Abstract
The purpose of this study was to test the hypothesis that remodeling of skeletal muscle extracellular matrix (ECM) is involved in protecting human muscle against injury. Biopsies were obtained from medial gastrocnemius muscles after a single bout of electrical stimulation (B) or a repeated bout (RB) 30 d later, or 30 d after a single stimulation bout (RBc). A muscle biopsy was collected from the control leg for comparison with the stimulated leg. Satellite cell content, tenascin C, and muscle regeneration were assessed by immunohistochemistry; real-time PCR was used to measure mRNA levels of collagens, laminins, heat-shock proteins (HSPs), inflammation, and related growth factors. The large responses of HSPs, CCL2, and tenascin C detected 48 h after a single bout were attenuated in the RB trial, indicative of protection against injury. Satellite cell content and 12 target genes, including IGF-1, were elevated 30 d after a single bout. Among those displaying the greatest difference vs. control muscle, ECM laminin-β1 and collagen types I and III were elevated ∼6- to 9-fold (P<0.001). The findings indicate that the sequenced events of load-induced early deadhesion and later strengthening of skeletal muscle ECM play a role in protecting human muscle against future injury.—Mackey, A. L., Brandstetter, S., Schjerling, P., Bojsen-Moller, J., Qvortrup, K., Pedersen, M. M., Doessing, S. Kjaer, M., Magnusson, S. P., Langberg, H. Sequenced response of extracellular matrix deadhesion and fibrotic regulators after muscle damage is involved in protection against future injury in human skeletal muscle.
Keywords: insulin-like growth factor-1, satellite cells, collagen, tenascin C, repeated bout effect
The adaptation of skeletal muscle after injury to resist future damage is not understood. Models inducing controlled and standardized damage to skeletal muscle have been employed to investigate muscle soreness, inflammation, and regeneration (e.g., 1, 2–4). The majority of these studies have used high-force lengthening (eccentric) contractions. However, we recently provided evidence for the capacity also of electrically stimulated isometric contractions to induce muscle damage and initiate regenerative events in humans, evidenced by infiltration of inflammatory cells, the presence of desmin− and dystrophin− fibers, z-line disruption, and activated satellite cells (SCs; refs. 5, 6).
Lengthening contractions have also been the traditional model of choice in investigations into understanding how one exercise bout could protect against muscle damage when a similar bout was repeated (the phenomenon known as the repeated-bout effect). The fact that a muscle can be protected from damage and soreness purely by a single bout of exercise performed from 1 wk up to several months (e.g., 7–9) prior to reexposure to the same exercise is intriguing and has implications not only for strategies in injury prevention but also potentially in understanding muscle plasticity in the context of acute and chronic muscle wasting. At the cellular level, heat shock proteins (HSPs) have received some attention, with support for and against a role in the repeated-bout effect (10–12). Similarly, evidence suggests that inflammatory cells might be involved (13, 14). While many candidates are likely, the connective tissue making up the endomysium and perimysium has received little attention, despite its many roles, including accommodation of nociceptors, transmission of force, registration and conversion of mechanical signals, and as a niche regulator of myogenic precursor cells (15–18). It has been shown clearly that physiological mechanical loading is associated with dynamic adaptation of collagen, the major adhesion-promoting component of muscle extracellular matrix (ECM; refs. 19–24). In addition, evidence is apparent for attenuation of contraction-induced injury with increased muscle collagen content in rats (25). Tenascin C, belonging to the matricellular class of ECM proteins (26), has been reported to demonstrate dramatic responses in skeletal muscle subjected to unaccustomed exercise (4, 27, 28) and indeed to be essential for full-scale muscle injury repair, providing deadhesive effects and potentially a coordinating role at least in stages of the regeneration process (29). In light of this, we hypothesized that coordinated deadhesion and strengthening of muscle ECM constitutes a vital adaptation in providing protection against contraction-induced injury in human skeletal muscle.
To investigate the role of ECM remodeling in protection of human skeletal muscle against damage, we subjected human volunteers to a single or repeated bout of electrically stimulated contractions and measured the response of factors involved in the early and late stages of muscle tissue regeneration (HSPs, ECM components, myogenic precursor cells, inflammatory cells, and related growth factors). We hypothesized that, along with lower soreness and circulating creatine kinase (CK) activity, muscle biopsies collected after a repeated bout of ES isometric contractions would demonstrate lower mRNA levels of HSPs, myogenic factors, matricellular proteins, inflammatory factors, and insulin-like growth factor 1 (IGF-1), together with a lesser extent of morphological and ultrastructural damage than after a single bout, reflecting an adaptation of the supporting ECM.
MATERIALS AND METHODS
Ethical approval
The study was approved by the Ethics Committees of the Municipalities of Copenhagen and Frederiksberg (ref. H-A-2007-0037) and conformed to the standards set by the Declaration of Helsinki. All volunteers gave written informed consent before inclusion.
Volunteers
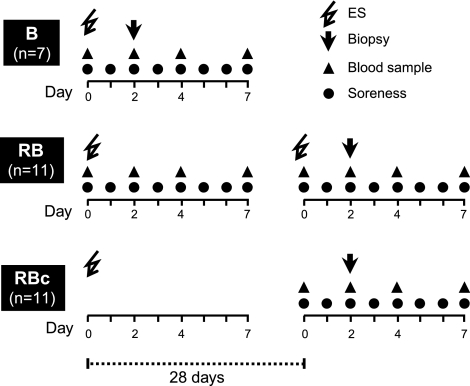
An overview of the participant groups is illustrated in Fig. 1. Twenty-two young healthy untrained men volunteered for the study and were divided into 2 groups of 11: repeated bout (RB; mean ± sd age 25±4 yrs; height 1.83±0.08 m; weight 76±7 kg) and repeated bout control (RBc; mean ± sd age 22±3 yrs; height 1.83±0.05 m; weight 74±10 kg). Participants assigned to the RB group were subjected to two bouts of electrical stimulation (ES), separated by 1 mo. RBc group participants followed a similar protocol but were not subjected to the second bout of ES. Single-bout (B) group participants, who were also only subjected to a single bout of ES, consisted of 7 young healthy untrained men (mean ± sd age 22±3 yrs; height 1.84±0.05 m; weight 78±6 kg) from a previous study (5).
Figure 1.
Outline of study design and timeline indicating the time points for muscle soreness measurements, blood samples, muscle biopsies and whether groups were subjected to a single bout (B) or repeated bout (RB) of electrical stimulation (ES). RBc, control group for the RB group.
Experimental design and setup
The experimental setup and ES protocol were identical to those described previously (5). Briefly, stimulation electrodes (Stimtrode, ST32D; Axelgaard Manufacturing Co., Fallbrook, CA USA; electrode size ∼25×25 mm, interelectrode center-center distance ∼35 mm) were positioned over the middle aspect of the gastrocnemius medialis muscle and connected to a stimulator (Elpha II 3000; Danmeter; Biotin, Odense, Denmark). During a short familiarization session, the muscle contraction was visually confirmed, after which intermittent muscle stimulation was performed for 30 min (60-Hz stimulation, pulse width 300 μs, duty cycle 40%, total cycle time 10 s, rise time 1 s, total stimulation time 4 s, descending time 1 s, rest time 6 s). Subjects held the stimulator themselves and were accordingly in control of the stimulation intensity (mA). They were instructed to increase the current as much as they could tolerate and were prompted to do this every 5 min throughout the 30-min period. The level of current was recorded every 5 min during the first bout, and each participant received the same level of current during the second bout as was delivered during the first bout.
Soreness
Muscle soreness was assessed prior to stimulation and on each of the 7 d postexercise with the aid of a visual-analog linear soreness scale, which ranged from 0 (normal, no pain) to 10 (extremely painful). Soreness was assessed in 3 different ways: by self-palpation in the belly of the muscle, active muscle contraction (heel-raise), and passive muscle stretch, as described previously (5). Soreness was recorded in the RB group after bout 1 and bout 2, and in the RBc group after biopsy alone.
Blood sampling
Blood samples were collected by venepuncture before and on d 2, 4, and 7 following both bouts of ES in the RB group, and at the corresponding time points in the RBc group, to monitor the effects of muscle biopsy sampling alone on circulating CK. Plasma CK levels were measured at the Department of Clinical Biochemistry at Bispebjerg Hospital, Copenhagen, as described in detail previously (5).
Muscle biopsies
Muscle biopsies were obtained from the belly of the gastrocnemius medialis by percutaneous needle biopsy with suction. None of the participants in this study had previously had biopsies taken from either gastrocnemius medialis muscle. Two biopsies were collected from each individual in the RB group 48 h after the second bout of ES, one from the leg that had been stimulated and one from the contralateral leg as a control. In the RBc group, which did not undergo a second bout of ES, biopsies were collected 30 d after the single bout of ES in order to investigate any residual regeneration at this time point. On extraction, part of the sample was immersed in 2% glutaraldehyde in 0.05 M sodium phosphate buffer (pH 7.2) for analysis by transmission electron microscopy. The remaining part of the sample was aligned, embedded in Tissue-Tek (Sakura Finetek Europe, Zoeterwoude, The Netherlands) and frozen by immersion in isopentane, precooled by liquid nitrogen. Serial transverse sections (10 μm) were cut at −24°C using a cryostat and stored at −80°C. Muscle biopsies from a previous study (5), stored in Tissue-Tek at −80°C since collection, were included for the mRNA analysis, and new immunohistochemical analyses were performed for the present study. These biopsies were collected 48 h after a single bout of ES identical to the one used in the current study and are referred to here as the B group. We have previously published data documenting muscle damage and SC activation in these biopsies (5, 6). The CD68 and laminin immunohistochemical double-staining performed for one of the previous studies (5) was reevaluated in the present study. All other stainings of biopsies in the B group described in the present study were performed in conjunction with staining biopsies from the RB and RBc groups.
