Abstract
We have developed a novel chemical modification of conventional nonsteroidal anti-inflammatory drugs to reduce their toxicity and enhance their efficacy. Phospho-ibuprofen [(PI) 2-(4-isobutyl-phenyl)-propionic acid-4-(diethoxy-phosphoryloxy)-butyl ester (MDC-917)], a novel derivative of ibuprofen, strongly inhibited the growth of human colon cancer cells in vitro and SW480 human colon cancer xenografts in nude mice. PI was metabolized minimally by cultured cells, but extensively by liver microsomes and mice, undergoing regioselective oxidation to produce 1-OH-PI and carboxyl-PI, which can be hydrolyzed to 1-OH-ibuprofen and carboxyl-ibuprofen, respectively. PI also can be hydrolyzed to release ibuprofen, which can generate 2-OH-ibuprofen, carboxyl-ibuprofen, and ibuprofen glucuronide. After a single oral administration (400 mg/kg) of PI, ibuprofen and ibuprofen glucuronide are the main plasma metabolites of PI; they have, respectively, Cmax of 530 and 215 μM, Tmax of 1 and 2 h, elimination t1/2 of 7.7 and 5.3 h, and area under the concentration-time curve (0–24 h) of 1816 and 832 μM × h. Intact PI was detected in several tissues but not in plasma; at a higher PI dose (1200 mg/kg), PI plasma levels were 12.4 μM. PI generated the same metabolites in mouse plasma as conventional ibuprofen, but with much lower levels, perhaps accounting for the enhanced safety of PI. The antitumor effect of PI was significantly associated with plasma ibuprofen levels (p = 0.016) but not with xenograft ibuprofen levels (p = 0.08), suggesting a complex anticancer effect. These results provide a pharmacological basis to explain, at least in part, the anticancer efficacy and safety of this promising compound and indicate that PI merits further evaluation as an anticancer agent.
Introduction
Inflammation is a critical component of tumor progression, and nonsteroidal anti-inflammatory drugs (NSAIDs) are efficacious in inhibiting early neoplastic progression and malignant conversion (Coussens and Werb, 2002). For example, the NSAID ibuprofen is effective in reducing the risk of various human cancers, including those of the colon (Meier et al., 2002), breast (Harris et al., 1996), lung (Marnett, 1995), and prostate (Harris et al., 2005). Indeed, NSAIDs inhibit cancer through cyclooxygenase (COX)-dependent and COX-independent mechanisms, and their COX-independent targets include nuclear factor-κB, peroxisome proliferator-activated receptor, lipoxygenase, and apoptosis signaling (Ulrich et al., 2006).
Conventional NSAIDs are associated with significant toxicity, which makes their long-term use for cancer prevention problematic (Piazza et al., 2009). For example, significant side effects are seen with ibuprofen, including those of the central nervous system (McElwee et al., 1990), gastrointestinal tract (Lanza, 1984), liver (Riley and Smith, 1998), and respiratory tract (Antonicelli and Tagliabracci, 1995). On the other hand, little has been accomplished over the same period of time for the treatment of advanced colon cancer, with its mortality remaining essentially unchanged for at least three decades (American Chemical Society, 2008). These considerations provide a compelling need to develop new effective agents for the control of cancer.
We developed a novel chemical modification of conventional NSAIDs to reduce their toxicity and enhance their efficacy (Mackenzie et al., 2010). One such modified NSAID is phospho-ibuprofen (PI), a derivative of ibuprofen, consisting of ibuprofen covalently attached to the diethylphosphate group via a spacer moiety (Fig. 1, inset). PI has strong anti-inflammatory properties both in vitro and in animal models of arthritis, and it was shown to have far better gastrointestinal safety than conventional ibuprofen (Huang et al., 2011). PI inhibited cancer cell growth 16 to 23 times more potently than its parent drug, ibuprofen. PI also inhibited the growth of human colon cancer xenografts in nude mice, while manifesting minimal or no animal toxicity.
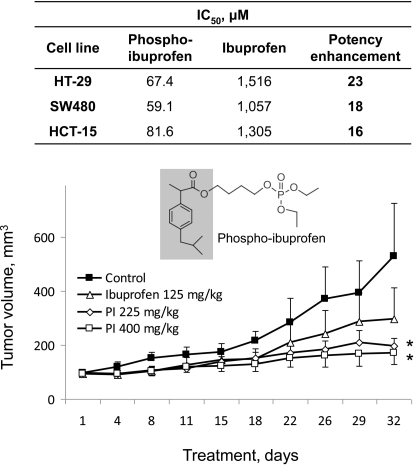
Fig. 1.
Effect of phospho-ibuprofen on colon cancer cell growth in vitro and in vivo. Top, IC50 values for colon cancer cells treated with PI or ibuprofen for 24 h. Bottom, PI inhibits the growth of SW480 human colon cancer xenografts in nude mice. The study was performed as described under Materials and Methods. The doses of ibuprofen (125 mg/kg) and PI (225 mg/kg) are equimolar. The structure of PI is also shown; the shaded part of the molecule corresponds to conventional ibuprofen. Values are mean ± S.E.M. *, p ≤ 0.05 compared with the corresponding control value.
Given the promise of PI, we studied its metabolism, pharmacokinetics, and pharmacodynamics in mice. Here, our results establish the metabolic pathways of PI in vitro and in vivo and provide an insight into the interplay between its metabolic transformations and pharmacological effects.
Materials and Methods
Reagents.
PI was provided by Medicon, Inc (Stony Brook, NY). Ibuprofen and acetonitrile of HPLC grade and porcine liver esterase were purchased from Sigma-Aldrich (St. Louis, MO). 1-OH-ibuprofen, 2-OH-ibuprofen, and ibuprofen glucuronide were purchased from U.S. Biological (Swampscott, MA). RPMI medium 1640 and antibiotics were obtained from Mediatech (Herndon, VA). Human colon cancer cell lines were purchased from American Type Culture Collection (Manassas, VA). Rat and human liver microsomes and NADPH-regenerating solution were purchased from BD Biosciences (San Jose, CA).
Cell Culture.
Human colon cancer cells were grown as monolayers as recommended by the American Type Culture Collection. Cell growth was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium assay (Promega, Madison, WI) following the manufacturer's instructions. To study PI metabolism in cultured cells, HCT-15 human colon cancer cells were seeded in 6-cm plates at a density of 105 cells/cm2 in 4 ml of culture media and allowed to attach for 24 h. The cells were treated with 100 μM PI for 6 h and subsequently washed three times with cold PBS and lysed by sonication. Acetonitrile was added to extract PI and its metabolites from cells, and the extracts were submitted for HPLC and LC-MS/MS analysis.
HPLC Analysis.
