Abstract
Pattern recognition receptors (PRRs) in innate immune cells play a pivotal role in the first line of host defense system. PRRs recognize pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) to initiate and regulate innate and adaptive immune responses. PRRs include Toll-like receptors (TLRs), RIG-I-like receptors (RLRs) and NOD-like receptors (NLRs), which have their own features in ligand recognition and cellular location. Activated PRRs deliver signals to adaptor molecules (MyD88, TRIF, MAL/TIRAP, TRAM, IPS-1) which act as important messengers to activate downstream kinases (IKK complex, MAPKs, TBK1, RIP-1) and transcription factors (NF-κB, AP-1, IRF3), which produce effecter molecules including cytokines, chemokines, inflammatory enzymes, and type I interferones. Since excessive PRR activation is closely linked to the development of chronic inflammatory diseases, the role of intrinsic and extrinsic regulators in the prevention of over- or unnecessary activation of PRRs has been widely studied. Intracellular regulators include MyD88s, SOCS1, TOLLIP, A20, and CYLD. Extrinsic regulators have also been identified with their molecular targets in PRR signaling pathways. TLR dimerization has been suggested as an inhibitory target for small molecules such as curcumin, cinnamaldehyde, and sulforaphane. TBK1 kinase can be a target for certain flavonoids such as EGCG, luteolin, quercetin, chrysin, and eriodictyol to regulate TRIF-dependent TLR pathways. This review focuses on the features of PRR signaling pathways and the therapeutic targets of intrinsic and extrinsic regulators in order to provide beneficial strategies for controlling the activity of PRRs and the related inflammatory diseases and immune disorders.
Keywords: Pattern recognition receptor, toll-like receptor, dimerization, TBK1, therapeutic target
INTRODUCTION
Innate and adaptive immunity is required to eliminate pathogens as host defense system. Pattern recognition receptors (PRRs), which are germ-line encoded receptors, play a critical role in initiating and regulating innate and adaptive immune responses by recognizing pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs).1-3 PRRs are quite ubiquitously expressed in a variety of cells including monocytes, dendritic cells, neutrophils, and epithelial cells. The best studied and characterized PRRs are Toll-like receptors (TLRs).4,5 TLRs are a family of type I transmembrane receptors with an extracellular domain that contains leucine-rich-repeat motifs, a transmembrane domain, and a conserved cytoplasmic domain known as the toll/interleukin-1 receptor homology domain.6 Another family of PRRs is the RIG-I-like receptors (RLRs), which include retinoic acid-inducible gene I (RIG-I), melanoma differentiation-associated gene-5 (MDA-5), and laboratory of genetics and physiology 2 (LGP2).7 RLRs are located in the cytoplasm and recognize RNA species that are generated by invading viruses producing type I IFNs and cytokines.8 The nucleotide binding and oligomerization domain (NOD)-like receptors (NLRs) are also cytoplasmic PRRs. NOD1 and NOD2 belong to the NLR family, and recognize bacterial components in order to protect the host from bacterial infection.
It is now well established that dysregulation of TLRs results in an increase of uncontrolled inflammation and metabolic syndromes, which contributes to the development and progression of chronic diseases, such as atherosclerosis, rheumatoid arthritis, asthma, and cancer.9-11 In this report, we intend to provide a review of what TLRs, RLRs, NODs, and their stimulators or inhibitors are, and show how the intracellular signaling pathways are composed (Summary is depicted in Fig. 1). This information contributes to the development of therapeutic intervention strategies for chronic inflammatory diseases and immune disorders, through the manipulation of PRR activation in a beneficial way.
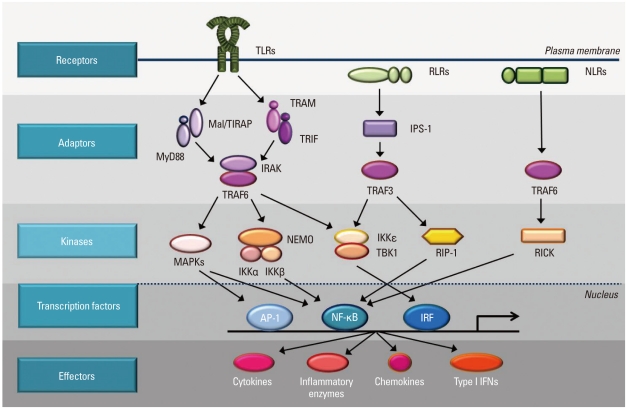
Fig. 1.
Intracellular signaling pathways of pattern-recognition receptors. TLRs are mostly present on the membrane. RLRs and NLRs are located in the cytosol. TLRs send signal through Mal/TIRAP and MyD88 or TRAM/TRIF to IRAK/TRAF6 to activate downstream kinases. RLRs use IPS-1 and TRAF3 as adaptor molecules and NLRs transmit activation signals through TRAF6. The signals from TLRs, RLRs, and NLRs are delivered to kinases such as MAPKs, IKKs, TBK1, RIP-1, and RICK to activate transcription factor, AP-1, NF-κB, and IRF. Transcription factors bind to specific DNA sequences and produce effecter molecules such as cytokines, inflammatory enzymes, chemokines, and type I interferons (IFNs).
PATTERN RECOGNITION RECEPTORS
Toll-like receptors
Toll protein, which plays an important role in antifungal defense, was first identified in Drosophila melanogaster (fruit-fly).12 Subsequently, the human homologue of Toll protein was discovered, and this analogue is referred to as the Toll-like receptor.13 So far, at least thirteen members of the TLR family have been identified and characterized in the mammalian system. TLR1 to TLR9 are conserved in both humans and mice. TLR10 is expressed in human, while TLR11 to TLR13 are present in mice.14 A study with mice deficient of TLRs 1-9, identified each TLR ligand, leaving the ligands for TLR10, TLR12, and TLR13 unknown. TLRs are expressed mainly in various immune cells, including monocytes, macrophages, dendritic cells and B cells; however, they are also present in non-immune cells, such as epithelial cells, endothelial cells, and fibroblasts. TLR1, TLR2, TLR4, TLR5, TLR6, and TLR11 are expressed on the cell surface; TLR3, TLR7, TLR8, and TLR9 are expressed in intracellular vesicles such as endosomes, lysosomes, and the endoplasmic reticulum.15 Epithelial TLR4 is expressed in phagosomes with a unique cellular expression profile.
Of the thirteen TLRs, TLR4 was characterized first.13 TLR4 recognizes lipopolysaccharide (LPS) in the outer membrane of Gram-negative bacteria, with the assistance of co-receptors such as CD14 and MD2.16,17 LPS binds first to LPS binding protein (LBP) and membrane-bound GPI (glycosylphosphatidylinositol)-anchored CD14, and is then transferred to the TLR4 and MD2 (myeloid differentiation proten-2) complexes.18,19 In the MD2 complex, LPS binds to a large hydrophobic pocket, through non-covalent interactions such as hydrogen bonding and hydrophobic and hydrophilic interactions, which results in the dimerization of the two TLR4/MD2 complexes. In addition, TLR4 recognizes mannan from Cadida albicans, glycoinositolphospholipids from Trypanosoma,20 and the envelope proteins from mouse mammary tumor virus (MMTV) and respiratory syncytial virus (RSV).21,22 TLR4 is also activated by endogenous molecules, including heat-shock proteins (HSP60, HSP70, and HSP gp96),23-25 fibrinogen,26 oligosaccharides of hyaluronic acid,27 extracellular domain A of fibronectin,28 heparan sulfate,29 myeloid-related proteins (Mrp8 and Mrp14),30 oxidized LDL,31 saturated fatty acid32 and amyloid-β.33 Furthermore, human TLR4 senses chemical elements such as nickel (Ni2+), conferring immunostimulatory activity to Ni2+.34 Non-conserved histidine residues in human TLR4 provide binding pockets for nickel and trigger an immune response and contact hypersensitivity.
