Abstract
Complement component C5a and ATP are potent effectors of microglial movement and are increased in diverse neurodegenerative diseases and at sites of injury. Apolipoprotein E (apoE) influences microglial function, and different human apoE isoforms confer variable risk for development of neurodegenerative disorders, especially Alzheimer's disease. The purpose of this investigation was to test the hypothesis that mouse apoE and human apoE isoforms influence microglial migration. Using primary wild-type and apoE-deficient microglia, we show that C5a- and ATP-stimulated chemotaxis are largely apoE-dependent processes with different molecular bases. Although the C5a-dependent chemotaxis of wild-type microglia was completely blocked by receptor-associated protein (RAP), suggesting apoE receptor involvement, ATP-stimulated migration was unaffected by RAP but was associated with differential ERK phosphorylation. Studies using primary microglia derived from targeted replacement mice “humanized” for the coding exons (protein isoform) of human ε2 (apoE2), ε3 (apoE3), or ε4 (apoE4) allele of APOE revealed that primary mouse microglia expressing apoE4 or apoE2 exhibited significantly reduced C5a- and ATP-stimulated migration compared with microglia expressing human apoE3. This study, for the first time, demonstrates apoE dependence and apoE isoform-specific modulation of microglial migration in response to distinct chemotactic stimuli commonly associated with neurodegenerative disease.—Cudaback, E., Li, X., Montine, K. S., Montine, T. J., Keene, C. D. Apolipoprotein E isoform-dependent microglia migration.
Keywords: C5a, ATP, ERK, receptor associated protein, targeted replacement
Microglia are the major innate immune cells of the brain and appear to be critical mediators of neuroprotection and neurotoxicity in several neurological diseases (reviewed in ref. 1). Although the ultimate outcomes might be highly contextual, multiple in vivo models have shown that depletion of microglia or inhibition of microglial function limits injury in models of Alzheimer's disease (AD), Parkinson's disease, stroke, spinal cord injury, multiple sclerosis, and amyotrophic lateral sclerosis (2–9). In vivo live imaging studies have shown that microglia actively migrate toward pathological stressors (10, 11), at which point the other critical activities of microglia, including elaboration of immunomodulatory substances and cytotoxic factors, phagocytosis, and coordination of the adaptive immune response, can occur (reviewed in ref. 12). The ability of microglia to migrate into and through brain parenchyma in response to various stressors is critical to their physiological and pathophysiological actions and is dependent on recognition of diverse external stimuli by cell surface receptors that mediate microglial migration and other functions via converging signaling pathways (13). Two such stimuli involve recognition of complement activation and release of extracellular nucleotides.
The complement system is activated by multiple pathways that culminate in the formation of C3 and C5 convertase enzymes, which catalyze the cleavage of C3 and C5 into active fragments, including anaphylatoxins C3a and C5a (reviewed in ref. 14). Complement proteins are synthesized by neurons, astrocytes, oligodendroglia, and microglia (15), and intracerebral levels of complement are increased in diseased regions of brain from patients with AD (16, 17). Aβ peptides, pleiotropic neurotoxins that exist in multiple higher-ordered forms and are thought to be central to the initiation and progression of AD, directly activate both classic (18) and alternative complement pathways with generation of C5a and interaction with CD88, the major C5a receptor (19). CD88 is primarily expressed on microglia in the vicinity of amyloid plaques in the central nervous system of transgenic mouse models of AD, suggesting a critical role for microglia in modulating plaque levels in AD mice (20). CD88 binding by C5a results in microglia activation and chemotaxis. Pharmacological blockade of C5a receptor results in a significant reduction of pathology in two mouse models of AD (21) as well as in CNS lupus (22), hypoxic-ischemic injury/stroke (23, 24), intracerebral hemorrhage (25), and traumatic brain injury (26). Thus, C5a activity in microglia may be an important factor in neurodegenerative disease.
ATP is released from stressed or injured cells, including neurons, astrocytes, and oligodendrocytes, and is rapidly metabolized to ADP, AMP, and adenosine (27, 28). ATP and its metabolites bind to both ionotropic (P2X) and metabotropic G-protein-coupled (P2Y) purinergic receptors that are variably expressed on microglia (for review, see ref. 29) and influence microglial chemotaxis. Classically, exposure of microglia to ATP/ADP results in membrane ruffling and purinergic receptor-dependent chemotaxis (30–32) (in addition to multiple other functions associated with microglial activation; ref. 33). More recent evidence suggests selective effects of purinergic receptor activation on human microglial vs. monocyte chemotaxis (27) and shows that ATP-induced migration is mediated at least in part by activation of matrix metalloproteinase 9 (34). Purinergic receptor modulation of microglial chemotaxis and activation appears to be a complex and highly regulated process that involves calcium-dependent signaling (28, 35) and functional antagonism (36). Purine metabolites are increased in cerebrospinal fluid from patients with AD (37), and in vitro activation of microglia by Aβ requires P2X7 receptors. Indeed, P2X7-knockout mice fail to increase expression of IL-1β in response to intrahippocampal Aβ injection (38). Thus, ATP-dependent microglial activity may play an important role in the pathophysiology of neurodegenerative disease.
Human APOE has 3 common alleles, ε2, ε3, and ε4, which encode 3 isoforms, apolipoprotein (apo) E2, apoE3, and apoE4. By far, the strongest known genetic risk factor for the development of late-onset AD appears to reside with APOE ε4, which displays a gene dosage effect (39–42). These strong genetic associations establish the relevance of apoE isoforms to the pathogenesis of AD but not the mechanisms by which apoE isoforms influence the initiation or progression of AD. Several groups have highlighted a role for apoE isoform-dependent trafficking and clearance of Aβ peptides (reviewed in refs. 43, 44). Others have demonstrated apoE isoform-specific regulation of the microglial innate immune response and paracrine damage to neurons in a variety of experimental models in vitro and in vivo (45–50).
In AD, microglia are increased in and around amyloid plaques (51), and studies with transgenic mouse models of AD show selective aggregation of bone marrow-derived microglia to plaques with associated reduction of soluble and insoluble Aβ (52, 53). Thus, the ability of microglia to migrate through brain parenchyma to areas of injury and senile plaque accumulation may be critical to both mechanisms proposed for apoE isoform-dependent effects of AD: Aβ clearance and innate immune activation. However, we are unaware of data concerning apoE- or apoE isoform-dependent regulation of microglial migration.
MATERIALS AND METHODS
Animals
Wild-type C57BL/6 and apoE−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Homozygous APOE ε2, APOE ε3, and APOE ε4 targeted replacement mice “humanized” at apoE were developed by Maeda and colleagues (54, 55). In brief, all targeted replacement mice contain intact 5′ regulatory elements for murine apoE, driving the expression of the coding exons for human isoforms apoE2, apoE3, or apoE4. Original strains were backcrossed >6 times to a C57BL/6 genetic background. Mice were housed in a temperature-controlled specific-pathogen-free facility with a strict 12-h light/dark cycle and with free access to food and water. All mice were used with approval of the University of Washington Animal Care and Use Committee.
