Abstract
Bombesin is a pruritogenic agent that causes intense itch-scratching activity in rodents. Bombesin has high affinity for the gastrin-releasing peptide (GRP) receptor (GRPr) and the neuromedin B (NMB) receptor (NMBr). The aim of this study was to investigate pharmacologically the ability of GRPr and NMBr to elicit scratching behavior in rats. The intracerebroventricular route was selected for drug delivery because the study focused on supraspinal sites of action. The magnitude and duration of scratching produced by the naturally occurring peptides GRP and NMB were characterized. Antagonists selective for GRPr [(d-Tpi6, Leu13Ψ(CH2-NH)-Leu14)Bombesin(6-14) (RC-3095)] and NMBr [(S)-α-methyl-α-[[[(4-nitrophenyl)amino]carbonyl]amino]-N-[[1-(2-pyridinyl)cyclohexyl]methyl]-1H-indole-3-propanamide (PD168368)] were used to define the role of GRPr and NMBr in the scratching response. After intracerebroventricular administration, GRP (0.03–0.3 nmol) and NMB (0.1–1 nmol) dose-dependently elicited marked scratching. There was a tolerance to scratching elicited by daily repeated administration of bombesin, GRP, or NMB. Presession administration of RC-3095 (0.1–1 nmol) and PD168368 (0.3–3 nmol) dose-dependently antagonized scratching elicited by GRP and NMB, respectively. More importantly, 1 nmol of RC-3095 failed to block NMB-elicited scratching, and 3 nmol of PD168368 failed to block GRP-elicited scratching. In addition, pretreatment with effective doses of RC-3095 or PD168368 alone or in combination did not block bombesin-elicited scratching. Through the use of the selective antagonists RC-3095 and PD168368, this study demonstrates that central GRPr and NMBr act independently to elicit scratching behavior and there is an additional, unidentified receptor mechanism underlying bombesin-elicited scratching.
Introduction
Itch (pruritus) is an unpleasant sensation that elicits a desire or reflex to scratch, and it is the most common symptom in skin diseases. According to a review of epidemiological data, itch is also one of key symptoms in patients suffering from a variety of systemic disorders including infectious, uremic, hepatic, and hematological diseases (Weisshaar and Dalgard, 2009). Although the causes of itch involve factors such as disease, age, and ethnicity, the symptoms of itch are highly prevalent and represent a medical burden in the global community (Weisshaar and Dalgard, 2009; Metz and Ständer, 2010; Patel and Yosipovitch, 2010). Thus, there is a strong need for more research into the cause and pharmacological treatment of itch.
Although animal models of itch may not simulate the exact dermatological diseases that can cause itch, laboratory animals can be used to study the role of receptors and neurons in the itch signaling transmission. This can help identify potential treatments for itch. For example, the μ-opioid receptor in the central nervous system has been identified as a mediator of itch sensation. Central administration of μ-opioid receptor agonists such as morphine can elicit scratching responses in primates (Ko and Naughton, 2000; Ko et al., 2004). Pretreatment with μ-opioid receptor antagonists, but not histamine receptor antagonists, attenuates opioid-induced scratching, indicating the effectiveness of μ-opioid receptor antagonists as antipruritics in this context (Ko et al., 2004; Ganesh and Maxwell, 2007). Therefore, it is valuable to evaluate potential pruritogenic agents in behaving animals to determine receptor-mediated itch responses and the therapeutic potential of agents that block these receptors. These findings may result in clinical trials and treatments in humans.
An intriguing finding from a previous study is that the central administration of bombesin elicits much more scratching response than morphine in rats (Lee et al., 2003). Bombesin, a tetradecapeptide originally isolated from frog skin (Anastasi et al., 1971), is a proposed pruritogenic agent that causes the most intense scratching activity after central administration in rodents (Katz, 1980; Gmerek and Cowan, 1983; Lee et al., 2003). Unlike other ligands such as substance P and nociception/orphanin FQ, which elicit scratching in rodents but not in monkeys (Frenk et al., 1988; Inoue et al., 1999; Ko and Naughton, 2009), bombesin is the only ligand reported other than μ-opioid receptor agonists that can elicit scratching across different species including monkeys (Cowan et al., 1985; Ko et al., 2004). Bombesin has relatively high binding affinity for both bombesin receptor subtypes: the neuromedin B (NMB) receptor (NMBr) and the gastrin-releasing peptide (GRP) receptor (GRPr) (Jensen et al., 2008). Using GRPr mutant mice, it has been demonstrated that the GRPr plays an important role in mediating itch sensation as opposed to pain in the dorsal spinal cord (Sun and Chen, 2007). More importantly, another study indicated that GRPr-expressing neurons in the spinal cord subserve itch sensation but are not involved in pain behaviors (Sun et al., 2009). There was no reduction of histamine-, endothelin-1-, or 5-hydroxytryptamine-induced scratching behavior in the GRPr mutant mice. In contrast, mice with ablated GRPr-expressing neurons showed nearly complete loss of scratching responses to these pruritogenic agents. Such findings indicate that GRPr-expressing neurons are crucial for itch processing (Sun et al., 2009).
