Abstract
Type 2 diabetes is highly prevalent in human populations, particularly in obese individuals, and is characterized by progressive pancreatic β-cell dysfunction and insulin resistance. Most mammals, including Old World primates, express two major kinds of sialic acids, N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc), typically found at the distal ends of glycoconjugate chains at the cell surface. Humans are uniquely unable to produce endogenous Neu5Gc due to an inactivating mutation in the CMP-Neu5Ac hydroxylase (CMAH) gene. The CMAH enzyme catalyzes the generation of CMP-Neu5Gc by the transfer of a single oxygen atom to the acyl group of CMP-Neu5Ac. Here, we show that mice bearing a human-like deletion of the Cmah gene exhibit fasting hyperglycemia and glucose intolerance following a high-fat diet. This phenotype is caused not by worsened insulin resistance but by compromised pancreatic β-cell function associated with a 65% decrease in islet size and area and 50% decrease in islet number. Obese Cmah-null mice also show an ∼40% reduction in response to insulin secretagogues in vivo. These findings show that human evolution-like changes in sialic acid composition impair pancreatic β-cell function and exacerbate glucose intolerance in mice. This may lend insight into the pathogenesis of type 2 diabetes in obese humans.—Kavaler, S., Morinaga, H., Jih, A., Fan, W. Q., Hedlund, M., Varki, A., Kim, J. J. Pancreatic β-cell failure in obese mice with human-like CMP-Neu5Ac hydroxylase deficiency.
Keywords: diabetes, sialic acid, pancreatic islet
Two hallmarks in the pathogenesis of type 2 diabetes are insulin resistance and failure of pancreatic β-cell compensation. Obesity in humans causes a state of insulin resistance, requiring increased insulin secretion to maintain relatively normal glucose levels. This increase in insulin secretion is usually accompanied by an increase in β-cell mass. In individuals that develop type 2 diabetes, β-cell compensation declines and relative insulin insufficiency develops, leading to glucose intolerance and eventually frank diabetes (1).
Sialic acids represent a class of 9-carbon monosaccharides that are abundantly expressed at the outermost region of the mammalian cellular glycocalyx. They are dominated by two major types: N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc). Cellular Neu5Gc is generated by the transfer of a single oxygen atom to the acyl group of Neu5Ac, catalyzed by the CMP-Neu5Ac hydroxylase (CMAH) enzyme in the cytosol (2). Both Neu5Ac and Neu5Gc are transported into the Golgi, where they may be used as donors to newly synthesized glycoproteins and glycolipids destined for the cell surface.
Although humans and chimpanzees share nearly 99% genetic identity, one rare difference is a human-specific inactivating mutation in the CMAH gene. The human mutation in CMAH consists of a 92-bp deletion in exon 6 that results in a frameshift and premature translation termination, producing a small 72-aa fragment. This deletion is universal in all human populations, but absent in all other mammals, including the African great apes (3). The mutation in the CMAH gene is thought to have occurred ∼2–3 × 106 yr ago, just prior to the emergence of the genus Homo (3), and resulted in the complete absence of endogenous Neu5Gc expression in all human tissues (4).
Humans demonstrate a marked propensity to develop type 2 diabetes. Although strong environmental factors such as diet certainly contribute to this phenotype, there is some evidence to suggest that intrinsic differences in pancreatic β-cell function exist between humans and other species. For example, although features of type 2 diabetes have been observed in obese rhesus monkeys and other Old World primates (5, 6), pancreatic β-cell function appears to be more severely impaired in humans. Adult rhesus monkeys have significantly lower fasting glucose levels, markedly higher fasting insulin levels, and a higher acute insulin response to glucose when compared to humans in both lean and obese states (7). Islet size and area are also significantly larger in monkeys (8). Insulin secretion is universally biphasic, with the first phase representing the first spike of insulin release occurring within 10 min after glucose exposure. While loss of first-phase insulin secretion is described as one of the earliest detectable defects in humans during the prodrome to overt diabetes (9), the loss of first-phase insulin release is a very late event in monkeys (10).
Although glycosylation of the Glut2 transporter has been shown to affect its expression in pancreatic β cells and impair insulin secretion (11), few other studies to date have addressed how changes in glycan structure at the cell surface modulate glucose homeostasis. To investigate the role of CMAH in glucose metabolism, we studied Cmah-null mice that were generated using Cre-mediated excision of exon 6, yielding a genotype essentially identical to the human mutation (4). We find that Cmah-null mice fed a high-fat diet (HFD) manifest worsened glucose tolerance secondary to pancreatic β-cell dysfunction. These data show that sialylation with Neu5Gc plays an important role in β-cell function and that its loss contributes to β-cell dysfunction in mice. We speculate that this human evolutionary loss of CMAH function may contribute to the development of type 2 diabetes in human obesity.
