Abstract
Maturation of NOS enzymes requires that they incorporate heme to become active, but how this cellular process occurs is unclear. We investigated a role for chaperone heat shock protein 90 (hsp90) in enabling heme insertion into the cytokine-inducible mouse NOS. We used macrophage cell line RAW 264.7 and human embryonic kidney HEK293T cells and studied insertion of native heme during iNOS expression and insertion of exogenous heme into preformed apo-iNOS. Pulldown experiments showed that the hsp90-iNOS complex was present in cells, but the extent of their association was inversely related to iNOS heme content. Hsp90 was primarily associated with apo-iNOS monomer and was associated 11-fold less with heme-containing iNOS monomer or dimer in cells. Kinetic studies showed that hsp90 dissociation occurred coincident with cellular heme insertion into apo-iNOS (0.8 h−1). The hsp90 inhibitor radicicol or coexpression of an ATPase-defective hsp90 blocked heme insertion into apo-iNOS by 90 and 75%, respectively. The ATPase activity of hsp90 was not required for complex formation with iNOS but was essential for heme insertion to occur. We conclude that hsp90 plays a primary role in maturation of iNOS protein by interacting with the apoenzyme in cells and then driving heme insertion in an ATP-dependent manner.—Ghosh, A., Chawla-Sarkar, M., Stuehr, D. J. Hsp90 interacts with inducible NO synthase client protein in its heme-free state and then drives heme insertion by an ATP-dependent process.
Keywords: heme-deficient, heme-replete, chaperone, cytokines, cardiovascular
Heat shock protein 90 (hsp90)and its homologs are widely expressed in biology and participate in a range of functions, including gene expression, signal transduction, oncogenesis, innate immunity, and shaping of evolutionary phenotypes (1–3). Hsp90 functions through protein-protein interactions, cochaperones, and its inherent ATPase activity to help control client protein maturation, trafficking, and lifetime in cells (2–5). Among the proteins known to interact with hsp90 are the 3 mammalian NOS enzymes [neuronal (nNOS), endothelial (eNOS), and inducible (iNOS); EC 1.14.13.39; refs. 6–9]. NOS enzymes are soluble heme proteins that catalyze conversion of l-arginine to citrulline and NO (10). They share between 50 and 60% protein sequence homology and are all active as obligate homodimers (11). Possible outcomes of their hsp90 interactions have been investigated primarily for eNOS and nNOS. Hsp90 affects these enzymes in various ways, including its acting as a scaffold protein to facilitate NOS phosphorylation and its influencing NOS enzymatic activities or protein stability in cells (8, 9, 12–15). The molecular basis for the different effects of hsp90 on NOS enzymes is still mostly unclear.
Heme insertion is a critical step during maturation of NOS enzymes because it allows the protein to form a homodimer and become active for NO generation (16–18). Despite its general importance, relatively little is known about heme transport and insertion into proteins in mammalian cells (19). Our group reported that cellular heme insertion into extramitochondrial heme proteins is generally blocked by NO (17, 20) and that heme insertion into iNOS involves GAPDH potentially acting as a heme delivery protein in mammalian cells (21). Osawa and colleagues (22–24) previously uncovered a role for hsp90 in nNOS heme insertion. They primarily used the hsp90 inhibitors radicicol and geldanamycin in their studies and worked with insect cell cultures that overexpressed rat nNOS. To further elucidate hsp90 action in this important cellular process, we investigated a role for hsp90 in heme insertion into iNOS. We used mammalian cell culture models exclusively, studied cellular heme insertion into preformed apo-iNOS and into iNOS during its expression induced by cytokines, and used mechanistically distinct hsp90 and iNOS pharmacologic inhibitors and an ATPase-inactive hsp90 mutant as tools. Our results uncover new aspects that define a primary role for hsp90 during maturation of iNOS protein in mammalian cells.
MATERIALS AND METHODS
Antibodies and reagents
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Fischer Chemicals (Gibbstown, NJ, USA). Gel-filtration protein standards, hemin, clotrimazole, and hsp90 inhibitor radicicol were purchased from Sigma-Aldrich. Pyrimidine imidazole (PI) was obtained from Berlex Biosciences (Wayne, NJ, USA). Stock solutions of clotrimazole and radicicol were prepared in DMSO, and PI was dissolved in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (EPPS) buffer. cDNAs for human hsp90β and D88N-hsp90β mutant were gifts from Dr. Bill Sessa (Yale University, New Haven, CT, USA). Mouse macrophage RAW 264.7 (RAW) cells and human embryonic kidney (HEK) 293T cells were purchased from American Type Culture Collection (Manassas, VA, USA). Mouse IFN-γ was purchased from Genentech (South San Francisco, CA, USA). Rabbit polyclonal hsp90 antibody was purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-iNOS antibody was obtained from BD Biosciences Transduction Laboratories (Lexington, KY, USA) and anti-hemagglutinin (HA) antibody was purchased from Sigma-Aldrich. These antibodies were used in Western blots and immunoprecipitation assays following procedures outlined by the manufacturers. Transfection reagent lipofectamine was purchased from Invitrogen (Carlsbad, CA, USA).
Cell culture and transient transfection
RAW cells were grown in DMEM containing 10% FBS and penicillin-streptomycin. To inhibit heme biosynthesis, the cells were treated with 250 μM succinyl acetone (SA) for 48 h. The cells were induced to express iNOS with Escherichia coli LPS (50 μg/ml) and IFN-γ (10 ng/ml) in the presence or absence of Nω-nitro-l-arginine methylester (l-NAME) as described previously (20). After 15 h postinduction, the cells were treated with cycloheximide (10 μg/ml) for 30 min and then incubated with different concentrations of hemin for a constant window of 3 h or at a constant hemin concentration (5 μM) at various time points wherever indicated. To study inhibition of iNOS dimerization, clotrimazole (30 μM) or PI (1 μM) was added to RAW cells during iNOS induction as described previously (25, 26). Confluent cultures (50–60%) of HEK cells or RAW cells were transfected with expression constructs (pcd-iNOS, hsp90β, or D88N-hsp90β as indicated) using Lipofectamine. After 28 h of transient transfection, the cells were either harvested or induced with IFN-γ (10 ng/ml) and LPS (50 μg/ml) for an additional 15 h before being harvested. For some HEK transfections using pcd-iNOS, radicicol (5 μM) was added after 8 h, and the cells were cultured for an additional 20 h before being harvested. In some cases HEK cells were pretreated with SA (250 μM) and transfected with pcd-iNOS for 25 h, followed by hemin (5 μM) addition for 3 h in the presence or absence of radicicol (20 μM). Similar transfections on RAW cells were also performed with D88N-hsp90β. All transfection experiments were performed in duplicate plates, and the experiments were repeated three times with similar results.