Immunohistochemical analysis of muscle biopsies
Immunohistochemical stainings were performed using the same antibodies as in a prior study (5). Briefly, visualization of primary antibody binding was achieved with a combination of two of the Molecular Probes Alexa Fluor 488/568 goat anti-mouse/rabbit secondary antibodies (A11031, A11034, A11036, A11029; Invitrogen A/S, Taastrup, Denmark). 4′,6-Diamidino-2-phenylindole (DAPI) in the mounting medium (P36931; Molecular Probes ProLong Gold; Invitrogen) stained the nuclei blue. As well as the rabbit anti-laminin antibody (Z0097; Dako Denmark A/S, Glostrup, Denmark) used as a general basement membrane marker, we also performed staining with a mouse antibody against laminin α5 chain (4C7, ab17107; Abcam, Cambridge, UK). To distinguish between these two, the general laminin antibody is referred to here as laminin, and the laminin α5 chain-specific antibody is referred to as laminin α5.
Myofiber damage
Macrophage infiltration of myofibers was evaluated from sections double-stained with primary antibodies for CD68 (M0718; Dako) and laminin (Z0097; Dako). Intermediate filament and sarcolemma disruption was investigated by double-staining with primary antibodies for desmin (18-0016; Zymed, San Francisco, CA, USA) and dystrophin (ab15277; Abcam), respectively.
Regeneration
Regeneration was investigated by double-staining serial sections with the following combinations of primary antibodies: embryonic myosin (F1.652; Developmental Studies Hybridoma Bank, Iowa City, IA, USA) and dystrophin; CD56 (347740; Becton Dickinson, San Jose, CA, USA) and laminin; laminin α5 (4C7, ab17107; Abcam) and laminin; myosin type I (A4.951; Developmental Studies Hybridoma Bank) and laminin; myosin type II (A4.74; Developmental Studies Hybridoma Bank) and laminin.
Tenascin C
Tenascin-C immunoreactivity was digitally evaluated from sections incubated with the primary antibody NCL-Tenas-C (Novocastra; Leica Microsystems, Newcastle On Tyne, UK), a biotinylated secondary antibody (E0433; Dako), Vector Elite ABC kit (PK6100; Vector Laboratories, Peterborough, UK) and visualized with Immpact diaminobenzidine (DAB) substrate (SK-4105, Vector Laboratories). The percentage of pixels demonstrating tenascin-C immunoreactivity was calculated from 2 nonoverlapping images of dimension 1323 × 1757 μm with the aid of the threshold function in ImageJ 1.42q (U.S. National Institutes of Health, Bethesda, MD, USA).
Collagen types I and III
Immunohistochemical staining for collagen types III (C7805, Sigma-Aldrich Denmark A/S, Copenhagen, Denmark) and type I (C2456, Sigma-Aldrich) was also performed to investigate the localization of these collagen types in regenerating muscle.
SCs
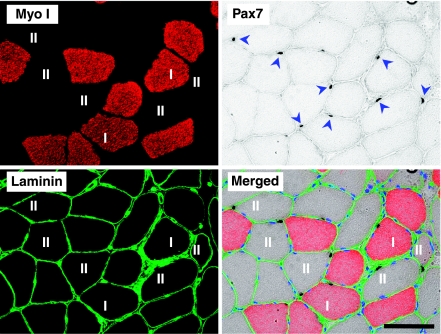
The number of SCs was determined from cross-sections stained with a combination of Pax7, Type I myosin, and laminin, as described recently in detail (30). Briefly, sections were incubated overnight with a mouse anti-Pax7 antibody (MO15020; Neuromics, Edina, MN, USA). A biotinylated secondary antibody (E0433; Dako) was then applied, followed by Vector Elite ABC kit (PK6100; Vector Laboratories). DAB substrate visualized antibody binding. The sections were then incubated with primary antibodies against type I myosin (A4.951; Developmental Studies Hybridoma Bank) and laminin. Alexa Fluor 488 goat anti-rabbit and 568 goat anti-mouse secondary antibodies rendered type I myosin red and laminin green. Sites of Pax7 antigenicity were stained with DAB, visible by light microscopy. See Fig. 2 for an example of this staining on one of the biopsies from this study. The number of Pax7 cells associated with type I or type II fibers was determined and expressed relative to the total number of type I or type II fibers included in the assessment.
Figure 2.
Immunohistochemical detection of Pax7 cells, type I myosin, and laminin on a single cross-section of regenerating healthy gastrocnemius medialis muscle 30 d after a single bout of ES (RBc group). In this series of images with an unusually high density of Pax7 cells, 8 Pax7 cells are clearly visible, 2 of which are associated with type I fibers (red) and 6 with type II fibers (unstained). Laminin staining (green) defines the fiber borders. Scale bar = 100 μm.
Macrophages and cellular activity
Macrophage staining was assessed in a similar manner to that described above for tenascin C from 3 nonoverlapping images of dimensions 664 × 882 μm, both to calculate the percentage of pixels demonstrating CD68 immunoreactivity and the number of CD68+ cells per square millimeter of tissue. As an indicator of general cellular activity, staining for Ki67 (CP249, Biocare Medical, Concord, CA, USA) was performed and the number of positive cells expressed relative to 100 fibers.
Transmission electron microscopy
Following 3 rinses in 0.15 M sodium phosphate buffer (pH 7.2), the specimens were postfixed in 1% OsO4 in 0.15 M sodium phosphate buffer (pH 7.2) for 2 h. The specimens were dehydrated in a graded series of ethanol, transferred to propylene oxide, and embedded in Epon (TAAB Laboratories Equipment Ltd., Aldermaston, UK) according to standard procedures. Ultrathin sections were cut with a Reichert-Jung Ultracut E microtome (Leica Microsystems), collected on 1-hole copper grids with Formvar supporting membranes (Merck, Darmstadt, Germany) and stained with uranyl acetate and lead citrate. The sections were examined using a Philips CM 100 transmission electron microscope (Philips, Amsterdam, The Netherlands) operated at an accelerating voltage of 80 kV. Digital images were obtained with an Olympus Soft Imaging Solutions (OSIS) Veleta, side-mounted digital slow scan 2000 × 2000 CCD camera (Olympus, Tokyo, Japan) and the AnalySIS ITEM software package (ITEM Software, Whitely,UK). Images from the RB and RBc groups were combined with the original images for the B group (5) and were assessed as described previously (5) in a masked procedure by an investigator not involved in any of the biopsy analyses.
Gene expression analysis
Muscle biopsies from all 3 groups were processed at the same time for this analysis. Biopsies were sectioned in batches (with biopsies from each of the 3 groups represented in each batch) at −24°C using a cryostat, and the frozen sections were collected into a precooled tube. After adding beads (five 2.3-mm steel beads and 1 silicium carbide grain; Biospec Products, Bartlesville, OK, USA) and 1000 μl TriReagent (Molecular Research Center, Cincinnati, OH, USA), the mixture was shaken (Fastprep24; MP Biomedicals, Solon, OH, USA) immediately for 15 s at speed 4 m/s and cooled on ice for ∼2 min. This procedure was repeated twice. 1-bromo-3-chloropropane (BCP; 100 μl; Molecular Research Center) was then added to separate the homogenized muscle samples into an aqueous and an organic phase. The aqueous phase (450 μl; containing the RNA) was used to precipitate RNA with isopropanol (450 μl). Following the first wash with 75% ethanol, the pellet was dissolved in 100 μl RNase-free water, precipitated again with the help of sodium-acetate (10 μl 3M NaAc pH 5.5) and 200 μl 96% ethanol. The obtained pellet was washed again with 75% ethanol, air-dried, and dissolved in 20 μl RNase-free water. RNA concentrations were determined by spectroscopy at 260 nm. In addition, absorbance at 240 and 280 nm was measured to ensure the purity of the sample (260/280- and 260/240-nm ratio). Furthermore, RNA quality was verified by RNA gel electrophoresis using a formaldehyde agarose gel.
Real-time PCR
Total RNA (450 ng)was reverse transcribed into cDNA in 20 μl using the OmniScript reverse transcriptase (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol. Following dilution of cDNA (1:20), 5 μl of diluted cDNA was amplified in a 25 μl reaction batch using 1× QuantiTect SYBR Green (Qiagen) Master Mix and 100 nM of each primer (Table 1). The amplification was monitored in real time using a Mx3005P real-time PCR machine (Stratagene, La Jolla, CA, USA). Cycle threshold (Ct) values were related to a standard curve made with the cloned PCR products. Specificity was ensured by melting curve analysis after the PCR run. Ct values ranged from 15 to 40. RPLP0 mRNA was chosen as an internal control because it was assumed to be expressed constitutively. To validate this assumption, another unrelated and constitutively expressed mRNA, GAPDH, was measured and normalized to RPLP0. GAPDH-RPLP0 ratio showed a nonconstitutive expression pattern, but RPLP0 was still considered the best choice, as described in Discussion. mRNA data are presented as fold changes relative to the mean of all control values for muscle. Samples were successfully analyzed from all groups: 5 pairs from the B group, 9 from the RB group, and 11 from the RBc group.
Table 1.
