Abstract
Purpose
Our previous study indicated that the presence of wheat-specific IgG1 and IgG4 antibodies was associated with work-related symptoms in workers exposed to wheat flour. We performed this study to investigate the genetic polymorphisms of β2-adrenergic receptors and wheat-specific antibodies in association with the clinical parameters of baker's asthma.
Materials and Methods
In total, 379 subjects working in a single industrial bakery were enrolled in this study. The skin prick test was performed with common inhalant allergens and wheat flour extract. The presence of serum- specific IgE, IgG1, and IgG4 antibodies to wheat flour were determined by ELISA. Whole blood samples were obtained for genotype analysis. Subjects were genotyped with regard to five candidate single nucleotide polymorphisms (SNPs) of the β2-adrenergic receptor gene (ADRB2; -47 T>C, 46 A>G, 79 C>G, 252 G>A, and 523 C>A) using a single-base extension method.
Results
No significant associations were observed between the genotype/allele frequencies of any of the SNPs tested and any clinical parameters. The haplotype of ADRB2 (GAA composed of 46 A>G, 252 G>A, and 523 C>A) was significantly associated with work-related symptoms (p<0.05). Moreover, in subjects with the AG or GG genotype at 46 A>G and haplotype [GAA] of ADRB2, the prevalence rates of wheat-specific IgG1 antibodies and lower respiratory symptoms increased significantly with exposure intensity (both p<0.05).
Conclusion
The findings of the present study suggest that ADRB2 genetic polymorphism may contribute to the development of work-related symptoms in workers exposed to wheat flour, which can lead to baker's asthma.
Keywords: Baker's asthma, ADRB2 polymorphism, haplotype, specific IgG antibody
INTRODUCTION
Baker's asthma and rhinitis are among the most common occupational respiratory disorders in Western countries.1-4 In the pathophysiology of occupational asthma, exposure intensity to occupational allergens is a widely known risk factor for work-related symptoms, and many studies have suggested close correlations between wheat flour allergen exposure and wheat flour sensitization.5-7 IgE-mediated response is a major pathogenic mechanism in the development of baker's asthma.8-10 In addition, wheat-specific IgG and IgG subclasses have also been suggested to be involved in the pathogenesis of baker's asthma.11-13 In our previous study, we demonstrated that the prevalence rates of serum wheat-specific IgG1 and IgG4 antibodies increased with exposure intensity and that they were also significantly associated with the presence of work-related symptoms.14
Although human leukocyte antigen (HLA) haplotypes and genetic polymorphisms are associated with some occupational asthma,15 no previous studies have reported any association between genetic polymorphisms and baker's asthma. However, several genetic studies have demonstrated that the β2-adrenergic receptor (ADRB2) polymorphism was associated with asthma phenotype16 and pharmacogenetic aspects.17,18 Based on our previous epidemiological and immunological data, we investigated ADRB2 genetic polymorphisms associated with the clinical findings of workers exposed to wheat flour in a single factory bakery.
MATERIALS AND METHODS
Study population
The protocols of this study were reviewed and approved by the Ajou University Institute Review Board. Informed consent was obtained from each participant.
In total, 379 subjects from our previous study who agreed to provide whole blood samples for genetic analysis were enrolled in the present study.14 All subjects completed a respiratory questionnaire regarding whether they had had work-related and/or lower-respiratory symptoms. Skin prick tests (SPT) were performed and included common inhalant allergens and wheat flour extract. Atopy was defined for subjects with more than one positive response to common inhalant allergens on the skin prick test. Serum total IgE levels were measured by the immunoCAP system (Phadia AB, Uppsala, Sweden). The presence of specific IgE, IgG1, and IgG4 antibodies to wheat extracts was determined by an enzyme-linked immunosorbent assay (ELISA), as described previously.19 Lung functions, including forced expiratory volume in 1 s (FEV1) were measured with a spirometer (Jaeger; MasterScope PC, Hoechberg, Germany).