TLR2 recognizes a variety of PAMPs derived from microbial organisms, including bacteria, fungi, virus, yeasts, and parasites.14 TLR2 detects peptidoglycan, lipoprotein, and lipoteichoic acid from Gram-positive bacteria, lipoarabinomannan from mycobacteria,35 glycosylphosphatidylinositol from Trypanosoma cruzi,36 a phenol-soluble modulin from Staphylococcus epidermises,37 hemagglutinin protein from the measles virus,38 and polysaccharides (known as zymosan) from Saccharamysec cerevisiae.39,40 TLR2 dimerizes with either TLR1 or TLR6. A study of macrophages from TLR1- or TLR6-deficient mice revealed that triacyl lipopeptide, from Gram-negative bacteria, is the ligand for the TLR1/TLR2 complex, and that diacyl lipopeptide, from mycoplasma, is the ligand for the TLR2/TLR6 complex.41,42 In addition, TLR2 forms a complex with non-TLR molecules such as CD36 and dectin-1. CD36, a member of the scavenger receptor type B family, has a role as a co-receptor for diacylglyceride recognition by the TLR2/TLR6 complex.43 Dectin-1, a C-type lectin receptor, recognizes β-glucan from fungal cell wall components, together with TLR2 triggering inflammatory responses.44 TLR2 is also activated by non-microbial molecules including HSP70 and HSP gp96,24,25 hyaluronan,45 and saturated fatty acids.46 In addition, TLR2 recognizes carboxyalkylpyrroles which are the end products of lipid oxidation.47 The wide responsiveness of TLR2 and TLR4 to danger signals, such as substances released from tissue injury and environmental toxicants, reinforces the theory that TLRs are strongly implicated in the development of chronic inflammatory diseases.
TLR5 recognizes flagellin, which is a monomeric constituent of bacterial flagella and an important structural protein for motile bacteria.48 TLR5 is mainly expressed on the luminar surface of epithelial cells in the mucosal tissues and respiratory tract.49,50
TLR11 recognizes profilins from the protozoan parasite Toxoplasma gondii51 and uropathogenic E. Coli.52 TLR11 is expressed on epithelial cells in the mouse bladder. TLR11-deficient mice have displayed an increased susceptibility to uropathogenic bacteria.52
TLR3, TLR7, TLR8, and TLR9 sense oligonucleotides derived from microbes and host cells. TLR3 recognizes double-stranded RNA (dsRNA) from the West Nile virus,53 RSV,54 and encephalomycarditis virus (EMCV)55; recognition results in the synthesis of type I interferons, such as IFNα and IFNβ which are important aspects of the antiviral response.56 TLR3 is expressed in myeloid dendritic cells, macrophages, B cells and NK cells; but not in plasmacytoid dendritic cells (pDCs).57 TLR7 and TLR8 detect viral and non-viral single-stranded RNA (ssRNA), and activate IRF3 and IRF7, leading to production of interferons and cytokines58,59; they also recognize imiquimod and its derivatives. TLR7 is highly expressed in pDCs, but TLR8 is mainly present in myeloid dendritic cells and macrophages. TLR9 recognizes DNA from the murine cytomegalovirus (MCMV)60,61 and Herpes simplex virus 1/2 (HSV1/HSV2),62,63 and unmethylated CpG motifs from bacteria and viruses, which induce inflammatory cytokines and type I IFNs.64 CpG DNA is a potent inducer of Type I IFNs in plasmacytoid dendritic cells, and is utilized as a vaccine adjuvant against viral infection.65
RIG-I-like receptors
RLRs are the primary sensor molecules for detecting viral RNA in the cytoplasm.7,66 Three RLRs have been identified: RIG-I (also known as DDX58), MDA5 (also known as Helicard), and LGP2. RIG-I and MDA5 contain both a caspase recruitment domain (CARD) and a RNA helicase domain.67
Activation of RIG-I generates type I IFNs in response to both viral infection and synthetic RNA introduced into the cytoplasm.68 RIG-I is essential for the recognition of ssRNA viruses, such as paramyxoviruses, the influenza virus, and VSV (vesicular stomatitis virus). Therefore, RIG-I deficiency disrupts immune responses to specific ssRNA viruses resulting in the increased susceptibility of mice exposed to RNA viruses.69 Host cells contain an abundance of their own RNA, but host RNA, unlike viral RNA, fails to be recognized by RIG-I. RIG-I binds to the 5'-triphosphate moiety, the signature of which is exposed in the process of viral entry or replication. This specificity explains the strict discrimination between self and non-self RNA by RIG-I; because most endogenous RNAs lose their 5'-triphosphate group during maturation, and thereby escape detection by RIG-I. Short dsRNA (<1 kb) also behaves as a RIG-I ligand in a sequence- and 5'-triphosphate-independent manner.70 Indeed, short segments of reovirus, a segmented dsRNA virus, and short polyI:C can activate RIG-I-mediated signaling.71 Infection by DNA viruses is also detected by RIG-I, through generation of dsRNA by polymerase III.72 RIG-I is coupled with signaling pathways that activate NF-κB, MAPKs, and IRFs, which result in production of type I IFNs with Interferon beta promoter stimulator-1 (IPS-1) as an adaptor. IPS-1 has an N-terminal CARD-like domain, sharing homology with RIG-I. The IPS-1 C-terminal domain contains a trans-membrane segment that targets mitochondria.73-76 IPS-1-deficient mice, exposed to RNA viruses, fail to activate NF-κB and IRF3, with the loss of type I IFN induction illustrating the critical role of IPS-1 in antiviral defense.77,78 However, in pDCs, IPS-1 deficiency did not affect type I IFN production, indicating that TLRs contribute more than RLRs to viral recognition by pDCs. In other cell types, such as macrophages and fibroblasts, RLRs play central roles in viral recognition. The C-terminal domain (CTD) was identified as the RNA recognition domain of RIG-I. Structural analysis revealed that CTD forms a cleft-like surface, with positively charged amino acids that specifically interact with A-form dsRNA.34 However, it remains to be understood how CTD specifically recognizes the 5'-triphosphate group in viral dsRNA. The recognition of an RNA ligand by CTD, induces a conformational change in RIG-I, which allows the N-terminal CARD to interact with the mitochondrial adaptor molecule IPS-1.77 The formation of a RIG-I/IPS-1 complex on the mitochondria triggers the assembly of downstream proteins to initiate signal transduction. TRAF3/6, caspase 8/10, RIP1, and Fas-associated death domain (FADD) have been demonstrated to be involved in RIG-I signaling.79
MDA5 is responsible for the detection of Picornaviridae, including the Encephalomyocarditis virus and Mengo virus.80 Since Picornaviridae is known to generate long double-stranded replication intermediates in infected cells,81 the double-stranded RNA structure has been predicted to be a ligand for MDA5. A relatively long poly I:C (>1 kb) is selectively recognized by MDA5, whereas a shorter poly I:C generated by enzyme digestion (<1 kb) is detected by RIG-I. Therefore, the dsRNAs appearing in virus-infected cells are recognized differentially by RIG-I and MDA5 depending on their length. Structural analysis of MDA5 CTD in solution and crystal has indicated that its global fold is similar to that of RIG-I CTD, suggesting that it plays a role in the recognition of dsRNA.82 However, the concave surface of MDA5 CTD adopts a relatively open structure, suggesting that access by dsRNA may be difficult. The affinity between MDA5 CTD and dsRNA was so low that recognition of dsRNA by MDA5 is likely to require additional adaptor molecules.