These neurobiological findings open a new avenue for pharmacological studies of central itch mediators in animals. In particular, the role of GRPr versus NMBr in the neurotransmission of itch has not been extensively elucidated. There is no pharmacological evidence showing the effectiveness of GRPr and NMBr antagonists to attenuate scratching elicited by bombesin-related peptides including bombesin, GRP, and NMB. Therefore, the aim of this study was to characterize pharmacologically the dose-response and duration of scratching elicited by bombesin-related peptides delivered centrally in rats. In addition, selective GRPr and NMBr antagonists, (d-Tpi6, Leu13Ψ(CH2-NH)-Leu14)Bombesin(6-14) (RC-3095) and (S)-α-methyl-α-[[[(4-nitrophenyl)amino]carbonyl]amino]-N-[[1- (2-pyridinyl)cyclohexyl]methyl]-1H-indole-3-propanamide (PD168368), respectively (González et al., 2009), were used to investigate the role of central GRPr and NMBr in the modulation of scratching behavior.
Materials and Methods
Animals.
Adult male Wistar rats, approximately 280 to 320 g, were purchased from BioLASCO Taiwan Co. Ltd. (Taipei, Taiwan) and housed individually. All animals had free access to food and water and were maintained on a 12-h light/dark cycle with light on at 8:00 AM in a temperature-controlled (22 ± 2°C) room. Each animal was used only once per dosing condition. All animal care and experimental procedures were conducted in accordance with the University Committee on the Use and Care of Animals at National Cheng Chi University and the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health (NIH-80-23).
Procedures.
The technique of intracerebroventricular administration was used to deliver drugs centrally because the study focused on the supraspinal site of action, which is highly relevant to the scratching response. Each animal was implanted with a stainless-steel cannula (Small Parts, Inc., Miramar, FL) in the right lateral cerebral ventricle. The detailed surgical procedure of intracerebroventricular implantation has been described previously (Lee et al., 2003; Zhang and Ko, 2009). Animals were allowed 6 days to recover from surgery. The placement of intracerebroventricular cannula was verified after the experiment by administration of methylene blue through the cannulae, followed by brain excision and location of the dye. Only data obtained from animals with correct intracerebroventricular cannula placement were used for data analysis.
The number of the scratching events was scored by individuals who were blind to the drug and dosing conditions. A scratch was defined as one short-duration episode of scraping contact by the hind paw on the skin surface of body parts around the neck and head. Rats were placed singly in Plexiglas observation boxes (56 cm long × 31 cm wide × 27 cm high) and allowed to habituate for at least 30 min. Each drug was slowly infused through the intracerebroventricular cannula. Behavioral observation started 10 min after drug administration. To distinguish scratching from grooming (i.e., movement by forepaws), scratching events were counted as those made only by the hind paws. As noted, previous studies have shown that morphine, a well known pruritogenic agent after central administration in humans (Ganesh and Maxwell, 2007), could elicit scratching, but not grooming, in rats (Thomas and Hammond, 1995; Lee et al., 2003). Therefore, the scratching event was used to quantify the itch/scratching-eliciting effects of drugs.
Experimental Designs.
All animals were randomly assigned to different dosing conditions (n = 7/condition). The first part of the study was to determine the characteristics of scratching behavior elicited by intracerebroventricular administration of GRP, NMB, and bombesin. Normally, 10 min after drug administration, the numbers of scratching events were counted in 5-min bins for 30 min. Because the dose-response curve for bombesin-elicited scratching has been established in rats (Lee et al., 2003), the initial effort was to establish dose-response curves for scratching elicited by GRP (0.03–1 nmol) and NMB (0.1–3 nmol). Then, the minimum dose that elicited the maximum possible scratching responses for each drug, i.e., GRP (0.1 nmol), NMB (1 nmol), and bombesin (0.1 nmol), was selected to determine the duration of scratching over a 3-h time course. For the time-course study, scratching responses were sampled for 5 min of every 30 min for 3 h after drug administration. In addition, the same doses were used to compare the development of tolerance to daily repeated administration for 4 days.