Primer sequences for PCR
| mRNA | Sense | Antisense |
|---|---|---|
| aB-crystallin | GTGTTGGGAGATGTGATTGAGGTG | CTGGGATCCGGTATTTCCTGTGG |
| CCL2 | GCCCTTCTGTGCCTGCTGCT | GCAGGTGACTGGGGCATTGATT |
| cmet | AACCCGAATACTGCCCAGACCC | TGATATCCGGGACACCAGTTCAG |
| Col1A1 | GGCAACAGCCGCTTCACCTAC | GCGGGAGGACTTGGTGGTTTT |
| Col3A1 | CACGGAAACACTGGTGGACAGATT | ATGCCAGCTGCACATCAAGGAC |
| Col4A1 | TCGCTGTGGATCGGCTACTCTT | CGATGAATGGCGCACTTCTAAAC |
| Collagen XII | CCCAGGTCCTCCTGGATACTGTGA | GCAGCACTGGCGACTTAGAAAATGT |
| CTGF | TGCGAAGCTGACCTGGAAGAGA | GCCGTCGGTACATACTCCACAGAA |
| FAK1 | CCAGTCCGAGGTCCAGCGAAG | CATGTGAACCAGGGTAGCCAGAA |
| GAPDH | CCTCCTGCACCACCAACTGCTT | GAGGGGCCATCCACAGTCTTCT |
| HGF | TGAAATATGTGCTGGGGCTGAAA | ACAAACAAGTGGGCCACCATAATCC |
| HSP27 | GCTGACGGTCAAGACCAAGGATG | TGAAGCACCGGGAGATGTAGCC |
| HSP70 | GTGGCTGGACGCCAACACCTT | TTACACACCTGCTCCAGCTCCTTC |
| IGF1a | GACATGCCCAAGACCCAGAAGGA | CGGTGGCATGTCACTCTTCACTC |
| IGF1b | GCCCCCATCTACCAACAAGAACAC | CAGACTTGCTTCTGTCCCCTCCTTC |
| IGF1c | GCCCCCATCTACCAACAAGAACAC | CGGTGGCATGTCACTCTTCACTC |
| IL-1b | TCCAGGGACAGGATATGGAGCA | AGGCCCAAGGCCACAGGTATTT |
| LAMB 1 | GGACAAGAGCAATGAGGAGCTGAGA | AGCAACTGCTTCAATGCTGTCCAA |
| LAMB 2 | TGAAATTGAAACGGGCAGGAAA | GATCACCCAGAGGACCGCGTAG |
| Myf6 | GGGCTCGTGATAACGGCTAAGGA | TGTCCACGATGGAAGAAAGGCA |
| Myogenin | CTGCAGTCCAGAGTGGGGCAGT | CTGTAGGGTCAGCCGTGAGCAG |
| p21 | CTCAGGGGAGCAGGCTGAAGG | AGCCGGCGTTTGGAGTGGTAG |
| RPLP0 | GGAAACTCTGCATTCTCGCTTCCT | CCAGGACTCGTTTGTACCCGTTG |
| Tenascin-C | CAACCATCACTGCCAAGTTCACAA | GGGGGTCGCCAGGTAAGGAG |
| TGF-1b | GAGGTCACCCGCGTGCTAATG | CACGGGTTCAGGTACCGCTTCT |
| TNFa | TTCCCCAGGGACCTCTCTCTAATC | GAGGGTTTGCTACAACATGGGCTAC |
Statistics
The level of statistical significance for all tests was P < 0.05. Unless otherwise specified, all data are presented as means ± se. mRNA data were log transformed and analyzed using SigmaPlot for Windows 11.0 (Systat Software Inc., San Jose, CA, USA) by 2-way repeated measures ANOVA. Where a significant group × leg interaction was detected, comparisons between control and ES legs within and across groups were performed using the Student-Newman-Keuls method. mRNA data are presented as geometric means ± back-transformed se. All other data were analyzed with GraphPad Prism for Macintosh 4.0c (GraphPad Software, San Diego, CA, USA). Force production under stimulation during bouts 1 and 2 in the RB group was compared using a 2-tailed paired t test. Soreness and CK data for bouts 1 and 2 in the RB group were analyzed using a 2-way repeated measures ANOVA with subsequent Bonferroni post hoc tests where a main interaction was observed. Soreness and CK data for the RBc group (effect of biopsying alone) were analyzed using a 1-way repeated measures ANOVA with subsequent Dunnett's multiple comparison tests of each post-treatment time point vs. baseline, where a significant main effect was detected. Soreness area under the curve was analyzed with a 1-way ANOVA test and Newman-Keuls multiple comparison post hoc tests. Tenascin-C, Ki67, z-line, and macrophage (RB and RBc group) data were analyzed by Kruskal-Wallis test, with Dunn's multiple comparison test where the main outcome was significant. Macrophage data from the B group were compared with the Wilcoxon signed rank test. Since the stability of the Pax7 antigen over time is unknown, the Pax7 analysis of the older biopsies from the B group were subject to separate statistical analysis (paired t test) from the RB and RBc groups. Pax7 data from the RB and RBc groups were analyzed by 2-way repeated measures ANOVA. Stimulation current for the B, RB, and RBc groups was also tested by 2-way repeated measures ANOVA.
RESULTS
Stimulation current and evoked force production
Levels of stimulation current during the first bout at 5, 10, 15, 20, 25, and 30 min were 32 ± 5, 37 ± 5, 40 ± 4, 43 ± 4, 45 ± 4, and 48 ± 6 mA, respectively, increasing continually with time. All subjects received exactly the same current at each 5-min interval during bout 2 as during bout 1 and could tolerate the stimulation current delivered. Force production under stimulation was similar for both bouts (bout 1 vs. 2: 101±41 vs. 91±44 N; P=0.47). The stimulation current over the 30 min period for the B, RB, and RBc groups was 29 ± 10, 41 ± 6, and 28 ± 7 mA, respectively. Significant main effects of time and group (P<0.0001) were detected, indicating that the mean stimulation current delivered to the RB group was greater than that delivered to the B and RBc groups.
Soreness
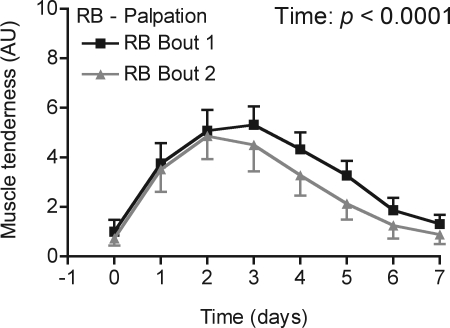
Muscle soreness was observed to increase in all individuals in the RB trial following both bout 1 and bout 2, peaking on d 2 or 3 (Fig. 3). A 2-way repeated measures ANOVA revealed a significant effect of time (P<0.0001), but not bout or interaction, for all 3 soreness measures (palpation, contraction, and stretching). The pattern for the contraction and stretching data was similar to the palpation data displayed in Fig. 3.
Figure 3.
Self-assessment of muscle soreness in the days following a single bout (bout 1) or a second bout (bout 2) of stimulated muscle contractions, performed by the same individuals 1 mo after bout 1 (RB). A significant main effect of time was detected, but not bout or interaction.
Participants in the RBc group reported increased muscle soreness after biopsy sampling alone, peaking on d 3 to 2 AU, 1 d after the biopsy procedure (P<0.001). To compare muscle soreness between bouts in the RB group with the effect of biopsy sampling alone (RBc group), the area under the curve from d 0 to 7 was calculated. Again, no significant differences were observed between bout 1 and bout 2 for the RB group. The active contraction area under the curve for biopsy sampling alone (5.6) was significantly smaller than that of RB group bout 2 (mean 13.8; P<0.05) and bout 1 (14.3; P<0.05). Soreness as assessed by muscle stretching followed a similar pattern, with biopsy sampling area under the curve (5.6) being smaller than RB bout 2 (12.9; P<0.05) and bout 1 (15.1; P<0.05). The area under the curve for palpation of the stimulated muscle with biopsy sampling (4.1) was also significantly smaller than both RB group bout 2 (16.9; P<0.01) and bout 1 (18.8; P<0.01). Accordingly, soreness as assessed by palpation, stretch, or active contraction following RB bout 2 was imilar to that observed following bout 1, both bouts resulting in a greater degree of soreness than that observed with biopsy sampling alone.
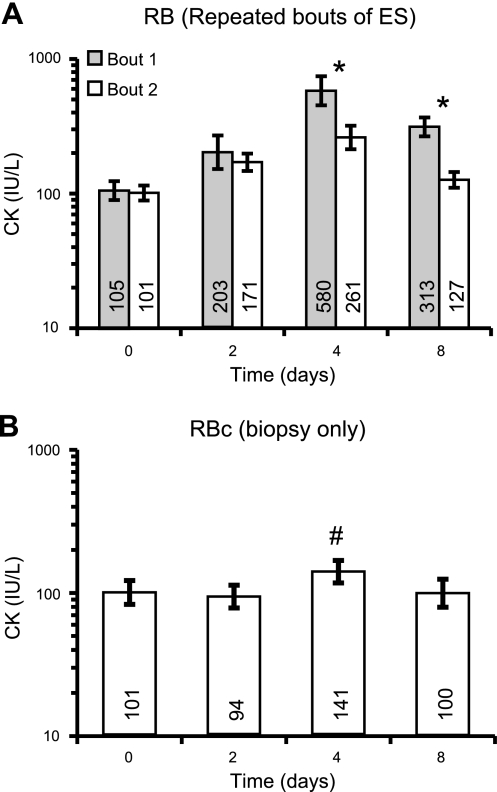
Circulating CK activity (Fig. 4)
Figure 4.
Circulating CK activity was measured in the RB group following 2 bouts of ES-induced isometric contractions of the medial gastrocnemius muscle (A), and in the RBc group following biopsying alone (B) on d 2. Biopsies were also collected from the RB group 2 d after bout 2. Data are back-transformed geometric means with back-transformed error bars, presented on a logarithmic scale. Actual mean values for each bar are given. *P < 0.05 vs. bout 1; #P < 0.05 vs. d 0.