NOD-like receptors
NOD-like receptors (NLRs) like RLRs, recognize intracellular PAMPs.83 NLRs include NOD1 and NOD2, which are differentiated by their ligand specificity. A ligand of NOD1 is dipeptide γ-D-glutamyl-meso-diaminopimelic acid (iE-DAP),84 which is derived from most Gram-negative and certain Gram-positive bacteria. NOD2 recognizes muramyl dipeptide (MDP), which is a component of peptidoglycan.85,86 When NOD1 and NOD2 are activated by ligands, NF-κB, MAP kinase p38, ERK, and JNK are activated through an signaling cascade, resulting in the production of cytokines.87,88 In order to activate MAP kinase, CARD9, a CARD-containing adaptor protein, acts as a downstream component of NOD2.89 The NF-κB and MAP kinase pathways cooperate, leading to the transcription of the proinflammatory genes.
INTRACELLULAR SIGNALING COMPONENTS OF PRR
Adaptor molecules of PRRs
TLRs, RLRs and NLRs act through adaptor molecules to activate various kinases and transcription factors. Adaptor molecules are very important messengers that deliver signals from the receptors to protect the host from infection.
MyD88 (Myeloid differentiation primary response gene 88) is one of the representative adaptor molecules in TLR signaling. 'MyD' refers to myeloid differentiation and '88' stands for the number of the gene. MyD88 is a protein that is induced by terminal differentiation of M1D+ myeloid precursors and responses to IL-6.90 MyD88 is located in the cytosol near the cytosolic part of TLRs and delivers an activation signal that is initiated by receptor activation. MyD88 is used by all TLR family members, except TLR3, to activate NF-κB. The structure of MyD88 is similar to that of TLR. MyD88 has an N-terminal death domain (DD), an intermediary domain (ID), and a C-terminal Toll-interleukin 1 receptor (TIR) domain. The TIR domain of MyD88 can bind to the TIR domain of TLR directly or indirectly.91 The N-terminal death domain of MyD88 binds to the death domains of other proteins, through homophilic DD-DD interaction, leading to the activation of NF-κB and JNK.92 In a past study, where MyD88 was knocked-out, treatment with ligands of TLR2, TLR5, TLR7, and TLR9 did not exhibit the proper immune responses.93 However, unlike other TLRs, TLR4 signals still exist in MyD88-deficient mice. This study led to the search for a MyD88-independent adaptor molecule, as it was suggested that TLR4 has another adaptor molecule, which was later discovered to be TRIF.
TRIF (TIR-domain containing adaptor protein inducing interferon-β) is another adaptor molecule associated with TLR signaling TRIF was found by database screening during the search for a TIR domain containing protein. TRIF interacts with TLRs through TIR-TIR interaction. In con-trast with MyD88, which is broadly used as an adaptor molecule in TLR signaling, TRIF is only involved in the signaling pathways of TLR3 and TLR4. TRIF is considered to be closely related to anti-viral signaling, since signals mediated by TRIF are linked to IRF activation and production of IFN.94 While TLR3 only uses TRIF as its adaptor molecule, TLR4 uses TRIF under limited conditions in a MyD88-independent manner. It has been questioned as to whether there is any regulatory mechanism for the preferential activation between MyD88- and TRIF-dependent signaling pathways in TLR4 signaling. A recent study suggests that LPS structure, and its relationship with CD14, could provide the answer. LPS structure can be differentiated into 'smooth LPS' and 'rough LPS'. Full-length O-chains render the 'smooth LPS' structure, whereas the reduction of O-chains produces the 'rough LPS' structure. 'Rough LPS' can bind to the TLR4/MD2 complex, while CD14 is required for 'smooth LPS' to bind to TLR4/MD2. When 'rough LPS' engages with a TLR4/MD2 complex in the absence of CD14, the complex initiates only MyD88-dependent responses. On the other hand, either 'smooth' or 'rough LPS', bound to TLR4, initiates both MyD88-dependent and MyD88-independent responses, in a CD14-dependent manner.95 TRIF recruits TRAF3 and TBK1 (TNF receptor-associated factor (TRAF) family member associated NF-κB activator binding kinase 1) in order to phosphorylate IRF3. A knockout study, using TRIF-deficient mice, revealed that production of type I IFNs, through TLR3 or TLR4, requires the presence of TRIF.94
MAL/TIRAP (MyD88-adapter-like protein/TIR domain-containing adapter protein) is an adaptor molecule essential to the TLR2 and TLR4 signaling pathways. MAL/TIRAP acts as a bridge between MyD88 and TLR. MAL/TIRAP has an N-terminus binding domain that binds to phosphatidylinositol-4,5-bisphosphate; this process mediates the recruitment of MAL/TIRAP to the plasma membrane and, in particular, to the microdomains that contain TLR4. MyD88 does not bind directly to TLR4, but instead interacts with MAL/TIRAP in association with TLR4.90 TLR2 and TLR4 signaling is impaired in cytokine production in MAL/TIRAP-deficient mice; however, TLR2 response is affected to a greater extent than TLR4 response.
TRAM (TRIF-related adaptor molecule) also known as TICAM2 plays an essential role in the MyD88-independent signaling pathway of TLR4. TRAM has a TIR domain, and acts as a bridge connecting TLR and TRIF, which allows for the activation of the TRIF dependent pathway in response to LPS.96 The activation of TRAM affects IRF3 and NF-κB activation as well. TRAM is regulated by myristoylation, which is required for the adaptor molecule to be localized within plasma membrane. Mutation of the myristoylation motif in TRAM abolishes the activation signal. Furthermore, protein kinase Cε (PKCε) phosphorylates TRAM. If phosphorylation is blocked, TRAM signals are impaired, which proves that PKCε is an essential component of the LPS-induced signaling pathway in macrophages.97
SARM (sterile α- and armadillo-motif-containing protein) consists of a sterile α motif (SAM) and a TIR domain. SARM has been shown to be a negative regulator of NF-κB and IRF in TLR signaling.98 Knockdown of SARM expression in primary human peripheral-blood mononuclear cells led to increased poly I:C- and LPS-induced chemokine and cytokine expression. Treatment of cells with LPS increased SARM protein levels, indicating negative feedback regulation of the TLR4/TRIF pathway. Since it is unclear how SARM inhibits TRIF function, it will be important to clarify this mechanism.