The second part of the study was to determine the receptor mechanism underlying the scratching behavior elicited by GRP, NMB, and bombesin. A selective GRPr antagonist, RC-3095 (0.1–1 nmol), was given intracerebroventricularly 10 min before intracerebroventricular administration of GRP (0.1 nmol). Likewise, a selective NMBr antagonist, PD168368 (0.3–3 nmol) was given 10 min before intracerebroventricular NMB (1 nmol). The number of scratching events was counted in 5-min bins for 30 min, starting 10 min after either GRP or NMB administration. In addition, a single dose of RC-3095 (1 nmol) and PD168368 (3 nmol) was used to cross-examine their antagonist effects against both GRP- and NMB-elicited scratching, to investigate whether GRP- and NMB-elicited scratching was mediated uniquely by GRPr and NMBr, respectively. Using effective doses of RC-3095 (1–3 nmol) and PD168368 (3–10 nmol), the final effort was to determine whether scratching elicited by bombesin, which has relatively high binding affinity for both GRPr and NMBr, could be blocked by pretreatment with RC-3095 or PD168368 alone or by administration of a mixture of RC-3095 and PD168368.
Data Analysis.
Mean values (mean ± S.E.M.) were calculated from individual values for each dosing condition. Data for the time course were analyzed by two-way analysis of variance (ANOVA), and data for the dose response were analyzed by one-way ANOVA followed by the Tukey test for post hoc comparisons (Statistica; StatSoft, Tulsa, OK). Data for the repeated administration were analyzed by repeated-measures ANOVA. The difference between conditions was considered significant at p < 0.05.
Drugs.
GRP [Ala-Pro-Val-Ser-Val-Gly-Gly-Gly-Thr-Val-Leu-Ala-Lys-Met-Tyr-Pro-Arg-Gly-Asn-His-Trp-Ala-Val-Gly-His-Leu-Met-NH2], NMB [Gly-Asn-Leu-Trp-Ala-Thr-Gly-His-Phe-Met-NH2], bombesin [pGlu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2] (Tocris Biosciences, Ellisville, MO), and RC-3095 (Sigma-Aldrich, St. Louis, MO) were dissolved in sterile water. PD168368 (Tocris Biosciences) was dissolved in 1% dimethyl sulfoxide (Thermo Fisher Scientific, Waltham, MA). For intracerebroventricular administration, GRP, NMB, and bombesin were slowly infused in a volume of 10 μl over 60 s. RC-3095 and PD168368 were slowly infused in a volume of 5 μl over 30 s.
Results
Figure 1 illustrates the scratching responses after intracerebroventricular administration of GRP. Within the 30-min observation period, GRP dose-dependently (F4,30 = 30.3; p < 0.05) elicited and maintained scratching. There was no time-dependent (F5,150 = 0.8; p > 0.05) effect because GRP (0.1 nmol)-induced scratching peaked in the first 5-min bin and there was no decline by the last 5-min bin. Over the dose range studied, GRP (0.03 nmol) slightly increased scratching activity, and GRP (0.1–1 nmol) elicited marked scratching responses compared with the vehicle condition (Fig. 1, bottom).
Fig. 1.
Effects of intracerebroventricular administration of GRP on scratching behavior. GRP was given 10 min before observation. Each value represents mean ± S.E.M. (n = 7). Symbols represent different dosing conditions. Because the magnitude and duration of GRP (1 nmol)-elicited scratching were similar to those of GRP (0.3 nmol), the top shows only the effects of GRP between 0.03 and 0.3 nmol for the sake of clarity. *, a significant difference from the vehicle condition (p < 0.05).
Figure 2 illustrates the scratching responses after intracerebroventricular administration of NMB. Within the 30-min observation period, NMB elicited scratching in both a dose-dependent (F4,30 = 7.9; p < 0.05) and time-dependent (F5,150 = 16.1; p < 0.05) manner. NMB (1 nmol)-elicited scratching peaked in the first 5-min bin and declined over the remaining 30 min. When scratching responses were summed over a 30-min period, NMB (1–3 nmol) elicited moderate scratching compared with the vehicle condition (Fig. 2, bottom).
Fig. 2.
Effects of intracerebroventricular administration of NMB on scratching behavior. NMB was given 10 min before observation. Each value represents mean ± S.E.M. (n = 7). Symbols represent different dosing conditions. Because the magnitude and duration of NMB (3 nmol)-elicited scratching were similar to those of NMB (1 nmol), the top shows only the effects of NMB between 0.1 and 1 nmol for the sake of clarity. *, a significant difference from the vehicle condition (p < 0.05).
Figure 3 compares the time course of intracerebroventricular bombesin-, GRP-, and NMB-elicited scratching. There were significant differences in the treatment conditions (F3,24 = 36.2; p < 0.05) and the duration of scratching (F5,120 = 17.8; p < 0.05). Bombesin (0.1 nmol)-elicited scratching lasted for 2 h. In contrast, GRP (0.1 nmol)-elicited scratching lasted for only 1 h, and NMB (1 nmol) did not elicit significantly scratching at any time point. It is worth noting that these doses of bombesin-related peptides did not significantly elicit facial wiping by the forelimbs (data not shown).
Fig. 3.