The HPLC system consisted of a Waters Alliance 2695 Separations Module equipped with a Waters 2998 photodiode array detector (220 nm) (Waters, Milford, MA) and a Thermo BDS Hypersil C18 column (150 × 4.6 mm, particle size 3 μm) (Thermo Fisher Scientific, Waltham, MA). The mobile phase followed a gradient between buffer A [formic acid, acetonitrile, H2O (95:4.9:0.1 v/v/v)] and buffer B (acetonitrile).
LC-MS/MS Analysis.
The LC-MS/MS system consisted of a Thermo TSQ Quantum Access triple quadrupole mass spectrometer (Thermo Fisher Scientific) interfaced by an electrospray ionization probe with an Ultimate 3000 HPLC system (Dionex Corporation, Sunnyvale, CA). Chromatographic separations were achieved on a Luna C18 column (150 × 2 mm) (Phenomenex Inc., Torrance, CA), and the mobile phase consisted of a gradient from 10 to 95% acetonitrile.
Metabolism of PI by Liver Microsomes.
PI (120 μM) was preincubated at 37°C for 5 min with NADPH-regenerating solution (1.3 mM NADP, 3.3 mM d-glucose 6-phosphate, 3.3 mM MgCl2, and 0.4 U/ml glucose-6-phosphate dehydrogenase) in 0.1 M potassium phosphate buffer, pH 7.4. Reaction was initiated by the addition of liver microsomes (protein concentration 0.5 mg/ml), and samples were maintained at 37°C for a given time. At the end of each incubation, 0.3-ml aliquots were mixed with 0.6 ml of CH3CN containing 2 M 1% phosphoric acid, vortexed, and then centrifuged for 10 min at 5000g. The supernatants were subjected to HPLC analysis.
Hydrolysis of Phospho-NSAIDs by Liver Esterases.
To release their NSAID moieties, phospho-NSAIDs (PI, 1-OH-PI, or carboxyl-PI) were treated with porcine liver esterase (10 mg/ml) at 37°C for 30 min in 0.1 M potassium phosphate buffer, pH 7.4,. After this, 0.3-ml aliquots were mixed with 0.6 ml of CH3CN, vortexed, and then centrifuged for 10 min at 5000g. The supernatants were subjected to HPLC analysis.
In Vivo Inhibition of Colon Cancer Growth in the Xenograft Mouse Model.
The animal study was approved by the Institutional Animal Care and Use Committee at Stony Brook University. SW480 human colon cancer cells (2 × 106 cells suspended in 100 μl of PBS) were injected subcutaneously in the left and right flanks of 5- to 6-week-old female NCr nude mice (Taconic Farms, Germantown, NY). After the average tumor volume reached ∼100 mm3, four groups of animals (n = 9/group) started receiving the following treatments intraperitoneally once daily five times a week (5 days continuously with a 2-day break) for 32 days: group 1 received vehicle; group 2 received 125 mg/kg per day ibuprofen; group 3 received 225 mg/kg per day PI; and group 4 received 400 mg/kg per day PI. The volume of each drug or vehicle dose was ∼100 μl. Tumor volume was measured every 3 or 4 days as specified and was calculated by measuring with a caliper its length (L) and width (W) and using the formula L × W × (L + W/2) × 0.56. At the endpoint, animals were euthanized following a standard protocol.
Pharmacokinetic Studies in Mice.
PI or ibuprofen dissolved in corn oil was administered to female BALB/c mice as a single equimolar dose by gastric gavage. Mice were sacrificed at various time points after drug administration, and the blood was collected and immediately centrifuged. The resulting plasma was deproteinized by immediately mixing it with 2-fold volume of acetonitrile. PI and its metabolites were analyzed by HPLC as described above.
PK parameters in plasma were determined by noncompartmental PK data analysis using PK Solutions 2.0 software (Summit Research Services, Montrose, CO). The PK parameters determined include maximal plasma concentration (Cmax), time to reach maximal plasma concentration (Tmax), half-life (t1/2), and the area under the concentration-time curve (AUC).
Tissue Distribution in Mice.
One hour after PI administration, various tissues of the sacrificed mice were collected, washed three times with 50% dimethyl sulfoxide, homogenized, and sonicated in PBS. PI and its metabolites were extracted by adding a 2-fold volume of acetonitrile. After centrifugation for 10 min at 5000g, the supernatants were subjected to HPLC analysis.
Statistical Analyses.
Results, obtained from at least three independent experiments, were expressed as mean ± S.E.M. and analyzed by one-factor analysis of variance followed by the Tukey test for multiple comparisons. p < 0.05 was considered statistically significant.
Results
PI Inhibits the Growth of SW480 Colon Cancer Cells In Vitro and In Vivo.
To evaluate the effect of PI on cell growth, we determined the 24-h IC50 values of PI and ibuprofen in HT-29, SW480, and HCT-15 human colon cancer cell lines (Fig. 1). The potency of PI was dramatically enhanced (approximately 16–23 times) compared with conventional ibuprofen.
We next evaluated the chemotherapeutic potential of PI and compared it with ibuprofen in a subcutaneous model of SW480 cell xenografts in nude mice. PI was administered intraperitoneally at two doses, 225 and 400 mg/kg, the former being equimolar to the dose of ibuprofen (125 mg/kg). As shown in Fig. 1, both PI and ibuprofen inhibited tumor growth throughout the observation period with the efficacy being 400 mg/kg PI > 225 mg/kg PI > 125 mg/kg ibuprofen. At sacrifice, the tumor volume of each study group was as follows: control = 530 ± 197 (mean± S.E.M. for this and subsequent values), ibuprofen = 297 ± 117 (not significantly different from control), 225 mg/kg PI = 196 ± 29 (p = 0.05 compared with control), and 400 mg/kg PI = 171 ± 42 mm3 (p < 0.05 compared with control). This indicates a reduction in tumor volume of 44, 63, and 68% by ibuprofen, 225 mg/kg PI, and 400 mg/kg PI, respectively. Whereas both PI doses were more effective than ibuprofen, the differences between either one of them and the effect of ibuprofen did not reach statistical significance. It is noteworthy that these treatments produced no apparent adverse effects in mice during the 32 days of administration.
The Metabolism of PI by Liver Microsomes.
Given the efficacy of PI, we explored its metabolism by rat liver microsomes (RLM), an enriched source of major metabolic enzymes (Bajrami et al., 2009). These enzymes, including oxidative enzymes, hydrolases, and transferases, function to make xenobiotic compounds more polar and more water-soluble, thus facilitating their elimination from the body.