Western blots, heme staining, and immunoprecipitation
For Western blotting, 40 μg of cell supernatants was loaded on 8% SDS-PAGE gels. Proteins were electroblotted to a PVDF membrane and probed with the respective antibodies. Heme staining of cell supernatants (250 μg) or of ADP-purified iNOS protein (20 μg) from RAW cells was performed according to the method of Klatt et al. (27). For immunoprecipitation, 500 μg of the total cell supernatants was precleared with 20 μl of protein G-Sepharose beads (Amersham Biosciences Corp., Piscataway, NJ, USA) for 1 h at 4°C, beads were pelleted, and the supernatants were incubated overnight at 4°C with 3 μg of anti-HA or anti-iNOS antibody. Protein G-Sepharose beads (20 μl) were then added and incubated for 1 h at 4°C. The beads were microcentrifuged (6000 rpm), washed 3 times with wash buffer (50 mM HEPES, pH 7.6; 100 mM NaCl; 1 mM EDTA; and 0.5% Nonidet P-40) and then boiled with SDS buffer. The supernatants were then loaded on SDS-PAGE gels for Western blotting with specific antibodies.
ADP resin binding assay and purification of iNOS
The ADP resin binding assay was performed on RAW cell supernatants under various heme deplete/replete conditions. RAW cell supernatants (500 μg) were applied to 50 μl of 2′,5′-ADP resin preequilibrated with the binding buffer (40 mM EPPS, pH 7.6; 10% glycerol; 150 mM NaCl; protease inhibitors; 3 mM DTT; 1 mM l-arginine; 4 μM H4biopterin; 4 μM FMN; and 4 μM FAD). The binding assay was performed at 4°C with orbital shaking for 1 h. The beads were collected by microcentrifugation (4000 rpm for 5 min) and then extensively washed 5 times with 1 ml of wash buffer (binding buffer containing 300 mM NaCl). The bound proteins were then stripped off the beads by boiling in Laemmli buffer (2 times the volume of beads) and loaded on SDS-PAGE gels for Western blotting with specific antibodies. Similar supernatants (500 μg) were added to 50 μl of ADP resin, bound, and washed after a similar procedure, and finally iNOS was eluted with 200 μl of elution buffer containing 8 mM NADPH. The eluted iNOS was concentrated to 50 μl on a micro-Centricon concentrator (Amicon Corp., Danvers, MA, USA) and then loaded on 8% SDS-PAGE gels and heme stained. Dimeric and monomeric iNOS was purified from various RAW cell supernatants using a 2′,5′-ADP column as described previously (16). In brief, 20 mg of cell supernatant was applied to a 0.5-ml ADP column bed that had been equilibrated with column buffer (40 mM EPPS, pH 7.6; 10% glycerol; 150 mM NaCl; protease inhibitors; 3 mM DTT; 1 mM l-arginine; 4 μM H4biopterin; 4 μM FMN; and 4 μM FAD). The column was washed with 10 ml of column buffer containing 0.3 M NaCl. iNOS was eluted with 2 ml of column buffer containing 8 mM NADPH. The eluted protein was concentrated to a 200-μl volume using a Centricon-30 Concentrator (Amicon) before injection on the gel-filtration column.
Gel-filtration chromatography
Size-exclusion chromatography was performed on purified iNOS or on cell supernatants at 4°C. The column was equilibrated at 0.5 ml/min with 40 mM EPPS buffer, pH 7.6, containing 3 mM DTT, 10% glycerol, and 150 mM NaCl. ADP resin-purified iNOS samples or transfected HEK supernatants were estimated by fractionating equal amounts of 100-μl samples on a Superdex 200 gel-filtration column at 4°C. Protein in the column effluent was detected at 280 nm using a flow-through detector. Aliquots of each column fraction were subjected to SDS-PAGE and Western blotting with specific antibodies. The molecular weights of the protein fractions were estimated relative to gel-filtration molecular weight standards. The dimer-monomer distribution in iNOS samples was estimated on the basis of relative peak heights on the chromatogram and band intensities on iNOS Western blots using ImageJ quantification software (U.S. National Institutes of Health, Bethesda, MD, USA).
In vitro reconstitution assay
iNOS activity was determined by measuring production of nitrite alone or nitrite plus nitrate (stable oxidation products of NO that accumulate quantitatively) in 60-min incubations run at 37°C. Aliquots (50 μl) of ADP resin-purified iNOS from FPLC column fractions or from RAW cell supernatants were transferred to microwells containing 40 mM Tris buffer (pH 7.8) supplemented with 3 mM DTT, 2 mM l-arginine, 1 mM NADPH, protease inhibitors, and a 4 μM concentration each of FAD, FMN, and H4biopterin, to give a final volume of 0.1 ml. Reactions were terminated by enzymatic depletion of the remaining NADPH (16). Nitrite production was quantitated by a colorimetric method based on the Griess reaction, as described previously (28).
Measurement of cellular NO synthesis
NO synthesis activity was determined using the spectrophotometric oxyhemoglobin assay. The NO-mediated conversion of oxyhemoglobin to methemoglobin was used to determine the rate of NO release from RAW or HEK cell supernatants at 37°C. The rate of NO release was calculated using the difference extinction coefficient of 38 mM−1 · cm−1 (29).
UV-visible spectroscopy
UV-visible wavelength scans were recorded at room temperature on a Cary/Shimadzu spectrophotometer (Shimadzu Scientific Instruments, Inc., Durham, NC, USA). Spectra for protein supernatants were recorded between 350 and 700 nm. Spectra were recorded before and after bubbling with CO and reduction by dithionite, and the difference spectrum was obtained by subtracting the prereduction spectrum from the postreduction spectrum.
RESULTS
Cellular Hsp90-iNOS interaction is inversely related to iNOS heme content
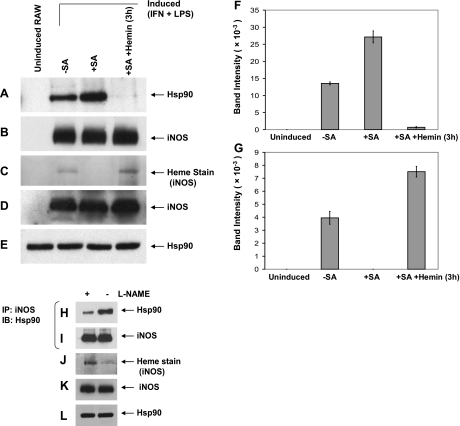
We first determined whether hsp90 was associated with iNOS in cells and whether the extent of their association was influenced by whether heme insertion into iNOS had taken place. Cells that were induced to express iNOS under typical culture conditions contained hsp90 and iNOS in a complex that was stable enough to be recovered from the cell supernatant by pulldown using 2′,5′-ADP affinity resin (Fig. 1A, B, F) or by immunoprecipitation (Fig. 1H, I). The amount of hsp90 that was associated with iNOS changed, depending on the culture conditions. It was increased 2 times when the iNOS was expressed in cells with SA-induced heme deficiency, which only expressed heme-free iNOS (apo-iNOS; Fig. 1A–C, F, G), and, conversely, was decreased 3 times when iNOS was expressed in cultures treated with l-NAME, which blocks NO synthesis and thus caused the cells to express a greater proportion of heme-containing iNOS (ref. 17; Fig. 1H–J). When heme was given to the SA-treated cells, it became incorporated into apo-iNOS, and this greatly diminished the amount of hsp90 found to be associated with iNOS in the cells (Fig. 1A–C, F, G). We obtained similar results when the hsp90-iNOS complex was recovered from the cell supernatants by immunoprecipitation using anti-iNOS Ab rather than with the affinity resin (Supplemental Fig. S1). Western blot analysis of the various cell supernatants showed that they contained equal contents of total hsp90 or iNOS proteins (Fig. 1D, E, K, L) and that the 2′,5′-ADP resin or the protein G-Sepharose isolated equal amounts of total iNOS (Fig. 1B, I). Taken together, the results indicate that hsp90 can form a relatively stable complex with iNOS in cells and that their association may be influenced by the iNOS heme content.