Among Toll-like receptors, endosomal TLRs require trafficking proteins, which transport TLRs from ER to endosomes. UNC93B ER membrane protein carries TLR3, TLR7 and TLR9. In addition to UNC93B, Adaptor protein 3 (AP-3) was also involved in TLR9 trafficking. AP-3 enhances the formation of the TLR9 complex with TRAF3 and IRF7. In the absence of AP-3, CpG-A DNA-induced type I IFN production through the stimulation of TLR9 is impaired.96
Interferon beta promoter stimulator-1 (IPS-1) contains an N-terminal CARD domain which is homologous with the domain in RIG-I. IPS-1 is localized in mitochondria, and initiates a signaling process that activates IRF3 and NF-κB, via TBK1/IKKε and IKKα/IKKβ, respectively. IPS-1 binds to RIG-I through CARD-CARD interaction. IPS-1-deficient mice fail to activate NF-κB and IRF3, with concomitant loss of type I IFN and inflammatory cytokine induction, after infection.99
Main kinases in PRR signaling pathways
Phosphorylation is one of the typical mechanisms that activate signaling cascades. Signals from adaptor molecules activate kinases, which can phosphorylate downstream molecules to regulate transcriptional factors.
A multiprotein complex, termed the IKK (IκB kinase) complex, consists of two catalytic components, IKKα and IKKβ, and a regulatory component, NF-κB essential modifier (NEMO, also known as IKKγ). IKKα and IKKβ are structurally similar, having a kinase domain, a leucine zipper domain, helix-loop-helix structures and a NEMO-binding domain (NBD). The IKK complex has a role in phosphorylating IκB. Phosphorylated IκB is degradated by ubiquitination. Then, NF-κB, which had been inhibited by IκB, is released to translocate into nucleus. The IKK complex is a common factor for activating NF-κB, while the regulator of the IKK complex is different in each pathway.100
In addition to IKKs, MAPKs act as important kinases. The expression of IL-6, IL-8, IL-12p40, and MCP-1 is regulated by MAPK signaling.101 There are three groups of MAPKs in mammals: extracellular signal regulated kinase 1/2 (ERK1/2), p38 proteins (α, β, γ, and δ), and c-Jun N-terminal kinases (JNKs). The upstream MAPK kinases (MAPKKs or MEKs) are MEK1/2, MKK3/6, MKK4/7, and MEK5, respectively.102 ERK1/2, p38, and JNK are activated by various TLR ligands. Through MyD88, TRAF6 activates a MAPK kinase kinase (MAPKKK) called transforming growth factor-activated kinase (TAK1). Activated TAK1 can phosphorylate MKK3 and MKK6, the kinases upstream of p38 MAPKs and JNK.103 TAK1 can also activate the IKK complex. The activation of the IKK complex by TAK1 appears to be indirect, and the identity of the kinase that is responsible for direct phosphorylation of the IKK complex remains unidentified.
TBK1 (TRAF family member-associated NF-κB activator (TANK) binding kinase-1) and IKKε (also known as IKKι) were initially implicated in IRF3 phosphorylation and activation, to produce type I IFN in the anti-viral response. Overexpression of IKKε and TBK1 markedly activates NF-κB, as IKKε and TBK1 also regulate NF-κB, in addition to IRF3. IKKε was originally isolated as an LPS-inducible protein in mouse macrophages and was shown to exhibit a similar sequence to canonical IKKs.104 TBK1 was identified as a protein kinase that interacts with TANK (also known as I-TRAF).105 TBK1 deficiency in mice results in embryonic lethality, around day 14.5, because of liver weakness.106 Given that the lethality of TBK1-deficient mice is nullified when TNFR is absent, TBK1 might be involved in TNFR signaling to NF-κB, especially in the liver.107
IPS-1 interacts with receptor-interacting protein-1 (RIP-1), which was originally shown to be associated with the TNF receptor family of death receptors. RIP-1 is a death domain kinase, and is implicated in virus infection-induced type I IFN induction.108 IPS-1 interacts with RIP-1 via the non-CARD region to facilitate NF-κB activation, rather than IRF3 activation. RIP-1 action is also facilitated by IPS-1 to activate NF-κB through activation of the IKK complex. RIP-1 is also involved in the TRIF pathway of TLR3 and TLR4.109 TRIF recruits RIP-1 upon TLR3 and TLR4 activation. In the absence of RIP-1, TLR3-induced NF-κB signaling is abolished.
The NLR proteins NOD1 and NOD2 interact with the serine-threonine kinase RICK (receptor-interacting protein (RIP)-like interacting caspase-like apoptosis regulatory protein kinase; also known as Ripk2 or RIP2), to induce NF-κB and MAPK signaling. Direct or indirect ligand recognition by NOD1 and NOD2 induces recruitment of RICK through CARD-CARD interactions.110 This CARD-containing serine-threonine kinase directly binds and promotes K63-type polyubiquitylation of the regulator IKKγ and activation of the kinase TAK1,111 a prerequisite for activation of the IKK complex. These events result in the degradation of the NF-κB inhibitor IκBα and the subsequent translocation of NF-κB to the nucleus, where transcription of the NF-κB-dependent target gene occurs.
Major transcription factors of PRRs
The stimulation of TLRs, RLRs or NLRs delivers signals through adaptor molecules and kinases. Ultimately, transcription factors, which trigger target gene transcription, are activated in the nucleus.
NF-κB is present in the cytoplasm, in an inactive form, captured by an inhibitor of NF-κB (IκB) proteins. Upon stimulation with various TLR ligands, IκBs are phosphorylated at serine residues by IKK complexes, which consist of IKKα and IKKβ protein kinases and a regulatory molecule, IKKγ/NEMO. Phosphorylation targets IκBs for ubiquitination and degradation, performed by the 26S proteasome, allowing NF-κB to be released into the nucleus and to bind to a response element, which starts transcription of the target genes.
AP-1 (Activator protein 1) has a dimeric basic region composed of members of the Jun, Fos, activating transcription factor (ATF), and Maf subfamilies. AP-1 may bind to TPA-response elements or cAMP-response elements. Among the AP-1 family proteins, c-Jun is thought to play a central role in inflammatory responses. AP-1 activation, in the TLR signaling pathway, is mostly mediated by MAP kinases, such as JNK, p38 and ERK, through phosphorylation. Many TLR ligands activate MAP kinases with similar kinetics.112
TBK1 and IKKε have central roles in the induction of type I IFN through phosphorylation and activation of its transcription factors, IRF3 and IRF7. In a resting state, IRF3 is located in the cytoplasm in an inactive form; however, either TLR3 and TLR4 ligands or viral infection cause TBK1- and IKKε-mediated phosphorylation of the C-terminal region of IRF3. This allows IRF3 to form a homodimer and translocate into the nucleus, where it can bind to the promoter regions of its target genes, such as the IFN-stimulated response element. Embryonic fibroblast cells from TBK1-deficient mice exhibit reduced IRF3 activation and IFN-β induction after stimulation with TLR3 and TLR4 ligands.113 While IKKε-deficient mice show no obvious changes with respect to IRF3 activation and IFN-β induction, cells deficient in both TBK1 and IKKε exhibit a complete loss of IRF3 activation and IFN-β induction, indicating a possible role of IKKε in IRF3 activation.107 Akt also participates in activation of IRF3 in TLR3 and -4 signaling pathways as Akt knockdown by siRNA resulted in the diminishment of IRF3 phosphorylation and dimerization.114 As TBK1 is able to enhance phosphorylation of Akt in response to TLR3 or -4 agonist, the interaction between TBK1 and Akt promotes IRF3 activation and IFNβ expression in TLR/TRIF-pathway. Notably, IRF3 activation by stimulation with TLR3 and TLR4 ligands is impaired in TRIF-deficient mice, but it is intact in MyD88-deficient mice, which suggests that IRF3 activation is controlled by the TRIF-dependent pathway. TBK1 and IKKε can also phosphorylate and activate IRF7, which is the member of the IRF family most closely related to IRF3.115 Whereas IRF3 is ubiquitously expressed and not inducible, IRF7 is expressed at low levels in most types of cells but is strongly induced in response to various stimuli.116 Therefore, IRF7 might be involved in positive feedback regulation of type I IFN induction.