Primary microglial culture
Primary cultures of mouse microglia were generated from 0- to 3-d-old pups as described previously (56). In brief, mixed glial cell cultures were plated in 162-cm2 flasks (Corning Inc., Corning, NY, USA) coated with poly-l-ornithine (Sigma-Aldrich, St. Louis, MO, USA) and maintained at 37°C and 5% CO2 in DME/F-12 supplemented with 10% FBS (both from HyClone, Logan, UT, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin (both from Invitrogen, Carlsbad, CA, USA). After 2 wk in culture, microglia were isolated from mixed glial cultures 1×/wk (for up to 3 wk) by gentle shaking.
Migration assay
Microglial migration was assayed using the higher-throughput modified Boyden chamber technique as described previously by Miller and Stella (57). In brief, microglia from each genotype were recovered in serum-free DME/F-12 and washed once to remove residual serum. Cells were resuspended in DME/F-12 at 106 cells/ml and stained with 700 nM DRAQ5 (Axxora, San Diego, CA, USA), a near-infrared nuclear dye, for 25 min at 4°C with gentle agitation. Stained cells were then washed twice in DME/F-12 in preparation for loading into the upper wells of the chemotaxis chamber (model 4A96-030; Neuroprobe, Cabin John, MD, USA). Mouse recombinant C5a (R&D Systems, Minneapolis, MN, USA) was serially diluted in serum-free DME/F-12 supplemented with modified G5 formulation (1 μg/ml biotin, 5 ng/ml basic fibroblast growth factor, 10 ng/ml epidermal growth factor, 50 μg/ml human transferrin, 3.6 ng/ml hydrocortisone, and 5.2 ng/ml selenite) and loaded into lower wells in triplicate. Dry polycarbonate filters (10-μm pore diameter) were attached to the chamber, washed microglia were resuspended in DME/F-12 with modified G5 formulation, and 7 × 104 cells (390 μl) were added into each of the upper wells. Basal migration was assessed by quantification of cells migrating toward C5a vehicle only (PBS containing 0.1% BSA). After an initial 10-min incubation on the bench top, cells were allowed to migrate for 3 h at 37°C and 5% CO2. Filters were then processed and analyzed on an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE, USA) according to the published protocol (57). After analysis, a representative filter from each genotype was stained using a Diff Quick stain kit (IMEB Inc., San Marcos, CA, USA), according to the manufacturer's protocol. Migrated cells from 3 high-powered fields (×320) per well were counted on a Zeiss Axiovert 25 microscope (Carl Zeiss, Thornwood, NY, USA) to rule out genotype-dependent differential uptake of the nuclear dye by microglia.
RT-PCR
Microglia were recovered in serum-free DME/F-12 as described above and pelleted by centrifugation. Total RNA was isolated from cell pellets using an RNeasy extraction kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol. RNA (1 μg) was reverse-transcribed using an Advantage RT-for-PCR Kit (Clontech, Mountain View, CA, USA), and gene-specific primers were used to amplify murine CD88 (forward, 5′-GATGCCACCGCCTGTATAGT-3′; reverse, 5′-ACGAAGGATGGAATGGTGAG-3′), P2X4 (forward, 5′-GCGTCTGTGAAGACCTGTGA-3′; reverse, 5′-GATTTGGCCAAGACGGAATA-3′), P2X7 (forward, 5′-CCCTGCACAGTGAACGAGTA-3′; reverse, 5′-CTTGCAGACTTTTCCCAAGC-3′), P2Y6 (forward, 5′-TTCCACATCACCAAGACAGC-3′; reverse, 5′-ACTTGGCTGTGAGCCTCTGT-3′), and P2Y12 (forward, 5′-CACCTCAGCCAATACCACCT-3′; reverse, 5′-AAATCCTCATTGCCAGCTG-3′) amplicons (35 cycles).
Western blot analysis
Microglia were plated into 12-well Falcon culture plates (BD, Franklin Lakes, NJ, USA) treated with poly-l-ornithine at 4 × 105 cells/well. After 48 h in DME/F-12 containing 10% FBS, cells were washed with PBS, their medium was replaced with serum-free DME/F-12 (1 ml/well), and cells were incubated for 24 h before treatment with C5a. Timed treatments were stopped by aspiration of medium (supernatants were collected for subsequent assay) with plates on ice and addition of 200 μl of NuPAGE LDS sample buffer (Invitrogen) supplemented with 2-mercaptoethanol (1%) to each well. Samples were loaded onto NuPAGE 4–12% bis-Tris precast gels (Invitrogen) and electrophoresed according to the manufacturer's protocol. Gels were electrophoretically transferred to an Immobilon-P transfer membrane (Millipore, Bedford, MA, USA) and blocked in Tris (50 mM)-buffered saline containing 0.1% Tween-20 and 5% nonfat dried milk.
Flow cytometry
Microglia were harvested from mixed glial cultures as described above, pelleted, and resuspended in HBSS containing 2% FBS and 1% host-specific IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Microglia were incubated for 30 min with antibody directed against CD88, P2X4, or P2Y12 (AbD Serotec, Oxford, UK; Alomone Labs Ltd., Jerusalem, Israel), washed with HBSS, and fixed. Microglia were assayed for cell surface receptor expression using a flow cytofluorometer LSR II (BD Biosciences, San Diego, CA, USA), and data were analyzed with FlowJo 7.2.2 software (Tree Star, Ashland, OR, USA). Target specificities were confirmed using IgG isotype and secondary-only antibody controls for each sample.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
C5a-stimulated microglial migration is apoE dependent
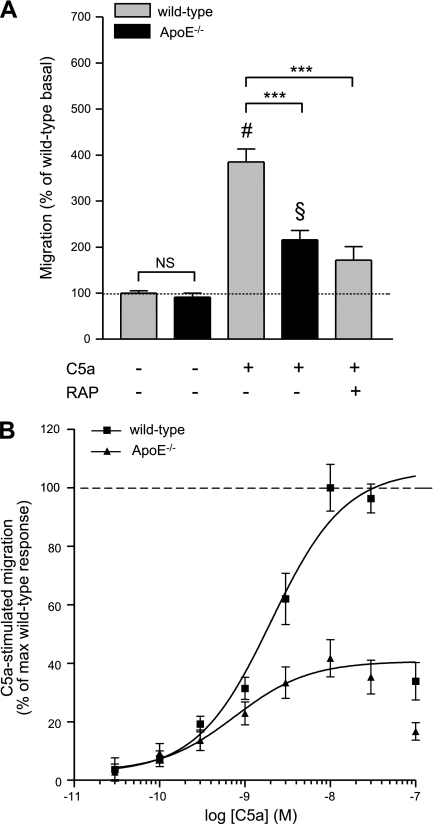
First, we sought to determine the extent to which microglial migration in vitro was dependent on expression of apoE. Migration of microglia isolated from newborn wild-type mice or those carrying a targeted disruption of murine apoE (apoE−/−) was assessed using a modified Boyden chamber assay as described previously (57), allowing the assessment of chemotaxis. Whereas basal migration (chemokinesis exhibited by cells in the absence of stimulant) of wild-type and apoE−/− microglia was not significantly different (Fig. 1A), stimulation of wild-type microglia with 30 nM C5a, a concentration previously shown to produce maximal chemotaxis in cultured microglia (57), resulted in a 385 ± 29% increase in migration over basal, whereas apoE−/− microglial migration was stimulated only to 216 ± 20%. As expected, the C5a-stimulated chemotactic response was dose dependent in both wild-type and apoE−/− microglia (Fig. 1B), and the concentration-response curve appeared as an inverted U, with the highest C5a concentrations eliciting submaximal effects.