The methacholine challenge test was performed in 16 subjects with suspected airway hyperresponsiveness, and the concentration of inhaled methacholine that causes a 20% fall in FEV1 (PC20) was measured. A bronchoprovocation test with wheat flour was performed in subjects with positive results in the methacholine challenge test as described previously.14 Briefly, normal saline was administered from a nebulizer connected to a dosimeter (646; Devilbiss Co, Somerset, PA, USA). The subject was asked to breathe each graded dosing of wheat extracts 5 times using dosimeter. The concentrations of inhaled wheat extract ranged from 1 mg/mL to 1 mg/mL, which were decided as the previous results of intradermal tests with wheat extracts. Lung function was measured with a spirometer (Jaeger) before and 10 minutes after each dosing of inhalation. Then, lung functions were measured every 10 minutes during the first hour, and every hour for 7 hours after the challenge. When the FEV1 level decreased more than 20% from the baseline value, it was regarded as a positive response.
All subjects were classified according to their dust exposure density as described previously.14 Subjects were briefly classified by their current task. The minimal exposure group included packaging, supplies, development, shipping, account, distribution, and other office jobs. The intermediate group included foaming, baking (oven), decoration and production manager. The high exposure group included mixing, weighing, and dividing tasks.
Genotyping
Genomic DNA was prepared from whole blood samples using a Puregene DNA purification kit (Gentra Systems, Minneapolis, MN, USA) according to the manufacturer's protocol. Genotyping of each polymorphism was performed using a single-base extension method. Five ADRB2 polymorphisms (-47, 46, 79, 252, and 523; numbers are relative to the first nucleotide of the start codon as +1) were screened using a single-base extension method. The used primer sequences are listed below: forward 5'-TGAATGAGGCTTCCAGGC-3', reverse 5'-GTRAGCGCACTGGCTGGG-3', and extension 5'-CXCGCAGAGCXCCGCCGTGGGTCCGCC-3' for -47 T>C; forward 5'-CAGGAAACAGCTATGACCGCACATAACGGGCAGAAC-3', reverse 5'-CCAGTGAAGTGATGAAGTAGTTG-3', and extension 5'-ACGTCRTGGTCCGGCGCATGGCTTC-3' for 46 A>G; forward 5'-TTCTTGCTGGCACCCAAT-3', reverse 5'-GA CATGACGATGCCCATG-3', and extension 5'- TGCGCCGGACCACGXCGXCACGCAG-3' for 79C>G; forward 5'-CAACTACTTCATCACTTCACTGR-3', reverse 5'-ACATTTTCATAAGAATATGGGCG-3', and extension 5'-CCTGTGCTGATCTGGTCATGGGCCT-3' for 252 G>A; and forward 5'-ATTGTGTCAGGCCTTACCTCR-3', reverse 5'-GTCTCATTGGCATAGCAGTTG-3', and extension 5'-CTTGCCCATTCAGATGCACTGGTAC-3' for 523 C>A.
Primer extension reactions were performed with a SNaP-shot ddNTP primer extension kit (Applied Biosystems, Foster City, CA, USA). PCR reactions were carried out as follows: denaturations were done as 1 cycle at 95℃ for 10 minutes, and 35 cycles at 95℃ for 30 seconds. Melting processes were done at 55℃ for -47T>C, 46A>G, 79C>G, and 523C>A SNPs, at 50℃ for 252G>A SNP for 1 minute. Extensions were done at 72℃ for 1 minute. The results were analyzed after completion of the study using the ABI Prism GeneScan and Genotyper software (Applied Biosystems).
Statistical analyses
All data are expressed as means±standard deviation. SPSS software (version 11.05; SPSS Inc., Chicago, IL, USA) was used for statistical analyses.
Estimation of haplotypes was performed using Haploview (version 3.32; Broad Institute, Cambridge, MA, USA), and three haplotype sets were derived from 46 A>G, 252 G>A, and 523 C>A of ADRB2 SNPs. Significant departures of the genotype frequency from Hardy-Weinberg equilibrium at the polymorphic site were tested by χ2 analysis. The clinical parameters of the subjects were compared with each genotype using ANOVA and χ2 analysis. The associations of each polymorphic genotype/haplotype in the study subjects with work-related symptoms were analyzed using Fisher's exact test. Power analysis was performed using PASS (version 2005; NCSS, Kaysville, UT, USA). All values were adjusted using Bonferroni's multiple comparison tests to correct for incidental significance.