A significant bout × time interaction (P=0.0096) was detected for log-transformed CK values following RB group bouts 1 and 2, with significantly lower CK activity levels on d 4 and 7 following bout 2 when compared to the same time points following bout 1. The biopsy procedure alone (RBc group) resulted in a very small mean 1.5-fold increase in CK levels (P<0.05), peaking on d 4 (P<0.05), 2 d after biopsy sampling.
Immunohistochemical analysis of muscle biopsies
Myofiber damage
Out of all the biopsies collected from the control legs in the B, RB, and RBc groups, only 1 affected fiber (desmin−, dystrophin−, infiltrated with CD68+ cells) was observed. In the biopsies collected from the muscles subjected to ES, desmin− fibers were observed in 2 subjects in the RB group and 2 subjects in the RBc group vs. 4 subjects in the B group (5). Fibers infiltrated with CD68+ cells were present in 4 biopsies from the RB group and 2 from the RBc group, compared to 5 of 7 biopsies in the B group (5).
Regeneration
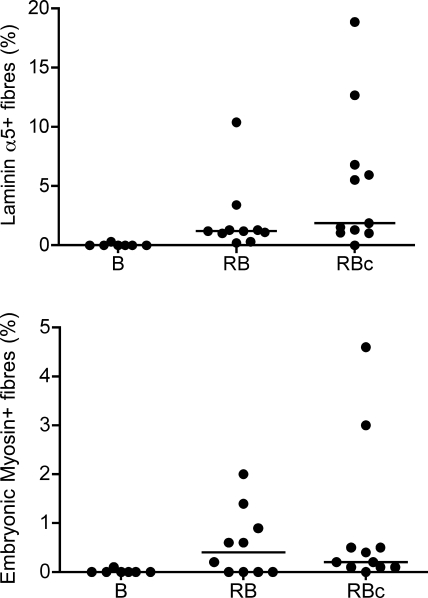
Figure 5 displays the proportion of fibers from the stimulated leg that were positive for embryonic myosin or laminin α5. Two biopsies from the control leg of the RBc trial contained 1 embryonic myosin (F1.652)+ fiber each. No laminin α5+ fibers were detected in any of the control leg biopsies. One biopsy from the stimulated leg of a member of the RBc group, clearly demonstrating ongoing regeneration, was investigated further (Fig. 6). The areas of laminin α5+ endomysium staining were associated with other signs of regeneration (see Fig. 5 for details).
Figure 5.
Scatter plot (with median bars) displaying the proportion of myofiber cross-sections that were positive for embryonic myosin or laminin α5, following single bout (B group) or repeated bout (RB group) of ES. Both markers of regeneration were clearly detectable in RB group and in RBc control group 30 d after a single bout. Higher values recorded for laminin α5 than embryonic myosin suggest that indications of regenerative processes are preserved in the fiber membrane for longer than in the myofibrillar component and signify that this marker is less sensitive to the number of fibers in the cross-section.
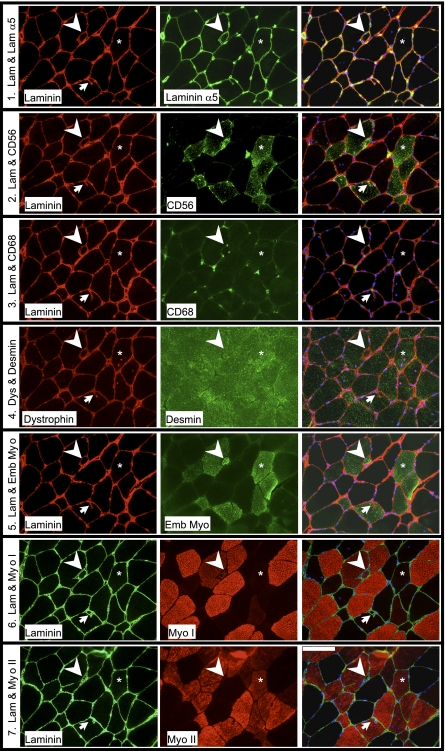
Figure 6.
Five consecutive 10-μm sections (rows 1–5) of a biopsy taken 30 d after a single bout of ES-induced isometric contractions (RBc group), clearly demonstrating ongoing regeneration. Each row is a series of images of 1 double-stained section, the 2 individual stainings in the left and middle panels merged digitally in the right panel. It was observed that the areas of laminin α5+ endomysial staining exhibited other signs of regeneration, such as CD56+ fibers, a high density of CD68+ cells in the ECM, embryonic myosin (Emb Myo)+ fibers, occasional small fibers (arrowheads), and central nuclei. A laminin+ dystrophin+ membrane (arrow), observed on these serial sections to encroach into one fiber, was seen to define a separate fiber on a section deeper into the biopsy (row 6). Most of the affected fibers (asterisks) were negative for type I myosin (Myo I; row 6) and positive for type II myosin (Myo II; row 7). Lam, laminin. Scale bar = 100 μm.
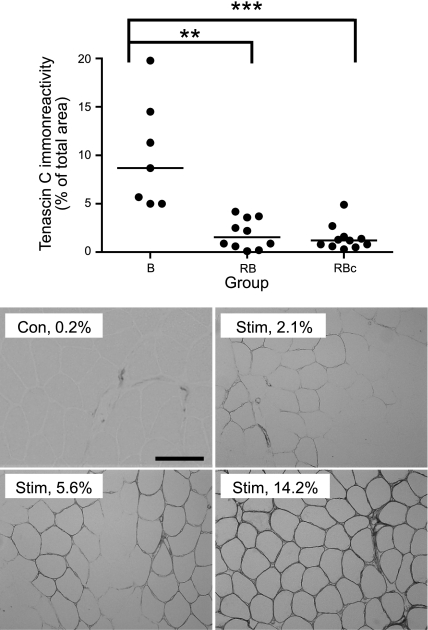
Tenascin C
Tenascin-C analysis revealed a greater extent of immunoreactivity in the B group when compared with the RB and RBc groups (Fig. 7). The mean value for control biopsies was 0.2%.
Figure 7.
Tenascin-C immunoreactivity. Top panel: scatter plot (with median bars) indicates significantly higher levels of tenascin-C immunoreactivity 48 h following a single bout (B group) compared to a repeated bout (RB group) of ES or the response 30 d after a single bout (RBc group). Bottom panels: images illustrate varying extents of tenascin-C immunoreactivity from control (Con) muscle and muscle subjected to ES (Stim). Percentage of immunoreactive area for each image was quantified; respective results for these representative images (a portion of each image is shown) are given. Scale bar = 200 μm. **P < 0.01, ***P < 0.001; Dunn's multiple comparison test.
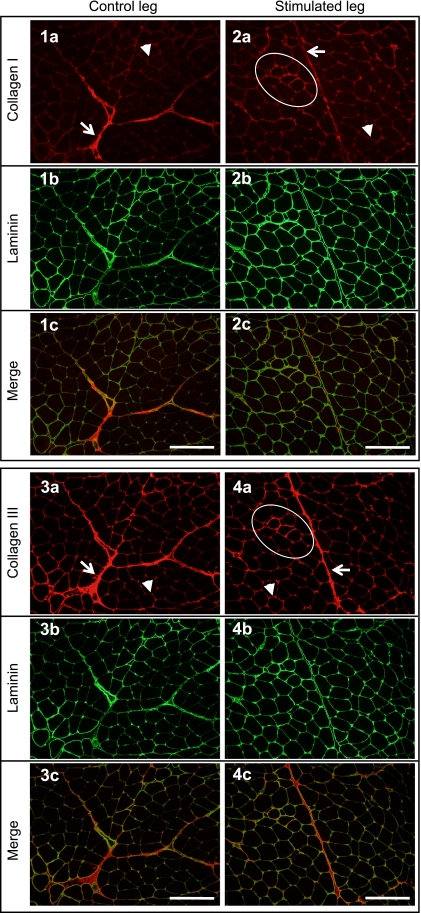
Collagen types I and III
Collagen type I and III staining patterns are displayed and described in Fig. 8. From comparisons of the control and stimulated muscle, a more intense immunoreactivity for both collagen types was apparent around small fibers, only present in the stimulated muscle and therefore likely to be regions of ongoing regeneration.
Figure 8.
Immunohistochemical staining pattern of collagen types I and III on cross-sections of skeletal muscle 30 d after a single bout of ES-induced isometric contractions (RBc group). Two serial sections from the stimulated and control legs of one individual were double-stained with laminin and type I (series 1 and 2) or type III (series 3 and 4) collagen. The collagen (a) and laminin (b) stainings are displayed separately and as computer-generated merged images (c). Both collagen types were observed in perimysium (arrows) and endomysium (arrowheads), with negligible staining of capillaries. Endomysium around regenerating fibers appeared to demonstrate more intense immunoreactivity for collagen types I and III (circled area). Scale bars = 200 μm.
SCs
No difference between ES and control treatment was observed in the B group (Table 2). For the RB and RBc groups, a main effect of leg (P<0.01), but not group or interaction, was observed for SC content per fiber, and when the SC content associated with type I or type II fibers was examined.
Table 2.
