortic dissection in undiagnosed Ehlers-Danlos syndrome type IV. Eur J Vasc Endovasc Surg 38:608–609 [DOI] [PubMed] [Google Scholar]
- Lindeman JH, Abdul-Hussien H, van Bockel JH, Wolterbeek R, Kleemann R. (2009) Clinical trial of doxycycline for matrix metalloproteinase-9 inhibition in patients with an abdominal aneurysm: doxycycline selectively depletes aortic wall neutrophils and cytotoxic T cells. Circulation 119:2209–2216 [DOI] [PubMed] [Google Scholar]
- Liu X, Wu H, Byrne M, Krane S, Jaenisch R. (1997) Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci USA 94:1852–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosorin M, Juvonen J, Biancari F, Satta J, Surcel HM, Leinonen M, Saikku P, Juvonen T. (2001) Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J Vasc Surg 34:606–610 [DOI] [PubMed] [Google Scholar]
- Ong KT, Perdu J, De Backer J, Bozec E, Collignon P, Emmerich J, Fauret AL, Fiessinger JN, Germain DP, Georgesco G, et al. (2010) Effect of celiprolol on prevention of cardiovascular events in vascular Ehlers-Danlos syndrome: a prospective randomised, open, blinded-endpoints trial. Lancet 376:1476–1484 [DOI] [PubMed] [Google Scholar]
- Pepin M, Schwarze U, Superti-Furga A, Byers PH. (2000) Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med 342:673–680 [DOI] [PubMed] [Google Scholar]
- Pope FM, Martin GR, Lichtenstein JR, Penttinen R, Gerson B, Rowe DW, McKusick VA. (1975) Patients with Ehlers-Danlos syndrome type IV lack type III collagen. Proc Natl Acad Sci USA 72:1314–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyeritz RE. (2000) Ehlers-Danlos syndrome. N Engl J Med 342:730–732 [DOI] [PubMed] [Google Scholar]
- Sastry PS. (2002) Matrix metalloproteinase inhibitor therapy to prevent complications as well as therapy for Ehler-Danlos syndrome. Med Hypotheses 59:314–315 [DOI] [PubMed] [Google Scholar]
- Schwarze U, Schievink WI, Petty E, Jaff MR, Babovic-Vuksanovic D, Cherry KJ, Pepin M, Byers PH. (2001) Haploinsufficiency for one COL3A1 allele of type III procollagen results in a phenotype similar to the vascular form of Ehlers-Danlos syndrome, Ehlers-Danlos syndrome type IV. Am J Hum Genet 69:989–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeland U, Selejan S, Engelhardt S, Müller P, Lohse MJ, Böhm M. (2007) Interstitial remodeling in β1-adrenergic receptor transgenic mice. Basic Res Cardiol 102:183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan B, Scheinfeld N. (2008) The use and safety of doxycycline hyclate and other second-generation tetracyclines. Expert Opin Drug Saf 7:571–577 [DOI] [PubMed] [Google Scholar]
- Smith LT, Schwarze U, Goldstein J, Byers PH. (1997) Mutations in the COL3A1 gene result in the Ehlers-Danlos syndrome type IV and alterations in the size and distribution of the major collagen fibrils of the dermis. J Invest Dermatol 108:241–247 [DOI] [PubMed] [Google Scholar]
- Thiboutot DM, Fleischer AB, Del Rosso JQ, Rich P. (2009) A multicenter study of topical azelaic acid 15% gel in combination with oral doxycycline as initial therapy and azelaic acid 15% gel as maintenance monotherapy. J Drugs Dermatol 8:639–648 [PubMed] [Google Scholar]
- Thompson RW, Baxter BT. (1999) MMP inhibition in abdominal aortic aneurysms. Rationale for a prospective randomized clinical trial. Ann NY Acad Sci 878:159–178 [DOI] [PubMed] [Google Scholar]
- Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, Welgus HG, Parks WC. (1995) Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest 96:318–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin S, Morales A, Sanchez JL, Rivera A. (2009) Safety and efficacy of doxycycline in the treatment of rosacea. Clin Cosmet Invest Dermatol 2:129–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Kosho T, Wada T, Sakai N, Fujimoto M, Fukushima Y, Shimada T. (2007) Genetic aspects of the vascular type of Ehlers-Danlos syndrome (vEDS, EDSIV) in Japan. Circ J 71:261–265 [DOI] [PubMed] [Google Scholar]
- Watanabe A, Shimada T. (2008) Vascular type of Ehlers-Danlos syndrome. J Nippon Med Sch 75:254–261 [DOI] [PubMed] [Google Scholar]
- Weinbrenner C, Nelles M, Herzog N, Sárváry L, Strasser RH. (2002) Remote preconditioning by infrarenal occlusion of the aorta protects the heart from infarction: a newly identified non-neuronal but PKC-dependent pathway. Cardiovasc Res 55:590–601 [DOI] [PubMed] [Google Scholar]
- Wennström JL, Newman HN, MacNeill SR, Killoy WJ, Griffiths GS, Gillam DG, Krok L, Needleman IG, Weiss G, Garrett S. (2001) Utilisation of locally delivered doxycycline in non-surgical treatment of chronic periodontitis. A comparative multi-centre trial of 2 treatment approaches. J Clin Periodontol 28:753–761 [DOI] [PubMed] [Google Scholar]
- Xiong W, Knispel RA, Dietz HC, Ramirez F, Baxter BT. (2008) Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J Vasc Surg 47:166–172; discussion 172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Noma T, Nakazawa A, Saito S, Fujioka K, Zempo N, Esato K. (2001) Enhanced expression of matrix metalloproteinase-9 in abdominal aortic aneurysms. World J Surg 25:259–265 [DOI] [PubMed] [Google Scholar]
- Yang HH, Kim JM, Chum E, van Breemen C, Chung AW. (2010) Effectiveness of combination of losartan potassium and doxycycline versus single-drug treatments in the secondary prevention of thoracic aortic aneurysm in Marfan syndrome. J Thorac Cardiovasc Surg 140:305–312.e2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.