Predictive probability was calculated for the presence of serum-specific IgG subclasses to wheat flour in subjects with ADRB2 genetic polymorphism or haplotype using logistic regression.20 All data was adjusted for age, sex, atopy status, and exposure density using logistic regression.
RESULTS
Subject characteristics and clinical findings
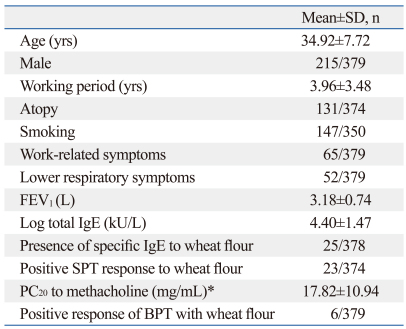
Table 1 shows the subject characteristics and clinical findings, including age, gender, working duration, prevalence rates of symptoms, results of skin prick tests, and presence of wheat-specific IgE antibody. The mean age of the subjects was 34.92±7.72 years, and 56.7% were male. The mean working duration in the baking industry was 3.96±3.48 years, and the prevalence rate of atopy was 34.6%. Sixty-five subjects (17.1%) had work-related symptoms and 52 (13.7%) exhibited lower-respiratory symptoms. The prevalence rates of wheat-specific IgE antibody and positive skin prick test results to wheat flour were 6.6% and 6.1%, respectively. The mean PC20 value was 17.82±10.94 mg/mL, and six subjects showed a positive response to the bronchoprovocation test with wheat flour.
Table 1.
Clinical Characteristics of the Subjects
*The methacholine challenge test was performed in only 16 subjects with suspected airway hyperresponsiveness.
PC20, the concentration of inhaled methacholine that causes a 20% fall in FEV1; BPT, bronchoprovocation test.
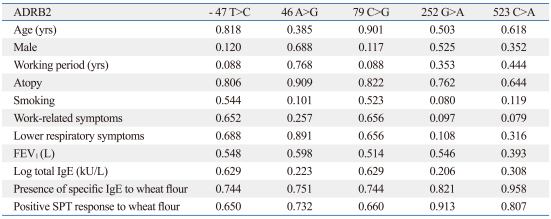
The associations of the clinical parameters according to each ADRB2 genotype are presented in Table 2. There were no significant associations between clinical parameters and genotypes.
Table 2.
Clinical Parameters According to Each ADRB2 Genotype
*Associations were presented as p value compared to each genotype using ANOVA and χ2-test.
Associations between SNPs and the haplotype of ADRB2
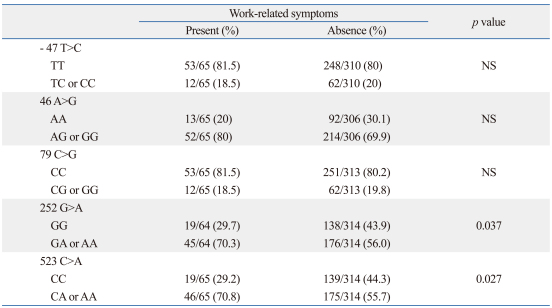
Table 3 summarizes the associations of five SNPs of ADRB2 with work-related symptoms. The prevalence of work-related symptoms was higher in subjects with GA or AA genotypes of 252 G>A, or the CA or AA genotypes of 523 C>A (p<0.05, respectively). However, the statistical significance disappeared after performing Bonferroni's multiple comparisons. The results of power analysis were 0.56 for 252 G>A (effect size 0.11), and 0.61 for 523 C>A (effect size 0.15).
Table 3.
Associations of ADRB2 SNPs with Work-Related Symptoms
SNP, single nucleotide polymorphism; WRS, work-related symptoms; NS, not significant; ADRB2, β2-adrenergic receptor 2; IL-13, interleukin-13; IFNGR2, interferon-γ receptor 2.