Assessment of Pax7 SCs on cross-sections of muscle biopsies from 3 groups of volunteers 48 h after a single bout (B group) or repeated bout (RB group) of ES-induced contractions separated by 1 mo
| Type | B group |
RB group |
RBc group |
|||
|---|---|---|---|---|---|---|
| Control | ES | Control | ES | Control | ES | |
| Pax7/f | 0.15 ± 0.03 | 0.14 ± 0.02 | 0.11 ± 0.01 | 0.16 ± 0.01 | 0.07 ± 0.01 | 0.13 ± 0.02* |
| Pax7/ft I | 0.10 ± 0.01 | 0.14 ± 0.01 | 0.07 ± 0.01 | 0.11 ± 0.01* | ||
| Pax7/ft II | 0.12 ± 0.01 | 0.18 ± 0.02 | 0.08 ± 0.01 | 0.16 ± 0.02* | ||
Control RBc group was biopsied 30 d after a single bout. Table shows number of SCs associated with the 2 main fiber types (ft I and ft II), or combined fiber types (f), in the ES leg and control leg. Values are means ± se. Statistical analysis of data from B group was performed separately from analysis of data from RB and RBc groups. SC content in control and ES legs of B group was found to be similar.
P < 0.01, main effect of leg.
Macrophages and cellular activity
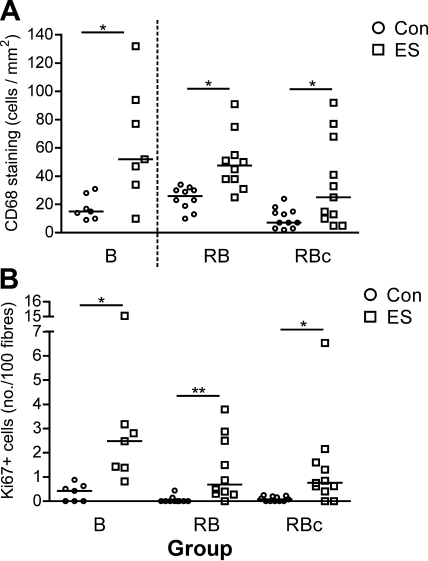
A significantly greater number of CD68+ cells per square millimeter of tissue (Fig. 9) was detected in the stimulated vs. control leg of B (P<0.05). Comparison of RB and RBc data revealed a significant overall effect (P=0.0002) with significant differences between ES and control in both groups (P<0.05). The fraction of CD68+ immunoreactive area demonstrated the same pattern (data not shown). A significant overall effect (P<0.0001) was detected for the number of Ki67+ cells per 100 fibers (Fig. 9), with significant differences between ES and control in all 3 groups (P<0.05).
Figure 9.
Scatter plots (with median bars) displaying the number of macrophages (CD68+ cells) per square millimeter of biopsy cross-section (A) and the number of Ki67+ cells per 100 fibers (B) from electrically stimulated (ES) or control (Con) muscle. Broken vertical line in panel A indicates that biopsies in B group were subject to separate immunohistochemical and statistical analyses from RB and RBc groups. *P < 0.05; **P < 0.01.
Transmission electron microscopy
A significantly greater extent of z-line disruption was observed in the stimulated leg of the B and RB vs. RBc trials (see Fig. 10). Out of the 26 control biopsies evaluated in the B, RB, and RBc groups, 3 were categorized under the grade 0.5 and all others were 0. It is worth noting for the stimulated leg of the RBc trial that discrete and inconsistent irregularities in z-line integrity, graded 0.5 to 1, were observed in 9 of the 11 samples. These include occasional deviation in the strict z-line alignment and z lines out of register with adjacent sarcomeres, as displayed in Fig. 10.
Figure 10.
Top panel: scatter plot (with median bars) displaying extent of z-line disruption (0 = none, 3 = severe disruption) following a single bout (B group) or repeated bout (RB group) of ES. Biopsies in RBc group were collected 30 d after a single bout. Bottom panels: transmission electron micrographs of longitudinal sections of human medial gastrocnemius from RBc group biopsies. Representative images from 2 subjects (Sub 1 and 2) are displayed, along with images from control (Con) legs of the same individuals. Images illustrate that, while strict z-line alignment and register have generally been restored, discrete and subtle differences are still visible at this time point. Scale bars = 1 μm. **P < 0.01; ***P < 0.001.
Gene expression
mRNA levels and details of statistical outcomes are presented in Figs. 11–14. Overall, of the 25 targets analyzed, 5 demonstrated an attenuated response to RB trial when compared to B trial: chemokine ligand 2 (CCL2), connective tissue growth factor (CTGF), HSPs; 12 demonstrated significantly elevated levels 30 d after a single bout of ES (RBc group) vs. their respective controls: CTGF, TGF-β, CCL2, IGF1-Ea, IGF1-Eb, IGF1-Ec, laminin-β1, laminin-β2, and collagen types I, III, IV, and XII; 5 targets displayed an attenuated response in the ES leg following RB trial when compared to RBc: CTGF, laminin-β2, and collagen types I, III, and XII.
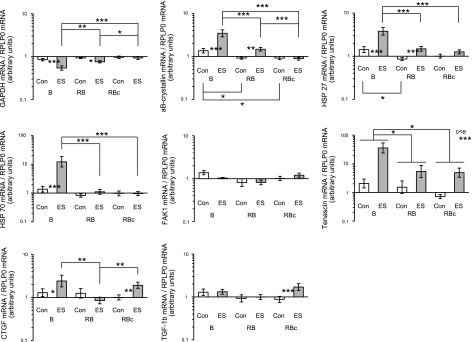
Figure 11.
HSP and matricellular protein gene expression levels in control (Con) muscle and muscle subjected to a single bout (B group) or a repeated bout (RB group) of ES 1 mo after first bout. Biopsies in RBc group were collected 30 d after a single bout of ES. mRNA levels of GAPDH, αβ-crystallin, HSP27, HSP70, FAK1, tenascin C, CTGF and TGF-β are presented, expressed relative to RPLP0 mRNA. Data are back-transformed geometric means ± se, displayed on a logarithmic scale y axis.
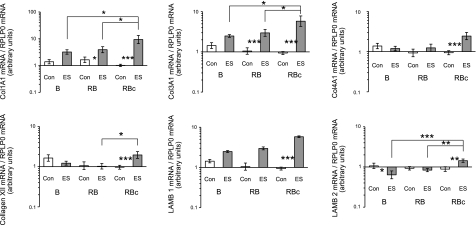
Figure 12.
Comparisons of extracellular matrix gene expression levels between control (Con) muscle and muscle subjected to a single bout (B group) or a repeated bout (RB group) of ES 1 mo after first bout. Biopsies in RBc group were collected 30 d after single bout of ES. mRNA levels of collagen types I (Col1A), III (Col3A1), IV (Col4A1), XII, and laminin-β1 (LAMB 1) and -β2 (LAMB 2) are presented, expressed relative to RPLP0 mRNA. Data are back-transformed geometric means ± se, displayed on a logarithmic scale y axis.
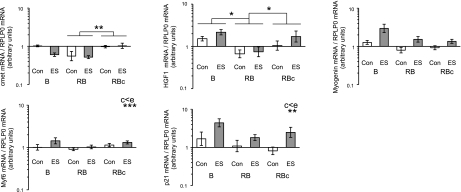
Figure 13.
Comparisons of SC-related gene expression levels between control (Con) muscle and muscle subjected to a single bout (B group) or a repeated bout (RB group) of ES 1 mo after first bout. Biopsies in RBc group were collected 30 d after a single bout of ES. mRNA levels of c-met, HGF (HGF1), myogenin, Myf6 (MRF4), and p21 are presented, expressed relative to RPLP0 mRNA. Data are back-transformed geometric means ± se, displayed on a logarithmic scale y axis.
Figure 14.
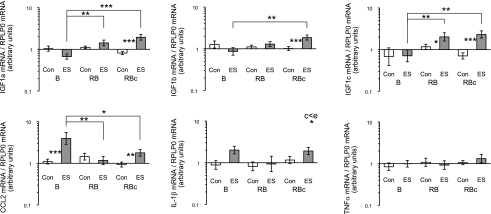
IGF-1 and inflammatory-related gene expression levels in control (Con) muscle and muscle subjected to a single bout (B group) or a repeated bout (RB group) of ES 1 mo after first bout. Biopsies in RBc group were collected 30 d after a single bout of ES. mRNA levels of IGF1-Ea (IGF1a), IGF1-Eb (IGF1b), IGF1-Ec (IGF1c; MGF), CCL2 (MCP-1), interleukin (Il)-1β, and tumor necrosis factor α (TNF-α) are presented, expressed relative to RPLP0 mRNA. Data are back-transformed geometric means ± se, displayed on a logarithmic scale y axis.
As can be seen in Fig. 11, HSPs demonstrated an attenuated response in the RB group when compared to the B group. At 48 h after a single bout of ES (B group), αβ-crystallin and HSP27 mRNA levels were ∼3-fold significantly higher in ES vs. control treatment groups, while HSP70 displayed a ∼10-fold elevation. Matricellular tenascin-C and CTGF values were also elevated in the B vs. RB or RBc groups. [A tendency for leg × group interaction (P=0.11) for tenascin C was detected.]
Extracellular matrix components (Fig. 12) generally demonstrated greatest mRNA levels 30 d after a single bout (RBc group), with the greatest ∼6- to 9-fold response observed in collagen types I and III and laminin-β1 at this time point. For collagen types IV and XII and laminin-β1, this was the only time point where a significant difference vs. control was detected. A tendency for elevated mRNA levels in the ES leg vs. control following B trial was seen for collagen type I (P=0.06) and type III (P=0.11).
Myogenic-related factors (Fig. 13) did not demonstrate a dramatic response at the time points examined in this study. Myf6 (MRF4) and p21 demonstrated a main effect of leg (control<ES), and a tendency for a main effect of leg (P=0.054) was observed for myogenin. A suppression of c-met and HGF gene expression levels was detected in the RB vs. RBc groups.