MATERIALS AND METHODS
Mutant mice
Cmah-knockout mice were created via Cre-LoxP-mediated deletion of 92 bp in exon 6 (4). This frameshift mutation produces a 72-aa nonfunctional peptide fragment that mimics the human mutation. Mice with the null mutation were backcrossed onto the C57/BL6 background for ≥10 generations prior to study. Wild-type (WT) C57/BL6 mice purchased from Harlan Laboratories (Indianapolis, IN, USA) were used as controls. Only male mice were used for study. All animal procedures adhered to University of California–San Diego institutional guidelines for the ethical treatment of animals.
In vivo metabolic testing
The study was initiated when mice were 3 mo of age. Mice were assigned to either a normal chow diet (NCD) containing 16% kcal from fat, or an HFD containing 60% kcal from fat (Research Diets, Inc., New Brunswick, NJ, USA). Body weight measurements were obtained weekly.
For the glucose tolerance test (GTT) or insulin tolerance test (ITT), mice were placed into weight-matched groups. Animals were allowed to feed overnight and then denied food for 6 h (GTT) or 4 h (ITT). After collection of basal blood work, either dextrose (1g/kg) or recombinant human insulin (0.50 U/kg; Novolog; Novo Nordisk, Bagsvaerd, Denmark) was injected intraperitoneally. Blood samples were drawn by tail vein sampling at 0, 15, 30, 60, 90, and 120 min after injection for whole-blood glucose and/or plasma insulin measurement. Blood glucose was measured using a OneTouch Ultra 2 glucometer (LifeScan Inc., Milpitas, CA, USA). Plasma insulin was quantified using the Ultra Sensitive Mouse Insulin ELISA kit (Alpco, Salem, NH, USA).
To evaluate first-phase insulin secretion, mice were denied access to food for 6 h, then subjected to dextrose (2 g/kg) or arginine (1 g/kg) challenge via intraperitoneal (i.p.) injection. Plasma insulin was measured at 0, 2, 5, 15, and 30 min after injection.
The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using glucose and insulin concentrations obtained after 6 h of food withdrawal, using the following formula: [fasting blood glucose (mg/dl) × fasting insulin (μU/ml)]/405 (ref. 12).
Glucose-stimulated insulin secretion studies in isolated islets
Primary mouse islets were isolated via intraductal collagenase (Roche Diagnostics, Mannheim, Germany) digestion and density centrifugation, as described previously (13). Following isolation, islets were hand picked and maintained in DMEM without phenol red, 5.5 mM glucose plus 10% (v/v) FBS (Sigma-Aldrich, St. Louis, MO, USA), and penicillin and streptomycin. Following overnight incubation, glucose-stimulated insulin secretion (GSIS) studies were performed. Briefly, islets were incubated first in Krebs Ringer HEPES buffer for a 1-h starvation period. Islets were then incubated in Krebs Ringer HEPES buffer containing either 2.8 or 16.7 mM glucose for 1 h. Insulin secretion was measured using the ultrasensitive insulin ELISA kit. All samples were run in triplicate. Values were adjusted to reflect insulin secretion per islet per hour in each sample.
Immunohistochemistry
To identify Neu5Gc expression in pancreatic tissues, deparaffinized tissue sections were blocked for endogenous peroxidase and endogenous biotin and overlaid with 0.5% fish gelatin in phosphate-buffered saline Tween 20. Sections were then incubated with chicken immunoglobulin Y or chicken anti-Neu5Gc antibody, followed by detection of specific binding using biotinylated anti-chicken and horseradish peroxidase-labeled streptavidin. Nuclei were counterstained with Mayers, and the slides were aqueous mounted for viewing and analysis.