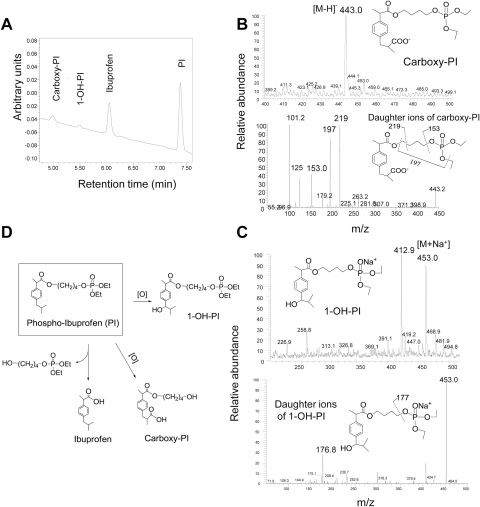
Three major metabolites of PI (ibuprofen, carboxyl-PI, and 1-OH-PI) were readily detected in the reaction mixture of PI and RLM (Fig. 2A). Ibuprofen was identified by comparing its HPLC retention time and UV spectrum with that of authentic compound; the other two metabolites were identified by LC-MS/MS analysis. The mass spectrum of carboxyl-PI showed a [M-H]+ ion at m/z 443.0, which was further fragmented at m/z 153, 197, and 219 (Fig. 2B). The mass spectrum of 1-OH-PI showed a [M+Na]+ ion at m/z 453.0, which was further fragmented at m/z 176.8. (Fig. 2C). To determine the position of the -OH group, 1-OH-PI was treated with porcine liver esterase as described under Materials and Methods, and it was completely hydrolyzed to release 1-OH-ibuprofen, indicating that the -OH group was at the 1-position of the isobutyl group. Thus PI undergoes hydrolysis and oxidation in RLM, and its oxidation regioselectively occurs at the 1- and 3-positions of the isobutyl group (Fig. 2D). Like 1-OH-PI, PI and carboxyl-PI were hydrolyzed by the same esterase to release ibuprofen and carboxyl-ibuprofen, respectively, indicating that the carboxyester bond of the phospho-NSAIDs is susceptible to hydrolysis by liver esterase. These results also support the identification of carboxyl-PI and 1-OH-PI.
Fig. 2.
Identification of the metabolites of PI by RLM. A, HPLC chromatogram of an extract of RLM incubated with PI for 30 min; PI and two of its metabolites are identified. PI and its metabolites were extracted and fractionated by HPLC as described under Materials and Methods. B, MS and MS/MS spectrum of hydroxyl-PI fraction collected from HPLC. C, MS and MS/MS spectrum of carboxyl-PI fraction collected from HPLC. D, metabolic transformations of PI by liver microsomes.
As shown in Fig. 3A, the level of PI in the reaction mixture continuously decreased during the entire period of observation, while, at the same time, the level of its hydrolysis product, ibuprofen, continuously increased. The levels of 1-OH-PI and carboxyl-PI increased in the first 30 min but slightly dropped afterward because they can also be hydrolyzed by RLM. The level of 1-OH-PI was much lower and decreased earlier than that of carboxyl-PI, suggesting that PI was oxidized preferentially at the 3-position over the 1-position of its isobutyl group.
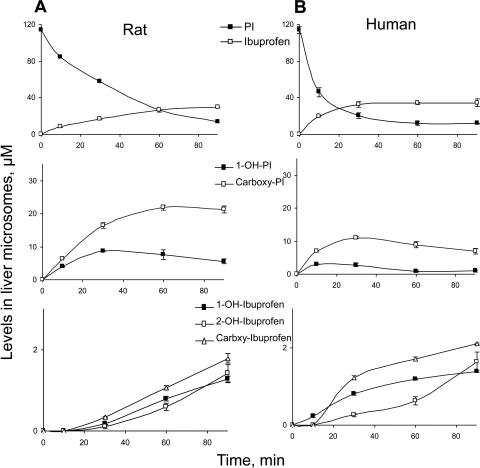
Fig. 3.
Kinetics of PI and its metabolites by rat and human liver microsomes. PI was incubated with rat (A) or human (B) liver microsomes at 37°C for up to 90 min. PI and its metabolites were extracted at the designated time points and assayed as described under Materials and Methods.
In addition, three minor metabolites (1-OH-ibuprofen, 2-OH-ibuprofen, and carboxyl-ibuprofen) were detected. They are derived from the oxidation of ibuprofen and the hydrolysis of 1-OH-PI and carboxyl-PI, and their levels are generally <10% of the major metabolites. These three minor metabolites shared similar kinetic behaviors in that their levels all were negligible in the first 10 min, after which they continued to increase until the end of the observation period.
Drug-metabolizing enzymes differ between humans and animals with respect to isoform composition, expression, and catalytic activities (Martignoni et al., 2006). In addition, testing drug metabolism by human liver microsomes (HLM) is required for the prediction of human pharmacokinetics of the drug (Liu et al., 2010). Therefore, we next explored the metabolism of PI by HLM. As was the case with RLM, three major metabolites (ibuprofen, 1-OH-PI, and carboxyl-PI) as well as the three minor metabolites (1-OH-ibuprofen, 2-OH-ibuprofen, and carboxyl-ibuprofen) were also detected in the reaction mixture of PI and HLM; this finding suggested that the two species share the same metabolic pathways in the metabolism of PI by liver microsomes.
The level of PI decreased rapidly and was cleared by 82% within the first 30 min of its reaction with HLM (Fig. 3B). Consistently, the levels of 1-OH-PI and carboxyl-PI increased rapidly during the initial reaction, suggesting that in the beginning the formation of these two metabolites was much faster than their hydrolysis because of the initial high concentration of PI. With the PI level decreasing rapidly, the formation rate of 1-OH-PI and carboxyl-PI decreased, and their consumption became dominant, resulting in the steady decrease of their levels during the later phase of the reaction. As was the case with RLM, the level of 1-OH-PI was much lower and decreased earlier than that of carboxyl-PI.
The level of ibuprofen increased initially and reached a plateau at 30 min, suggesting equilibrium between its formation from PI and oxidation to other metabolites. Similar to the case with RLM, all of the minor metabolites (1-OH-ibuprofen, 2-OH-ibuprofen, and carboxyl-ibuprofen) continued to increase during the entire period of observation.
Even though PI followed the same metabolic pathway in RLM and HLM, the kinetics of metabolite formation in the two species was distinct. PI seems to be more stable in RLM than in HLM. The t1/2 of PI in RLM was 4.5 times longer than that in HLM (28.5 versus 6.4 min). PI was hydrolyzed more rapidly in HLM than in RLM, because the rate constant of the formation of ibuprofen was 3.8 times higher in HLM that in RLM (0.088 versus 0.023 min−1). Thus, PI was metabolized more extensively and rapidly in HLM than in RLM. Because of the slow clearance of PI in RLM, 1-OH-PI and carboxyl-PI were formed more rapidly in RLM than in HLM, and the observed highest concentrations of 1-OH-PI and carboxyl-PI in RLM were 2.9 and 2.0 times higher than those in HLM, respectively.
The Metabolism of PI by Cultured Cancer Cells.