The greatest mRNA levels of the 3 IGF-1 isoforms (Fig. 14) were observed in the stimulated leg of the RBc trial, with IGF1-Ec displaying the largest (∼3-fold) elevation vs. control treatment. No elevation was seen in any of the IGF-1 isoforms with ES vs. control at 48 h after a single bout (B group). With regard to inflammatory targets (Fig. 14), CCL2 [monocyte chemoattractant protein 1 (MCP-1)] was the only one to demonstrate a response in the B group, where a ∼4-fold elevation vs. control was detected.
DISCUSSION
The present findings indicate that, in human skeletal muscle, acutely induced damage by ES results in an initiation of deadhesive, disassembly, and disorganization responses in the contractile connective tissue, followed by a delayed anabolic response in the supporting ECM, providing the basis for a prolonged strengthening of the muscle matrix (Fig. 15). This results in a diminished degradation of the skeletal muscle and its connective tissue when subjected to a similar damaging exercise at a later stage. The fact that sustained enhancement of SC content was observed in damaged muscle beyond muscle fiber regeneration suggests that crosstalk between muscle stem cell activity and the matrix components of skeletal muscle exists, with the overall goal to protect the individual muscle fiber from damage on reexposure to extreme loading. We suggest a sequential series of responses to damage in skeletal muscle, with an early response favoring muscle damage, inflammation, disassembly, and disorganization. Following this, a more anabolic matrix-oriented response is seen, which includes growth factors and collagen, in concert with a prolonged elevation of SC content. The outcome of the delayed response is an attenuated early response following subsequent reexposure to a damaging stimulus.
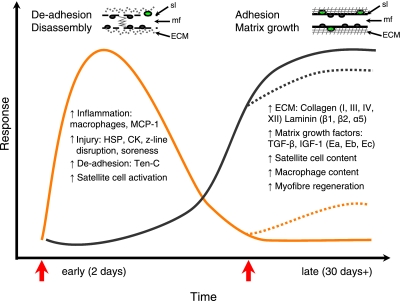
Figure 15.
Schematic illustration of human skeletal muscle responses to damaging ES (red arrows). In response to the first bout, marked muscle damage and associated early responses are observed (orange line), followed by a delayed anabolic response of the muscle ECM (black line). This late response raises the resistance toward muscle damage and disorganization after a second repeated bout in the same muscle. Solid lines represent response to the first bout; broken lines illustrate how a repeated bout may alter this response. At top left, a muscle fiber (mf) with myonuclei (dark gray circles) and SCs (green circles) is depicted during the early response, illustrating the disorganized ECM and damage to the myofibrils and sarcolemma (sl). Myofiber at top right represents the late phase, characterized by a higher SC content, a repaired sarcolemma, and a strengthened ECM, factors that are likely to be involved in protecting the muscle from damage on exposure to subsequent injuring stimuli.
Normalization of gene expression values
In an attempt to validate RPLP0 mRNA as an internal reference for mRNA levels throughout the experiment, we first normalized another housekeeping mRNA, GAPDH, with RPLP0. However, the GAPDH-RPLP0 ratio showed an alteration in the expression pattern resulting in apparent lower GAPDH mRNA levels in the ES leg vs. control leg of the B and RB groups. (P<0.05; Fig. 11). mRNA levels of ES and control in the RBc group were similar. GAPDH mRNA levels in muscle tissue are usually ∼10 times higher than in other tissues of the body (31). Furthermore, a huge amount of invading cells was observed after ES. Taken together, we argue that in muscle cell lysates with high GAPDH mRNA levels, invading cells with lower GAPDH mRNA levels, such as macrophages, would lead to a dilution of total GAPDH mRNA within this cell lysate, thus resulting in lower concentrations of GAPDH mRNA, as was clearly observed after each bout of stimulation (B and RB groups) but not after a longer period of recovery (RBc group). Hence, normalization of GAPDH with RPLP0 showed a changing expression of a housekeeping gene, which we assume to be caused by a simple dilution effect of GAPDH. We therefore conclude that, although not perfect, the most reasonable was to normalize all other targets to RPLP0. It should be noted, though, that other muscle-specific mRNA might be subject to the same dilution effect (seen as a 2-fold drop in B). For example, the similar drop in laminin-β2 in the B group might accordingly be explained by the dilution effect, whereas the increase in the RBc group is likely to be a “real” effect.
Attenuated early damage response to a repeated damaging stimulus
This is the first study of human muscle biopsies subjected to 2 bouts of electrically stimulated isometric contractions. We observed an attenuated increase in circulating CK following the second bout when compared to the first bout, in line with the only other human study (32) investigating repeated bouts of electrically stimulated isometric contractions. Measurement of muscle HSP gene expression in the 3 sets of biopsies in the present study provides further evidence for a repeated bout effect with a large up-regulation following B treatment and an attenuated increase in the small HSPs (HSP27 and αβ-crystallin) following RB treatment. Previous studies have either reported an attenuated HSP response with a repeated bout of exercise (11, 13), or no attenuation with the second bout (10–13), providing an unclear picture with regard to the response of HSPs to repeated bouts. While our HSP results do not provide support for a protective role of HSPs against muscle damage with electrically stimulated isometric contractions, they do, together with the data on CK, CCL2, and the qualitative assessment of macrophage-infiltrated and desmin− fibers, provide strong support for the occurrence of less muscle damage after the second bout in this model. The observation of a similar extent of z-line disruption after the single and repeated bouts, though, suggests more intricate adaptations in providing protection against ES-induced damage at the level of the sarcomere.
Tenascin C rapidly creates a deadhesive ECM environment
The response of tenascin C at protein and mRNA levels in loaded human muscle has not been investigated previously. The immunohistochemistry data generally mirror the gene expression findings and not only suggest a greater tenascin-C response following a single bout than a repeated bout but also reveal sustained tenascin-C up-regulation 30 d into muscle regeneration. The presence of tenascin-C protein, as well as during embryogenesis (33), has been observed in response to mechanical stress, such as previously reported in loaded human (4, 24, 28) and chicken muscle (27), or at mRNA level (28). In contrast to the adhesion-promoting ECM proteins, the role of matricellular tenascin C in skeletal muscle is in deadhesion, the process of disassembly of focal adhesion complexes, which is believed to promote a more favorable environment for cell motility, survival, and repair (26, 29). In line with these data, we also detected up-regulated gene expression of CTGF (CCN2), a matricellular protein facilitating migration and chemotaxis in injured environments (34). It is possible that altered activity of matricellular proteins, central modulators of the ECM, and its resident or migrating cells, plays an important role in repair and protection of human skeletal muscle on re-exposure to a muscle-damaging stimulus.
ECM remodeling is a late event in regenerating human muscle
Our analysis of adhesion-promoting matrix components revealed a general pattern for a stronger up-regulation at 30 d than at 2 d postexercise. Accordingly, it appears that ECM remodeling is not prioritized immediately after exercise. When compared to the B group, the RBc group displayed a stronger up-regulation in gene expression of collagen types I and III and laminin-β2, suggesting that while ECM remodeling, rather than a marked synthesis, might be initiated in the early phase of recovery, it is not until the later stages that matrix structure synthesis is augmented. Furthermore, the lower levels of ECM mRNA in the RB group compared to the RBc group suggest a partial suppression after the second bout of exercise, supporting a complex reordering at the level of gene expression. This finding is in line with a matrix synthesis suppressing response in the early stage after damage, and thus the response is diminished after the second bout of exercise, where the structural changes induced by the first bout have already taken place. While we cannot rule out that changes in type IV collagen and laminin might be related to capillary remodeling, the prevalence of collagen types I and III in endomysium and perimysium (confirmed by immunohistochemistry in the present study), together with the apparent intensified staining around regenerating fibers, suggests that the increased collagen gene expression reflects remodeling of connective tissue surrounding individual fibers, as reported earlier (21), as well as fiber fascicles. Further support for this finding was provided by rarely reported staining of laminin-α5 around some fibers. This staining pattern has been described previously in the muscle of power lifters (35) and likely indicates processes reminiscent of development (36), which, together with the ECM gene expression data, provides strong new evidence of ongoing endomysial remodeling 30 d after a muscle injuring stimulus.
A delayed IGF-1 response mirrors the ECM time course
The response of IGF-1 to repeated bouts of exercise in humans and at a relatively late stage of muscle regeneration has not been documented previously. Interestingly, the mRNA response for IGF-1 in this study was delayed following the damaging exercise compared to the more rapid response observed with more physiological nondamaging exercise (37–39). Thus, it seems that the degree of muscle damage and associated inflammation in the present experiment resulted in signaling that suppresses the usual immediate IGF-1 response and does not allow any increased expression until later stages of regeneration when accelerated formation of new matrix tissue is needed. While it has been reported that IGF-1Ec (MGF) in particular responds within hours of the stimulus, IGF-1Ea (and perhaps also IGF-1-Eb) is typically more prominent 1 to several days later (37, 39–43). The elevated levels of IGF-1Ea mRNA observed in the present study at such a late stage after damage are perhaps not surprising in light of the extent of ongoing muscle remodeling and the suggested role of this IGF-1 isoform, not only in differentiation (41, 43) but perhaps more importantly in moderating inflammatory and fibrotic activity during the later stages of muscle regeneration (44). Alternatively, it has been suggested that macrophages are the source of IGF-1 in regenerating muscle (45), in line with our heightened numbers of macrophages at this time. Surprisingly though, mRNA levels of MGF were also elevated at 30 d compared to the control leg, either implying that proliferation of some cell types, potentially SCs, is still required at this stage, or suggesting that MGF plays a role in the regeneration of muscle ECM. In support of the latter, increased gene expression of MGF has been reported in loaded rat tendon, where a role for this IGF-1 isoform in collagen adaptation was suggested (46) and also more recently in mouse skeletal muscle (47). Taken together, the data presented here provide new insight into the magnitude, time course, and potential role of IGF-1 isoform responses in regenerating human skeletal muscle.