For islet morphometric study, paraffin-embedded pancreatic tissues were first stained with rabbit anti-insulin (N1542; Dako, Carpenteria, CA, USA) and anti-glucagon (A0565; Dako) antibodies. Secondary antibodies for immunofluorescence detection were Cy3-conjugated anti-rabbit (111-165-144; Jackson Laboratories, Bar Harbor, ME, USA) and Alexa 488-nm conjugated anti-guinea pig (S-40174A; Molecular Probes, Eugene, OR, USA). Specimens were viewed on a Zeiss AxioObserver Z1 microscope, and 24-bit TIFF images were acquired with a Zeiss AxioCam digital camera driven by Zeiss AxioVision 3.1 software (Carl Zeiss, Oberkochen, Germany). Images were processed with Adobe Photoshop CS2 9.0 (Adobe Systems, San Jose, CA, USA). Morphometry was performed on a minimum of 5 mutants and 5 WT controls using Image-Pro Plus v.5.0.1 (Media Cybernetics, Silver Spring, MD, USA). Relative pancreatic areas of β cells were then calculated. Mean islet size and number were determined using Image J software (U.S. National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All values are expressed as means ± se unless otherwise noted. We used ANOVA to determine differences between groups, and repeated-measures ANOVA testing for comparisons over time. Values of P < 0.05 were considered significant.
RESULTS
Cmah-null mice demonstrate fasting hyperglycemia and impaired glucose tolerance
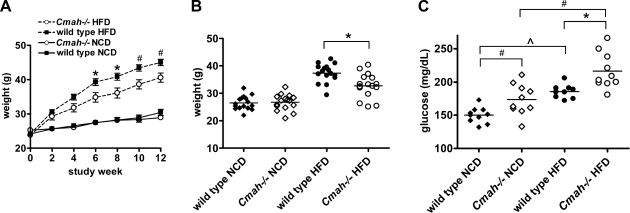
The study was initiated when Cmah-null and WT control mice were 3 mo of age. Mice were fed either NCD containing 16% kcal from fat or HFD containing 60% kcal from fat. As shown in Fig. 1, HFD-fed Cmah−/− mice gained less weight than WT controls, with a significant difference in body weight by 5 wk of HFD feeding (Cmah−/− vs. WT: 33.5±1.4 vs. 37.4±0.85 g, P=0.04; Fig. 1A, B). Food intake measured over a 4-wk period in HFD-fed mice showed that Cmah−/− mice ate a smaller quantity than WT mice (Cmah−/− vs. WT: 2.33±0.03 vs. 2.50±0.08 g/mouse/d, P=0.03), although this difference was not significant when normalized to body weight.
Figure 1.
Effect of Cmah inactivation on body weight and fasting glucose levels. A) Weights of Cmah-null and WT mice fed NCD or HFD containing 60% kcal from fat (n=16/group). B) Body weight distribution of Cmah-null and WT mice after 5 wk of HFD feeding. C) Fasting blood glucose values in Cmah-null and WT mice fed both NCD and HFD following 4 h food withdrawal after 5 wk of HFD feeding (n=10/group). Data are represented as means ± se. *P < 0.05, #P < 0.01; ∧P < 0.001.
We conducted in vivo metabolic studies after 5 wk of either HFD or NCD, when Cmah−/− and WT mice were ∼4 mo of age. Although mice fed NCD had similar body weights, Cmah−/− mice demonstrated significantly higher blood glucose after 4 h food withdrawal (Cmah−/− vs. WT: 174±4 vs. 150±4 mg/dl, P=0.01; Fig. 1C). HFD mice were placed in weight-matched groups in order to determine the effect of genotype independent of weight. HFD significantly increased fasting blood glucose values in both genotypes, consistent with obesity-induced insulin resistance. However, HFD-fed Cmah−/− mice exhibited further worsening in fasting hyperglycemia when compared to diet-matched controls (Cmah−/− vs. WT: 217±9 vs. 194±9 mg/dl, P=0.04; Fig. 1C).
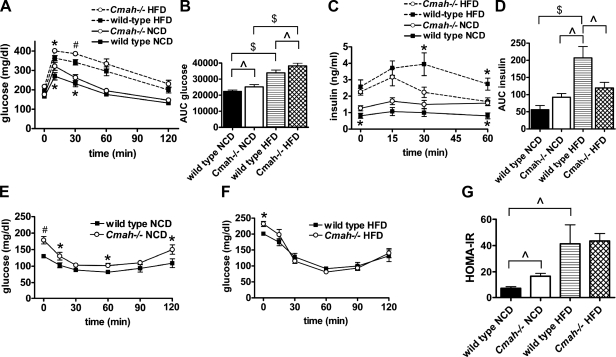
To further explore these differences in fasting glucose levels, we subjected all cohorts to GTT. NCD-fed Cmah−/− mice were mildly glucose intolerant, with an increase in area under the curve (AUC) glucose excursion when compared to WT controls following i.p. dextrose injection (Fig. 2A, B). HFD-fed Cmah−/− and WT mice demonstrated higher glucose values compared to NCD-fed lean mice, consistent with obesity-induced insulin resistance. However, HFD-fed Cmah−/− mice exhibited more severely impaired glucose tolerance when compared to HFD-fed WT controls (Fig. 2A, B). Plasma insulin measured concurrently during GTT showed no differences in AUC insulin secretion between NCD-fed groups. Insulin values were significantly raised in HFD-fed groups, reflecting hyperinsulinemia in response to obesity-induced insulin resistance. Interestingly, insulin secretion was reduced in HFD-fed Cmah−/− mice when compared to diet-matched controls (Figs. 2C, D), suggesting that impaired insulin secretion contributed to glucose intolerance in these mice.