Figure 1.
The hsp90-iNOS interaction correlates with the heme content of iNOS. A–G) RAW cells were pretreated with or without SA for 48 h and then induced for 15 h to express iNOS. Cells were then given buffer or 5 μM hemin for an additional 3 h. Cell supernatants were prepared, and equivalent protein amounts were subject to ADP resin pulldown and/or SDS-PAGE. A, B) Western blot detection of hsp90 (A) and iNOS (B) in the ADP resin pulldowns. C) Heme staining of the iNOS band in 500 μg of protein of each cell supernatant. D, E) Western blot detection of total iNOS (D) and hsp90 protein (E) in the supernatants. F, G) Densitometric measures of bound hsp90 (F) and iNOS heme (G) from A and C, respectively. H–L) RAW cells were induced to express iNOS for 15 h, with (+) or without (−) l-NAME. H, I) Western blot detection of immunoprecipitated hsp90 (H) and iNOS (I) (input 20%) from supernatants. J–L) Heme stain of iNOS in supernatants (J) and the expression levels of hsp90 (K) and iNOS (L). IB, immunoblot; IP, immunoprecipitation.
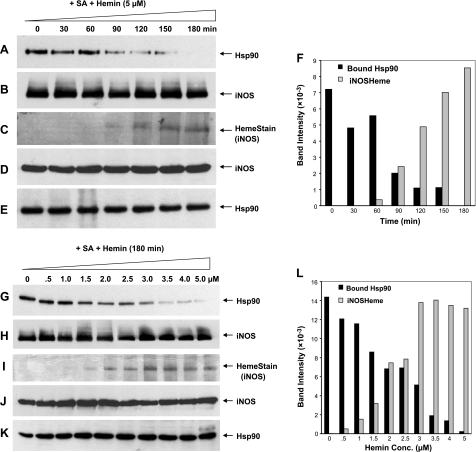
To further refine the relationship, we monitored the level of hsp90 associated with iNOS over the time frame of a typical heme-insertion reaction, using cells that had accumulated a preformed pool of apo-iNOS (Fig. 2A–F). We also performed a hemin dose-response study over a concentration range known to control the extent of heme insertion into the accumulated apo-iNOS (ref. 20; Fig. 2G–L). Under both circumstances, we observed an inverse correlation between the level of hsp90 associated with iNOS and the amount of heme that became incorporated into iNOS during the experiment. The inverse relationship is most apparent from the gel band densitometry scans (Fig. 2F, L). Results from a replica hemin-dosing experiment in which the hsp90-iNOS complex was recovered by immunoprecipitation yielded similar results (Supplemental Fig. S2). Cellular expression of hsp90 or iNOS proteins was similar across the experiments, and an equivalent amount of total iNOS was recovered from each sample (Fig. 2B, D, E, H, J, K). Our results suggest that hsp90 exists in a stable complex with apo-iNOS in cells, and this complex becomes destabilized when heme is inserted into iNOS.
Figure 2.
The hsp90-iNOS interaction during heme insertion and the heme concentration dependence. RAW cells were treated with SA and then induced for 15 h to express apo-iNOS. A–F) Hemin (5 μM) was added to the cells, supernatants were prepared at the indicated times, and equal protein amounts were subjected to ADP resin pulldown and/or SDS-PAGE. G–L) Indicated hemin concentrations were added, supernatants were prepared after 3 h, and equal protein amounts were subjected to ADP resin pulldown and/or SDS-PAGE. A, G) Western blots showing hsp90 in each ADP pulldown. B, H) Western blots showing iNOS in each ADP pulldown (input 25%). C, I) Heme stain of the iNOS band in the ADP pulldowns. D, E, J, K) Western blots showing expression levels of total iNOS (D, J) and hsp90 (E, K) in the supernatants. F, L) Densitometric quantification of bands in A and C (F) and G and I (L).
Factors regulating hsp90 interaction with apo-iNOS
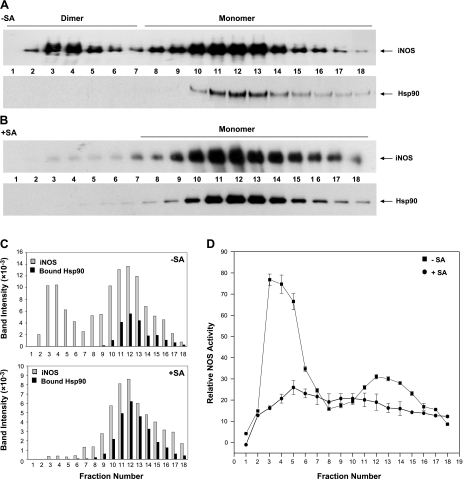
During the process of iNOS maturation in cells, heme must become inserted into apo-iNOS monomers before they can form a dimer and become an active enzyme (17). To understand how hsp90 association is related to iNOS dimer assembly, we isolated iNOS from activated cells using 2′,5′-ADP resin and then performed size-exclusion chromatography to determine how the level of associated hsp90 changes relative to the iNOS dimer (heme-containing) to monomer (heme-free) ratio. Figure 3A shows that the iNOS was recovered from activated RAW cells as a mixture of dimers and monomers (estimated to be a 2:3 ratio by densitometry), consistent with previous reports (17) and also had some associated hsp90. We know that the hsp90 present in these fractions had been associated with iNOS because identical processing of an iNOS-free supernatant from noninduced cells showed that no hsp90 was captured by the 2′,5′-ADP resin in that case (Supplemental Fig. S3). In addition, the elution profiles of the iNOS and hsp90 proteins in Fig. 3A indicate that the two proteins dissociated from one another before our running of the pulldown samples on the size-exclusion column.
Figure 3.
Hsp90 complexes with monomeric iNOS. ADP resin pulldowns of iNOS from SA- or vehicle-pretreated, activated RAW cells (+SA and −SA) were run on an FPLC size-exclusion column, and the fractions were assayed for iNOS activity and analyzed for iNOS and associated hsp90 by SDS-PAGE and Western blotting. A, B) Western blot analysis of iNOS dimers and monomers (top panel) and associated hsp90 (bottom panel) present in the vehicle-treated (A) and SA-treated (B) fractions. C) Densitometric quantification for iNOS and associated hsp90 protein bands in the fractions of A and B. D) Relative iNOS NO synthesis activities in the column fractions from A and B.