Time course of intracerebroventricular bombesin-related ligand-induced scratching. Each value represents mean ± S.E.M. (n = 7). Symbols represent different treatment conditions. Top, scratching responses were sampled as a 5-min bin at each time point illustrated on the horizontal axis. Bottom, total scratching responses sampled at six time points were summed for comparison. *, a significant difference from the vehicle condition (p < 0.05).
Figure 4 compares the development of tolerance to daily administration of these ligands. Three groups of subjects received repeated intracerebroventricular administration of bombesin (0.1 nmol), GRP (0.1 nmol), or NMB (1 nmol) four times at a 24-h interval. Each ligand's total scratching responses within a 30-min observation period were scored for comparison. There were significant differences in the treatment day for bombesin-, GRP-, and NMB-treated groups (i.e., F3,18 = 34.0, p < 0.05; F3,18 = 13.2, p < 0.05; F3,18 = 4.5, p < 0.05, respectively). Total scratching responses of bombesin significantly decreased after the second administration. Likewise, GRP-elicited scratching significantly decreased after the second administration. Although the second administration of NMB elicited similar scratching responses as the first administration, scratching responses elicited by each of these three ligands gradually subsided after the daily dosing regimen.
Fig. 4.
Development of tolerance to repeated intracerebroventricular administration of bombesin-related peptides. Each peptide (in nmol) was administered repeatedly in the same group of animals on a daily basis, i.e., with a 24-h interval. Each value represents mean ± S.E.M. (n = 7). Bars represent different repeated administration of the peptides. *, a significant difference from the responses observed on day 1 (p < 0.05). See Figs. 1 and 2 for other details.
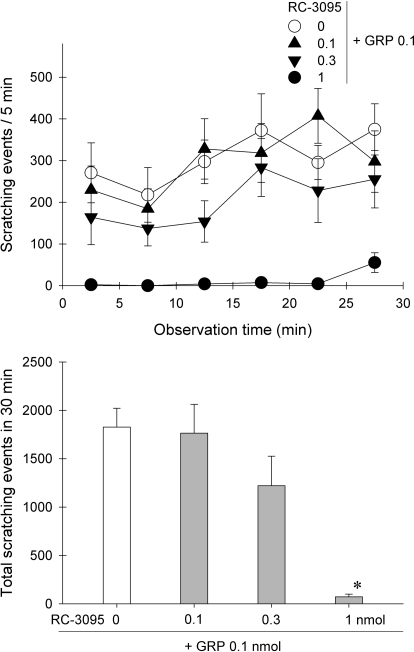
Figure 5 shows the effects of the GRPr antagonist RC-3095 against GRP-elicited scratching. After the same intracerebroventricular route, pretreatment with RC-3095 dose-dependently attenuated GRP-induced scratching (F3,24 = 11.9; p < 0.05). In particular, pretreatment with 1 nmol of RC-3095 completely blocked GRP (0.1 nmol)-elicited scratching. RC-3095 (1 nmol) did not produce motor impairment under this condition (data not shown).
Fig. 5.
Effects of the GRPr antagonist RC-3095 on intracerebroventricular GRP-elicited scratching. Pretreatment with RC-3095 (in nmol) was given through the intracerebroventricular route 10 min before administration of GRP (0.1 nmol). Each value represents mean ± S.E.M. (n = 7). Symbols represent different dosing conditions. *, a significant difference from the vehicle pretreatment group (p < 0.05).
Figure 6 shows the effects of the NMBr antagonist PD168368 against NMB-elicited scratching. After intracerebroventricular administration, pretreatment with PD168368 dose-dependently decreased NMB-induced scratching (F3,24 = 5.1; p < 0.05). Specifically, pretreatment with 3 nmol of PD168368 significantly blocked NMB (1 nmol)-elicited scratching. PD168368 (3 nmol) after intracerebroventricular administration did not produce motor impairment under this condition (data not shown).
Fig. 6.
Effects of the NMBr antagonist PD168368 on intracerebroventricular NMB-elicited scratching. Pretreatment with PD168368 (in nmol) was given through the intracerebroventricular route 10 min before administration of NMB (1 nmol). Each value represents mean ± S.E.M. (n = 7). Symbols represent different dosing conditions. *, a significant difference from the vehicle pretreatment group (p < 0.05).
Figure 7 compares the effectiveness of RC-3095 and PD168368 against both GRP- and NMB-elicited scratching. After intracerebroventricular administration, GRP (0.1 nmol)-induced scratching was significantly blocked by 1 nmol of RC-3095, but not by 3 nmol of PD168368 (F2,18 = 20.1; p < 0.05) (Fig. 7, left). In contrast, NMB (1 nmol)-induced scratching was significantly blocked by 3 nmol of PD168368, but not by 1 nmol of RC-3095 (F2,18 = 8.1; p < 0.05) (Fig. 7, right). There was no difference in scratching behavior between intracerebroventricular 1% dimethyl sulfoxide and sterile water.
Fig. 7.