Figure 2.
Impaired glucose tolerance in Cmah-null mice. A) I.p. GTT in both NCD and HFD mice. Whole-blood glucose was measured following i.p. dextrose (1 g/kg; n=7/group). *P < 0.05, #P < 0.01 vs. diet-matched WT. B) AUC glucose during i.p. GTT. ∧P < 0.05, $P < 0.001. C) Plasma insulin values measured during i.p. GTT (n=7/group). *P < 0.05 vs. diet-matched WT. D) AUC insulin during i.p. GTT. ∧P < 0.05, $P < 0.001. E, F) ITT in NCD-fed (E; n=7/group) and HFD-fed (F; n=6/group) mice. Whole-blood glucose was measured following i.p. insulin (0.50 U/kg). *P < 0.05 vs. diet-matched WT. Data are represented as means ± se. G) HOMA-IR (n=7/group). ∧P < 0.05.
We then performed ITT in NCD and HFD mice. NCD-fed Cmah-null mice showed higher blood glucose values when compared to WT following i.p. insulin injection (Fig. 2E). However, HFD-fed mice showed no differences between genotypes following i.p. insulin injection when measuring either absolute glucose values (Fig. 2F) or percentage glucose decrease (data not shown), confirming that differences in glucose tolerance in the obese state were not secondary to differences in insulin sensitivity. When we performed homeostatic model assessment (HOMA) analysis as a measure of insulin resistance (12), fasting HOMA-IR values were higher in NCD-fed Cmah−/− mice, indicating the presence of moderate insulin resistance (Cmah−/− vs. WT: 16.70±2.16 vs. 7.49±1.30, P=0.04). HFD significantly increased HOMA-IR values in both genotypes, consistent with severe obesity-induced insulin resistance. However, HOMA-IR values in HFD groups were similar between genotypes, indicating that insulin resistance was not further worsened in obese Cmah−/− mice (Fig. 2G).
Cmah-null mice show impaired insulin secretion in response to glucose and arginine stimulation in vivo
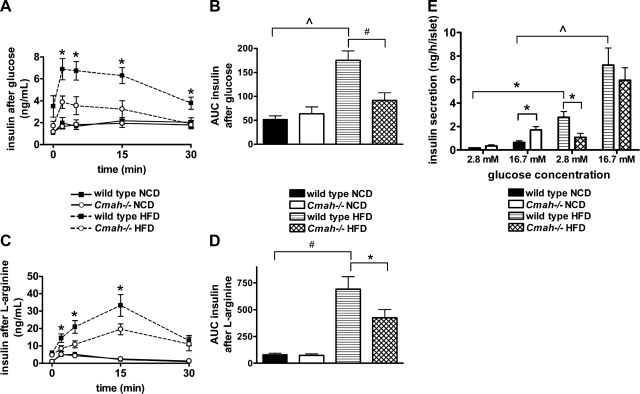
Because we did not observe significant differences in insulin sensitivity between genotypes fed HFD, we next conducted in vivo measurements of acute insulin secretion in response to i.p. glucose or arginine to assess pancreatic β-cell function. There were no genotype-specific differences in NCD-fed mice in insulin secretory responses to either secretagogue. HFD enhanced the response of WT mice to glucose stimulation, indicating pancreatic β-cell compensation in the face of increased peripheral insulin resistance. By contrast, the insulin secretory response to glucose was significantly diminished in HFD-fed Cmah−/− mice compared to diet-matched WT controls (Fig. 3A, B).
Figure 3.