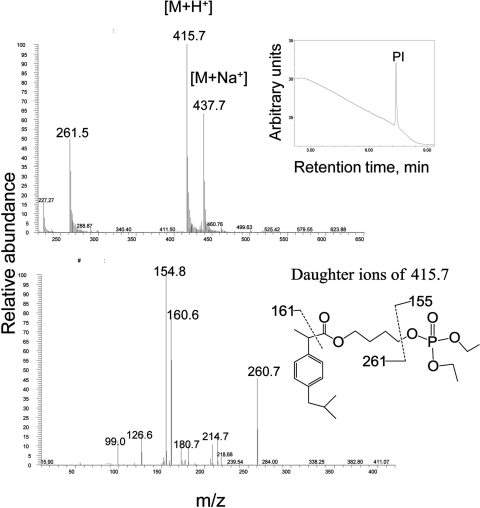
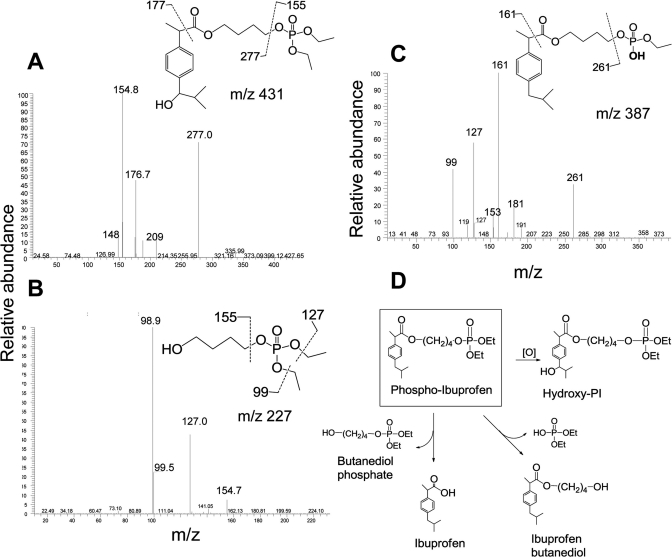
Given its potency in inhibiting cancer cell growth, we also explored the metabolism of PI by cultured HCT-15 human colon cancer cells. Cell lysates were prepared as described under Materials and Methods and analyzed using HPLC and MS. HPLC analysis using UV detection showed that PI remained primarily intact 6 h after treatment (Fig. 4, inset). Consistent with this result, LC-MS analysis showed that the dominant ion of the highest intensity was at m/z 415, which further generated fragment ions at m/z 155, 161, and 261 (Fig. 4). This spectrum was identical to that of authentic PI, confirming that the majority of PI taken up by the cells stays intact. This result also confirmed the chemical structure of PI.
Fig. 4.
PI metabolism by cultured colon cancer cells. PI and its metabolites were extracted from the PI-treated HCT-15 cell lysates and analyzed using HPLC and LC/MS/MS as described under Materials and Methods. Top, MS spectrum of PI obtained from LC separation. Inset, HPLC chromatogram of extract from HCT-15 cells treated with PI; only PI was detected by UV detector. Bottom, MS/MS spectrum of fragment ions of the PI.
Multiple minor metabolites of PI were detected and identified in cultured cells using LC-MS/MS analysis. The mass spectrum of the hydroxy-PI showed a [M+H]+ ion at m/z 431, which was further fragmented at m/z 155, 177, and 277 (Fig. 5A). Butanediol phosphate, the carboxyester hydrolysis product of PI, gave an [M+H]+ ion at m/z 227 in the MS spectrum, which further generated fragment ions at 99, 127, and 155 (Fig. 5B). Desethyl-PI, the phosphate ester hydrolysis product of PI, showed an [M+H]+ ion at m/z 387, which was further fragmented to 161 and 261 (Fig. 5C). Thus PI undergoes hydrolysis and oxidation transformations in cultured cells (Fig. 5D), which are generally similar to its metabolism by liver microsomes.
Fig. 5.
Identification of the metabolites from PI-treated cancer cells. The metabolites of PI were extracted from the PI-treated HCT-15 cells and analyzed using LC/MS/MS as described under Materials and Methods. A, MS/MS spectrum of fragment ions of hydroxyl-PI. B, MS/MS spectrum of fragment ions of butanediol phosphate. C, MS/MS spectrum of fragment ions of desethyl-PI. D, proposed metabolic transformations of PI by liver microsomes.
We assessed the pharmacological activity of the various metabolites of PI in cultured cells. As shown in Table 1, each of the three structural components of PI (plain ibuprofen, spacer, and diethyl phosphate) tested individually for its cell growth inhibitory effect was essentially inactive compared with PI. The same applied to three additional metabolites of PI (2-OH-ibuprofen, carboxyl-ibuprofen, and ibuprofen glucuronide), all of which had IC50 values in the millimolar range. These results suggested that the limited metabolism of PI by cells may be important for its growth inhibitory effect.
TABLE 1.
IC50 values of phospho-ibuprofen and its components/metabolites in cultured HT-29 cancer cells
| Compound | IC50 |
|---|---|
| μM | |
| Phospho-ibuprofen | 67 |
| Ibuprofen | 1516 |
| 1,4-Butanediol (spacer) | >10,000 |
| Diethyl phosphate | 10,229 |
| 2-OH-ibuprofen | >4500 |
| Carboxyl-ibuprofen | >4500 |
| Ibuprofen glucuronide | >4500 |
Pharmacokinetics of PI in the Mouse.
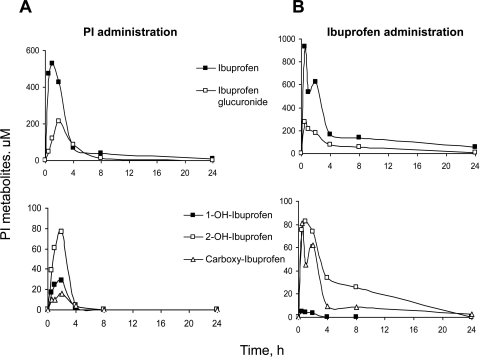
We studied the PK properties of PI after its single oral administration to mice. The two major metabolites in plasma were ibuprofen and ibuprofen glucuronide. As shown in Fig. 6A, the plasma levels of ibuprofen increased rapidly and reached the maximum concentration of 530 μM 1 h after oral gavage, suggesting that PI was rapidly absorbed, metabolized, and distributed to the blood. The level of ibuprofen decreased substantially 4 h after drug administration and became negligible at 8 h. The plasma levels of ibuprofen glucuronide followed a similar pattern, but the peak value was much smaller.
Fig. 6.
Comparison of pharmacokinetics of PI and ibuprofen in mice. Equimolar doses (1 mmol/kg) of PI (A) and ibuprofen (B) dissolved in corn oil were administered to mice as a single dose by gastric gavage. Plasma levels of PI metabolites at the indicated time points were determined as described under Materials and Methods.
As was the case with PI metabolism by liver microsomes, the three minor metabolites (1-OH-ibuprofen, 2-OH-ibuprofen, and carboxyl-ibuprofen) were detected in mouse plasma after PI administration. These three metabolites shared similar kinetic behavior because they are derived from ibuprofen. The levels of these metabolites reached their peak values at 2 h after PI administration, but decreased dramatically afterward. The Tmax of each of these metabolites was 1 h later than that of ibuprofen (Table 2), which is reasonable because they are derived from ibuprofen.