Figure 1.
C5a-stimulated microglial migration is apoE and RAP dependent. A) Migration of microglia from wild-type or apoE−/− mice was measured using a modified Boyden chamber in the presence or absence of C5a and RAP. Microglial migration in untreated/vehicle-treated wild-type cells was set as 100%. There was no significant difference in basal migration (unstimulated) in wild-type compared with apoE−/− mice. However, 30 nM C5a was less effective at stimulating migration of apoE−/− microglia in a Boyden chamber assembly after 3 h. Coadministration of RAP (1 μM) significantly reduced C5a-stimulated migration of wild-type microglia. Data are expressed as the mean ± se percentage of wild-type or apoE−/− basal migration; n = 9–15 (i.e., 3–5 independent experiments performed in triplicate). ***P < 0.001; #P < 0.001 vs. wild-type; §P < 0.001 vs. knockout; ANOVA with Bonferroni multiple comparison test. B) C5a-stimulated migration of wild-type and apoE−/− microglia is dose dependent. Data are expressed as mean ± se percentage of the maximum wild-type response to C5a; n = 9–12 (i.e., 3–4 independent experiments were performed in triplicate).
We attempted to validate our finding of diminished C5a-stimulated migration by apoE−/− primary microglia with wild-type microglia exposed to a recombinant peptide inhibitor, receptor-associated protein (RAP), which is known to inhibit a subset of apoE receptors functionally expressed by microglia, including LDL receptor (LDLR) and LDLR-related protein (LRP) (58–60). RAP (1 μM) significantly suppressed C5a-stimulated wild-type microglia migration to a level that was similar to that of apoE−/− microglia (Fig. 1A) These complementary genetic and pharmacological data from primary mouse microglia highlight a quantitatively important contribution of apoE and its interaction with LDLR or LRP in C5a-stimulated migration.
Reduced C5a-stimulated migration by apoE−/− microglia is not associated with MAPK and Akt signal transduction
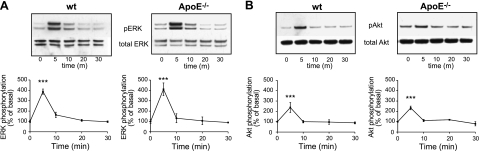
Activation of MAPK and PI3K/Akt pathways, specifically ERK and Akt, has been implicated in microglial chemotaxis (61–63). We therefore investigated whether the reduced C5a-stimulated migration seen in apoE−/− microglia was associated with disruption in these signal transduction pathways. Cultured wild-type microglia treated with 30 nM C5a showed a significant increase in ERK and Akt phosphorylation after 5 min compared with unstimulated controls (380 ± 25.1 and 249.3 ± 41.7%, respectively; P<0.001; Fig. 2). apoE−/− microglia similarly treated with C5a showed 5-min peak phosphorylation of ERK and Akt (411.8±61.0 and 278.4±26.5%, respectively; P<0.001), as well as a time course not significantly different from that of the wild-type (Fig. 2). These data confirm microglial ERK and Akt phosphorylation with C5a stimulation but reveal no differences in these signaling pathways associated with the presence or absence of apoE.
Figure 2.
C5a stimulates signal transduction pathways similarly in wild-type (wt) and apoE−/− microglia. A) Western blot analysis shows time course of ERK activation (pERK) in whole-cell lysates prepared from cultured murine microglia stimulated with 30 nM C5a over 30 min. Quantification reveals peak ERK activation at 5 min in both genotypes. B) Akt activation was likewise time dependent, with peak activation in both genotypes observed at 5 min. Western blots are representative of ≥3 independent experiments. Data are expressed as the percentage of ERK or Akt phosphorylation over the unstimulated control. ***P < 0.001 vs. corresponding basal; ANOVA with Dunnett's multiple comparison test.
ApoE-targeted replacement microglia show isoform-specific differences in C5a-stimulated migration
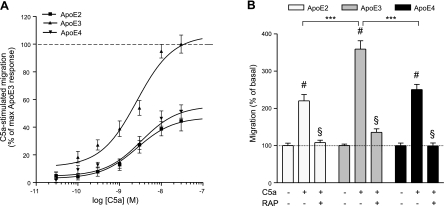
Our next focus was on the effect of apoE isoforms, rather than on the presence or absence of apoE, on C5a-stimulated migration of microglia. We used microglia isolated from targeted replacement (TR) mice in which the coding exons have been replaced with APOE ε2 (TR APOE2), ε3 (TR APOE3), or ε4 (TR APOE4). Although C5a-stimulated dose-dependent chemotaxis in microglia humanized for each of the three genotypes, stimulated migration of TR APOE2 and TR APOE4 microglia was greatly reduced compared with that of TR APOE3 (Fig. 3). Indeed, although EC50 values for C5a-stimulated migration were comparable among the three genotypes, maximal chemotactic responses to C5a by TR APOE2 and TR APOE4 microglia were approximately half that (44.5±7.3 and 51.3±4.8%, respectively) of TR APOE3 microglia (Fig. 3B and Table 1). In addition, absolute migration data from Figs. 1A and 3B show that RAP was equally effective at reducing maximally C5a-stimulated microglial migration to near basal levels in all 3 TR genotypes (Fig. 3B). As with the signaling data for wild-type and apoE−/− microglia, ERK and Akt activation was uniformly similar over the same 30-min time course for the 3 TR microglia genotypes (data not shown). Of importance, basal migration of all TR microglia was similar to that of wild-type and apoE−/− microglia, and, likewise, dose responses to C5a produced an inverted U (data not shown).
Figure 3.
Humanized microglia differentially migrate in response to C5a. A) Murine microglia expressing apoE2, apoE3, or apoE4 display differential C5a-stimulated, dose-dependent chemotaxis. Data are expressed as the percentage of the maximum apoE3 response to C5a. B) C5a-stimulated migration of humanized microglia is RAP dependent; 1 μM RAP added to both upper and lower wells of the Boyden chamber blocked microglial migration toward 30 nM C5a (bottom well only). Whereas no significant difference was seen in the level of basal migration among genotypes, the extent of maximal stimulation was greatest in microglia humanized for apoE3. Data are expressed as percentage of corresponding basal migration; n = 9 (i.e., 3–5 independent experiments performed in triplicate). ***P < 0.001; #P < 0.001 vs. basal; §P < 0.001 vs. maximally stimulated; ANOVA with Bonferroni multiple comparison test.
Table 1.