Figure 3B shows a similar gel-filtration analysis of an affinity resin pulldown sample that contained apo-iNOS expressed in heme-deficient cells. In this case, almost all of the iNOS was present as a heme-free monomer, and the sample contained a relatively greater amount of associated hsp90 than the sample pulldown from heme-replete cells (Fig. 3C). The measured NO synthesis activities of the column samples (Fig. 3D) are consistent with the monomeric iNOS fractions being either mostly (−SA) or completely (+SA) heme free. Taken together, the data suggest that hsp90 may only form a stable complex with monomeric iNOS in cells.
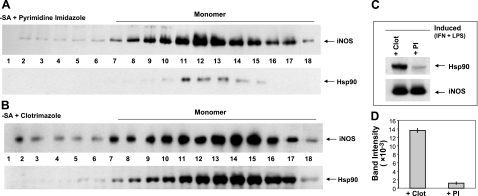
To test this suggestion further, we used two mechanistically distinct iNOS dimerization inhibitors, clotrimazole and PI (25, 30). Clotrimazole prevents cells from inserting heme into apo-iNOS (25) and thus blocks any subsequent iNOS dimer formation by default. In contrast, PI allows cells to insert heme into the apo-iNOS monomer but then binds very tightly to the heme-containing iNOS monomers and sterically prevents their dimerization (26, 30). We induced expression of iNOS in the presence of either inhibitor, isolated the iNOS with 2′,5′-ADP resin, and performed gel-filtration analysis. Both samples contained iNOS in a predominantly monomeric form (Fig. 4A, B). However, the two samples differed greatly with respect to their content of associated hsp90, which was considerable in the clotrimazole-treated cells but almost undetectable in the PI-treated cells (11-fold decrease; Fig. 4). These data establish the fact that hsp90 formed a more stable complex with apo-iNOS monomer in cells and, furthermore, suggest that hsp90 dissociates from iNOS monomer once heme insertion occurs, before subsequent dimer formation.
Figure 4.
Heme-containing and heme-free iNOS monomers display markedly different hsp90 association. iNOS expression in RAW cells was induced in the presence of 30 μM clotrimazole (Clot) or 1 μM PI, supernatants were prepared, and equivalent protein amounts were subjected to ADP pulldown. Pulldowns were then fractionated by gel filtration, and column fractions were analyzed by SDS-PAGE and Western blotting. A, B) Western blot analysis of iNOS (top panel, input 25%) and associated hsp90 (bottom panel) present in the IP-treated (A) and Clot-treated (B) fractions. C) SDS-PAGE and Western blot analysis of the iNOS and hsp90 proteins present in equal total protein amounts of ADP pulldown samples. D) Densitometric quantification of bound hsp90 from C.
Radicicol inhibits heme insertion into iNOS
Radicicol binds to hsp90 and inhibits its ATPase activity, thus blocking the hsp90 chaperone function for a number of client proteins (31, 32). We tested whether radicicol would inhibit iNOS heme insertion if it was added to cells during expression of iNOS in either transfected HEK cells or in cytokine-activated RAW cells (Fig. 5A, B). Addition of radicicol had no effect on total iNOS protein expression but did inhibit heme insertion into iNOS by 75–90% under both circumstances, as judged by measuring the iNOS NO synthesis activity or by a spectroscopic measure of its bound heme (Fig. 5A, B). Radicicol also inhibited iNOS dimer formation in the same experiments (Supplemental Fig. S4), consistent with its inhibiting heme insertion into iNOS.
Figure 5.
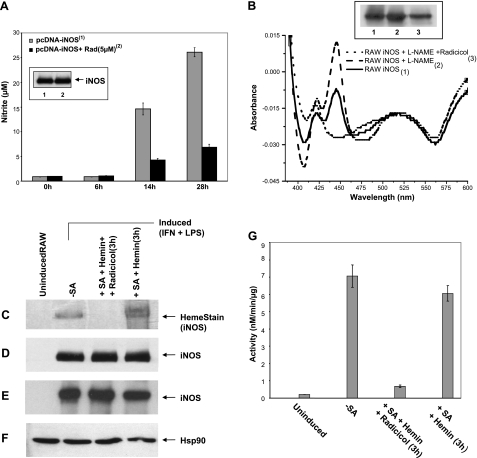
Radicicol inhibits cells from inserting native or exogenous heme into iNOS. A) HEK293T cells were transiently transfected to express iNOS in the presence or absence of radicicol (RAD, 5 μM) and active iNOS dimer content vs. time was determined by nitrite accumulation in the culture medium. Inset: total iNOS protein expression at 28 h determined by SDS-PAGE and Western blotting. B) RAW cells were induced to express iNOS with or without radicicol (5 μM) and with or without the NO synthesis inhibitor l-NAME. Supernatants were prepared, and equivalent protein contents were subjected to ADP pulldown. iNOS heme content (444 nm absorbance) and total iNOS protein (inset) were measured. C–G) RAW cells pretreated with vehicle or SA were induced to express iNOS and then were treated for 3 h with hemin (5 μM) or hemin plus radicicol (20 μM). Supernatants were prepared, and equivalent protein was subjected to ADP pulldown and/or SDS-PAGE and Western blot analysis. C) Heme stain of the iNOS band after ADP pulldown from each supernatant and SDS-PAGE. D) Total iNOS in ADP pulldown as determined by SDS-PAGE and Western blotting (input 25%). E, F) Expression levels of iNOS (E) and hsp90 (F) in the supernatants, as determined by SDS-PAGE and Western blotting. G) iNOS NO synthesis activity in the supernatants.
We next tested whether radicicol would inhibit heme insertion into preformed apo-iNOS protein that had accumulated in heme-deficient cells. RAW or HEK cells were induced or transfected, respectively, under heme-deficient conditions to express apo-iNOS, and the cells were then treated with hemin alone or with hemin plus radicicol for an additional 3 h. Radicicol blocked heme insertion into the preexisting apo-iNOS by 90% in both cases (Fig. 5C–G; Supplemental Fig. S5). The ability of radicicol to quickly inhibit heme insertion into preformed apo-iNOS suggests that the ATPase activity of the iNOS-associated hsp90 is critical for the heme-insertion process.
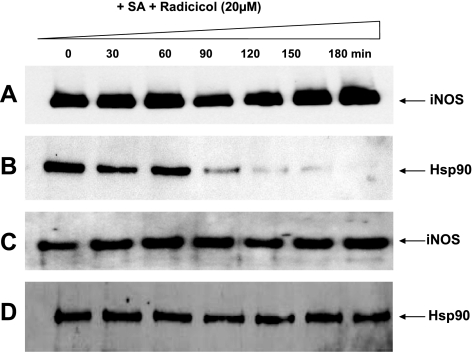
To probe a possible alternative mechanism of action, we examined whether radicicol has an impact on the stability of the hsp90-apo-iNOS complex in the absence of heme insertion. We used 2′,5′-ADP resin to pull down apo-iNOS from heme-deficient cells at various times after addition of radicicol and compared the extent of hsp90 association. As shown in Fig. 6, radicicol caused disruption of the hsp90-apo-iNOS complex, but significant dissociation was only seen after 90 min of radicicol exposure. There was no accompanying effect on the levels of iNOS or hsp90 protein expression. The results suggest that ATPase activity is needed for hsp90 to maintain its interaction with apo-iNOS in cells. However, the destabilizing effect of radicicol was delayed and too slow to explain how radicicol can almost completely inhibit heme insertion into apo-iNOS in the 3-h heme insertion experiment. This result suggests that hsp90 ATPase activity is key when in complex with apo-iNOS to drive the heme insertion process.