Cmah-null mice show impaired insulin secretion in response to glucose and arginine stimulation. A, B) In vivo insulin secretion following glucose injection. A) Serum insulin was collected following i.p. dextrose (2 g/kg) in NCD-fed and HFD-fed mice. B) Data represent mean ± se AUC insulin (n=7 males/group). C, D) In vivo insulin secretion following l-arginine injection. C) Serum insulin was collected following i.p. arginine (1 g/kg) in NCD-fed and HFD-fed mice. D) Data represent mean ± se AUC insulin (n=6 mice/group). E) Glucose-stimulated insulin secretion (GSIS) studies in isolated islets from NCD-fed and HFD-fed mice. Insulin release was measured following stimulation with low (2.8 mM) or high (16.7 mM) glucose. Data represent means ± se (n=3/group). *P < 0.05, #P < 0.01, ∧P < 0.005.
To investigate potential defects in distal steps of the stimulus-secretion pathway, we administered arginine in vivo. Arginine is known to trigger insulin secretion by inducing membrane depolarization, subsequent calcium influx, and insulin exocytosis (14), while bypassing glucose transport and glycolysis. Again, acute insulin secretion was markedly higher in response to arginine in HFD-fed WT mice when compared to NCD-fed groups, consistent with compensatory hyperinsulinemia. Moreover, we again observed a significant decrease in insulin secretion in HFD-fed Cmah−/− mice when compared to HFD-fed WT controls (Fig. 3C, D).
Glucose-stimulated insulin secretion in isolated islets in Cmah-null mice
In an effort to further characterize the nature of pancreatic β-cell dysfunction, pancreatic islets of mice were isolated and subjected to either low (2.8 mM) or high (16.7 mM) concentrations of glucose. Insulin secretion was then measured and corrected for the total number of islets in each sample. Glucose-stimulated insulin secretion was higher in islets isolated from NCD-fed Cmah−/− mice when compared to diet-matched controls following exposure to high glucose concentrations (Cmah−/− vs. WT: 1.72±0.28 vs. 0.65±0.13 ng/h/islet, P=0.02; Fig. 3E), perhaps in response to their moderately insulin resistant state. Insulin secretion was considerably higher in HFD-fed WT mice when compared to their NCD-fed counterparts, consistent with severe obesity-associated insulin resistance and compensatory hyperinsulinemia. In contrast, insulin secretion in response to low glucose dosage was significantly lower in islets of HFD-fed Cmah−/− mice compared to their diet-matched controls in response to low glucose concentrations (Cmah−/− vs. WT: 1.03±0.32 vs. 2.79±0.50 ng/h/islet, P=0.03) but was not significantly changed in response to high glucose concentrations (Fig. 3E).
Islet size and area are significantly reduced in obese Cmah-null mice
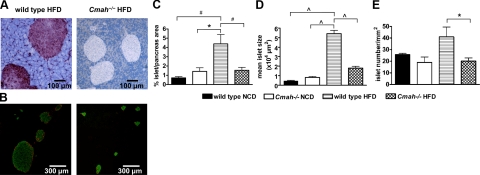
To further investigate the divergence in insulin secretory responses between WT and “human-like” Cmah−/− mice, we examined pancreata by immunohistochemical analysis when the mice were 7 mo of age and had been fed HFD for 12 wk. Prior studies of Cmah−/− mice have shown no evidence of Neu5Gc expression in null tissues (4). As predicted from the underlying genetic differences, we observed abundant expression of Neu5Gc in pancreatic islets of WT mice using an antibody specific for Neu5Gc (15) and no evidence of endogenous Neu5Gc expression in pancreatic tissues of Cmah-null mice (Fig. 4A).
Figure 4.
Islet size and area are significantly reduced in obese Cmah-null mice. A) Immunohistochemical analysis of pancreata using antibody specific for Neu5Gc in HFD-fed mice. Images are representative. B) Pancreatic islet sections from HFD-fed Cmah-null and WT mice analyzed by fluorescence microscopy for insulin (green) and glucagon (red). Images are representative. C) Islet area, expressed as a percentage of total pancreatic area, in NCD-fed and HFD-fed mice (n=4–6 mice/group). D) Mean islet size in NCD-fed and HFD-fed Cmah-null and WT mice (n=4–5 mice/group). E) Islet numbers, normalized for pancreatic area, in NCD-fed and HFD-fed mice (n=4–6 mice/group). Data are represented as means ± se. *P < 0.05, #P < 0.01, ∧P < 0.005.