ApoE-dependent microglia chemotaxis to C5a is modulated by human ApoE genetic isoforms
| Genotype | C5a |
|
|---|---|---|
| EC50 (nM) | Maximum response (% of max) | |
| Wild-type | 2 ± 1.3 | 100 ± 8 |
| apoE−/− | 0.8 ± 1.8 | 41.7 ± 6.4* |
| apoE2 | 2.3 ± 1.2 | 44.5 ± 7.3# |
| apoE3 | 1.1 ± 0.4 | 100 ± 6.5 |
| apoE4 | 4.1 ± 3 | 51.3 ± 4.8† |
P < 0.001 vs. wild-type maximum response;
P < 0.001 vs. apoE3 maximum response; unpaired t test.
ATP-stimulated microglial migration is apoE dependent
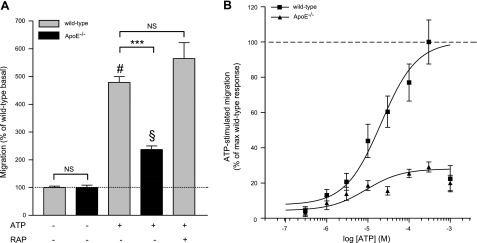
We next tested whether the apoE- and RAP-dependent reductions in C5a-stimulated microglial migration were generalizable to all directed migration by using ATP, a chemotactic agent known to differ mechanistically from C5a (57). As before, basal migration of wild-type and apoE−/− microglia was not significantly different (Fig. 4A). ATP yielded dose-dependent stimulation of wild-type microglia migration (Fig. 4B) similar to previously published reports (57), with an EC50 of 21 ± 1.5 μM (Fig. 4B and Table 2) and 300 μM ATP increasing migration to 479 ± 21.6% over basal (Fig. 4A). Comparably, 300 μM ATP was significantly less effective at stimulating migration of apoE−/− microglia, increasing cell migration to only 200.2 ± 12% over basal (Fig. 4A). In addition, maximal stimulation of apoE−/− microglia by ATP was only 29.1 ± 2.9% that of wild-type, with EC50 values for wild-type and apoE−/− microglia differing significantly (21±1.5 and 9.1±1.8 μM, respectively; Fig. 4B and Table 2). In contrast with C5a-stimulated microglia migration, 1 μM RAP failed to reduce ATP-stimulated migration of wild-type microglia (Fig. 4A). These results show that C5a- and ATP-stimulated mouse primary microglia migration are both apoE dependent but apparently through different mechanisms.
Figure 4.
ATP-stimulated microglial migration is apoE dependent. A) Basal migration of wild-type and apoE−/− microglia was similar, although 300 μM ATP was less effective at stimulating migration of apoE−/− microglia in a Boyden chamber assembly. ATP-stimulated migration of wild-type microglia was RAP independent (1 μM). Data are expressed as percentage of wild-type basal migration; n = 9–15 (i.e., 3–5 independent experiments performed in triplicate). ***P < 0.001; #P < 0.001 vs. basal wild-type; §P < 0.001 vs. basal knockout; ANOVA with Bonferroni multiple comparison test. B) ATP-stimulated migration of wild-type and apoE−/− microglia is dose dependent. Data are expressed as the percentage of the maximum wild-type response to ATP; n = 9–12 (i.e., 3–4 independent experiments were performed in triplicate).
Table 2.
ApoE-dependent microglia chemokinesis in the presence of ATP is modulated by human apoE genetic isoforms
| Genotype | ATP |
|
|---|---|---|
| EC50 (μM) | Maximum response (% of max) | |
| Wild-type | 21 ± 1.5 | 100 ± 12.5 |
| apoE−/− | 9.1 ± 1.8* | 29.1 ± 2.9# |
| apoE2 | 37.4 ± 1.5@ | 50.6 ± 11.6§ |
| apoE3 | 15.6 ± 1.4 | 100 ± 8 |
| apoE4 | 125 ± 2.3@ | 39.4 ± 9.2§ |
P < 0.001 vs. wild-type EC50;
P < 0.001 vs. wild-type maximum response;
P < 0.001 vs. apoE3 EC50;
P < 0.001 vs. apoE3 maximum response; unpaired t test.
Reduced ATP-stimulated migration by apoE−/− microglia is associated with altered ERK activation
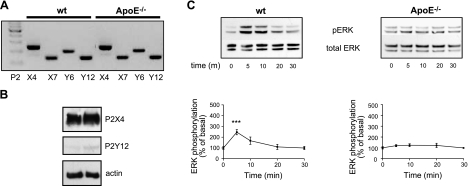
Because ATP-stimulated migration is apoE dependent but RAP independent, we next investigated whether reduced ATP-stimulated migration by apoE−/− microglia resulted from altered purinergic signaling. Wild-type and apoE−/− microglia equally express mRNAs for P2X4, P2X7, P2Y6, and P2Y12 (Fig. 5A), purinergic cell-surface receptors shown to be critical for appropriate immune responses by microglia, equally (64). Protein expression of P2X4 and P2Y12, the two principle ATP receptors associated with microglial migration (30, 65), was corroborated by Western blot analysis (Fig. 5B) and flow cytometry (Supplemental Fig. S3B), with levels not differing significantly between wild-type and apoE−/− microglia. Because activation of MAPK and PI3K/Akt pathways have been previously implicated in ATP-mediated microglial migration, we determined whether ATP-stimulated ERK and Akt activation was comparable in wild-type and apoE−/− microglia. Whereas treatment of wild-type microglia with 300 μM ATP resulted in a 5-min peak for ERK activation of 246.7 ± 29.9% over basal, ATP failed to activate ERK in apoE−/− microglia over the same 30-min time course (Fig. 5C). ATP-stimulated activation of Akt followed a similar time course and was not significantly different among genotypes (data not shown). Taken together, these data suggest that the lack of apoE in microglia suppresses ATP-stimulated ERK activation and migration through RAP-independent mechanisms.
Figure 5.
ATP failed to activate ERK in apoE−/− microglia. A) RT-PCR analysis of mRNAs from wild-type (wt) and apoE−/− microglia shows expression of purinergic receptors. B) P2X4 and P2Y12 receptor expression was confirmed using Western blot analysis of whole-cell lysates isolated from untreated cultured microglia. C) Western blot analysis shows time course of ERK activation (pERK) in whole-cell lysates prepared from cultured murine microglia stimulated with 300 μM ATP over 30 min. Quantification revealed failure of ATP to induce ERK activation in apoE−/− microglia over 30 min. Western blots are representative of ≥3 independent experiments. Data are expressed as percentage of ERK over time 0 control. ***P < 0.001 vs. corresponding basal; ANOVA withy Dunnett's multiple comparison test.