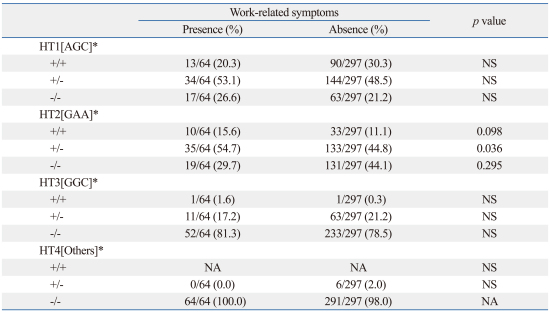
Table 4 shows the associations of these haplotypes with WRS. When the haplotype of ADRB2 was composed of 46 A>G, 252 G>A, and 523 C>A, the prevalence rate of work-related symptoms was significantly higher in subjects with than in those without HT2 [GAA] (p<0.05). The power analysis result was 0.56 for HT2 [GAA] (effect size 0.11).
Table 4.
Associations between β2-Adrenergic Receptor Gene Haplotypes and Work-Related Symptoms
*The haplotype was composed of 46A>G, ADRB2 252G>A, and 523C>A β2-adrenergic receptor polymorphisms.
WRS, work-related symptoms; NS, not significant; HT, haplotype; NA, not available.
Predictive probabilities for serum-specific IgG subclasses to wheat flour
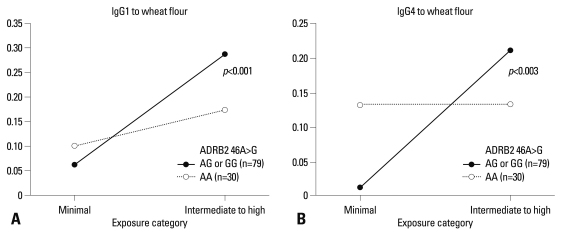
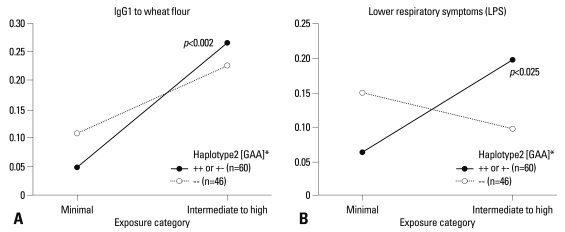
No significant associations were observed between the genotypes of five SNPs and prevalence rates of serum-specific IgE, IgG1, and IgG4 antibodies (all p>0.05, data not shown). However, the predicted probabilities for the presence of serum-specific IgG1 and IgG4 antibodies to wheat flour were calculated according to the exposure intensity (minimal and intermediate to high) by a logistic regression model as shown in Figs. 1 and 2. In subjects with AG or GG genotype of ADRB2 46 A>G, prevalence rates of serum-specific IgG1 and IgG4 antibodies to wheat flour increased significantly with higher exposure intensity [odds ratio (OR) 2.524, p<0.001, Fig. 1A; OR 5.097, p=0.002, Fig. 1B]. No significant changes were found in those with the AA genotype, which was replicated in the results of the haplotype analysis, as the subjects with HT2 [GAA] had a significantly higher prevalence rate of serum-specific IgG1 antibody to wheat flour in relation to exposure intensity (OR 2.652, p=0.002) (Fig. 2A). The subjects with this HT2 haplotype also showed a higher risk of having LRS in relation to exposure intensity (OR 2.019, p=0.014) (Fig. 2B).
Fig. 1.
Predictive probabilities for the presence of serum-specific IgG antibodies to wheat flour in relation to exposure, according to the ADRB2 46A>G polymorphism.
Fig. 2.
Predictive probabilities for the presence of serum-specific IgG antibodies to wheat flour in relation to exposure density, according to the genotype of ADRB2. *The haplotype was composed of 46A>G, 252G>A, and 523C>A of the β2-adrenergic receptor genetic polymorphism.
DISCUSSION
Environmental and genetic backgrounds play important roles in the development of bronchial asthma. Occupational asthma is defined as a disease characterized by variable airflow limitation with airway hyperresponsiveness that is attributable to a particular occupational environment.21 Baker's asthma is a common occupational disease, with wheat flour being one of the common causative agents of occupational asthma.22,23 The working environment is known to be the most important factor associated with baker's asthma, but no previous studies have examined genetic polymorphisms in subjects with this condition. This is the first study to investigate genetic susceptibility in association with immune responses to wheat flour and work-related symptoms.