We detected no genotype differences in islet morphology of mice fed NCD. Islet area was markedly increased by HFD in WT mice, consistent with β-cell compensation and the observed increase in insulin secretion following glucose and arginine challenge in vivo. However, Cmah−/− mice failed to expand islet area when challenged with HFD. We noted that islet area was significantly smaller in HFD-fed Cmah−/− mice when compared to HFD-fed controls (Cmah−/− vs. WT: 1.54±0.25 vs. 4.39±0.81%, P=0.01; Fig. 4B, C), consistent with diminished β-cell compensation. Mean islet size in NCD-fed Cmah−/− mice was not significantly increased when compared to WT controls. However, HFD-fed Cmah−/− mice showed markedly reduced mean islet size when compared to HFD-fed controls (Cmah−/− vs. WT: 1.80±0.19×104 vs. 5.43±0.34×104 μm2, P=0.0001; Fig. 4D and Supplemental Fig. S1). Furthermore, islet number normalized for pancreatic area was significantly lower in HFD-fed Cmah−/− mice when compared to diet-matched controls (Cmah−/− vs. WT: 20.1±2.7 vs. 41.0±8.5 mm−2 pancreatic area, P=0.02; Fig. 4E). We did not detect evidence of increased β-cell proliferation or apoptosis by BrDU, Ki67, or TUNEL assay (data not shown), perhaps because of the extremely slow rates of β-cell turnover observed in older mice (16). Immunohistochemical staining of islets with anti-CD45 antibodies detected no lymphocyte infiltrates, which would have implicated inflammation in the β-cell failure of this model (data not shown).
DISCUSSION
Prospective studies of metabolic control in humans with type 2 diabetes indicate that, whereas insulin resistance is relatively constant, β-cell failure is progressive in nature (17). The role of CMAH deletion in glucose metabolism and obesity has not been previously explored. In this study, we investigated the metabolic consequences of modifying sialic acid composition in the mammalian glycocalyx by using mice bearing a human-like mutation in the Cmah gene.
The CMAH enzyme catalyzes the conversion of Neu5Ac to Neu5Gc. Both Neu5Ac and Neu5Gc sialic acids are endogenously produced in all nonhuman vertebrates, including primates. In humans, an inactivating mutation of the CMAH gene has resulted in the evolutionary loss of Neu5Gc synthesis. Humans, therefore, express only Neu5Ac sialic acids at the cell surface. Here, we find that Cmah-null mice with HFD-induced obesity demonstrate fasting hyperglycemia and impaired glucose tolerance. Although NCD-fed mice exhibit moderate insulin resistance, the interaction of CMAH deletion and HFD produced significant defects in insulin secretion with markedly reduced pancreatic islet size and area.
Prior studies in using Cmah-null mice with the exon 6 deletion have shown that these mice have impaired hearing and delayed wound healing (4), as well as worsened neuromuscular disease when crossed with the mdx-null model for Duchenne muscular dystrophy (18). Another knockout model of Cmah, using neocassette insertion into exon 5 (19), has been studied in the context of xenotransplantation, producing robust anti-Neu5Gc antibody levels in response to immunization with Neu5Gc-expressing thymocytes (20). In this case, Neu5Gc-containing islets from syngeneic WT mice were transplanted into immunized Cmah-null mice with streptozotocin-induced diabetes. These null mice demonstrated hyperglycemia due to the rejection of transplanted islets and poor β-cell engraftment. However, glucose metabolism was not studied in nontransplanted mice.
In the present study, our primary finding is that pancreatic islet mass appears to be significantly smaller in HFD-fed Cmah−/− mice, indicating failure of β-cell compensation in response to severe obesity-induced insulin resistance. It is yet unclear how changes in sialic acid composition contribute to the expansion of β-cell mass. Ongoing studies will determine whether this reduction in islet size and area in HFD-fed Cmah−/− mice accounts entirely for the decreased phenotype in vivo, or whether cellular defects in β-cell function also exist. Prior work has shown that impaired glycosylation of Glut2 reduces its expression at the β-cell surface, resulting in impaired glucose-stimulated insulin secretion but preserved arginine-stimulated insulin secretion (11). In our model, the insulin secretory response was reduced to both secretagogues. This could merely be explained by reduced islet mass, although it could also reflect possible defects in β-cell function at more distal steps in the stimulus-secretion pathway.
Interestingly, activity of both the insulin receptor and IGF-1 receptor can be modified via desialylation by endogenous neuraminidase-1, which alters their ability to transduce net proliferative responses to insulin in skeletal myoblasts (21). β-Cell-specific mutations of the insulin receptor and IGF-1 receptor are also associated with reduced first-phase insulin secretion and impaired glucose tolerance (22–24). Defective insulin signaling has also been shown to diminish β-cell replication and survival (25–27). Further work will elucidate whether Cmah inactivation and loss of Neu5Gc expression alter insulin receptor expression or action in β cells of obese mice.