TABLE 2.
Pharmacokinetic parameters of phospho-ibuprofen (400 mg/kg) and ibuprofen (200 mg/kg) in mice
The doses of PI and ibuprofen are equimolar. Experiments were done independently three times; values were generally within 15%.
| Drug Administered | Measurement |
||||
|---|---|---|---|---|---|
| Cmax | Tmax | Elimination t1/2 | AUC0–24h | Sum of AUC0–24h | |
| μM | h | μM × h | |||
| Phospho-ibuprofen | |||||
| Ibuprofen | 530 | 1.0 | 7.7 | 1816 | 2963 |
| 1-OH-ibuprofen | 28 | 2.0 | N.D. | 74 | |
| 2-OH-ibuprofen | 77 | 2.0 | N.D. | 193 | |
| Carboxyl-ibuprofen | 16 | 2.0 | N.D. | 48 | |
| Ibuprofen glucuronide | 215 | 2.0 | 5.3 | 832 | |
| Ibuprofen | |||||
| Ibuprofen | 932 | 0.5 | 12.8 | 4146 | 6437 |
| 1-OH-ibuprofen | 5 | 0.5 | N.D. | 11 | |
| 2-OH-ibuprofen | 83 | 2.0 | N.D. | 572 | |
| Carboxyl-ibuprofen | 81 | 0.5 | 10.0 | 311 | |
| Ibuprofen glucuronide | 274 | 0.5 | 6.3 | 1398 | |
N.D., not determined.
When PI was administered at a dose of 400 mg/kg, intact PI was detected in mouse plasma by the MS detector (data not shown), but was undetectable by the UV detector, suggesting high carboxyesterase activity in mouse plasma, as has been shown previously (Li et al., 2005). However, PI at a dose of 1200 mg/kg generated a significant plasma level of intact PI (12.4 μM). These results indicated that PI can indeed survive in the blood.
For purposes of comparison, we performed side-by-side PK studies of conventional ibuprofen and PI administered at equimolar doses. As shown in Fig. 6B, ibuprofen was metabolized to produce ibuprofen glucuronide, 1-OH-ibuprofen, 2-OH-ibuprofen, and carboxyl-ibuprofen in mouse plasma; this metabolic profile was the same as that after PI administration. The plasma levels of ibuprofen and its metabolites increased rapidly and reached their peak values within ∼0.5 h after ibuprofen administration (Fig. 6B), indicating that ibuprofen was rapidly absorbed, metabolized, and distributed to the blood. Compared with the plasma levels of 2-OH-ibuprofen and carboxyl-ibuprofen, those of 1-OH-ibuprofen were negligible (Fig. 6B).
Compared with PI, ibuprofen generated higher levels of metabolites with the AUC0–24h of “total metabolites” (the sum of the five metabolites) being 2.2-fold higher (Table 2); this probably reflects the higher hydrophobicity of PI and hence lower solubility in plasma. The Tmax of each metabolite after ibuprofen administration was much shorter than those after PI administration (Table 2). This finding suggests that the absorption and distribution of PI are slower than those of ibuprofen, also probably caused by its higher hydrophobicity.
Biodistribution of PI in the Mouse Model.
We also measured the levels of PI and its metabolites in liver, heart, kidney, and gastrointestinal tissues of mice after its oral administration (Table 3). Significant levels of intact PI were detected in all of the tissues examined, confirming that PI can partially survive in mouse plasma and therefore be distributed to other tissues. High levels of intact PI were detected in gastrointestinal tissues, whereas its liver concentration was the lowest, probably because of the high metabolic capacity of this organ.
TABLE 3.
PI metabolite levels in mouse tissues 1 h after oral administration of 400 mg/kg PI
Experiments were done independently three times; values were generally within 15%.
| PI | 1-OH-PI | Carboxyl-PI | Ibuprofen | 1-OH-Ibuprofen | 2-OH-Ibuprofen | Carboxyl-Ibuprofen | Ibuprofen Glucuronide | |
|---|---|---|---|---|---|---|---|---|
| pmol/mg protein | ||||||||
| Liver | 82 | 22 | 1013 | 739 | 19 | 33 | 13 | 408 |
| Heart | 101 | N.D. | N.D. | 2409 | 34 | 96 | N.D. | 55 |
| Kidney | 121 | N.D. | N.D. | 1946 | 75 | 220 | 104 | 322 |
| Stomach | 421 | N.D. | N.D. | 462 | N.D. | N.D. | N.D. | N.D. |
| Small intestine | 521 | N.D. | 36 | 913 | N.D. | N.D. | N.D. | 50 |
| Colon | 414 | N.D. | 20 | 431 | N.D. | N.D. | N.D. | 64 |
| Blood* | N.D. | N.D. | N.D. | 530 | 24 | 60 | 10 | 121 |
N.D., not detected.
Metabolite levels in the blood were expressed as μM in plasma.
As expected, the five metabolites detected in mouse plasma (ibuprofen, ibuprofen glucuronide, 1-OH-ibuprofen, 2-OH-ibuprofen, and carboxyl-ibuprofen) were also detected in these tissues. High levels of ibuprofen glucuronide were detected in the liver and kidney, suggesting that most of the glucuronidation of ibuprofen occurs in these organs (Rudy et al., 1991). Similar to PI metabolism by liver microsomes, the level of 1-OH-PI in liver tissue was much lower than that of carboxyl-PI.
Blood and Xenograft Levels of PI Metabolites in Mice: Correlations with Tumor Size.
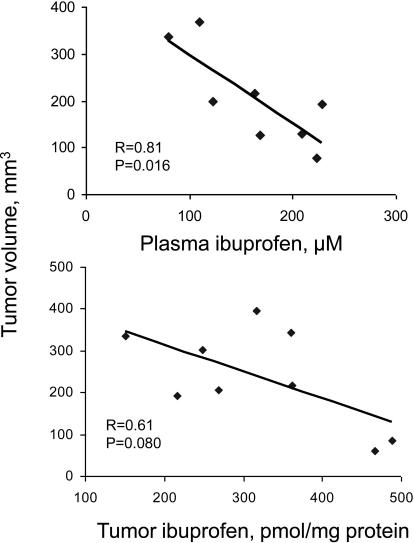
To gain an insight into the interplay between PI's metabolic transformations and pharmacological effects, we measured the levels of PI metabolites in mouse blood and tumor tissue from the efficacy study. The major metabolite detected was ibuprofen, and there was a statistically significant association between the tumor volume and ibuprofen level in plasma (p = 0.02), underscoring the relative importance of the metabolites of PI in cancer control (Fig. 7). However, the association between the anticancer efficacy and tumor levels of ibuprofen was statistically significant only for the trend (p = 0.08), suggesting that metabolite-independent mechanisms are also involved in inhibiting cancer growth.