Effects of GRPr and NMBr antagonists on intracerebroventricular GRP- and NMB-induced scratching. The GRPr antagonist (RC-3095, 1 nmol) or the NMBr antagonist (PD168368, 3 nmol) was given by the intracerebroventricular route 10 min before GRP or NMB. Left, effects of both antagonists on intracerebroventricular GRP (0.1 nmol)-elicited scratching. Right, effects of both antagonists on intracerebroventricular NMB (1 nmol)-elicited scratching. Each value represents mean ± S.E.M. (n = 7). *, a significant difference from the vehicle pretreatment group (p < 0.05). See Figs. 5 and 6 for other details.
Figure 8 further illustrates the effects of RC-3095 and PD168368 on bombesin-elicited scratching. After intracerebroventricular administration, pretreatment with 1 nmol of RC-3095, 3 nmol of PD168368, or a combination of RC-3095 (1 nmol) + PD168368 (3 nmol) was not effective in blocking bombesin (0.1 nmol)-induced scratching (F3,24 = 0.7; p > 0.05) (Fig. 8, A and B). In an attempt to antagonize bombesin-induced scratching, doses of RC-3095 and PD168368 were increased to three times the fully effective doses in producing GRPr and NMBr antagonist effects shown in Figs. 5 and 6. However, intracerebroventricular pretreatment with 3 nmol of RC-3095, 10 nmol of PD168368, or a combination of RC-3095 (3 nmol) + PD168368 (10 nmol) was not able to block bombesin-induced scratching (F3,24 = 0.6; p > 0.05) (Fig. 8, C and D).
Fig. 8.
Effects of GRPr and NMBr antagonists on intracerebroventricular bombesin-induced scratching. The GRPr antagonist (RC-3095) or the NMBr antagonist (PD168368) was given by the intracerebroventricular route 10 min before bombesin (0.1 nmol). A and B, effects of RC-3095 (1 nmol) or PD168368 (3 nmol) alone or in combination (1 nmol of RC-3095 and 3 nmol of PD168368) on bombesin-elicited scratching. C and D, effects of RC-3095 (3 nmol) or PD168368 (10 nmol) alone or in combination (3 nmol of RC-3095 and 10 nmol of PD168368) on bombesin-elicited scratching. Each value represents mean ± S.E.M. (n = 7). See Figs. 5 and 6 for other details.
Discussion
The first part of the study showed the basic characteristics of intracerebroventricular GRP- and NMB-elicited scratching responses in rats. Both GRP and NMB dose-dependently elicited scratching, and the scratching activity reached a plateau after administration of larger doses (Figs. 1 and 2). There are few studies characterizing the scratching-eliciting effects of bombesin-related peptides. One early study (Masui et al., 1993) showed a similar plateau effect for intracerebroventricular GRP-elicited scratching within the dose range of 0.3 and 10 μg in rats. In addition, by testing the same dose, intracerebroventricular administration of 1 μg of bombesin-related peptides all elicited scratching responses, but the degrees of scratching activities varied across different peptides (Masui et al., 1993).
By testing multiple doses, the present study established dose-response curves of both GRP and NMB and identified the minimum doses that produced maximum responses to compare the durations of action. Bombesin-elicited scratching lasted for 2 h, GRP-elicited scratching lasted for 1 h, and NMB-elicited scratching lasted for less than 30 min (Fig. 3). Although no previous study revealed the differential durations of action of these peptides in rats, one early mouse study showed similar findings (Bishop et al., 1986). Namely, intraspinal administration of bombesin produced the longest-lasting response, GRP produced an intermediate response, and NMB produced the shortest-lasting response. Bombesin has relatively high binding affinity for both GRPr and NMBr (Jensen et al., 2008; González et al., 2009). It is not clear whether bombesin compared with GRP and NMB is more resistant to degradation that contributes to a longer duration of action.
What is more interesting is that the development of tolerance to bombesin-, GRP-, and NMB-elicited scratching occurred in rats after daily repeated administration (Fig. 4). Animal studies have shown that intradermal administration of histamine exhibited significant tachyphylaxis in mice (Akiyama et al., 2009). This is consistent with the clinical evidence indicating that antihistamines are not effective in treating patients suffering from chronic itch (Feramisco et al., 2010; Metz and Ständer, 2010; Patel and Yosipovitch, 2010). On the other hand, opioid receptor antagonists are used to treat chronic itch in patients affected by various liver diseases such as cholestasis, indicating the potential involvement of endogenous opioids in some types of itch (Jones et al., 2002; Bergasa, 2008). Future studies are warranted to determine the role of GRPr and NMBr in chronic itch. For example, it is valuable to determine the effectiveness of GRPr and NMBr antagonists in the model of chronic scratching behaviors exhibited in NC/Nga mice (Matsuda et al., 1997; Tominaga et al., 2009).