Figure 6.
The Hsp90-iNOS complex is slowly disrupted in the presence of radicicol. SA-pretreated RAW cells were induced to express iNOS for 15 h and then were treated with radicicol (20 μM). Supernatants were prepared at the indicated times, and equivalent protein amounts were subjected to ADP pulldown and/or SDS-PAGE and Western blot analysis. A, B) iNOS in ADP pulldowns (A; input 25%) and associated hsp90 (B). C, D) Expression levels of iNOS (C) and hsp90 (D) in the supernatants.
An ATPase-defective hsp90 mutant (D88N) antagonizes heme insertion
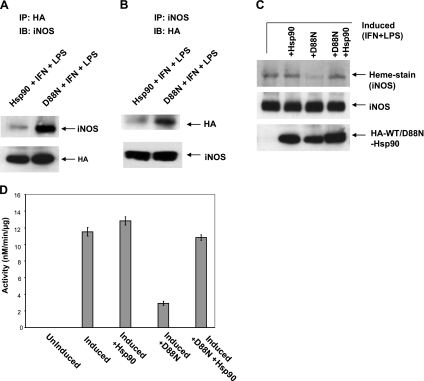
To further examine the role of hsp90 ATPase activity in heme insertion, we used the D88N mutant of hsp90, which has no ATPase activity but is known to form in-cell complexes with client proteins such as eNOS (33). We transfected the D88N mutant and/or wild-type hsp90β (both HA-tagged) into RAW cells for 28 h and then induced the cells to express iNOS for an additional 15 h. Immunoprecipitation showed that the HA-tagged D88N-hsp90 formed a complex with iNOS in cells, possibly to a greater extent than did the HA-tagged wild-type hsp90 (Fig. 7A, B). There was an accompanying 75% reduction in iNOS heme incorporation associated with D88N-hsp90 expression as determined by three independent measures, without any impact on the iNOS protein expression levels (Fig. 7C, D; Supplemental Fig. S6A). Moreover, the inhibition caused by the D88N mutant could be overcome by coexpressing the wild-type hsp90 (Fig. 7C, D; Supplemental Fig. S6A). We obtained similar results by transfecting HEK with iNOS in combination with wild-type hsp90 or D88N mutant or both (Supplemental Fig. S7A, B). Again, the inhibition of iNOS heme insertion by the D88N mutant (as measured by NO synthesis activity) was rescued by coexpression of wild-type hsp90. We also studied how D88N-hsp90 would have an impact on heme insertion into preformed apo-iNOS. In this case, the hsp90-transfected cells were induced to express apo-iNOS under heme-deficient conditions, heme was added, and its incorporation was assessed after an additional 3 h. Expression of D88N-hsp90 inhibited heme incorporation into apo-iNOS relative to the control (Supplemental Fig. S6B). Together, these results show that hsp90 does not require a functional ATPase activity to complex with apo-iNOS, but once they associate the ATPase activity is required to drive heme insertion.
Figure 7.
An ATPase-defective hsp90 mutant (D88N) associates with apo-iNOS but does not support heme insertion. Supernatants were prepared from RAW cells that were induced to express iNOS and in some cases also transfected to coexpress HA-tagged D88N-hsp90β, hsp90β, or both. Equivalent protein amounts were then used in immunoprecipitations and/or analyzed by SDS-PAGE and Western blotting. A, B) Immunoprecipitated hsp90β and D88N-hsp90β with associated iNOS (input 10%) and vice versa as indicated. C) Heme stain of iNOS bands from supernatants and expression levels of iNOS and HA-tagged wild-type or mutant hsp90. D) iNOS NO synthesis activity of the supernatants. IP, immunoprecipitation; IB, immunoblot; WT, wild-type.
DISCUSSION
Our study reveals that hsp90 has an essential role in maturation of iNOS protein in mammalian cells. Hsp90 performs a key post-translational step by assisting heme insertion into apo-iNOS, which is required before iNOS subunits can form a dimer and become an active NO synthase. The requirement for hsp90 was demonstrated using both pharmacologic and molecular biologic approaches and was manifest in cells that were inserting native heme during iNOS expression and in cells that were inserting added heme into a preexisting pool of apo-iNOS. Several of the time- and context-specific aspects of the hsp90-iNOS interaction that we observed during heme insertion shed new light on how hsp90 affects NO biology, as discussed below.
Working model for hsp90 function
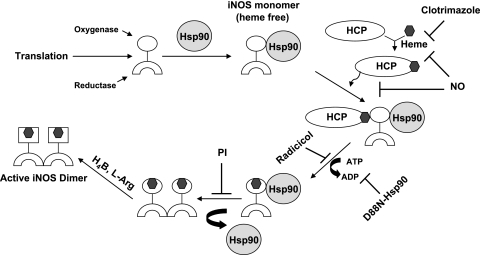
Our current data and results from the literature suggest that hsp90 functions in the following manner during maturation of iNOS protein in mammalian cells (Fig. 8). Hsp90 first binds to newly formed apo-iNOS monomers, possibly by associating with the NOS oxygenase domain (12, 34), to form a relatively stable protein complex. Our data with D88N-hsp90 indicates that formation of a stable complex does not require hsp90 to have an intact ATPase activity. This can also be the case for hsp90 complex formation with other client proteins (35). Complex formation may protect apo-iNOS against proteosomal degradation as it does for nNOS (36) and may explain why apo-iNOS monomers can accumulate in cells despite their oxygenase domains being partly unfolded and susceptible to proteolysis in the heme-free state (36, 37). When hsp90 is bound to apo-iNOS, it may also stabilize the oxygenase domain in a conformation that is amenable to heme insertion. This would mimic a proposed role for hsp90 in stabilizing open cleft forms of several client proteins, including the steroid-binding glucocorticoid receptor and apo-nNOS (22). It is unclear whether any cochaperone proteins associate or are required during the heme insertion process, but this is conceivable based on general mechanisms of hsp90 function (4). Heme is likely to be delivered to the hsp90-apo-iNOS complex by a separate cytosolic heme transfer protein such as GAPDH (21). Heme insertion into apo-iNOS is a gradual process in mammalian cells (it takes place over 90 to 180 min at 37°C; ref. 20) and seems to involve at least 3 proteins (apo-iNOS, hsp90, and a heme chaperon such as GAPDH; ref. 21). The heme insertion can be inhibited by various means by NO (17, 20) or by the pharmacologic agents clotrimazole (25) or radicicol. The hsp90 associated with apo-iNOS must have functional ATPase activity for heme insertion to take place. This requirement is clear from results with the D88N-hsp90 mutant, which formed a stable hsp90-apo-iNOS complex in cells but did not support heme insertion. Conceivably, the ATPase activity might provide the energy needed to stabilize an open cleft form of the apo-iNOS and/or to drive a related energy-requiring step in the heme transfer process, much as what occurs during heme transfer among bacterial protein systems (38, 39). Once heme becomes inserted into iNOS, it appears to destabilize the hsp90-iNOS complex and lead to hsp90 dissociation. Our results with the PI inhibitor suggest that heme insertion itself promotes hsp90 to dissociate from iNOS, because dissociation still occurred despite an inability of the heme-containing iNOS monomers to dimerize. This behavior may be reminiscent of hsp90 dissociating from the steroid receptor after ligand insertion (40). It is also possible that hsp90 must dissociate from the heme-replete iNOS monomers to remove a steric block for their dimerization. Once iNOS dimers form, there is a distinct structural rearrangement that can occur within their oxygenase domains (37, 41) that allows H4biopterin and l-Arg to bind with high affinity and enable function as an NO synthase. The iNOS enzyme is active in the absence of hsp90, and so far there is no compelling evidence that hsp90 associates with iNOS dimer in cells to modulate its activity.