Fig. 7.
Correlation of PI metabolite levels with tumor volume. Mice with SW480 colon cancer xenografts were sacrificed 1 h after the last dosing, and ibuprofen levels in mouse blood and tumors were determined as described under Materials and Methods. A significant correlation (p = 0.016) between plasma levels of ibuprofen and tumor volume (top) and a trend between tumor levels of ibuprofen and tumor volume (bottom) were observed.
Discussion
Our work demonstrated the preclinical anticancer efficacy of PI, a novel derivative of ibuprofen, established its metabolism in vitro and in vivo, defined its PK in mice, and explored its PD in tumor xenografts. Collectively, these findings provide strong support for the anticancer potential of this agent and set the stage for its further pharmacological evaluation.
PI inhibited the growth of colon cancer cell lines much more potently than ibuprofen and also inhibited the growth of xenografts of one of these cell lines in nude mice. These effects are quantitatively significant and, considering the efficacy of PI on pancreatic and breast cancer from our unpublished data, constituted a firm justification for studying its metabolism and PK/PD. Compared with ibuprofen, the two PI doses inhibited tumor growth approximately 1.5 times more effectively. The differences in efficacy between the two were statistically not significant; such lack of significance is probably caused by a lack of power. Nonetheless, we deem these differences as clinically relevant, especially when the greater safety of PI is factored in (current data and Huang et al., 2011).
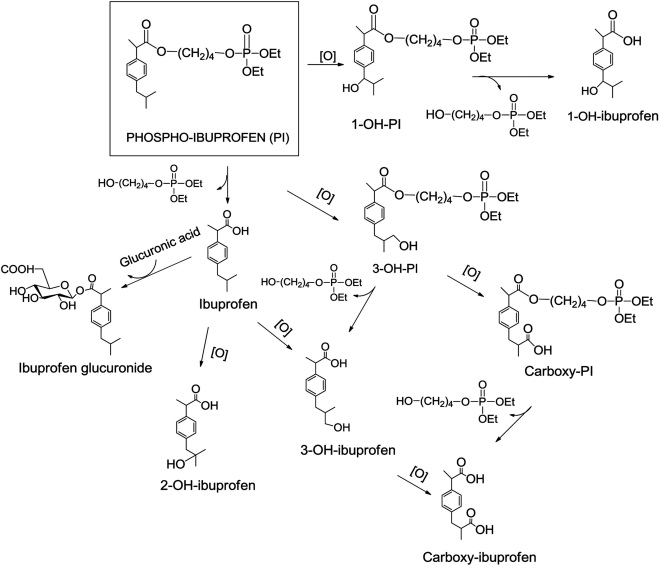
As summarized in the metabolic diagram of Fig. 8, PI undergoes extensive biotransformations, leading to at least nine products. Three distinct pathways of PI metabolism are readily recognizable: 1) oxidation at the 1-position of the isobutyl group to form 1-OH-PI, which can be hydrolyzed to form 1-OH-ibuprofen, 2) oxidation at the 3-position of the isobutyl group to form carboxyl-PI, probably through 3-OH-PI, and 3) carboxyl-ester hydrolysis to form ibuprofen, which, in turn, can follow three metabolic routes: 1) conjugation with glucuronic acid to form ibuprofen glucuronide, 2) oxidation to form 2-OH-ibuprofen, or 3) oxidation at the 3-position of the isobutyl group to form carboxyl-ibuprofen, probably through 3-OH-ibuprofen. In general, hydrolysis, oxidation, and glucuronidation of drugs represent mechanisms used by an organism to inactivate and/or eliminate them (Ulrich et al., 2006). Given that all PI metabolites are much weaker than PI at inhibiting cancer cell growth and their aqueous solubility is enhanced (evidenced by their retention times on reverse-phase HPLC), it seems that PI follows the general fate of compounds that are inactivated and eliminated by the mice.
Fig. 8.
PI metabolism in vitro and in vivo. PI can be metabolized through three distinct pathways: 1) oxidation to form 1-OH-PI, 2) oxidation to form carboxyl-PI, or 3) hydrolysis to form ibuprofen, which, in turn, can be metabolized through: 1) glucuronidation to form ibuprofen glucuronide, 2) oxidation to form 2-OH-ibuprofen, or 3) oxidation to form carboxyl-ibuprofen.
The metabolic fate of PI is largely context-dependent. Cultured colon cancer cells preserve PI essentially intact, with only a minor fraction (<10%) metabolized to mostly biologically inactive metabolites. Of these, the most active metabolite of PI is ibuprofen, which is 22-fold less potent than PI in inhibiting cell growth; all others are >67-fold less potent than PI. Liver microsomes generate a different profile of metabolites; at 90 min the four most abundant metabolites are (in descending order) ibuprofen, carboxyl-PI, PI, and 1-OH-PI. Human and rat liver microsomes generate the same metabolic profile, although the ratio between the four most abundant metabolites is different between the two. Finally, a markedly different metabolite profile is obtained in mice. AUC quantification shows that ibuprofen and ibuprofen glucuronide make up 90% of the total plasma metabolites, while PI is conspicuous by its absence.
Both liver and mice microsomes oxidized PI at the 1- and 3-positions of its isobutyl group to form 1-OH-PI and 3-OH-PI, respectively, with the latter further oxidized to carboxyl-PI. In contrast, ibuprofen, generated as a metabolite of PI, was oxidized at the 2- and 3-positions of its isobutyl group, generating 2-OH-ibuprofen and 3-OH-ibuprofen, the latter leading to carboxyl-ibuprofen. The metabolism of ibuprofen generated from PI is identical to that of conventional ibuprofen (Hamman et al., 1997; Chang et al., 2008). The differential regioselectivity of the oxidation of ibuprofen and PI is reflected in our finding that PI generated 15.6 times more 1-OH-ibuprofen in murine plasma than ibuprofen (2.5 versus 0.16%).
The CYP450 superfamily, and particularly CYP2C9, plays a major role in the regioselective oxidation of ibuprofen (Hamman et al., 1997; Chang et al., 2008). The molecular basis of regioselectivity of CYP2C9 has been examined in the case of the anticoagulant warfarin using molecular dynamics simulations (Seifert et al., 2006). Warfarin binds to the active site of CYP450 and is preferentially oxidized at its C-H bond close to the active oxygen of the heme moiety of CYP450 (Seifert et al., 2006). By analogy, we propose that the phosphate moiety of PI may affect its binding to CYP450, resulting in geometric preference of position 1 over position 2 for its oxidation by CYP450.