An emerging literature shows that changes in sialic acid composition may affect cell function through several mechanisms. The evolutionary loss of Neu5Gc in humans has been studied in immunity, potentially determining human resistance or susceptibility to certain Neu5Gc- or Neu5Ac-preferring pathogens (28). Sialic acid modifications also affect their recognition and binding by Siglecs (sialic acid binding Ig-like lectins) at the cell surface. For example, Siglec-1, which is expressed specifically by resident and inflammatory macrophages, strongly prefers to bind Neu5Ac over Neu5Gc structures (29). Extracellular matrix proteins, such as laminins and agrins, also preferentially bind Neu5Ac rather than Neu5Gc (18).
In this study, we have expanded our understanding of CMAH in vivo by showing that it plays a role in systemic glucose metabolism in response to HFD. HFD-fed Cmah-null mice manifest glucose intolerance secondary to pancreatic β-cell dysfunction. These data suggest that human-like changes in sialic acid composition contribute to impaired glucose tolerance and β-cell dysfunction in obese mice, which may lend insight into the development of type 2 diabetes in obese humans. Given the high prevalence of type 2 diabetes and its comorbidities, it is clear that further study is necessary to understand how the evolutionary loss of CMAH and changes in sialic acid composition affect the pathogenesis and prevention of metabolic disease in humans.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health grants DK075479 (J.K.), DK063491 DERC Pilot and Feasibility Award (J.K.), and GM32373 (A.V.). The authors thank Nissi Varki [University of California–San Diego (UCSD) Histology Core] for immunohistochemistry studies on pancreatic tissues. Thanks are due also to Jerrold Olefsky (UCSD) for helpful discussions and support.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Perley M. J., Kipnis D. M. (1967) Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects. J. Clin. Invest. 46, 1954–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Varki A. (2007) Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature 446, 1023–1029 [DOI] [PubMed] [Google Scholar]
- 3. Varki A. (2010) Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proc. Natl. Acad. Sci. U. S. A. 107(Suppl. 2), 8939–8946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hedlund M., Tangvoranuntakul P., Takematsu H., Long J. M., Housley G. D., Kozutsumi Y., Suzuki A., Wynshaw-Boris A., Ryan A. F., Gallo R. L., Varki N., Varki A. (2007) N-glycolylneuraminic acid deficiency in mice: implications for human biology and evolution. Mol. Cell. Biol. 27, 4340–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mordes J. P., Rossini A. A. (1981) Animal models of diabetes. Am. J. Med. 70, 353–360 [DOI] [PubMed] [Google Scholar]
- 6. Jones S. M. (1974) Spontaneous diabetes in monkeys. Lab. Anim. 8, 161–166 [DOI] [PubMed] [Google Scholar]
- 7. LeRoith D., Taylor S. I., Olefsky J. M. (2004) Diabetes Mellitus: A Fundamental and Clinical Text, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 8. Kim A., Miller K., Jo J., Kilimnik G., Wojcik P., Hara M. (2009) Islet architecture: a comparative study. Islets 1, 129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pimenta W., Korytkowski M., Mitrakou A., Jenssen T., Yki-Jarvinen H., Evron W., Dailey G., Gerich J. (1995) Pancreatic beta-cell dysfunction as the primary genetic lesion in NIDDM. Evidence from studies in normal glucose-tolerant individuals with a first-degree NIDDM relative. JAMA 273, 1855–1861 [PubMed] [Google Scholar]
- 10. Hansen B. C., Bodkin N. L. (1990) Beta-cell hyperresponsiveness: earliest event in development of diabetes in monkeys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 259, R612–R617 [DOI] [PubMed] [Google Scholar]
- 11. Ohtsubo K., Takamatsu S., Minowa M. T., Yoshida A., Takeuchi M., Marth J. D. (2005) Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell 123, 1307–1321 [DOI] [PubMed] [Google Scholar]
- 12. Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 [DOI] [PubMed] [Google Scholar]