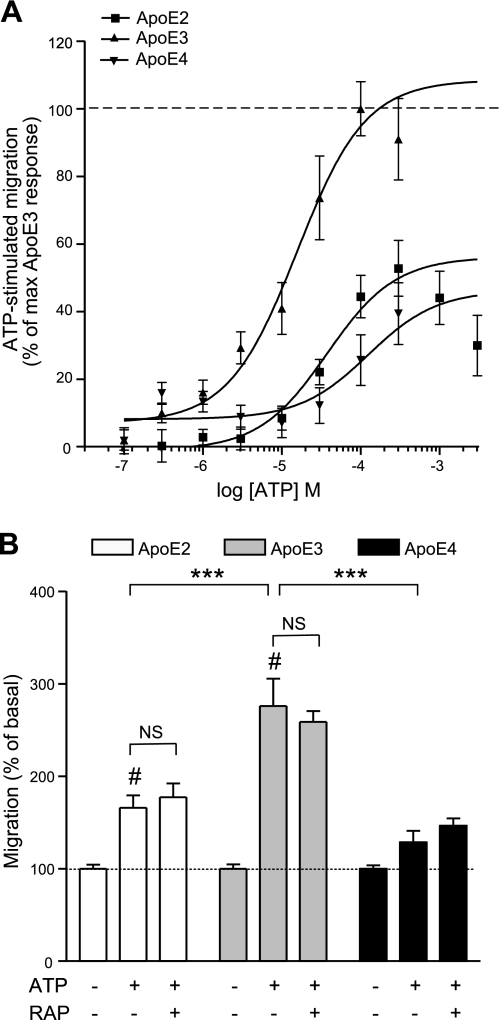
ApoE-targeted replacement microglia show isoform-specific differences in ATP-stimulated migration
Because of our C5a and ATP findings, we hypothesized that apoE isoform-specific differences might also exist for ATP-stimulated migration of TR APOE microglia. Indeed, whereas ATP stimulated dose-dependent chemotaxis in TR APOE microglia of each genotype, microglia isolated from TR APOE2 and TR APOE4 mice showed a greatly reduced migratory response compared with that of TR APOE3 microglia (Fig. 6). Unlike C5a-stimulated TR APOE microglia, however, EC50 values for ATP-stimulated migration of these cells were significantly different among the three genotypes (Fig. 6B and Table 2). In addition, maximal chemotactic responses to ATP differed significantly, with ATP-stimulated migration of TR APOE2 microglia being 50.6 ± 11.6% that of TR APOE3 cells and TR APOE4 microglial migration reduced to 39.4 ± 9.2% (Fig. 6B and Table 2). As with wild-type and apoE−/− microglia, ATP-stimulated migration of TR APOE microglia was RAP independent (Fig. 6B), and TR APOE2, TR APOE3, and TR APOE4 microglia showed no significant difference in mRNA or cell surface expression of relevant purinergic receptors (Supplemental Fig. S3A, B). Surprisingly, signaling data for ATP-stimulated TR APOE2, TR APOE3, and TR APOE4 microglia showed similar activation of both ERK and Akt over the same 30-min time course (data not shown).
Figure 6.
Humanized microglia differentially migrate in response to ATP. A) Murine microglia expressing either apoE2, apoE3, or apoE4 display differential ATP-stimulated, dose-dependent chemotaxis. Data are expressed as percentage of maximum apoE3 response to ATP. B) ATP-stimulated migration of humanized microglia occurs independent of 1 μM RAP treatment. Although no significant difference was seen in the level of basal migration among genotypes, the extent of maximal stimulation was greatest in microglia humanized for apoE3. Data are expressed as percentage of corresponding basal migration; n = 9 (i.e., 3–5 independent experiments performed in triplicate). ***P < 0.001; #P < 0.001 vs. basal; ANOVA with Bonferroni multiple comparison test.
DISCUSSION
Increased numbers of microglia and a proinflammatory environment are characteristic features of many neurological disorders, including AD, Parkinson's disease, stroke, and traumatic brain injury. Although microglia can serve neurotoxic as well as neuroprotective functions, both depend on their ability to migrate toward an injury focus. Understanding the mechanisms of microglial migration in brain is critical for the development of strategies aimed to elucidate their role in neuropathological states and identification of therapeutic targets in neurodegenerative diseases. In addition, the apoE genotype strongly influences a patient's risk of developing AD and may confer risk in other neurodegenerative diseases, but the mechanism by which different apoE isoforms mediate this risk remains unknown. To that end, we show for the first time that apoE is required for mouse microglial migration in vitro to select stimuli and that human apoE isoforms differentially modulate mouse microglial migration. This is a novel finding that may contribute to the pathology of apoE genotype-dependent neurodegenerative diseases.
It is important to stress that basal migration of mouse primary microglia was not significantly different among wild-type, apoE−/−, and TR APOE cells, but rather migration diverged among these microglial genotypes only when stimulated by C5a or ATP. These data reveal significant apoE-dependent and apoE isoform-specific influences on microglia migration stimulated by C5a or ATP.
C5a activates the receptor CD88, a G-protein-coupled receptor that acts via increased intracellular calcium to stimulate a variety of downstream signal transduction pathways (for review, see ref. 66). C5a-stimulated chemotaxis was largely apoE dependent and similarly RAP dependent, suggesting that apoE interaction with LDLR or LRP or some other apoE receptor that is inhibited by RAP is necessary for most of the C5a-stimulated primary microglial migration. Experiments with microglia expressing different human apoE isoforms demonstrated a significantly decreased maximal migratory response to C5a by TR APOE2 or TR APOE4 microglia compared with that of TR APOE3 microglia, and, again, C5a-stimulated migration was completely blocked by RAP in all three TR APOE microglia. Although these data suggest that C5a-stimulated migration is somehow related to apoE (isoforms) interactions with RAP-dependent receptors, we did not observe differences in the activation of downstream signaling molecules thought to be central to C5a-stimulated microglia migration, raising the possibility that still other second messenger systems underlie the effects of apoE (isoforms) in C5a-stimulated migration. In addition, all microglial genotypes tested similarly expressed CD88 mRNA and protein (Supplemental Fig. S2). Furthermore, TR APOE2, TR APOE3, and TR APOE4 microglia secreted apoE isoforms to the same level in the presence or absence of C5a over the same 3-h time frame in which migration experiments were conducted, suggesting that differential C5a-stimulated migration did not result from different apoE isoform levels (Supplemental Fig. S1A). ApoE also was necessary for ATP-stimulated microglial migration because apoE−/− microglia failed to migrate in response to ATP. However, in contrast with C5a-stimulated migration, ATP-stimulated migration was not RAP dependent in wild-type microglia, and the EC50 for ATP, not simply the maximal response, was reduced in apoE−/− microglia. Microglia respond to ATP/ADP by increasing net cellular movement (chemokinesis), a response that is dependent on activation of a diverse set of purinergic receptors (64). Although we did not see a difference in P2X and P2Y receptor expression between wild-type and apoE−/− microglia, ATP-stimulated ERK phosphorylation was significantly different among genotypes. ERK is a downstream effector of purinergic receptor activation, and differential phosphorylation of ERK suggests that, unlike with C5a, ATP receptor activation or downstream signal transduction cascades are modulated by apoE acting through mechanisms that do not include RAP-sensitive receptors. Because wild-type cells exposed to RAP did not replicate the effect of apoE−/− microglia, it is possible that that apoE−/− microglia somehow have adapted to the lack of apoE, and this adaptation suppresses ERK signaling and migration on ATP stimulation. However, this possibility seems unlikely because TR APOE microglia that expressed different human apoE isoforms, and so never lacked apoE, also had an altered response to ATP stimulation. The effect of apoE isoforms in ATP-stimulated microglia demonstrated a clear effect of apoE4 and apoE2, similar to that observed with C5a stimulation. However, not only did TR APOE2 microglia exhibit decreased maximal motility, they also showed less sensitivity to ATP. The mechanism for this result is unclear but may provide additional clues as to the disparate effects of apoE isoforms in human biology.