Figure 8.
Model for hsp90 function during heme insertion into iNOS in mammalian cells. Hsp90 binds to heme-free iNOS monomer, and the complex probably interacts with a heme carrier protein (HCP). Hsp90 uses its ATPase activity to help drive heme insertion into the oxygenase domain of iNOS. Hsp90 then dissociates, and the heme-containing iNOS monomers can form active homodimers. The heme insertion process can be blocked at different points by NO, clotrimazole, or radicicol. See text for further details.
Relationship to other hsp90 functions
Pharmacologic inhibition of hsp90 over a relatively long time (i.e., >10 h) or knockdown of hsp90 expression often leads to enhanced degradation of client proteins, including the NOS enzymes and soluble guanylyl cyclase (42–46). However, such changes in protein stability did not affect our study, because we observed no significant difference in iNOS protein expression among the experimental groups in our experiments and in some cases performed experiments in time frames so short (3-h heme insertion into apo-iNOS) that this possibility would be minimized or eliminated (26, 47). Therefore, the role of hsp90 in iNOS heme insertion appears to be separate from, and perhaps in addition to, its role in determining client protein stability in cells.
Hsp90 can associate with all three NOS isoforms in cells (6–8), and for eNOS and nNOS the extent of their hsp90 association can change in a dynamic way in response to external stimuli or stress (6, 13, 15, 40, 48, 49). In previous studies, it was assumed that hsp90 associated with the heme-replete NOS dimmer; consequently, what portion of hsp90 was associated with the heme-replete vs. apo-NOS protein or whether hsp90 might exhibit a preference for either form was not distinguished. Despite this, there is ample evidence in the literature that hsp90 can interact with the heme-containing eNOS and nNOS in cells. Examples include hsp90 association with eNOS that enables an Akt-1-dependent phosphorylation that activates NO synthesis (12) and the hsp90 association with nNOS that is lost after the heme site of the enzyme becomes damaged during catalysis, leading to polyubiquination and proteosomal degradation (43, 50). In light of our current findings, these hsp90 interactions with heme-replete NOS enzymes should be considered “mature-phase” interactions that may be distinct from the “early-phase” interaction of hsp90 with apo-NOS that is required for heme insertion during protein maturation. Thus, hsp90 appears capable of interacting with NOS proteins at two different points during their lifetime in cells. The early-phase hsp90 interaction that is required for heme insertion has now been demonstrated for two of the three NOS isoforms (nNOS and iNOS) and is probably also required for eNOS. In contrast, the mature-phase hsp90 interactions with heme-replete NOS enzymes are context- and isoform-specific and so may not always occur. Given this circumstance, more emphasis should be placed on the early-phase hsp90 interaction that is critical for NOS protein maturation to better understand how hsp90 and inhibitors could affect NO biosynthesis and NO signaling. This is particularly relevant, given the many current drug discovery programs that aim to develop hsp90 inhibitors as anticancer agents (51, 52). On the other hand, hsp90 inhibitors that block iNOS heme insertion might also prove to be valuable for down-modulating NO production during inflammation or pathologic disorders.
Potential crosstalk between NO and hsp90 in NOS heme insertion
NO generated from NOS enzymes or by pharmacologic agents can generally inhibit heme-insertion reactions in cells (20). For at least one heme protein (iNOS), the mechanism of NO action has been shown to involve S-nitrosylation of GAPDH, which appears to impair GAPDH function in cellular heme delivery (21). Hsp90 can also become S-nitrosylated in cells that generate NO (53). The modification occurs within the C-terminal domain of hsp90 and appears to cause a conformational change that inhibits hsp90 interaction with aha-1, which is responsible for up-regulating hsp90 ATPase activity (49, 54). We did not check whether hsp90 becomes S-nitrosylated in cell cultures in which iNOS was expressed. However, in our study it is important to note that the hsp90-iNOS interaction and its role in heme insertion were established in the absence of active iNOS or any NO production by the cells. This result precludes the possibility that S-nitrosylation of hsp90 was regulating its interaction with apo-iNOS or was modulating the hsp90 ATPase activity during heme insertion in our study. However, it is entirely possible that S-nitrosylation of hsp90 or its cochaperones (55) could occur in cells that generate or become exposed to NO during the heme insertion process, which would create another level of NO feedback regulation of cellular heme insertion and should now be investigated.
Implications
We found that hsp90 interacts almost exclusively with heme-free monomeric iNOS in cells to drive heme insertion into iNOS in an ATP-dependent process. Overall, our work places a primary emphasis on the hsp90 interaction that occurs during iNOS maturation in cells. This is a new concept, given that previous work in the field has emphasized (or assumed) hsp90 interactions with the heme-replete, mature NOS enzymes. Thus, our findings shift and broaden the focus concerning hsp90 interactions with the NOS enzymes. Because hsp90-driven heme insertion is essential for iNOS to generate NO, our findings may connect hsp90 and its potential inhibition to important biologic functions of iNOS in the immune and cardiovascular systems.
Supplementary Material
Acknowledgments
The authors thank Drs. W. Sessa and A. Papapetropoulos for providing hsp90 constructs.