- 13. Carter J. D., Dula S. B., Corbin K. L., Wu R., Nunemaker C. S. (2009) A practical guide to rodent islet isolation and assessment. Biol. Proced. Online 11, 3–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marcelli-Tourvieille S., Hubert T., Pattou F., Vantyghem M. C. (2006) Acute insulin response (AIR): review of protocols and clinical interest in islet transplantation. Diabetes Metab. 32, 295–303 [DOI] [PubMed] [Google Scholar]
- 15. Tangvoranuntakul P., Gagneux P., Diaz S., Bardor M., Varki N., Varki A., Muchmore E. (2003) Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc. Natl. Acad. Sci. U. S. A. 100, 12045–12050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teta M., Long S. Y., Wartschow L. M., Rankin M. M., Kushner J. A. (2005) Very slow turnover of beta-cells in aged adult mice. Diabetes 54, 2557–2567 [DOI] [PubMed] [Google Scholar]
- 17. Levy J., Atkinson A. B., Bell P. M., McCance D. R., Hadden D. R. (1998) Beta-cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10-year follow-up of the Belfast Diet Study. Diabet. Med. 15, 290–296 [DOI] [PubMed] [Google Scholar]
- 18. Chandrasekharan K., Yoon J. H., Xu Y., deVries S., Camboni M., Janssen P. M., Varki A., Martin P. T. (2010) A human-specific deletion in mouse Cmah increases disease severity in the mdx model of Duchenne muscular dystrophy. Sci. Transl. Med. 2, 42–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naito Y., Takematsu H., Koyama S., Miyake S., Yamamoto H., Fujinawa R., Sugai M., Okuno Y., Tsujimoto G., Yamaji T., Hashimoto Y., Itohara S., Kawasaki T., Suzuki A., Kozutsumi Y. (2007) Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation. Mol. Cell. Biol. 27, 3008–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tahara H., Ide K., Basnet N. B., Tanaka Y., Matsuda H., Takematsu H., Kozutsumi Y., Ohdan H. (2010) Immunological property of antibodies against N-glycolylneuraminic acid epitopes in cytidine monophospho-N-acetylneuraminic acid hydroxylase-deficient mice. J. Immunol. 184, 3269–3275 [DOI] [PubMed] [Google Scholar]
- 21. Arabkhari M., Bunda S., Wang Y., Wang A., Pshezhetsky A. V., Hinek A. (2010) Desialylation of insulin receptors and IGF-1 receptors by neuraminidase-1 controls the net proliferative response of L6 myoblasts to insulin. Glycobiology 20, 603–616 [DOI] [PubMed] [Google Scholar]
- 22. Kulkarni R. N., Bruning J. C., Winnay J. N., Postic C., Magnuson M. A., Kahn C. R. (1999) Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96, 329–339 [DOI] [PubMed] [Google Scholar]
- 23. Kulkarni R. N., Holzenberger M., Shih D. Q., Ozcan U., Stoffel M., Magnuson M. A., Kahn C. R. (2002) Beta-cell-specific deletion of the Igf1 receptor leads to hyperinsulinemia and glucose intolerance but does not alter beta-cell mass. Nat. Genet. 31, 111–115 [DOI] [PubMed] [Google Scholar]
- 24. Xuan S., Kitamura T., Nakae J., Politi K., Kido Y., Fisher P. E., Morroni M., Cinti S., White M. F., Herrera P. L., Accili D., Efstratiadis A. (2002) Defective insulin secretion in pancreatic beta cells lacking type 1 IGF receptor. J. Clin. Invest. 110, 1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim J. J., Kido Y., Scherer P. E., White M. F., Accili D. (2007) Analysis of compensatory beta-cell response in mice with combined mutations of Insr and Irs2. Am. J. Physiol. Endocrinol. Metab. 292, E1694–E1701 [DOI] [PubMed] [Google Scholar]
- 26. Okamoto H., Hribal M. L., Lin H. V., Bennett W. R., Ward A., Accili D. (2006) Role of the forkhead protein FoxO1 in beta cell compensation to insulin resistance. J. Clin. Invest. 116, 775–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Withers D. J., Burks D. J., Towery H. H., Altamuro S. L., Flint C. L., White M. F. (1999) Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat. Genet. 23, 32–40 [DOI] [PubMed] [Google Scholar]
- 28. Martin M. J., Rayner J. C., Gagneux P., Barnwell J. W., Varki A. (2005) Evolution of human-chimpanzee differences in malaria susceptibility: relationship to human genetic loss of N-glycolylneuraminic acid. Proc. Natl. Acad. Sci. U. S. A. 102, 12819–12824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brinkman-Van der Linden E. C., Sjoberg E. R., Juneja L. R., Crocker P. R., Varki N., Varki A. (2000) Loss of N-glycolylneuraminic acid in human evolution. Implications for sialic acid recognition by siglecs. J. Biol. Chem. 275, 8633–8640 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.