A persistent elevation of SC content at 30 d
SC content of the regenerating muscle at 30 d was significantly greater than the corresponding control muscle, which is a novel observation on the time course of stem cell activity in human skeletal muscle. It is not known how long this enhancement of the SC pool would be retained and indeed if the unneeded cells would be permitted to remain as SCs, or be forced to differentiate or undergo apoptosis as the muscle reestablishes its predamage environment. It is possible that the elevated levels of tenascin C, CTGF, and the 3 IGF-1 isoforms contribute to the survival of the new SCs. In addition, contact between SCs and a mature myofiber has been reported to be a potent factor signaling SC quiescence (15) and the analysis of regenerating markers in the present study indicates that many myofibers had not yet reached full maturity and thus potentially lacked the quiescence-signaling factor. While further investigation is required to clarify the reason behind the elevated SC content, it is interesting, in light of the delayed ECM remodeling, to consider that crosstalk between the muscle ECM and the SC exists.
Slow resolution of local inflammation in regenerating muscle
Levels of MCP-1 (CCL2) were higher in the stimulated leg of RBc vs. control, indicating sustained chemotactic signaling of the muscle at this time point. The immunohistochemical analysis of macrophages confirmed this activity and, in line with the SC data, indicated continuing enhanced cellular activity in the regenerating muscle. Over 30 yr ago it was reported that collagen serves as a chemotactic stimulus for monocytes (48), contributing to evidence for an active role of muscle ECM, in concert with inflammatory cells, in adaptation to loading. An additional finding of the present study was the strong indication of incomplete repair 30 d after a damaging stimulus to the muscle, providing novel documentation of the time course of regeneration of human skeletal muscle.
Strengths and limitations of the model
In general terms, given that ES does not respect the order of motor unit recruitment known for voluntary contractions, further work is required to confirm to what extent the current findings can be transferred to a model of voluntary muscle contraction. However, it is likely that the more indiscriminate recruitment during ES when compared to voluntary contractions would result in initiation of a stronger, but not necessarily dissimilar, repair response (4). The reason for using our ES model was to minimize the interindividual variation in muscle damage observed with voluntary contraction models. A further strength of the model is that, due to its relatively small size, the medial gastrocnemius muscle can be stimulated wholly and uniformly (49), thus eliminating concerns about biopsy sampling from an unaffected part of the muscle, as is often a concern with biopsying larger human muscles. With regard to subjecting the same muscle to repeated bouts of ES, direct evidence for the occurrence of a lesser extent of damage after the second bout was provided by the attenuation in response of circulating CK, while indirect evidence was apparent in the comparison of biopsy markers in the B and RB groups. Despite this, no attenuation in muscle soreness after the second bout was observed in this study—a finding that was unexpected and is in contrast to a previous ES study (32). While this finding can perhaps be explained by the differences between the two studies in the number of contractions, the muscle group stimulated, and the length of time between the two bouts, further work is required to explain why muscle soreness was not attenuated with our study design. Examination of the response of pain-related substances such as bradykinin and nerve growth factor in our model may provide some insight (50). In relation to comparing the 3 groups, force measurements would have provided a solid base for comparison. However, a limitation of our model is that the medialis gastrocnemius muscle only comprises 20% of the total physiological cross-sectional area of the triceps surae muscles (51), resulting in a minor contribution to the total force produced by the triceps surae muscle group during a voluntary contraction. While stimulation current is generally not considered a good indication of contraction intensity due to such variables as the thickness of subcutaneous fat, skin impedance, and the positioning of the electrodes with respect to the motor point, we cannot rule out the possibility that the RB group was subjected to greater contraction intensity than the B and RBc groups. However, the analyses performed on these biopsies do not indicate a greater extent of damage in this group and, given that the individuals in the RB group were stimulated equally during bouts 1 and 2, this potential limitation does not detract from the main findings of this study, summarized below. Lastly, collecting a muscle biopsy from the control leg at the time of sampling from the stimulated leg, instead of repeatedly biopsying the stimulated leg, not only removes the potential for signal contamination from a previous biopsy (52) but also allows us to interpret differences between the legs as arising from the ES and not from diurnal or dietary variation, or altered levels of daily physical activity (52).
CONCLUSIONS
This study confirms the occurrence of a protective effect of an initial muscle-damaging stimulus against later injury in human skeletal muscle by an attenuated response in circulating and biopsy markers following the second bout. We observed at 30 d after 1 bout of ES previously undocumented dramatic and subtle changes in the structure and activity of muscle and its surrounding connective tissue and identified new potential players in this protection, which sheds light on the time course of muscle regeneration and its resistance toward future damage. Together, these findings demonstrate an ordered response to a damaging stimulus (Fig. 15). An initial muscle fiber damaging, deadhesive, and disassembly response with early elevated CK, soreness, z-line disruption, HSPs, tenascin, and inflammation markers occurs, which favors a disorganization of the muscle-matrix tissue. This was time-wise followed by a delayed elevation in expression of anabolic matrix growth factors and collagen, favoring ECM strengthening, as well as a continued elevation of SC content. When the muscle was reexposed to the same damaging stimulus 1 mo later, the disorganizing responses were markedly reduced compared to the initial response, which suggests a protective role of the intramuscular connective tissue against future pronounced muscle injury. Furthermore, the partial suppression of gene expression of CTGF and 4 components of the ECM following the repeated bout suggests a complex and continual reordering of remodeling events of skeletal muscle and its connective tissue, depending on exposure to periods of loading or recovery. Finally, the delayed SC response coupled to the increased anabolic ECM response raises the possibility that SCs are involved in crosstalk between myofibrillar damage and matrix adaptation in human skeletal muscle.
Acknowledgments
Casper Mortensen and Christine Bodilsen are gratefully acknowledged for help with recruiting subjects and collecting data. The excellent technical assistance involved in taking the blood samples and preparing the muscle biopsies (Camilla Hansen) and the preparation of samples for electron microscopy (Mary-Ann Gleie) is warmly acknowledged. The F1.652, A4.951, and A4.74 antibodies developed by Helen M. Blau were obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA, USA), developed under the auspices of the U.S. National Institute of Child Health and Human Development and maintained by the Department of Biological Sciences, University of Iowa. Funding is gratefully acknowledged from the Nordea Foundation (Healthy Aging grant), the Danish Agency for Science Technology and Innovation (Medical Research Council), the Danish Rheumatism Association (R74-A1088), and the Lundbeck Foundation.
REFERENCES
- 1. Round J. M., Jones D. A., Cambridge G. (1987) Cellular infiltrates in human skeletal muscle: exercise induced damage as a model for inflammatory muscle disease? J. Neurol. Sci. 82, 1–11 [DOI] [PubMed] [Google Scholar]
- 2. Hikida R. S., Staron R. S., Hagerman F. C., Sherman W. M., Costill D. L. (1983) Muscle fiber necrosis associated with human marathon runners. J. Neurol. Sci. 59, 185–203 [DOI] [PubMed] [Google Scholar]
- 3. Jones D. A., Newham D. J., Round J. M., Tolfree S. E. (1986) Experimental human muscle damage: morphological changes in relation to other indices of damage. J. Physiol. 375, 435–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crameri R. M., Aagaard P., Qvortrup K., Langberg H., Olesen J., Kjaer M. (2007) Myofibre damage in human skeletal muscle: effects of electrical stimulation vs voluntary contraction. J. Physiol. (Lond.) 583, 365–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mackey A. L., Bojsen-Moller J., Qvortrup K., Langberg H., Suetta C., Kalliokoski K. K., Kjaer M., Magnusson S. P. (2008) Evidence of skeletal muscle damage following electrically stimulated isometric muscle contractions in humans. J. Appl. Physiol. 105, 1620–1627 [DOI] [PubMed] [Google Scholar]
- 6. Mackey A. L., Kjaer M., Charifi N., Henriksson J., Bojsen-Moller J., Holm L., Kadi F. (2009) Assessment of satellite cell number and activity status in human skeletal muscle biopsies. Muscle Nerve 40, 455–465 [DOI] [PubMed] [Google Scholar]
- 7. Nosaka K., Sakamoto K., Newton M., Sacco P. (2001) How long does the protective effect on eccentric exercise-induced muscle damage last? Med. Sci. Sports Exerc. 33, 1490–1495 [DOI] [PubMed] [Google Scholar]
- 8. McHugh M. P. (2003) Recent advances in the understanding of the repeated bout effect: the protective effect against muscle damage from a single bout of eccentric exercise. Scand. J. Med. Sci. Sports. 13, 88–97 [DOI] [PubMed] [Google Scholar]