Glucuronidation reactions are an important part of the detoxification process (Zheng et al., 2002). The only glucuronide derivative that we detected was ibuprofen glucuronide. Such a transformation is well known for conventional ibuprofen, whose carboxylic acid group is conjugated with the hydroxyl group of UDP-glucuronic acid; this reaction is catalyzed by UDP-glucuronosyltransferases (UGTs), especially UGT2B7 and UGT1A9 (Kepp et al., 1997; Kuehl et al., 2005). Glucuronidation of PI is unlikely to occur because of the lack of a free carboxylic acid group. It is noteworthy that ibuprofen glucuronide can undergo spontaneous chemical reactions, resulting in acyl migration and irreversible binding to endogenous proteins, which can have toxicological consequences (Johnson et al., 2007).
HLM and RLM metabolize PI in much the same manner, generating an identical array of metabolites. The principal differences between them concern the rate and extent of each metabolic step, with HLM metabolizing PI faster and generating more ibuprofen and less carboxyl-PI than RLM. The faster metabolism of PI by HLM (evidenced by the much faster formation of ibuprofen by HLM) is probably caused by differences in esterase activity between HLM and RLM. Differential metabolic kinetics in RLM and HLM has been reported for other drugs. For example, p-nitrophenylacetate was hydrolyzed more rapidly by HLM than RLM, whereas the opposite was observed for isocarboxazid (Hosokawa et al., 1990; Imai et al., 2003). Taken together, these findings indicate significant differences in esterase activity between animals and humans (Kremers et al., 1981).
We determined the basic PK parameters of PI in mice and also assessed its biodistribution. PI is rapidly absorbed, metabolized, and distributed to the blood and other tissues. PI, administered at the dose of 400 mg/kg, was not detected in plasma, whereas the Cmax of its main metabolites was in the high micromolar range. All PI metabolites reached Tmax within 2 h, and their t1/2 was relatively short (<8 h), indicating an efficient elimination process; the high degree of glucuronidation probably accounts to a large extent for this rapid elimination. Even though PI was not detectable in plasma, remarkably, it was found in all other tissues that we evaluated, albeit at low concentrations; its liver concentration was the lowest, probably reflecting the high metabolic capacity of this organ. The simplest interpretation of this apparent paradox is that PI partially and briefly escapes blood esterases and that once taken up by other tissues its degradation becomes much slower. This is supported by the finding that PI at a 3-fold higher dose generated significant plasma levels of PI (12.4 μM). These results indicated that PI can indeed survive in the blood.
As a corollary, it is conceivable that protection of PI from plasma esterases would lead to higher levels of intact PI in target tissues and higher anticancer efficacy, because PI is much more potent against cancer cells than any of its metabolites, including ibuprofen. Nonspecific carboxylic ester hydrolases, the suspected culprit in the hydrolysis of PI in plasma, are grouped into carboxyesterase, arylesterase, and acetylesterase, based on their substrate specificity (Xie et al., 2008); carboxyesterase can efficiently catalyze the hydrolysis of carboxyester drugs to release free acids (Imai et al., 2003). It is of great interest that in human plasma carboxyesterase activity is either undetectable or very low compared with mice and rats (Li et al., 2005). Thus, it is reasonable to predict that in humans, who have very low plasma carboxyesterase activity, the plasma and tissue levels of PI will be high. If this proves to be the case, PI should have a stronger anticancer efficacy in humans. In agreement with this notion, irinotecan was reported to undergo much less hydrolysis by carboxyesterase in human plasma than in mouse plasma (Mathijssen et al., 2002).
The side-by-side comparison of PI to conventional ibuprofen in which these compounds were administered at equimolar doses, corresponding to the lower dose of PI was of interest: 1) both compounds generated the same metabolites in plasma; 2) the relative abundance of the two major metabolites of PI, ibuprofen and ibuprofen glucuronide, was similar between the two (89.4 versus 86.1%); and 3) of the quantitatively minor metabolites, the most striking difference concerned 1-OH-ibuprofen and to a lesser extent carboxyl-ibuprofen; PI generated 15.6-fold more 1-OH-ibuprofen and 3-fold less carboxyl-ibuprofen than ibuprofen. As discussed above, the difference in 1-OH-ibuprofen was expected. Perhaps the mechanistically most interesting observation was that PI, even though equimolar to ibuprofen, generated lower plasma AUC0–24h of ibuprofen (2.3-fold) and fewer total metabolites (2.2-fold). Characteristically, the Cmax value of ibuprofen derived from PI is approximately half that of ibuprofen derived from conventional ibuprofen administered at the equimolar dose. The explanation of these differences is not readily apparent. However, it is conceivable that these differences account, at least partially, for the greater safety of PI compared with ibuprofen.
Pharmacodynamically, there was a statistically significant association between plasma levels of ibuprofen and the antitumor effect of PI, determined as the volume of the xenograft. The association between ibuprofen levels in the xenografts and the antitumor effect of PI, however, did not reach statistical significance (p = 0.08). Whether this is caused by our small sample size or an effect by additional PI metabolites and/or effects triggered by intact PI has yet to be determined.
In conclusion, our work established the metabolic pathway of PI in vitro and in vivo, defined its PK and biodistribution in mice, and explored its PD in tumor xenografts. These results provide a pharmacological basis to explain the anticancer efficacy and safety of this promising compound, suggest areas of potential improvements in terms of drug formulation and administration, and set the stage for its further evaluation.
Acknowledgments
We thank R. Rieger and T. Koller (Stony Brook University) for the expert LC-MS/MS analysis of our samples.
This work was supported by the National Institutes of Health National Cancer Institute [Grants STTR-CA156920A, R01-CA153662 and Contract 1N01-CN43302WA22].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.180224.
- NSAID
- nonsteroidal anti-inflammatory drug
- COX
- cyclooxygenase
- PI
- phospho-ibuprofen
- RLM
- rat liver microsomes
- HLM
- human liver microsomes
- PK
- pharmacokinetics
- PD
- pharmacodynamics
- HPLC
- high-performance liquid chromatography
- UGT
- UDP-glucuronosyltransferase
- MS
- mass spectrometry
- MS/MS
- tandem MS
- AUC
- area under the concentration-time curve
- PBS
- phosphate-buffered saline.
Authorship Contributions
Participated in research design: Xie, Sun, and Rigas.
Conducted experiments: Xie, Sun, Nie, Mackenzie, and Huang.
Contributed new reagents or analytic tools: Kopelovich and Komninou.
Performed data analysis: Xie, Sun, Nie, Mackenzie, Huang, and Rigas.
Wrote or contributed to the writing of the manuscript: Xie, Kopelovich, Komninou, and Rigas.