This work was supported by U.S. National Institutes of Health grants CA53914, GM51491, and HL076491 (to D.J.S.).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Taipale M., Jarosz D. F., Lindquist S. (2010) HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 11, 515–528 [DOI] [PubMed] [Google Scholar]
- 2. Dezwaan D. C., Freeman B. C. (2008) HSP90: the Rosetta stone for cellular protein dynamics? Cell Cycle 7, 1006–1012 [DOI] [PubMed] [Google Scholar]
- 3. Joly A. L., Wettstein G., Mignot G., Ghiringhelli F., Garrido C. (2010) Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J. Innate Immun. 2, 238–247 [DOI] [PubMed] [Google Scholar]
- 4. Vaughan C. K., Neckers L., Piper P. W. (2010) Understanding of the Hsp90 molecular chaperone reaches new heights. Nat. Struct. Mol. Biol. 17, 1400–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wandinger S. K., Richter K., Buchner J. (2008) The Hsp90 chaperone machinery. J. Biol. Chem. 283, 18473–18477 [DOI] [PubMed] [Google Scholar]
- 6. Garcia-Cardena G., Fan R., Shah V., Sorrentino R., Cirino G., Papapetropoulos A., Sessa W. C. (1998) Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 392, 821–824 [DOI] [PubMed] [Google Scholar]
- 7. Yoshida M., Xia Y. (2003) Heat shock protein 90 as an endogenous protein enhancer of inducible nitric-oxide synthase. J. Biol. Chem. 278, 36953–36958 [DOI] [PubMed] [Google Scholar]
- 8. Kone B. C., Kuncewicz T., Zhang W., Yu Z. Y. (2003) Protein interactions with nitric oxide synthases: controlling the right time, the right place, and the right amount of nitric oxide. Am. J. Physiol. Renal Physiol. 285, F178–F190 [DOI] [PubMed] [Google Scholar]
- 9. Song Y., Cardounel A. J., Zweier J. L., Xia Y. (2002) Inhibition of superoxide generation from neuronal nitric oxide synthase by heat shock protein 90: implications in NOS regulation. Biochemistry 41, 10616–10622 [DOI] [PubMed] [Google Scholar]
- 10. Stuehr D. J., Santolini J., Wang Z. Q., Wei C. C., Adak S. (2004) Update on mechanism and catalytic regulation in the NO synthases. J. Biol. Chem. 279, 36167–36170 [DOI] [PubMed] [Google Scholar]
- 11. Daff S. (2010) NO synthase: structures and mechanisms. Nitric Oxide 23, 1–11 [DOI] [PubMed] [Google Scholar]
- 12. Fontana J., Fulton D., Chen Y., Fairchild T. A., McCabe T. J., Fujita N., Tsuruo T., Sessa W. C. (2002) Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ. Res. 90, 866–873 [DOI] [PubMed] [Google Scholar]
- 13. Clapp K. M., Peng H. M., Morishima Y., Lau M., Walker V. J., Pratt W. B., Osawa Y. (2010) C331A mutant of neuronal nitric-oxide synthase is labilized for Hsp70/CHIP (C terminus of HSC70-interacting protein)-dependent ubiquitination. J. Biol. Chem. 285, 33642–33651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fleming I. (2010) Molecular mechanisms underlying the activation of eNOS. Pflügers Arch. 459, 793–806 [DOI] [PubMed] [Google Scholar]
- 15. Sud N., Sharma S., Wiseman D. A., Harmon C., Kumar S., Venema R. C., Fineman J. R., Black S. M. (2007) Nitric oxide and superoxide generation from endothelial NOS: modulation by HSP90. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L1444–L1453 [DOI] [PubMed] [Google Scholar]
- 16. Baek K. J., Thiel B. A., Lucas S., Stuehr D. J. (1993) Macrophage nitric oxide synthase subunits. Purification, characterization, and role of prosthetic groups and substrate in regulating their association into a dimeric enzyme. J. Biol. Chem. 268, 21120–21129 [PubMed] [Google Scholar]
- 17. Albakri Q. A., Stuehr D. J. (1996) Intracellular assembly of inducible NO synthase is limited by nitric oxide-mediated changes in heme insertion and availability. J. Biol. Chem. 271, 5414–5421 [DOI] [PubMed] [Google Scholar]
- 18. Hemmens B., Gorren A. C., Schmidt K., Werner E. R., Mayer B. (1998) Haem insertion, dimerization and reactivation of haem-free rat neuronal nitric oxide synthase. Biochem. J. 332, 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Severance S., Hamza I. (2009) Trafficking of heme and porphyrins in metazoa. Chem. Rev. 109, 4596–4616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waheed S. M., Ghosh A., Chakravarti R., Biswas A., Haque M. M., Panda K., Stuehr D. J. (2010) Nitric oxide blocks cellular heme insertion into a broad range of heme proteins. Free Radic. Biol. Med. 48, 1548–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chakravarti R., Aulak K. S., Fox P. L., Stuehr D. J. (2010) GAPDH regulates cellular heme insertion into inducible nitric oxide synthase. Proc. Natl. Acad. Sci. U. S. A. 107, 18004–18009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Billecke S. S., Draganov D. I., Morishima Y., Murphy P. J. M., Dunbar A. Y., Pratt W. B., Osawa Y. (2004) The role of hsp90 in heme-dependent activation of apo-neuronal nitric-oxide synthase. J. Biol. Chem. 279, 30252–30258 [DOI] [PubMed] [Google Scholar]
- 23. Bender A. T., Silverstein A. M., Demady D. R., Kanelakis K. C., Noguchi S., Pratt W. B., Osawa Y. (1999) Neuronal nitric-oxide synthase is regulated by the Hsp90-based chaperone system in vivo. J. Biol. Chem. 274, 1472–1478 [DOI] [PubMed] [Google Scholar]
- 24. Bender A. T., Nakatsuka M., Osawa Y. (2000) Heme insertion, assembly, and activation of apo-neuronal nitric-oxide synthase in vitro. J. Biol. Chem. 275, 26018–26023 [DOI] [PubMed] [Google Scholar]
- 25. Sennequier N., Wolan D., Stuehr D. J. (1999) Antifungal imidazoles block assembly of inducible NO synthase into an active dimer. J. Biol. Chem. 274, 930–938 [DOI] [PubMed] [Google Scholar]
- 26. Panda K., Chawla-Sarkar M., Santos C., Koeck T., Erzurum S. C., Parkinson J. F., Stuehr D. J. (2005) Visualizing inducible nitric-oxide synthase in living cells with a heme-binding fluorescent inhibitor. Proc. Natl. Acad. Sci. U. S. A. 102, 10117–10122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klatt P., Pfeiffer S., List B. M., Lehner D., Glatter O., Bachinger H. P., Werner E. R., Schmidt K., Mayer B. (1996) Characterization of heme-deficient neuronal nitric-oxide synthase reveals a role for heme in subunit dimerization and binding of the amino acid substrate and tetrahydrobiopterin. J. Biol. Chem. 271, 7336–7342 [DOI] [PubMed] [Google Scholar]
- 28. Miranda K. M., Espey M. G., Wink D. A. (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5, 62–71 [DOI] [PubMed] [Google Scholar]
- 29. Panda K., Haque M. M., Garcin-Hosfield E. D., Durra D., Getzoff E. D., Stuehr D. J. (2006) Surface charge interactions of the FMN module govern catalysis by nitric-oxide synthase. J. Biol. Chem. 281, 36819–36827 [DOI] [PubMed] [Google Scholar]