- 9. Clarkson P. M., Byrnes W. C., Gillisson E., Harper E. (1987) Adaptation to exercise-induced muscle damage. Clin. Sci. (Lond.) 73, 383–386 [DOI] [PubMed] [Google Scholar]
- 10. Thompson H. S., Clarkson P. M., Scordilis S. P. (2002) The repeated bout effect and heat shock proteins: intramuscular HSP27 and HSP70 expression following two bouts of eccentric exercise in humans. Acta Physiol. Scand. 174, 47–56 [DOI] [PubMed] [Google Scholar]
- 11. Vissing K., Bayer M. L., Overgaard K., Schjerling P., Raastad T. (2009) Heat shock protein translocation and expression response is attenuated in response to repeated eccentric exercise. Acta Physiol. (Oxf.) 196, 283–293 [DOI] [PubMed] [Google Scholar]
- 12. Paulsen G., Lauritzen F., Bayer M. L., Kalhovde J. M., Ugelstad I., Owe S. G., Hallen J., Bergersen L. H., Raastad T. (2009) Subcellular movement and expression of HSP27, alphaB-crystallin, and HSP70 after two bouts of eccentric exercise in humans. J. Appl. Physiol. 107, 570–582 [DOI] [PubMed] [Google Scholar]
- 13. Hubal M. J., Chen T. C., Thompson P. D., Clarkson P. M. (2008) Inflammatory gene changes associated with the repeated-bout effect. Am. J. Physiol. 294, R1628–1637 [DOI] [PubMed] [Google Scholar]
- 14. Lapointe B. M., Fremont P., Cote C. H. (2002) Adaptation to lengthening contractions is independent of voluntary muscle recruitment but relies on inflammation. Am. J. Physiol. 282, R323–R329 [DOI] [PubMed] [Google Scholar]
- 15. Bischoff R. (1990) Interaction between satellite cells and skeletal muscle fibers. Development 109, 943–952 [DOI] [PubMed] [Google Scholar]
- 16. Sarasa-Renedo A., Chiquet M. (2005) Mechanical signals regulating extracellular matrix gene expression in fibroblasts. Scand. J. Med. Sci. Sports 15, 223–230 [DOI] [PubMed] [Google Scholar]
- 17. Kuang S., Gillespie M. A., Rudnicki M. A. (2008) Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell 2, 22–31 [DOI] [PubMed] [Google Scholar]
- 18. Alexakis C., Partridge T., Bou-Gharios G. (2007) Implication of the satellite cell in dystrophic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am. J. Physiol. Cell Physiol. 293, C661–C669 [DOI] [PubMed] [Google Scholar]
- 19. Miller B. F., Olesen J. L., Hansen M., Dossing S., Crameri R. M., Welling R. J., Langberg H., Flyvbjerg A., Kjaer M., Babraj J. A., Smith K., Rennie M. J. (2005) Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J. Physiol. 567, 1021–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suominen H., Heikkinen E. (1975) Enzyme activities in muscle and connective tissue of M. Vastus lateralis in habitually training and sedentary 33 to 70-year-old men. Eur. J. Appl. Physiol. Occup. Physiol. 34, 249–254 [DOI] [PubMed] [Google Scholar]
- 21. Mackey A. L., Donnelly A. E., Turpeenniemi-Hujanen T., Roper H. P. (2004) Skeletal muscle collagen content in humans after high-force eccentric contractions. J. Appl. Physiol. 97, 197–203 [DOI] [PubMed] [Google Scholar]
- 22. Kjaer M. (2004) Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 84, 649–698 [DOI] [PubMed] [Google Scholar]
- 23. Mackey A. L., Donnelly A. E., Roper H. P. (2005) Muscle connective tissue content of endurance-trained and inactive individuals. Scand. J. Med. Sci. Sports 15, 402–408 [DOI] [PubMed] [Google Scholar]
- 24. Crameri R. M., Langberg H., Teisner B., Magnusson P., Schroder H. D., Olesen J. L., Jensen C. H., Koskinen S., Suetta C., Kjaer M. (2004) Enhanced procollagen processing in skeletal muscle after a single bout of eccentric loading in humans. Matrix Biol. 23, 259–264 [DOI] [PubMed] [Google Scholar]
- 25. Lapier T. K., Burton H. W., Almon R., Cerny F. (1995) Alterations in intramuscular connective tissue after limb casting affect contraction-induced muscle injury. J. Appl. Physiol. 78, 1065–1069 [DOI] [PubMed] [Google Scholar]
- 26. Murphy-Ullrich J. E. (2001) The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J. Clin. Invest. 107, 785–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fluck M., Tunc-Civelek V., Chiquet M. (2000) Rapid and reciprocal regulation of tenascin-C and tenascin-Y expression by loading of skeletal muscle. J. Cell Sci. 113, 3583–3591 [DOI] [PubMed] [Google Scholar]
- 28. Mikkelsen U. R., Schjerling P., Helmark I. C., Reitelseder S., Holm L., Skovgaard D., Langberg H., Kjaer M., Heinemeier K. M. (2010) Local NSAID infusion does not affect protein synthesis and gene expression in human muscle after eccentric exercise. [E-pub ahead of print] Scand. J. Med. Sci. Sports doi: 10.1111/j.1600-0838.2010.01170.x [DOI] [PubMed] [Google Scholar]
- 29. Fluck M., Mund S. I., Schittny J. C., Klossner S., Durieux A. C., Giraud M. N. (2008) Mechano-regulated tenascin-C orchestrates muscle repair. Proc. Natl. Acad. Sci. U. S. A. 105, 13662–13667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mackey A. L., Andersen L. L., Frandsen U., Suetta C., Sjogaard G. (2010) Distribution of myogenic progenitor cells and myonuclei is altered in women with vs. those without chronically painful trapezius muscle. J. Appl. Physiol. 109, 1920–1929 [DOI] [PubMed] [Google Scholar]
- 31. Barber R. D., Harmer D. W., Coleman R. A., Clark B. J. (2005) GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol. Genomics 21, 389–395 [DOI] [PubMed] [Google Scholar]
- 32. Aldayel A., Jubeau M., McGuigan M. R., Nosaka K. (2010) Less indication of muscle damage in the second than initial electrical muscle stimulation bout consisting of isometric contractions of the knee extensors. Eur. J. Appl. Physiol. 108, 709–717 [DOI] [PubMed] [Google Scholar]
- 33. Jones F. S., Jones P. L. (2000) The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev. Dyn. 218, 235–259 [DOI] [PubMed] [Google Scholar]
- 34. Chen C. C., Lau L. F. (2009) Functions and mechanisms of action of CCN matricellular proteins. Int. J. Biochem. Cell Biol. 41, 771–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eriksson A., Lindström M., Carlsson L., Thornell L. E. (2006) Hypertrophic muscle fibers with fissures in power-lifters; fiber splitting or defect regeneration? Histochem. Cell Biol. 126, 409–417 [DOI] [PubMed] [Google Scholar]
- 36. Gullberg D., Tiger C. F., Velling T. (1999) Laminins during muscle development and in muscular dystrophies. Cell. Mol. Life Sci. 56, 442–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hameed M., Orrell R. W., Cobbold M., Goldspink G., Harridge S. D. R. (2003) Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J. Physiol. (Lond.) 547, 247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bamman M. M., Petrella J. K., Kim J. S., Mayhew D. L., Cross J. M. (2007) Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J. Appl. Physiol. 102, 2232–2239 [DOI] [PubMed] [Google Scholar]
- 39. McKay B. R., O'Reilly C. E., Phillips S. M., Tarnopolsky M. A., Parise G. (2008) Co-expression of IGF-1 family members with myogenic regulatory factors following acute damaging muscle lengthening contractions in humans. J. Physiol. 586, 5549–5560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haddad F., Adams G. R. (2002) Exercise effects on muscle insulin signaling and action: selected contribution: acute cellular and molecular responses to resistance exercise. J. Appl. Physiol. 93, 394–403 [DOI] [PubMed] [Google Scholar]
- 41. Yang S. Y., Goldspink G. (2002) Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett. 522, 156–160 [DOI] [PubMed] [Google Scholar]
- 42. Yang S., Alnaqeeb M., Simpson H., Goldspink G. (1996) Cloning and characterization of an IGF-1 isoform expressed in skeletal muscle subjected to stretch. J. Muscle Res. Cell Motil. 17, 487–495 [DOI] [PubMed] [Google Scholar]
- 43. Hill M., Wernig A., Goldspink G. (2003) Muscle satellite (stem) cell activation during local tissue injury and repair. J. Anat. 203, 89–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pelosi L., Giacinti C., Nardis C., Borsellino G., Rizzuto E., Nicoletti C., Wannenes F., Battistini L., Rosenthal N., Molinaro M., Musaro A. (2007) Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J. 21, 1393–1402 [DOI] [PubMed] [Google Scholar]
- 45. Lu H., Huang D., Saederup N., Charo I. F., Ransohoff R. M., Zhou L. (2011) Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 25, 358–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Heinemeier K. M., Olesen J. L., Schjerling P., Haddad F., Langberg H., Baldwin K. M., Kjaer M. (2007) Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J. Appl. Physiol. 102, 573–581 [DOI] [PubMed] [Google Scholar]
- 47. Barton E. R., DeMeo J., Lei H. (2010) The insulin-like growth factor (IGF)-I E-peptides are required for isoform-specific gene expression and muscle hypertrophy after local IGF-I production. J. Appl. Physiol. 108, 1069–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Postlethwaite A. E., Kang A. H. (1976) Collagen-and collagen peptide-induced chemotaxis of human blood monocytes. J. Exp. Med. 143, 1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kalliokoski K. K., Bojsen-Moller J., Seppanen M., Johansson J., Kjaer M., Teras M., Magnusson S. P. (2007) Contraction-induced [18F]-fluoro-deoxy-glucose uptake can be measured in human calf muscle using high-resolution PET. Clin. Physiol. Funct. Imaging 27, 239–241 [DOI] [PubMed] [Google Scholar]
- 50. Murase S., Terazawa E., Queme F., Ota H., Matsuda T., Hirate K., Kozaki Y., Katanosaka K., Taguchi T., Urai H., Mizumura K. (2010) Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise (delayed-onset muscle soreness). J. Neurosci. 30, 3752–3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fukunaga T., Roy R. R., Shellock F. G., Hodgson J. A., Day M. K., Lee P. L., Kwong-Fu H., Edgerton V. R. (1992) Physiological cross-sectional area of human leg muscles based on magnetic resonance imaging. J. Orthop. Res. 10, 928–934 [DOI] [PubMed] [Google Scholar]
- 52. Vissing K., Andersen J. L., Schjerling P. (2005) Are exercise-induced genes induced by exercise? FASEB J. 19, 94–96 [DOI] [PubMed] [Google Scholar]