INTRINSIC AND EXTRINSIC REGULATION OF PRR SIGNALING
Endogenous regulators
There exists a cellular device to prevent over- or unnecessary activation of PRRs. Several intracellular negative regulators include MyD88s (the short form of MyD88), SOCS1, TOLLIP, A20, and CYLD (Fig. 2).
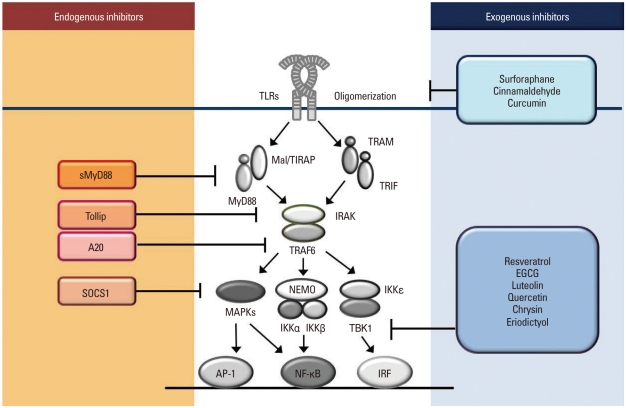
Fig. 2.
Endogenous and exogenous regulators of TLR activation. Endogenous negative regulators of TLRs are revealed. The short form of MyD88 (sMyD88) substitutes MyD88 but cannot send signals downstream. Tollip interacts with IRAK to decrease phosphorylation. A20 deubiquitylates TRAF6. SOCS1 regulates phosphoryltation of IκBα, p38, and JNK. Exogenous TLR regulators have been identified. Small molecules such as surforaphane, cinnamaldehyde, and curcumin block oligomerization of the receptor, whereas resveratrol, EGCG, and certain flavonoids such as lutelolin, quercetin, chrysin, and eriodictyol have the ability to decrease kinase activity of TBK1. In addition, ligand binding to the receptor complex can be another target for regulation of the TLR signaling pathway.
The most universal adaptor molecule in TLR signaling is MyD88, which is employed by TLR2, TLR4, TLR5, TLR7, TLR8 and TLR9. MyD88s lacks the intermediary domain that mediates DD interaction between IRAK4 and MyD88, which is present in wild-type MyD88. Although MyD88 is ubiquitously expressed, expression of MyD88s has only been detected in the spleen and, less strongly, in the brain. However, expression of MyD88s was upregulated in the human monocytic cell line (THP-1) following 16 hours of stimulation with LPS.117 In the presence of MyD88s, IRAK1 was still recruited, through a death-domain interaction with MyD88s, but was no longer phosphorylated.118 The presence of MyD88s prevents IRAK4 from phosphorylating IRAK1, since D88s cannot associate with IRAK4. This indicates that MyD88s might be involved in a negative-feedback regulatory mechanism that controls excessive TLR activation.119
Macrophages from SOCS1-deficient mice exhibited enhanced phosphorylation of STAT1, IκBα, p38, and JNK, and produced high levels of nitric oxide and proinflammatory cytokines, in response to the presence of TLR4 and TLR9 ligands, LPS and CpG DNA, respectively.120 SOCS1-deficient mice die within three weeks of birth from multi-organ inflammation and high susceptibility to sepsis. Furthermore, LPS and CpG DNA induced SOCS1 expression in macrophages, which indicates that SOCS1 is a non-redundant negative regulator of TLR signaling, and may participate in the termination and resolution processes of inflammation.
Tollip (Toll-interacting protein) was originally identified through a yeast-two-hybrid screening process. The process used the cytoplasmic tail of the IL-1R associated protein (residues 385-570) as bait to isolate a murine complementary DNA, which encodes a protein of 274 amino acids, in order to find a new component or IL-1R pathway. Further study showed that Tollip was also able to interact with several members of the TIR superfamily, including TLR2 and TLR4.121 Overexpression of Tollip has been shown to result in inhibition of TLR2- and TLR4-mediated NF-κB activation. Tollip interacts with IRAK1, and the level of IRAK1 autophosphorylation is reduced in the presence of Tollip. IRAK1 causes phosphorylation of Tollip upon TLR stimulation. Although the physiological importance of this is unclear, it is possible that phosphorylation of Tollip facilitates the ubiquitinylation of IRAK1 and its subsequent degradation. In addition, Tollip expression is elevated in intestinal epithelial cells that are hypo-responsive to TLR2 ligands. Therefore, phosphorylation and dephosphorylation of Tollip and IRAK, in the TLR signaling pathway, may be a switch for TLR4- and TLR2-mediated responses.
A20 was initially identified as a TNF-induced zinc-finger protein that suppresses TNF-mediated NF-κB activation.122 A20 expression is rapidly induced by both TLR4 ligands, LPS and TNF, and is expressed in many cell types, which suggests that it is involved in regulating TLR function. Macrophages from A20-deficient mice produced elevated levels of pro-inflammatory cytokines when stimulated with the TLR2 ligands (peptidoglycan and lipoteichoic acid), the TLR3 ligand (poly I:C), and the TLR9 ligand (CpG DNA).123 A20 is important in preventing the host from developing endotoxic shock; however, A20 deficiency does not play an important part in LPS tolerance. A20 is a cysteine protease deubiquitylating enzyme that blocks TLR mediated signaling by deubiquitylating TRAF6. A20 is a negative regulator that can control both MyD88-dependent and MyD88-independent TLR-signaling pathways.
The tumor suppressor CYLD (cylindromatosis) is a negative regulator of the RIG-I-mediated innate antiviral response.124 Ectopic expression of CYLD inhibits both the IRF3 signaling pathway and IFN production triggered by RIG-I; conversely, CYLD knockdown enhances the RIG-I-induced IFN production. CYLD is closely related, in its function, to a deubiquitinating enzyme that removes Lys 63-linked polyubiquitin chains, which suggests a functional association between the two molecules. CYLD removes polyubiquitin chains from RIG-I as well as TBK1, which is the kinase that phosphorylates IRF3, inhibiting the IRF3 signaling pathway. Furthermore, CYLD protein level is re-duced by tumor necrosis factor or viral infection, concomitant with enhanced IFN production.