The apoE isoform-specific risk for AD is ε2 < ε3 < ε4. However, our results showed that the ranking of EC50 for ATP-stimulated migration was TR APOE3 < TR APOE2 < TR APOE4 (C5a EC50 was not significantly different among TR APOE microglia), and the ranking of maximal migration after C5a or ATP stimulation was TR APOE3 < TR APOE2 ≈ TR APOE4. It is not possible to directly reconcile these two sets of data, because apoE is pleiotropic, with profound effects on astrocytes and neurons and perhaps other cells in the brain important to Aβ clearance. However, note that humans appear to have evolved the ε3 from the ε4 allele and the ε2 from the ε3 allele. Of course, the selection pressures that led to these common alleles of APOE in humans worldwide is not known but has been proposed to be related to immune function (67). From this perspective, our results suggest that the mutation that generated ε3 significantly altered stimulated microglia migration with respect to the ε4 allele and that the subsequent mutation that generated ε2 shifted stimulated microglia migration back closer to that of apoE4. We do not yet know whether these findings with microglia will be relevant to other migratory inflammatory cells, but they do nevertheless demonstrate one mechanism by which apoE isoforms may influence immune function.
In summary, for the first time, we show that stimulated microglial migration in response to C5a and ATP is apoE dependent and operates through different intracellular signaling pathways. We also show that microglial migration is variably influenced by the TR APOE genotype in a pattern that partially recapitulates disease risk in humans. These findings have important implications for understanding the pathogenesis of neurological diseases, particularly those in which the apoE genotype is associated with risk of disease, most notably AD. Although neonatal microglia are widely used as a model of microglia migration, changes in microglia with age suggest caution in overinterpreting results. Within the constraints of our model system, it was not possible to isolate adult microglia in the absence of confounding growth factor supplementation or other manipulation. Further studies addressing these challenges will be needed to better characterize apoE-dependent differences in adult microglia migration in vivo. Further studies will also be required to elucidate the specific molecular mechanisms responsible for differential regulation of microglia migration by apoE isoforms and to determine additional functional characteristics of microglia that are influenced by apoE.
Supplementary Material
Acknowledgments
We thank Dr. Yue Yang, Meilany Wijaya, Amy Look, and Lisa Keene for technical assistance and Carol Arnold for managerial support. We also thank Dr. Nobuyo Maeda for the generous gift of apoE2-, apoE3-, and apoE4-targeted replacement mice.
This work was supported by U.S. National Institutes of Health grants AG05136 and ES16754 as well as 5T32 AG000258 (Dr. Suzanne Craft) and the Nancy and Buster Alvord Endowment.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Amor S., Puentes F., Baker D., van der Valk P. Inflammation in neurodegenerative diseases. Immunology 129, 154–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Festoff B. W., Ameenuddin S., Arnold P. M., Wong A., Santacruz K. S., Citron B. A. (2006) Minocycline neuroprotects, reduces microgliosis, and inhibits caspase protease expression early after spinal cord injury. J. Neurochem. 97, 1314–1326 [DOI] [PubMed] [Google Scholar]
- 3. Howell O. W., Rundle J. L., Garg A., Komada M., Brophy P. J., Reynolds R. Activated microglia mediate axoglial disruption that contributes to axonal injury in multiple sclerosis. J. Neuropathol. Exp. Neurol. 69, 1017–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malm T. M., Magga J., Kuh G. F., Vatanen T., Koistinaho M., Koistinaho J. (2008) Minocycline reduces engraftment and activation of bone marrow-derived cells but sustains their phagocytic activity in a mouse model of Alzheimer's disease. Glia 56, 1767–1779 [DOI] [PubMed] [Google Scholar]
- 5. Neumann J., Gunzer M., Gutzeit H. O., Ullrich O., Reymann K. G., Dinkel K. (2006) Microglia provide neuroprotection after ischemia. FASEB J. 20, 714–716 [DOI] [PubMed] [Google Scholar]
- 6. Seabrook T. J., Jiang L., Maier M., Lemere C. A. (2006) Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia 53, 776–782 [DOI] [PubMed] [Google Scholar]
- 7. Wu D. C., Jackson-Lewis V., Vila M., Tieu K., Teismann P., Vadseth C., Choi D. K., Ischiropoulos H., Przedborski S. (2002) Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 22, 1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yrjanheikki J., Keinanen R., Pellikka M., Hokfelt T., Koistinaho J. (1998) Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc. Natl. Acad. Sci. U. S. A. 95, 15769–15774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Den Bosch L., Tilkin P., Lemmens G., Robberecht W. (2002) Minocycline delays disease onset and mortality in a transgenic model of ALS. Neuroreport 13, 1067–1070 [DOI] [PubMed] [Google Scholar]
- 10. Peri F., Nusslein-Volhard C. (2008) Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 133, 916–927 [DOI] [PubMed] [Google Scholar]
- 11. Venneti S., Wiley C. A., Kofler J. (2009) Imaging microglial activation during neuroinflammation and Alzheimer's disease. J. Neuroimmune Pharmacol. 4, 227–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lull M. E., Block M. L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 7, 354–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hickman S. E., El Khoury J. Mechanisms of mononuclear phagocyte recruitment in Alzheimer's disease. CNS Neurol. Disord. Drug Targets 9, 168–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Woodruff T. M., Ager R. R., Tenner A. J., Noakes P. G., Taylor S. M. The role of the complement system and the activation fragment C5a in the central nervous system. Neuromol. Med. 12, 179–192 [DOI] [PubMed] [Google Scholar]
- 15. Morgan B. P., Gasque P. (1996) Expression of complement in the brain: role in health and disease. Immunol. Today 17, 461–466 [DOI] [PubMed] [Google Scholar]
- 16. McGeer P. L., Akiyama H., Itagaki S., McGeer E. G. (1989) Activation of the classical complement pathway in brain tissue of Alzheimer patients. Neurosci. Lett. 107, 341–346 [DOI] [PubMed] [Google Scholar]
- 17. Eikelenboom P., Stam F. C. (1982) Immunoglobulins and complement factors in senile plaques. An immunoperoxidase study. Acta Neuropathol. 57, 239–242 [DOI] [PubMed] [Google Scholar]