Several genetic studies have investigated ADRB2 with regard to phenotype and the pharmacogenetic aspects of asthma, particularly in association with the bronchodilating effect of β-agonist use,17 and reports have described the presence of common functional polymorphic variants of the ADRB2 gene.18 Thirteen polymorphisms in this gene and its transcriptional regulator β upstream peptide have been identified to date.24 Among these, many have reported on the associations of 46 A>G polymorphisms and indicated that the Gly mutation at this position increases the risk of severe asthma16 and airway hyperresponsiveness.25 In addition, pharmacogenetic studies have shown that ADRB2 polymorphism can modify the bronchodilator response to β-agonists.17,26
Caucasian individuals with the Gly/Gly genotype of ADRB2 46 A>G have a greater bronchodilator response to the use of β-agonists. However, different findings have been reported in Korean populations, in that patients with asthma who had the homozygous Arg/Arg genotype showed a significantly greater response to short-acting β2-agonist.27 Such differing results were reported in other ethnic groups.28 Therefore, ethnic differences play a major role in determining the bronchodilator responses to β-agonist.
With regard to other ADRB2 SNPs, polymorphisms at ADRB2 -47 T>C located in the β upstream peptide were reported to be associated with the bronchodilator response in African-American patients with asthma.29 Polymorphism at ADRB2 523 C>A was also shown to be associated with the bronchodilator response in an American population.30 In addition, there have been no data collected regarding the associations between allele frequency and gender difference, since the ADRB2 gene is located at 5q31-q32, not on the X chromosome. In this study, we could not find any significant gender differences in allele frequency (data not shown).
In the present study, HT2 [GAA], including the ADRB2 46 G allele, was significantly associated with the presence of work-related symptoms. Considering the importance of genetic susceptibility and environmental exposure to the development of baker's asthma, the subjects in this study were classified into two groups according to the intensity of exposure. The results indicated that subjects with HT2 [GAA] showed significantly greater lower-respiratory symptoms with increasing intensity of exposure to wheat flour. Moreover, the subjects with the G allele at ADRB2 46 A>G had significantly higher prevalence rates of serum-specific IgG1 and IgG4 to wheat flour with increasing exposure intensity. In our previous study, the presence of serum wheat-specific IgG4 antibodies was associated with the presence of work-related symptoms and work-related lower-respiratory symptoms.14 The presence of serum-specific IgG1 to wheat may represent exposure to wheat flour in exposed workers. These findings suggest that workers with the AG/GG genotype of ADRB2 46 A>G are at a higher risk of generating wheat-specific IgG1 and IgG4 antibodies with increasing exposure intensity. Subjects with this haplotype of ADRB2 HT2 [GAA] may have greater genetic susceptibility to developing work-related lower-respiratory symptoms with increasing exposure intensity.
However, there are some limitations to this study. Ethnic differences should be considered. Since this study is limited to a Korean population, the results may not be applicable to other populations because genotypic distribution appears to differ among races. The prevalence of baker's asthma and the number of study subjects were also relatively low in this study population.