Poly(ADP-ribose) polymerases (PARPs), which regulate cell survival, cell death, and other biological functions, consist of at least 17 members. Among them, PARP-13 (ZAP) is known to be involved in IFN production against viral infection. The shorter form of PARP-13 (ZAPS) is an especially strong stimulator of the RIG-I-signaling pathway, as it responds to 5'-triphosphate-modified RNA (3pRNA). ZAPS promotes the activation of IRF3 and NF-κB through its association with RIG-I. The production of not only IFN but also of other inflammatory cytokines such as IL-6, TNF-α and CXCL10 is regulated in a ZAPS-dependent manner.125
Regulation of PRR activation by exogenous substances
Since the activation of PRRs is closely associated with the risk of chronic inflammatory diseases and immune disorders, the identification of therapeutic target points in PRR signaling could provide critical information for the prevention and treatment of these diseases. IKKβ and NF-κB have long been popular targets for anti-inflammation studies. However, there remain unrevealed mechanisms for wellknown anti-inflammatory agents. This leads us to search for new therapeutic targets for the treatment of inflammatory diseases and immune disorders.
Receptor oligomerization is an initial step of TLR signaling, which triggers the association of intracellular domains to provide a platform for the recruitment of downstream molecules. When dimerization is blocked, the signal cannot be delivered to the adaptor molecules and downstream signaling cascades. Recently, the suppression of TLR dimerization has been suggested as the inhibitory target for small molecules such as curcumin, cinnamaldehyde, and sulforaphane, which have been reported to have anti-inflammatory effects (Fig. 2).126,127 Thiol-modifying activity appears to be related to the action of these phytochemicals since a supplement of thiol-donors reversed the inhibitory effects of the phytochemicals on TLR4 activation. Indeed, the study using LC-MS/MS analysis has revealed that sulforaphane binds directly to cysteine residues in the TLR4 extracellular domain and inhibits TLR4-TLR4 interaction. These results suggest that receptor clustering, especially the dimerization step, could be a novel target for TLR regulators, and that the modification of cysteine residues could be a promising strategy for modulating TLR activation.127
The representative kinase found in TRIF-dependent TLR signaling is TBK1. TBK1 acts as a critical kinase for IRF3 activation and type I IFNs production by phosphorylating IRF3. Resveratol and its structural analog stilbene specifically inhibit TRIF signaling in the TLR3 and TLR4 pathway by targeting TBK1. Resveratol directly blocks TBK1 kinase activity, as demonstrated by an in vitro kinase assay (Fig. 2).128 Certain flavonoids such as EGCG, luteolin, quercetin, chrysin, and eriodictyol also inhibit TBK1 kinase activity, resulting in a decrease in IRF3 activation and target gene expression, while naringenin and hesperetin had no such effect (Fig. 2). This proves that kinases, especially TBK1, can be a regulatory target in TLR signaling, and provide a potential base for developing an inflammation inhibitor.129,130
In case of TLR4, MD2 is the essential partner in receptor cluster forming two TLR4-MD2 complexes upon engagement of LPS. Understanding the structure of MD2 and the interaction between MD2 and LPS can suggest a therapeutic strategy for regulating TLR4 activation. A free cysteine residue at the 133 position within a binding pocket of MD2 has been suggested as an important site for modulating the interaction between MD2 and LPS. Binding of MD2 Cys133 by thiol-reactive compounds decreases LPS signaling, such as NO production and NF-κB activation, possibly by preventing LPS access to the MD2 pocket.131 Other small molecules that occupy this binding pocket in MD2 will prevent the ligand from engaging the receptor and the subsequent activation of intracellular signaling pathways.
CONCLUSION
The search for new innate immune receptors, and their signaling pathways, is still ongoing. The PYHIN proteins, AIM2 and IFI16, have been proposed as members of the AIM-like receptor (ALRs) family, which senses bacterial and viral DNA in the cytoplasm.132,133 In addition, LRRFIP1 has been identified as another cytosolic sensor for intracellular DNA.134 The three receptors have different intracellular signaling pathways: AIM2 couples with ASC and caspase-1 to cleave pro-IL-1β producing mature IL-1β; IFI16 associates with STING and TBK1 to activate IRF3 and NF-κB; LRRFIP1 promotes phosphorylation of β-catenin, which activates IRF3 and produces IFNβ. Along with the discovery of new receptors, new regulators of immune response are also being revealed. TREX1 (Three prime repair exonulease 1) degrades IFN-stimulatory DNA derived from virus, which blocks the recognition of viral DNA by sensors and suppresses anti-viral immunity.135
Some PRRs are coupled with other receptors. The inflammasome system, which activates caspase-1, requires a primary signal from other PRRs in order to initiate the transcription of pro-IL-1β, the caspase-1 substrate. It is well recognized that inflammasome plays a critical role in sensing danger signals. Nalp3 inflammasome is activated by endogenous and environmental toxicants such as uric acid, amyloid protein, alum, asbestos, and silica. Therefore, it is expected that inflammasomes regulate autoimmune diseases. Representative diseases that occur due to abnormalities in inflammasome function are systemic onset juvenile idiopathic arthritis (SOJIA), urate crystal arthritis (Gout), and type 2 diabetes.136 The cause of these diseases is a mutation in the inflammasome-related genes. Malfunction of the inflammasome results in inappropriate regulation of the immune system and the production of excess IL-1β, which leads to the development of chronic inflammatory diseases such as arthritis.