References
- American Cancer Society (2008) Cancer Facts and Figures 2008. American Cancer Society; Atlanta, GA [Google Scholar]
- Antonicelli L, Tagliabracci A. (1995) Asthma death induced by ibuprofen. Monaldi Arch Chest Dis 50:276–278 [PubMed] [Google Scholar]
- Bajrami B, Zhao L, Schenkman JB, Rusling JF. (2009) Rapid LC-MS drug metabolite profiling using microsomal enzyme bioreactors in a parallel processing format. Anal Chem 81:9921–9929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SY, Li W, Traeger SC, Wang B, Cui D, Zhang H, Wen B, Rodrigues AD. (2008) Confirmation that cytochrome P450 2C8 (CYP2C8) plays a minor role in (S)-(+)- and (R)-(−)-ibuprofen hydroxylation in vitro. Drug Metab Dispos 36:2513–2522 [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. (2002) Inflammation and cancer. Nature 420:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamman MA, Thompson GA, Hall SD. (1997) Regioselective and stereoselective metabolism of ibuprofen by human cytochrome P450 2C. Biochem Pharmacol 54:33–41 [DOI] [PubMed] [Google Scholar]
- Harris RE, Beebe-Donk J, Doss H, Burr Doss D. (2005) Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review). Oncol Rep 13:559–583 [PubMed] [Google Scholar]
- Harris RE, Namboodiri KK, Farrar WB. (1996) Nonsteroidal antiinflammatory drugs and breast cancer. Epidemiology 7:203–205 [DOI] [PubMed] [Google Scholar]
- Hosokawa M, Maki T, Satoh T. (1990) Characterization of molecular species of liver microsomal carboxylesterases of several animal species and humans. Arch Biochem Biophys 277:219–227 [DOI] [PubMed] [Google Scholar]
- Huang L, Mackenzie G, Ouyang N, Sun Y, Xie G, Johnson F, Komninou D, Rigas B. (2011) The novel phospho-non-steroidal anti-inflammatory drugs, OXT-328, MDC-22 and MDC-917, inhibit adjuvant-induced arthritis in rats. Br J Pharmacol 162:1521–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Yoshigae Y, Hosokawa M, Chiba K, Otagiri M. (2003) Evidence for the involvement of a pulmonary first-pass effect via carboxylesterase in the disposition of a propranolol ester derivative after intravenous administration. J Pharmacol Exp Ther 307:1234–1242 [DOI] [PubMed] [Google Scholar]
- Johnson CH, Wilson ID, Harding JR, Stachulski AV, Iddon L, Nicholson JK, Lindon JC. (2007) NMR spectroscopic studies on the in vitro acyl glucuronide migration kinetics of Ibuprofen ((+/−)-(R,S)-2-(4-isobutylphenyl) propanoic acid), its metabolites, and analogues. Anal Chem 79:8720–8727 [DOI] [PubMed] [Google Scholar]
- Kepp DR, Sidelmann UG, Hansen SH. (1997) Isolation and characterization of major phase I and II metabolites of ibuprofen. Pharm Res 14:676–680 [DOI] [PubMed] [Google Scholar]
- Kremers P, Beaune P, Cresteil T, de Graeve J, Columelli S, Leroux JP, Gielen JE. (1981) Cytochrome P-450 monooxygenase activities in human and rat liver microsomes. Eur J Biochem 118:599–606 [DOI] [PubMed] [Google Scholar]
- Kuehl GE, Lampe JW, Potter JD, Bigler J. (2005) Glucuronidation of nonsteroidal anti-inflammatory drugs: identifying the enzymes responsible in human liver microsomes. Drug Metab Dispos 33:1027–1035 [DOI] [PubMed] [Google Scholar]
- Lanza FL. (1984) Endoscopic studies of gastric and duodenal injury after the use of ibuprofen, aspirin, and other nonsteroidal anti-inflammatory agents. Am J Med 77:19–24 [DOI] [PubMed] [Google Scholar]
- Li B, Sedlacek M, Manoharan I, Boopathy R, Duysen EG, Masson P, Lockridge O. (2005) Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem Pharmacol 70:1673–1684 [DOI] [PubMed] [Google Scholar]
- Liu D, Zheng X, Tang Y, Zi J, Nan Y, Wang S, Xiao C, Zhu J, Chen C. (2010) Metabolism of tanshinol borneol ester in rat and human liver microsomes. Drug Metab Dispos 38:1464–1470 [DOI] [PubMed] [Google Scholar]
- Mackenzie GG, Sun Y, Huang L, Xie G, Ouyang N, Gupta RC, Johnson F, Komninou D, Kopelovich L, Rigas B. (2010) Phospho-sulindac (OXT-328), a novel sulindac derivative, is safe and effective in colon cancer prevention in mice. Gastroenterology 139:1320–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marnett LJ. (1995) Aspirin and related nonsteroidal anti-inflammatory drugs as chemopreventive agents against colon cancer. Prev Med 24:103–106 [DOI] [PubMed] [Google Scholar]
- Martignoni M, Groothuis GM, de Kanter R. (2006) Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol 2:875–894 [DOI] [PubMed] [Google Scholar]
- Mathijssen RH, Verweij J, de Bruijn P, Loos WJ, Sparreboom A. (2002) Effects of St. John's wort on irinotecan metabolism. J Natl Cancer Inst 94:1247–1249 [DOI] [PubMed] [Google Scholar]
- McElwee NE, Veltri JC, Bradford DC, Rollins DE. (1990) A prospective, population-based study of acute ibuprofen overdose: complications are rare and routine serum levels not warranted. Ann Emerg Med 19:657–662 [DOI] [PubMed] [Google Scholar]
- Meier CR, Schmitz S, Jick H. (2002) Association between acetaminophen or nonsteroidal antiinflammatory drugs and risk of developing ovarian, breast, or colon cancer. Pharmacotherapy 22:303–309 [DOI] [PubMed] [Google Scholar]
- Piazza GA, Keeton AB, Tinsley HN, Gary BD, Whitt JD, Mathew B, Thaiparambil J, Coward L, Gorman G, Li Y, et al. (2009) A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prev Res (Phila) 2:572–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley TR, 3rd, Smith JP. (1998) Ibuprofen-induced hepatotoxicity in patients with chronic hepatitis C: a case series. Am J Gastroenterol 93:1563–1565 [DOI] [PubMed] [Google Scholar]
- Rudy AC, Knight PM, Brater DC, Hall SD. (1991) Stereoselective metabolism of ibuprofen in humans: administration of R-, S- and racemic ibuprofen. J Pharmacol Exp Ther 259:1133–1139 [PubMed] [Google Scholar]
- Seifert A, Tatzel S, Schmid RD, Pleiss J. (2006) Multiple molecular dynamics simulations of human p450 monooxygenase CYP2C9: the molecular basis of substrate binding and regioselectivity toward warfarin. Proteins 64:147–155 [DOI] [PubMed] [Google Scholar]
- Ulrich CM, Bigler J, Potter JD. (2006) Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer 6:130–140 [DOI] [PubMed] [Google Scholar]
- Xie G, Liu M, Zhu H, Lei B. (2008) Esterase SeE of Streptococcus equi ssp. equi is a novel nonspecific carboxylic ester hydrolase. FEMS Microbiol Lett 289:181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Fang JL, Lazarus P. (2002) Glucuronidation: an important mechanism for detoxification of benzo[a]pyrene metabolites in aerodigestive tract tissues. Drug Metab Dispos 30:397–403 [DOI] [PubMed] [Google Scholar]