Several studies have demonstrated that GRPr and NMBr are widely distributed in the central nervous system, including several brain regions and the spinal cord, as an indication of their potential role in central sensory processing (Panula et al., 1983; O'Donohue et al., 1984; Moody and Merali, 2004). However, there is no pharmacological study illustrating the interactions between agonists and antagonists selective for GRPr or NMBr. The second part of the present study is the first to demonstrate that RC-3095 and PD168368 dose-dependently antagonized GRP- and NMB-elicited scratching, respectively (Figs. 5 and 6). During a period of active research on bombesin-related peptides in the 1980s, there were no selective antagonists for GRPr and NMBr to permit pharmacological definition of the role of these receptors in any physiological function. With the availability of RC-3095 and PD168368 (Moody et al., 2000; Bajo et al., 2004; Jensen et al., 2008), such selective antagonists allow us to illustrate the complete reversal of agonist-induced scratching, which is similar to the interaction of μ-opioid receptor agonists and antagonists in modulating itch scratching responses (Ko and Naughton, 2000; Ko et al., 2004).
More importantly, cross-examination of RC-3095 and PD168368 against GRP- and NMB-elicited scratching revealed that GRPr and NMBr may act independently to mediate scratching behavior (Fig. 7). In particular, pretreatment with an effective dose of RC-3095 (1 nmol), completely blocked GRP-elicited scratching, but did not block NMB-elicited scratching. The opposite was true for PD168368. These findings indicate that the GRPr antagonism of scratching produced by RC-3095 is selective for GRPr-mediated behavior and is not confounded by motor impairment. It is noteworthy that GRPr mutant mice displayed less reduction in scratching responses to various pruritogenic ligands compared with mice with ablated GRPr neurons. This may indicate that there are additional, unidentified receptors mediating itch sensation on sensory neurons in the central nervous system of rodents (Sun and Chen, 2007; Sun et al., 2009). Supporting this notion, the present study provides the first pharmacological evidence in vivo showing that both GRPr and NMBr can mediate scratching behavior independently.
Based on the antagonist potency of RC-3095 and PD168368 obtained from the second part of the study, we further investigated what receptor mechanisms underlie bombesin-elicited scratching. We were surprised to find that pretreatment with either effective doses of RC-3095 or PD168368 alone or in combination were not effective in blocking bombesin-elicited scratching (Fig. 8, A and B). Larger doses of RC-3095 and PD168368 alone or in combination still failed to block bombesin-elicited scratching (Fig. 8, C and D). Bombesin has a relatively high binding affinity (i.e., 4–34 nM) for both GRPr and NMBr (Jensen et al., 2008). It is puzzling that the combination of RC-3095 and PD168368 in effective doses failed to block bombesin-elicited scratching. In a previous study attempting to develop a bombesin-blocking agent, a novel phyllolitorin analog, [desTrp3,Leu8]phyllolitorin, has been shown to block bombesin-elicited scratching, but this ligand did not have measurable binding affinity for bombesin receptors (Johnson et al., 1999). Antagonists selective for μ-, κ-, and δ-opioid receptors are also ineffective in blocking bombesin-elicited scratching (Lee et al., 2003). It is possible that bombesin itself or/and its metabolites act on other unidentified receptors to produce scratching. Although bombesin has a very low binding affinity for the bombesin receptor subtype 3 (Jensen et al., 2008), it is worth verifying whether an agonist selective for the bombesin receptor subtype 3 (Guan et al., 2011) after central administration can elicit scratching.
If bombesin-induced scratching responses were not GRPr-mediated, abolishing GRPr positive neurons by bombesin-saporin (Sun et al., 2009) might not be directly linked to the antipruritic effects. In other words, bombesin-recognized neurons, not GRPr-expressing neurons, mediate the itch signaling transmission. Future experiments can be initiated to determine whether there are differences in scratching responses of rodents treated with bombesin-saporin versus GRP-saporin. In particular, the scratching-eliciting effects of centrally administered bombesin, GRP, and NMB can be further investigated and compared between GRP-saporin/NMB-saporin-treated rodents and GRPr/NMBr mutant mice. These experiments can help to clarify the role of bombesin-recognized neurons versus GRPr-expressing neurons in the neurotransmission of itch. Although this study chose the intracerebroventricular rather than the intrathecal route, it is worth noting that the potency, effectiveness, and duration of action of bombesin, but not the scratching sites, are the same between intracerebroventricular and intrathecal routes in rats (Lee et al., 2003). In addition, intracerebroventricular bombesin did not affect the rat's nociceptive threshold and responses (Lai et al., 2009). Future experiments administering bombesin-saporin into the rat's brain and spinal cord can be conducted to determine whether bombesin-binding sites in the brain versus spinal cord exhibit similar physiological functions.