Dysregulation of innate immune receptors leads to uncontrolled and improper immune responses against infection and danger signals, and causes severe diseases. Therefore, discovery of new receptors and investigation into their downstream signal activation mechanisms, are essential in order to understand how to efficiently regulate the immune system and, eventually, to advance the quality of human life. As the effort to develop new therapeutic agents modulating PRRs is being extensively pursued,137 identification of novel regulatory targets provides an important information on constructing beneficial therapeutic strategies.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Korea Food and Drug Administration for Studies on the Standardization of Herbal Medicine (2009, 2010).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Janeway CA., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- 7.Meylan E, Tschopp J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol Cell. 2006;22:561–569. doi: 10.1016/j.molcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Yoneyama M, Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol Rev. 2009;227:54–65. doi: 10.1111/j.1600-065X.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee JY, Zhao L, Hwang DH. Modulation of pattern recognition receptor-mediated inflammation and risk of chronic diseases by dietary fatty acids. Nutr Rev. 2010;68:38–61. doi: 10.1111/j.1753-4887.2009.00259.x. [DOI] [PubMed] [Google Scholar]
- 10.O'Neill LA, Bryant CE, Doyle SL. Therapeutic targeting of Toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol Rev. 2009;61:177–197. doi: 10.1124/pr.109.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JY, Hwang DH. The modulation of inflammatory gene expression by lipids: mediation through Toll-like receptors. Mol Cells. 2006;21:174–185. [PubMed] [Google Scholar]
- 12.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 13.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 14.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 17.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 18.Miyake K. Roles for accessory molecules in microbial recognition by Toll-like receptors. J Endotoxin Res. 2006;12:195–204. doi: 10.1179/096805106X118807. [DOI] [PubMed] [Google Scholar]
- 19.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 20.Gazzinelli RT, Ropert C, Campos MA. Role of the Toll/interleukin-1 receptor signaling pathway in host resistance and pathogenesis during infection with protozoan parasites. Immunol Rev. 2004;201:9–25. doi: 10.1111/j.0105-2896.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 21.Burzyn D, Rassa JC, Kim D, Nepomnaschy I, Ross SR, Piazzon I. Toll-like receptor 4-dependent activation of dendritic cells by a retrovirus. J Virol. 2004;78:576–584. doi: 10.1128/JVI.78.2.576-584.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 23.Ohashi K, Burkart V, Flohé S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 24.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 25.Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, et al. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 2002;277:20847–20853. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- 26.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 27.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 29.Johnson GB, Brunn GJ, Platt JL. Cutting edge: an endogenous pathway to systemic inflammatory response syndrome (SIRS)-like reactions through Toll-like receptor 4. J Immunol. 2004;172:20–24. doi: 10.4049/jimmunol.172.1.20. [DOI] [PubMed] [Google Scholar]
- 30.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 31.Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- 32.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 33.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt M, Raghavan B, Müller V, Vogl T, Fejer G, Tchaptchet S, et al. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat Immunol. 2010;11:814–819. doi: 10.1038/ni.1919. [DOI] [PubMed] [Google Scholar]
- 35.Wieland CW, Knapp S, Florquin S, de Vos AF, Takeda K, Akira S, et al. Non-mannose-capped lipoarabinomannan induces lung inflammation via toll-like receptor 2. Am J Respir Crit Care Med. 2004;170:1367–1374. doi: 10.1164/rccm.200404-525OC. [DOI] [PubMed] [Google Scholar]
- 36.Campos MA, Almeida IC, Takeuchi O, Akira S, Valente EP, Procópio DO, et al. Activation of Toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J Immunol. 2001;167:416–423. doi: 10.4049/jimmunol.167.1.416. [DOI] [PubMed] [Google Scholar]
- 37.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bieback K, Lien E, Klagge IM, Avota E, Schneider-Schaulies J, Duprex WP, et al. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol. 2002;76:8729–8736. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato M, Sano H, Iwaki D, Kudo K, Konishi M, Takahashi H, et al. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J Immunol. 2003;171:417–425. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- 40.Frasnelli ME, Tarussio D, Chobaz-Péclat V, Busso N, So A. TLR2 modulates inflammation in zymosan-induced arthritis in mice. Arthritis Res Ther. 2005;7:R370–R379. doi: 10.1186/ar1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, et al. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi O, Kawai T, Mühlradt PF, Morr M, Radolf JD, Zychlinsky A, et al. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 43.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 44.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 46.Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, et al. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 47.West XZ, Malinin NL, Merkulova AA, Tischenko M, Kerr BA, Borden EC, et al. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature. 2010;467:972–976. doi: 10.1038/nature09421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 49.Hawn TR, Verbon A, Lettinga KD, Zhao LP, Li SS, Laws RJ, et al. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires' disease. J Exp Med. 2003;198:1563–1572. doi: 10.1084/jem.20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 51.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 52.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 53.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 54.Groskreutz DJ, Monick MM, Powers LS, Yarovinsky TO, Look DC, Hunninghake GW. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J Immunol. 2006;176:1733–1740. doi: 10.4049/jimmunol.176.3.1733. [DOI] [PubMed] [Google Scholar]
- 55.Hardarson HS, Baker JS, Yang Z, Purevjav E, Huang CH, Alexopoulou L, et al. Toll-like receptor 3 is an essential component of the innate stress response in virus-induced cardiac injury. Am J Physiol Heart Circ Physiol. 2007;292:H251–H258. doi: 10.1152/ajpheart.00398.2006. [DOI] [PubMed] [Google Scholar]
- 56.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 57.Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420:1–16. doi: 10.1042/BJ20090272. [DOI] [PubMed] [Google Scholar]
- 58.Moynagh PN. TLR signalling and activation of IRFs: revisiting old friends from the NF-kappaB pathway. Trends Immunol. 2005;26:469–476. doi: 10.1016/j.it.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 59.Kawai T, Sato S, Ishii KJ, Coban C, Hemmi H, Yamamoto M, et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 2004;5:1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- 60.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 62.Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004;103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 63.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 65.Wagner H. The immunogenicity of CpG-antigen conjugates. Adv Drug Deliv Rev. 2009;61:243–247. doi: 10.1016/j.addr.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 66.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 67.Nallagatla SR, Hwang J, Toroney R, Zheng X, Cameron CE, Bevilacqua PC. 5'-triphosphate-dependent activation of PKR by RNAs with short stem-loops. Science. 2007;318:1455–1458. doi: 10.1126/science.1147347. [DOI] [PubMed] [Google Scholar]