- 18. Velazquez P., Cribbs D. H., Poulos T. L., Tenner A. J. (1997) Aspartate residue 7 in amyloid β-protein is critical for classical complement pathway activation: implications for Alzheimer's disease pathogenesis. Nat. Med. 3, 77–79 [DOI] [PubMed] [Google Scholar]
- 19. Bradt B. M., Kolb W. P., Cooper N. R. (1998) Complement-dependent proinflammatory properties of the Alzheimer's disease β-peptide. J. Exp. Med. 188, 431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ager R. R., Fonseca M. I., Chu S. H., Sanderson S. D., Taylor S. M., Woodruff T. M., Tenner A. J. Microglial C5aR (CD88) expression correlates with amyloid-β deposition in murine models of Alzheimer's disease. J. Neurochem. 113, 389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fonseca M. I., Ager R. R., Chu S. H., Yazan O., Sanderson S. D., LaFerla F. M., Taylor S. M., Woodruff T. M., Tenner A. J. (2009) Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer's disease. J. Immunol. 183, 1375–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jakob A. (2010) CNS lupus. J. Neuroinflammation 221, 46–52 [Google Scholar]
- 23. Wang Y., Li Y., Dalle Lucca S. L., Simovic M., Tsokos G. C., Dalle Lucca J. J. Decay accelerating factor (CD55) protects neuronal cells from chemical hypoxia-induced injury. J. Neuroinflammation 7, 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim G. H., Mocco J., Hahn D. K., Kellner C. P., Komotar R. J., Ducruet A. F., Mack W. J., Connolly E. S., Jr. (2008) Protective effect of C5a receptor inhibition after murine reperfused stroke. Neurosurgery 63, 122–125; discussion 125–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garrett M. C., Otten M. L., Starke R. M., Komotar R. J., Magotti P., Lambris J. D., Rynkowski M. A., Connolly E. S. (2009) Synergistic neuroprotective effects of C3a and C5a receptor blockade following intracerebral hemorrhage. Brain Res. 1298, 171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sewell D. L., Nacewicz B., Liu F., Macvilay S., Erdei A., Lambris J. D., Sandor M., Fabry Z. (2004) Complement C3 and C5 play critical roles in traumatic brain cryoinjury: blocking effects on neutrophil extravasation by C5a receptor antagonist. J. Neuroimmunol. 155, 55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lambert C., Ase A. R., Seguela P., Antel J. P. Distinct migratory and cytokine responses of human microglia and macrophages to ATP. Brain Behav. Immun. 24, 1241–1248 [DOI] [PubMed] [Google Scholar]
- 28. Samuels S. E., Lipitz J. B., Dahl G., Muller K. J. Neuroglial ATP release through innexin channels controls microglial cell movement to a nerve injury. J. Gen. Physiol. 136, 425–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. James G., Butt A. M. (2002) P2Y and P2X purinoceptor mediated Ca2+ signaling in glial cell pathology in the central nervous system. Eur. J. Pharmacol. 447, 247–260 [DOI] [PubMed] [Google Scholar]
- 30. Honda S., Sasaki Y., Ohsawa K., Imai Y., Nakamura Y., Inoue K., Kohsaka S. (2001) Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J. Neurosci. 21, 1975–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haynes S. E., Hollopeter G., Yang G., Kurpius D., Dailey M. E., Gan W. B., Julius D. (2006) The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 9, 1512–1519 [DOI] [PubMed] [Google Scholar]
- 32. Kurpius D., Nolley E. P., Dailey M. E. (2007) Purines induce directed migration and rapid homing of microglia to injured pyramidal neurons in developing hippocampus. Glia 55, 873–884 [DOI] [PubMed] [Google Scholar]
- 33. Inoue K. (2002) Microglial activation by purines and pyrimidines. Glia 40, 156–163 [DOI] [PubMed] [Google Scholar]
- 34. Choi M. S., Cho K. S., Shin S. M., Ko H. M., Kwon K. J., Shin C. Y., Ko K. H. ATP induced microglial cell migration through non-transcriptional activation of matrix metalloproteinase-9. Arch Pharm. Res. 33, 257–265 [DOI] [PubMed] [Google Scholar]
- 35. Newman E. A. (2001) Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J. Neurosci. 21, 2215–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gyoneva S., Orr A. G., Traynelis S. F. (2009) Differential regulation of microglial motility by ATP/ADP and adenosine. Parkinsonism Relat. Disord. 15(Suppl. 3), S195–S199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Degrell I., Niklasson F. (1988) Purine metabolites in the CSF in presenile and senile dementia of Alzheimer type, and in multi infarct dementia. Arch. Gerontol. Geriatr. 7, 173–178 [DOI] [PubMed] [Google Scholar]
- 38. Sanz J. M., Chiozzi P., Ferrari D., Colaianna M., Idzko M., Falzoni S., Fellin R., Trabace L., Di Virgilio F. (2009) Activation of microglia by amyloid β requires P2X7 receptor expression. J. Immunol. 182, 4378–4385 [DOI] [PubMed] [Google Scholar]
- 39. Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., Roses A. D., Haines J. L., Pericak-Vance M. A. (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 261, 921–923 [DOI] [PubMed] [Google Scholar]
- 40. Seshadri S., Fitzpatrick A. L., Ikram M. A., DeStefano A. L., Gudnason V., Boada M., Bis J. C., Smith A. V., Carassquillo M. M., Lambert J. C., Harold D., Schrijvers E. M., Ramirez-Lorca R., Debette S., Longstreth W. T., Jr., Janssens A. C., Pankratz V. S., Dartigues J. F., Hollingworth P., Aspelund T., Hernandez I., Beiser A., Kuller L. H., Koudstaal P. J., Dickson D. W., Tzourio C., Abraham R., Antunez C., Du Y., Rotter J. I., Aulchenko Y. S., Harris T. B., Petersen R. C., Berr C., Owen M. J., Lopez-Arrieta J., Varadarajan B. N., Becker J. T., Rivadeneira F., Nalls M. A., Graff-Radford N. R., Campion D., Auerbach S., Rice K., Hofman A., Jonsson P. V., Schmidt H., Lathrop M., Mosley T. H., Au R., Psaty B. M., Uitterlinden A. G., Farrer L. A., Lumley T., Ruiz A., Williams J., Amouyel P., Younkin S. G., Wolf P. A., Launer L. J., Lopez O. L., van Duijn C. M., Breteler M. M. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA 303, 1832–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harold D., Abraham R., Hollingworth P., Sims R., Gerrish A., Hamshere M. L., Pahwa J. S., Moskvina V., Dowzell K., Williams A., Jones N., Thomas C., Stretton A., Morgan A. R., Lovestone S., Powell J., Proitsi P., Lupton M. K., Brayne C., Rubinsztein D. C., Gill M., Lawlor B., Lynch A., Morgan K., Brown K. S., Passmore P. A., Craig D., McGuinness B., Todd S., Holmes C., Mann D., Smith A. D., Love S., Kehoe P. G., Hardy J., Mead S., Fox N., Rossor M., Collinge J., Maier W., Jessen F., Schurmann B., van den Bussche H., Heuser I., Kornhuber J., Wiltfang J., Dichgans M., Frolich L., Hampel H., Hull M., Rujescu D., Goate A. M., Kauwe J. S., Cruchaga C., Nowotny P., Morris J. C., Mayo K., Sleegers K., Bettens K., Engelborghs S., De Deyn P. P., Van Broeckhoven C., Livingston G., Bass N. J., Gurling H., McQuillin A., Gwilliam R., Deloukas P., Al-Chalabi A., Shaw C. E., Tsolaki M., Singleton A. B., Guerreiro R., Muhleisen T. W., Nothen M. M., Moebus S., Jockel K. H., Klopp N., Wichmann H. E., Carrasquillo M. M., Pankratz V. S., Younkin S. G., Holmans P. A., O'Donovan M., Owen M. J., Williams J. (2009) Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat. Genet. 