Taken together, this study establishes basic characteristics of centrally administered bombesin-related peptides for eliciting scratching responses in rats. Although bombesin has a longer duration of scratching than GRP and NMB, there is a tolerance to scratching elicited by daily repeated administration of bombesin, GRP, or NMB. This study is the first to provide the pharmacological evidence that central GRPr and NMBr independently mediate scratching behavior. Although the receptor mechanisms underlying bombesin-elicited scratching continues to elude us, this study not only improves our knowledge of GRPr and NMBr independently in the neurotransmission of itch, but also validates the therapeutic potential of GRPr and NMBr antagonists as antipruritics in this context. It further provides a pharmacological basis for additional, unidentified receptors in bombesin-recognized neurons in the neurotransmission of itch.
Acknowledgments
We thank Chia-Chen Cheng and Chang-Ming Chen for technical assistance with data collection; Dr. Gail Winger for assistance in the editing of the article; and Dr. John Traynor for helpful comments on the revision of the article.
This study was supported by the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases [Grant R01-AR-059193] and the Taiwan National Science Council [Grant NSC-97-2628-H-004-089-MY2].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.111.178970.
- NMB
- neuromedin B
- NMBr
- NMB receptor
- GRP
- gastrin-releasing peptide
- GRPr
- GRP receptor
- RC-3095
- (d-Tpi6,Leu13Ψ(CH2-NH)-Leu14)Bombesin(6-14)
- PD168368
- (S)-α-methyl-α-[[[(4-nitrophenyl)amino]carbonyl]amino]-N-[[1-(2-pyridinyl)cyclohexyl]methyl]-1H-indole-3-propanamide
- ANOVA
- analysis of variance.
Authorship Contributions
Participated in research design: Su and Ko.
Conducted experiments: Su.
Performed data analysis: Su and Ko.
Wrote or contributed to the writing of the manuscript: Su and Ko.
Other: Ko acquired funding for the research.
References
- Akiyama T, Merrill AW, Zanotto K, Carstens MI, Carstens E. (2009) Scratching behavior and Fos expression in superficial dorsal horn elicited by protease-activated receptor agonists and other itch mediators in mice. J Pharmacol Exp Ther 329:945–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasi A, Erspamer V, Bucci M. (1971) Isolation and structure of bombesin and alytesin, 2 analogues active peptides from the skin of the European amphibians Bombina and Alytes. Experientia 27:166–167 [DOI] [PubMed] [Google Scholar]
- Bajo AM, Schally AV, Groot K, Szepeshazi K. (2004) Bombesin antagonists inhibit proangiogenic factors in human experimental breast cancers. Br J Cancer 90:245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergasa NV. (2008) Pruritus in primary billary cirrhosis: pathogenesis and therapy. Clin Liver Dis 12:385–406 [DOI] [PubMed] [Google Scholar]
- Bishop JF, Moody TW, O'Donohue TL. (1986) Peptide transmitters of primary sensory neurons: similar actions of tachykinins and bombesin-like peptides. Peptides 7:835–842 [DOI] [PubMed] [Google Scholar]
- Cowan A, Khunawat P, Zhu XZ, Gmerek DE. (1985) Effects of bombesin on behavior. Life Sci 37:135–145 [DOI] [PubMed] [Google Scholar]
- Feramisco JD, Berger TG, Steinhoff M. (2010) Innovative management of pruritus. Dermatol Clin 28:467–478 [DOI] [PubMed] [Google Scholar]
- Frenk H, Bossut D, Urca G, Mayer DJ. (1988) Is substance P a primary afferent neurotransmitter for nociceptive input? I. Analysis of pain-related behaviors resulting from intrathecal administration of substance P and 6 excitatory compounds. Brain Res 455:223–231 [DOI] [PubMed] [Google Scholar]
- Ganesh A, Maxwell LG. (2007) Pathophysiology and management of opioid-induced pruritus. Drugs 67:2323–2333 [DOI] [PubMed] [Google Scholar]
- Gmerek DE, Cowan A. (1983) Studies on bombesin-induced grooming in rats. Peptides 4:907–913 [DOI] [PubMed] [Google Scholar]
- González N, Mantey SA, Pradhan TK, Sancho V, Moody TW, Coy DH, Jensen RT. (2009) Characterization of putative GRP- and NMB-receptor antagonist's interaction with human receptors. Peptides 30: 1473–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan XM, Metzger JM, Yang L, Raustad KA, Wang SP, Spann SK, Kosinski JA, Yu H, Shearman LP, Faidley TD, et al. (2011) Antiobesity effect of MK-5046, a novel bombesin receptor subtype-3 agonist. J Pharmacol Exp Ther 336:356–364 [DOI] [PubMed] [Google Scholar]
- Inoue M, Shimohira I, Yoshida A, Zimmer A, Takeshima H, Sakurada T, Ueda H. (1999) Dose-related opposite modulation by nociception/orphanin FQ of substance P nociception in the nociceptors and spinal cord. J Pharmacol Exp Ther 291:308–313 [PubMed] [Google Scholar]