- 68.Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 69.Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 70.Takahasi K, Yoneyama M, Nishihori T, Hirai R, Kumeta H, Narita R, et al. Nonself RNA-sensing mechanism of RIG-I helicase and activation of antiviral immune responses. Mol Cell. 2008;29:428–440. doi: 10.1016/j.molcel.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 71.Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald KA, Hornung V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009;10:1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 74.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 75.Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 76.Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 77.Sun Q, Sun L, Liu HH, Chen X, Seth RB, Forman J, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Kumar H, Kawai T, Kato H, Sato S, Takahashi K, Coban C, et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med. 2006;203:1795–1803. doi: 10.1084/jem.20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oganesyan G, Saha SK, Guo B, He JQ, Shahangian A, Zarnegar B, et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 80.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 81.Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J Virol. 2006;80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li X, Lu C, Stewart M, Xu H, Strong RK, Igumenova T, et al. Structural basis of double-stranded RNA recognition by the RIG-I like receptor MDA5. Arch Biochem Biophys. 2009;488:23–33. doi: 10.1016/j.abb.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 83.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 84.Chamaillard M, Hashimoto M, Horie Y, Masumoto J, Qiu S, Saab L, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 85.Girardin SE, Hugot JP, Sansonetti PJ. Lessons from Nod2 studies: towards a link between Crohn's disease and bacterial sensing. Trends Immunol. 2003;24:652–658. doi: 10.1016/j.it.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 86.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 87.Girardin SE, Yaniv M. A direct interaction between JNK1 and CrkII is critical for Rac1-induced JNK activation. EMBO J. 2001;20:3437–3446. doi: 10.1093/emboj/20.13.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pauleau AL, Murray PJ. Role of nod2 in the response of macrophages to toll-like receptor agonists. Mol Cell Biol. 2003;23:7531–7539. doi: 10.1128/MCB.23.21.7531-7539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hsu YM, Zhang Y, You Y, Wang D, Li H, Duramad O, et al. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol. 2007;8:198–205. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
- 90.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 91.Li C, Zienkiewicz J, Hawiger J. Interactive sites in the MyD88 Toll/interleukin (IL) 1 receptor domain responsible for coupling to the IL1beta signaling pathway. J Biol Chem. 2005;280:26152–26159. doi: 10.1074/jbc.M503262200. [DOI] [PubMed] [Google Scholar]
- 92.Burns K, Martinon F, Esslinger C, Pahl H, Schneider P, Bodmer JL, et al. MyD88, an adapter protein involved in interleukin-1 signaling. J Biol Chem. 1998;273:12203–12209. doi: 10.1074/jbc.273.20.12203. [DOI] [PubMed] [Google Scholar]
- 93.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 94.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 95.Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, et al. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- 96.Oshiumi H, Sasai M, Shida K, Fujita T, Matsumoto M, Seya T. TIR-containing adapter molecule (TICAM)-2, a bridging adapter recruiting to toll-like receptor 4 TICAM-1 that induces interferon-beta. J Biol Chem. 2003;278:49751–49762. doi: 10.1074/jbc.M305820200. [DOI] [PubMed] [Google Scholar]
- 97.Castrillo A, Pennington DJ, Otto F, Parker PJ, Owen MJ, Boscá L. Protein kinase Cepsilon is required for macrophage activation and defense against bacterial infection. J Exp Med. 2001;194:1231–1242. doi: 10.1084/jem.194.9.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carty M, Goodbody R, Schröder M, Stack J, Moynagh PN, Bowie AG. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat Immunol. 2006;7:1074–1081. doi: 10.1038/ni1382. [DOI] [PubMed] [Google Scholar]
- 99.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 100.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 101.Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 102.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 103.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 104.Suzuki N, Suzuki S, Duncan GS, Millar DG, Wada T, Mirtsos C, et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416:750–756. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- 105.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 107.Hemmi H, Takeuchi O, Sato S, Yamamoto M, Kaisho T, Sanjo H, et al. The roles of two IkappaB kinase-related kinases in lipopolysaccharide and double stranded RNA signaling and viral infection. J Exp Med. 2004;199:1641–1650. doi: 10.1084/jem.20040520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Balachandran S, Thomas E, Barber GN. A FADD-dependent innate immune mechanism in mammalian cells. Nature. 2004;432:401–405. doi: 10.1038/nature03124. [DOI] [PubMed] [Google Scholar]
- 109.Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 110.Inohara N, Koseki T, del Peso L, Hu Y, Yee C, Chen S, et al. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kappaB. J Biol Chem. 1999;274:14560–14567. doi: 10.1074/jbc.274.21.14560. [DOI] [PubMed] [Google Scholar]
- 111.Abbott DW, Wilkins A, Asara JM, Cantley LC. The Crohn's disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol. 2004;14:2217–2227. doi: 10.1016/j.cub.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 112.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 113.McWhirter SM, Fitzgerald KA, Rosains J, Rowe DC, Golenbock DT, Maniatis T. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc Natl Acad Sci U S A. 2004;101:233–238. doi: 10.1073/pnas.2237236100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Joung SM, Park ZY, Rani S, Takeuchi O, Akira S, Lee JY. Akt contributes to activation of the TRIF-dependent signaling pathways of TLRs by interacting with TANK-binding kinase 1. J Immunol. 2011;186:499–507. doi: 10.4049/jimmunol.0903534. [DOI] [PubMed] [Google Scholar]
- 115.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 116.Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 117.Janssens S, Burns K, Tschopp J, Beyaert R. Regulation of interleukin-1- and lipopolysaccharide-induced NF-kappaB activation by alternative splicing of MyD88. Curr Biol. 2002;12:467–471. doi: 10.1016/s0960-9822(02)00712-1. [DOI] [PubMed] [Google Scholar]
- 118.Burns K, Janssens S, Brissoni B, Olivos N, Beyaert R, Tschopp J. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J Exp Med. 2003;197:263–268. doi: 10.1084/jem.20021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Janssens S, Burns K, Vercammen E, Tschopp J, Beyaert R. MyD88S, a splice variant of MyD88, differentially modulates NF-kappaB- and AP-1-dependent gene expression. FEBS Lett. 2003;548:103–107. doi: 10.1016/s0014-5793(03)00747-6. [DOI] [PubMed] [Google Scholar]
- 120.Kinjyo I, Hanada T, Inagaki-Ohara K, Mori H, Aki D, Ohishi M, et al. SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity. 2002;17:583–591. doi: 10.1016/s1074-7613(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 121.Bulut Y, Faure E, Thomas L, Equils O, Arditi M. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J Immunol. 2001;167:987–994. doi: 10.4049/jimmunol.167.2.987. [DOI] [PubMed] [Google Scholar]
- 122.Opipari AW, Jr, Boguski MS, Dixit VM. The A20 cDNA induced by tumor necrosis factor alpha encodes a novel type of zinc finger protein. J Biol Chem. 1990;265:14705–14708. [PubMed] [Google Scholar]
- 123.Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- 124.Friedman CS, O'Donnell MA, Legarda-Addison D, Ng A, Cárdenas WB, Yount JS, et al. The tumour suppressor CYLD is a negative regulator of RIG-I-mediated antiviral response. EMBO Rep. 2008;9:930–936. doi: 10.1038/embor.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hayakawa S, Shiratori S, Yamato H, Kameyama T, Kitatsuji C, Kashigi F, et al. ZAPS is a potent stimulator of signaling mediated by the RNA helicase RIG-I during antiviral responses. Nat Immunol. 2011;12:37–44. doi: 10.1038/ni.1963. [DOI] [PubMed] [Google Scholar]
- 126.Youn HS, Lee JK, Choi YJ, Saitoh SI, Miyake K, Hwang DH, et al. Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem Pharmacol. 2008;75:494–502. doi: 10.1016/j.bcp.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 127.Youn HS, Kim YS, Park ZY, Kim SY, Choi NY, Joung SM, et al. Sulforaphane suppresses oligomerization of TLR4 in a thiol-dependent manner. J Immunol. 2010;184:411–419. doi: 10.4049/jimmunol.0803988. [DOI] [PubMed] [Google Scholar]
- 128.Youn HS, Lee JY, Fitzgerald KA, Young HA, Akira S, Hwang DH. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: molecular targets are TBK1 and RIP1 in TRIF complex. J Immunol. 2005;175:3339–3346. doi: 10.4049/jimmunol.175.5.3339. [DOI] [PubMed] [Google Scholar]
- 129.Youn HS, Lee JY, Saitoh SI, Miyake K, Kang KW, Choi YJ, et al. Suppression of MyD88- and TRIF-dependent signaling pathways of Toll-like receptor by (-)-epigallocatechin-3-gallate, a polyphenol component of green tea. Biochem Pharmacol. 2006;72:850–859. doi: 10.1016/j.bcp.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 130.Lee JK, Kim SY, Kim YS, Lee WH, Hwang DH, Lee JY. Suppression of the TRIF-dependent signaling pathway of Toll-like receptors by luteolin. Biochem Pharmacol. 2009;77:1391–1400. doi: 10.1016/j.bcp.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 131.Mancek-Keber M, Gradisar H, Iñigo Pestaña M, Martinez de Tejada G, Jerala R. Free thiol group of MD-2 as the target for inhibition of the lipopolysaccharide-induced cell activation. J Biol Chem. 2009;284:19493–19500. doi: 10.1074/jbc.M109.003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang P, An H, Liu X, Wen M, Zheng Y, Rui Y, et al. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a beta-catenin-dependent pathway. Nat Immunol. 2010;11:487–494. doi: 10.1038/ni.1876. [DOI] [PubMed] [Google Scholar]
- 135.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hennessy EJ, Parker AE, O'Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010;9:293–307. doi: 10.1038/nrd3203. [DOI] [PubMed] [Google Scholar]