- 30. Blasko E., Glaser C. B., Devlin J. J., Xia W., Feldman R. I., Polokoff M. A., Phillips G. B., Whitlow M., Auld D. S., McMillan K., Ghosh S., Stuehr D. J., Parkinson J. F. (2002) Mechanistic studies with potent and selective inducible nitric-oxide synthase dimerization inhibitors. J. Biol. Chem. 277, 295–302 [DOI] [PubMed] [Google Scholar]
- 31. Roe S. M., Prodromou C., O'Brien R., Ladbury J. E., Piper P. W., Pearl L. H. (1999) Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 42, 260–266 [DOI] [PubMed] [Google Scholar]
- 32. Schulte T. W., Akinaga S., Soga S., Sullivan W., Stensgard B., Toft D., Neckers L. M. (1998) Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones 3, 100–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miao R. Q., Fontana J., Fulton D., Lin M. I., Harrison K. D., Sessa W. C. (2008) Dominant-negative Hsp90 reduces VEGF-stimulated nitric oxide release and migration in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 28, 105–111 [DOI] [PubMed] [Google Scholar]
- 34. Xu H., Shi Y., Wang J, Jones D., Weilrauch D., Ying R., Wakim B., Pritchard K. A., Jr. (2007) A heat shock protein 90 binding domain in endothelial nitric-oxide synthase influences enzyme function. J. Biol. Chem. 282, 37567–37574 [DOI] [PubMed] [Google Scholar]
- 35. Walerych D., Gutkowska M., Klejman M. P., Wawrzynow B., Tracz Z., Wiech M., Zylicz M., Zylicz A. (2010) ATP binding to Hsp90 is sufficient for effective chaperoning of p53 protein. J. Biol. Chem. 285, 32020–32028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Osawa Y., Lowe E. R., Everett A. C., Dunbar A. Y., Billecke S. S. (2003) Proteolytic degradation of nitric oxide synthase: effect of inhibitors and role of hsp90-based chaperones. J. Pharmacol. Exp. Ther. 304, 493–497 [DOI] [PubMed] [Google Scholar]
- 37. Ghosh D. K., Wu C., Pitters E., Moloney M., Werner E. R., Mayer B., Stuehr D. J. (1997) Characterization of the inducible nitric oxide synthase oxygenase domain identifies a 49 amino acid segment required for subunit dimerization and tetrahydrobiopterin interaction. Biochemistry 36, 10609–10619 [DOI] [PubMed] [Google Scholar]
- 38. Burkhard K. A., Wilks A. (2008) Functional characterization of the Shigella dysenteriae heme ABC transporter. Biochemistry 47, 7977–7979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Feissner R. E., Richard-Fogal C. L., Frawley E. R., Kranz R. G. (2006) ABC transporter-mediated release of a haem chaperone allows cytochrome c biogenesis. Mol. Microbiol. 61, 219–231 [DOI] [PubMed] [Google Scholar]
- 40. Pratt W. B., Morishima Y., Osawa Y. (2008) The Hsp90 chaperone machinery regulates signaling by modulating ligand binding clefts. J. Biol. Chem. 283, 22885–22889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pant K., Crane B. R. (2005) Structure of a loose dimer: an intermediate in nitric oxide synthase assembly. J. Mol. Biol. 352, 932–940 [DOI] [PubMed] [Google Scholar]
- 42. Pratt W. B., Morishima Y., Peng H. M., Osawa Y. (2010) Proposal for a role of the Hsp90/Hsp70-based chaperone machinery in making triage decisions when proteins undergo oxidative and toxic damage. Exp. Biol. Med. 235, 278–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dunbar A. Y., Kamada Y., Jenkins G. J., Lowe E. R., Billecke S. S., Osawa Y. (2004) Ubiquitination and degradation of neuronal nitric-oxide synthase in vitro: dimer stabilization protects the enzyme from proteolysis. Mol. Pharmacol. 66, 964–969 [DOI] [PubMed] [Google Scholar]
- 44. Papapetropoulos A., Zhou Z., Gerassimou C., Yetik G., Venema R. C., Roussos C., Sessa W. C., Catravas J. D. (2005) Interaction between the 90-kDa heat shock protein and soluble guanylyl cyclase: physiological significance and mapping of the domains mediating binding. Mol. Pharmacol. 68, 1133–1141 [DOI] [PubMed] [Google Scholar]
- 45. Nedvetsky P. I., Meurer S., Opitz N., Nedvetskaya T. Y., Müller H., Schmidt H, H. (2008) Heat shock protein 90 regulates stabilization rather than activation of soluble guanylate cyclase. FEBS Lett. 582, 327–331 [DOI] [PubMed] [Google Scholar]
- 46. Fairchild T. A., Fulton D., Fontana J. T., Gratton J. P., McCabe T. J., Sessa W. C. (2001) Acidic hydrolysis as a mechanism for the cleavage of the Glu298→Asp variant of human endothelial nitric-oxide synthase. J. Biol. Chem. 276, 26674–26679 [DOI] [PubMed] [Google Scholar]
- 47. Kolodziejski P. J., Koo J. S., Eissa N. T. (2004) Regulation of inducible nitric oxide synthase by rapid cellular turnover and cotranslational down-regulation by dimerization inhibitors. Proc. Natl. Acad. Sci. U. S. A. 101, 18141–18146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aschner J. L., Zeng H., Kaplowitz M. R., Zhang Y., Slaughter J. C., Fike C. D. (2009) Heat shock protein 90-eNOS interactions mature with postnatal age in the pulmonary circulation of the piglet. Am. J. Physiol. Lung Cell. Mol. Physiol. 296, L555–L564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Antonova G., Lichtenbeld H., Xia T., Chatterjee A., Dimitropoulou C., Catravas J. D. (2007) Functional significance of hsp90 complexes with NOS and sGC in endothelial cells. Clin. Hemorheol. Microcirc. 37, 19–35 [PubMed] [Google Scholar]
- 50. Noguchi S., Jianmongkol S., Bender A. T., Kamada Y., Demady D. R., Osawa Y. (2000) Guanabenz-mediated inactivation and enhanced proteolytic degradation of neuronal nitric-oxide synthase. J. Biol. Chem. 275, 2376–2380 [DOI] [PubMed] [Google Scholar]
- 51. Prodromou C. (2009) Strategies for stalling malignancy: targeting cancer's addiction to Hsp90. Curr. Top. Med. Chem. 9, 1352–1368 [DOI] [PubMed] [Google Scholar]
- 52. Porter J. R., Fritz C. C., Depew K. M. (2010) Discovery and development of Hsp90 inhibitors: a promising pathway for cancer therapy. Curr. Opin. Chem. Biol. 14, 412–420 [DOI] [PubMed] [Google Scholar]
- 53. Martínez-Ruiz A., Villanueva L., González de Orduña C., López-Ferrer D., Higueras M. A., Tarín C., Rodríguez-Crespo I., Vázquez J., Lamas S. (2005) S-Nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc. Natl. Acad. Sci. U. S. A. 102, 8525–8530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Retzlaff M., Stahl M., Eberl H. C., Lagleder S., Beck J., Kessler H., Buchner J. (2009) Hsp90 is regulated by a switch point in the C-terminal domain. EMBO Rep. 10, 1147–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Marozkina N. V., Yemen S., Borowitz M., Liu L., Plapp M., Sun F., Islam R., Erdmann-Gilmore P., Townsend R. R., Lichti C. F., Mantri S., Clapp P. W., Randell S. H., Gaston B., Zaman K. (2010) Hsp70/Hsp90 organizing protein as a nitrosylation target in cystic fibrosis therapy. Proc. Natl. Acad. Sci. U. S. A. 107, 11393–11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.