- Jensen RT, Battey JF, Spindel ER, Benya RV. (2008) International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev 60:1–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Ko M, Choo KS, Traynor JR, Mosberg HI, Naughton NN, Woods JH. (1999) The effects of the phyllolitorin analogue [deTrp3, Leu8]phyllolitorin on scratching induced by bombesin and related peptides in rats. Brain Res 839:194–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EA, Neuberger J, Bergasa NV. (2002) Opiate antagonist therapy for the pruritus of cholestasis: the avoidance of opioid withdrawal-like reactions. QJM 95:547–552 [DOI] [PubMed] [Google Scholar]
- Katz R. (1980) Grooming elicited by intracerebroventricular bombesin and eledoisin in the mouse. Neuropharmacology 19:143–146 [DOI] [PubMed] [Google Scholar]
- Ko MC, Naughton NN. (2000) An experimental itch model in monkeys: characterization of intrathecal morphine-induced scratching and antinociception. Anesthesiology 92:795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Naughton NN. (2009) Antinociceptive effects of nociception/orphanin FQ administered intrathecally in monkeys. J Pain 10:509–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Song MS, Edwards T, Lee H, Naughton NN. (2004) The role of central μ opioid receptors in opioid-induced itch in primates. J Pharmacol Exp Ther 310:169–176 [DOI] [PubMed] [Google Scholar]
- Lai CP, Chen CM, Lin AP, Ko MC. (2009) The roles of mu opioid receptors and bombesin receptors in the modulation of pain and itch sensation, at the 24th Joint Annual Conference of Biomedical Science; 2009 March 21–22; Taipei, Taiwan p. 448 Pharmacological Society of Taiwan, Taipei, Taiwan [Google Scholar]
- Lee H, Naughton NN, Woods JH, Ko MC. (2003) Characterization of scratching responses in rats following centrally administered morphine or bombesin. Behav Pharmacol 14:501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui A, Kato N, Itoshima T, Tsunashima K, Nakajima T, Yanaihara N. (1993) Scratching behavior induced by bombesin-related peptides. Comparison of bombesin, gastrin-releasing peptide and phyllolitorins. Eur J Pharmacol 238:297–301 [DOI] [PubMed] [Google Scholar]
- Matsuda H, Watanabe N, Geba GP, Sperl J, Tsudzuki M, Hiroi J, Matsumoto M, Ushio H, Saito S, Askenase PW, et al. (1997) Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol 9:461–466 [DOI] [PubMed] [Google Scholar]
- Metz M, Ständer S. (2010) Chronic pruritus–pathogenesis, clinical aspects and treatment. J Eur Acad Dermatol Venereol 24:1249–1260 [DOI] [PubMed] [Google Scholar]
- Moody TW, Jensen RT, Garcia L, Leyton J. (2000) Nonpeptide neuromedin B receptor antagonists inhibit the proliferation of C6 cells. Eur J Pharmacol 409:133–142 [DOI] [PubMed] [Google Scholar]
- Moody TW, Merali Z. (2004) Bombesin-like peptides and associated receptors within the brain: distribution and behavioral implications. Peptides 25:511–520 [DOI] [PubMed] [Google Scholar]
- O'Donohue TL, Massari VJ, Pazoles CJ, Chronwall BM, Shults CW, Quirion R, Chase TN, Moody TW. (1984) A role for bombesin in sensory processing in the spinal cord. J Neurosci 4:2956–2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P, Hadjiconstantinou M, Yang HY, Costa E. (1983) Immunohistochemical localization of bombesin/gastrin-releasing peptide and substance P in primary sensory neurons. J Neurosci 3:2021–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T, Yosipovitch G. (2010) Therapy of pruritus. Expert Opin Pharmacother 11:1673–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. (2007) A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448:700–703 [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. (2009) Cellular basis of itch sensation. Science 325:1531–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DA, Hammond DL. (1995) Microinjection of morphine into the rat medullary dorsal horn produces a dose-dependent increase in facial scratching. Brain Res 695:267–270 [DOI] [PubMed] [Google Scholar]
- Tominaga M, Ogawa H, Takamori K. (2009) Histological characterization of cutaneous nerve fibers containing gastrin-releasing peptide in NC/Nga mice: an atopic dermatitis model. J Invest Dermatol 129:2901–2905 [DOI] [PubMed] [Google Scholar]
- Weisshaar E, Dalgard F. (2009) Epidemiology of itch: adding to the burden of skin morbidity. Acta Derm Venereol 89:339–350 [DOI] [PubMed] [Google Scholar]
- Zhang HN, Ko MC. (2009) Seizure activity involved in the up-regulation of BDNF mRNA expression by activation of central mu opioid receptors. Neuroscience 161:301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]