41, 1088–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beecham G. W., Martin E. R., Li Y. J., Slifer M. A., Gilbert J. R., Haines J. L., Pericak-Vance M. A. (2009) Genome-wide association study implicates a chromosome 12 risk locus for late-onset Alzheimer disease. Am. J. Hum. Genet. 84, 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim J., Basak J. M., Holtzman D. M. (2009) The role of apolipoprotein E in Alzheimer's disease. Neuron 63, 287–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee C. Y., Landreth G. E. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. 117, 949–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Colton C. A., Needham L. K., Brown C., Cook D., Rasheed K., Burke J. R., Strittmatter W. J., Schmechel D. E., Vitek M. P. (2004) APOE genotype-specific differences in human and mouse macrophage nitric oxide production. J. Neuroimmunol. 147, 62–67 [DOI] [PubMed] [Google Scholar]
- 46. Barger S. W., Harmon A. D. (1997) Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature 388, 878–881 [DOI] [PubMed] [Google Scholar]
- 47. Belinson H., Michaelson D. M. (2009) ApoE4-dependent Aβ-mediated neurodegeneration is associated with inflammatory activation in the hippocampus but not the septum. J. Neural Transm. 116, 1427–1434 [DOI] [PubMed] [Google Scholar]
- 48. Maezawa I., Nivison M., Montine K. S., Maeda N., Montine T. J. (2006) Neurotoxicity from innate immune response is greatest with targeted replacement of E4 allele of apolipoprotein E gene and is mediated by microglial p38MAPK. FASEB J. 20, 797–799 [DOI] [PubMed] [Google Scholar]
- 49. Maezawa I., Zaja-Milatovic S., Milatovic D., Stephen C., Sokal I., Maeda N., Montine T. J., Montine K. S. (2006) Apolipoprotein E isoform-dependent dendritic recovery of hippocampal neurons following activation of innate immunity. J. Neuroinflammation 3, 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pocivavsek A., Burns M. P., Rebeck G. W. (2009) Low-density lipoprotein receptors regulate microglial inflammation through c-Jun N-terminal kinase. Glia 57, 444–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Egensperger R., Kosel S., von Eitzen U., Graeber M. B. (1998) Microglial activation in Alzheimer disease: Association with APOE genotype. Brain Pathol. 8, 439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Simard A. R., Soulet D., Gowing G., Julien J. P., Rivest S. (2006) Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron 49, 489–502 [DOI] [PubMed] [Google Scholar]
- 53. Keene C. D., Chang R. C., Lopez-Yglesias A. H., Shalloway B. R., Sokal I., Li X., Reed P. J., Keene L. M., Montine K. S., Breyer R. M., Rockhill J. K., Montine T. J. Suppressed accumulation of cerebral amyloid β peptides in aged transgenic Alzheimer's disease mice by transplantation with wild-type or prostaglandin E2 receptor subtype 2-null bone marrow. Am. J. Pathol. 177, 346–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xu P. T., Schmechel D., Rothrock-Christian T., Burkhart D. S., Qiu H. L., Popko B., Sullivan P., Maeda N., Saunders A. M., Roses A. D., Gilbert J. R. (1996) Human apolipoprotein E2, E3, and E4 isoform-specific transgenic mice: human-like pattern of glial and neuronal immunoreactivity in central nervous system not observed in wild-type mice. Neurobiol. Dis. 3, 229–245 [DOI] [PubMed] [Google Scholar]
- 55. Sullivan P. M., Mezdour H., Aratani Y., Knouff C., Najib J., Reddick R. L., Quarfordt S. H., Maeda N. (1997) Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J. Biol. Chem. 272, 17972–17980 [DOI] [PubMed] [Google Scholar]
- 56. Cimino P. J., Sokal I., Leverenz J., Fukui Y., Montine T. J. (2009) DOCK2 is a microglial specific regulator of central nervous system innate immunity found in normal and Alzheimer's disease brain. Am. J. Pathol. 175, 1622–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Miller A. M., Stella N. (2009) Microglial cell migration stimulated by ATP and C5a involve distinct molecular mechanisms: quantification of migration by a novel near-infrared method. Glia 57, 875–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Christie R. H., Freeman M., Hyman B. T. (1996) Expression of the macrophage scavenger receptor, a multifunctional lipoprotein receptor, in microglia associated with senile plaques in Alzheimer's disease. Am. J. Pathol. 148, 399–403 [PMC free article] [PubMed] [Google Scholar]
- 59. Marzolo M. P., von Bernhardi R., Bu G., Inestrosa N. C. (2000) Expression of α2-macroglobulin receptor/low density lipoprotein receptor-related protein (LRP) in rat microglial cells. J. Neurosci. Res. 60, 401–411 [DOI] [PubMed] [Google Scholar]
- 60. Zhang C., An J., Strickland D. K., Yepes M. (2009) The low-density lipoprotein receptor-related protein 1 mediates tissue-type plasminogen activator-induced microglial activation in the ischemic brain. Am. J. Pathol. 174, 586–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Egger M., Beer A. G., Theurl M., Schgoer W., Hotter B., Tatarczyk T., Vasiljevic D., Frauscher S., Marksteiner J., Patsch J. R., Schratzberger P., Djanani A. M., Mahata S. K., Kirchmair R. (2008) Monocyte migration: a novel effect and signaling pathways of catestatin. Eur. J. Pharmacol. 598, 104–111 [DOI] [PubMed] [Google Scholar]
- 62. Irino Y., Nakamura Y., Inoue K., Kohsaka S., Ohsawa K. (2008) Akt activation is involved in P2Y12 receptor-mediated chemotaxis of microglia. J. Neurosci. Res. 86, 1511–1519 [DOI] [PubMed] [Google Scholar]
- 63. Calvo M., Zhu N., Tsantoulas C., Ma Z., Grist J., Loeb J. A., Bennett D. L. Neuregulin-ErbB signaling promotes microglial proliferation and chemotaxis contributing to microgliosis and pain after peripheral nerve injury. J. Neurosci. 30, 5437–5450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Inoue K. (2008) Purinergic systems in microglia. Cell. Mol. Life Sci. 65, 3074–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Horvath R. J., DeLeo J. A. (2009) Morphine enhances microglial migration through modulation of P2X4 receptor signaling. J. Neurosci. 29, 998–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ward P. A. (2009) Functions of C5a receptors. J. Mol. Med. 87, 375–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mahley R. W., Rall S. C. (2000) Apolipoprotein E: Far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 01, 507–537 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.