In conclusion, we speculate that ADRB2 polymorphism may be associated with the presence of specific IgG and work-related symptoms in workers who have high exposure to wheat flour, which can lead to the development of baker's asthma.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Korea Science and Engineering Foundation, funded by the Korean government (2009-0078646).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Baur X, Degens PO, Sander I. Baker's asthma: still among the most frequent occupational respiratory disorders. J Allergy Clin Immunol. 1998;102:984–997. doi: 10.1016/s0091-6749(98)70337-9. [DOI] [PubMed] [Google Scholar]
- 2.Meredith S. Reported incidence of occupational asthma in the United Kingdom, 1989-90. J Epidemiol Community Health. 1993;47:459–463. doi: 10.1136/jech.47.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siracusa A, Kennedy SM, DyBuncio A, Lin FJ, Marabini A, Chan-Yeung M. Prevalence and predictors of asthma in working groups in British Columbia. Am J Ind Med. 1995;28:411–423. doi: 10.1002/ajim.4700280310. [DOI] [PubMed] [Google Scholar]
- 4.Smith TA, Smith PW. Respiratory symptoms and sensitization in bread and cake bakers. Occup Med (Lond) 1998;48:321–328. doi: 10.1093/occmed/48.5.321. [DOI] [PubMed] [Google Scholar]
- 5.Houba R, Heederik D, Doekes G. Wheat sensitization and work-related symptoms in the baking industry are preventable. An epidemiologic study. Am J Respir Crit Care Med. 1998;158:1499–1503. doi: 10.1164/ajrccm.158.5.9803055. [DOI] [PubMed] [Google Scholar]
- 6.Cullinan P, Lowson D, Nieuwenhuijsen MJ, Gordon S, Tee RD, Venables KM, et al. Work related symptoms, sensitisation, and estimated exposure in workers not previously exposed to laboratory rats. Occup Environ Med. 1994;51:589–592. doi: 10.1136/oem.51.9.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Houba R, Van Run P, Heederik D, Doekes G. Wheat antigen exposure assessment for epidemiological studies in bakeries using personal dust sampling and inhibition ELISA. Clin Exp Allergy. 1996;26:154–163. doi: 10.1111/j.1365-2222.1996.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 8.Baur X. Baker's asthma: causes and prevention. Int Arch Occup Environ Health. 1999;72:292–296. doi: 10.1007/s004200050377. [DOI] [PubMed] [Google Scholar]
- 9.Brisman J. Baker's asthma. Occup Environ Med. 2002;59:498–502. doi: 10.1136/oem.59.7.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palosuo K. Update on wheat hypersensitivity. Curr Opin Allergy Clin Immunol. 2003;3:205–209. doi: 10.1097/00130832-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Tiikkainen U, Klockars M. Clinical significance of IgG subclass antibodies to wheat flour antigens in bakers. Allergy. 1990;45:497–504. doi: 10.1111/j.1398-9995.1990.tb00525.x. [DOI] [PubMed] [Google Scholar]
- 12.Battais F, Pineau F, Popineau Y, Aparicio C, Kanny G, Guerin L, et al. Food allergy to wheat: identification of immunogloglin E and immunoglobulin G-binding proteins with sequential extracts and purified proteins from wheat flour. Clin Exp Allergy. 2003;33:962–970. doi: 10.1046/j.1365-2222.2003.01592.x. [DOI] [PubMed] [Google Scholar]
- 13.Pfeil T, Schwabl U, Ulmer WT, König W. Western blot analysis of water-soluble wheat flour (Triticum vulgaris) allergens. Int Arch Allergy Appl Immunol. 1990;91:224–231. doi: 10.1159/000235121. [DOI] [PubMed] [Google Scholar]
- 14.Hur GY, Koh DH, Kim HA, Park HJ, Ye YM, Kim KS, et al. Prevalence of work-related symptoms and serum-specific antibodies to wheat flour in exposed workers in the bakery industry. Respir Med. 2008;102:548–555. doi: 10.1016/j.rmed.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Malo JL, Chan-Yeung M. Occupational asthma. J Allergy Clin Immunol. 2001;108:317–328. doi: 10.1067/mai.2001.116432. [DOI] [PubMed] [Google Scholar]
- 16.Reihsaus E, Innis M, MacIntyre N, Liggett SB. Mutations in the gene encoding for the beta 2-adrenergic receptor in normal and asthmatic subjects. Am J Respir Cell Mol Biol. 1993;8:334–339. doi: 10.1165/ajrcmb/8.3.334. [DOI] [PubMed] [Google Scholar]
- 17.Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack R, Craig TJ, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004;364:1505–1512. doi: 10.1016/S0140-6736(04)17273-5. [DOI] [PubMed] [Google Scholar]
- 18.Green SA, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human beta 2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994;33:9414–9419. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- 19.Bahn JW, Lee JY, Jang SH, Kim SH, Kim HM, Park HS. Sensitization to Empynase(pronase B) in exposed hospital personnel and identification of the Empynase allergen. Clin Exp Allergy. 2006;36:352–358. doi: 10.1111/j.1365-2222.2006.02434.x. [DOI] [PubMed] [Google Scholar]
- 20.Mehl A, Verstege A, Staden U, Kulig M, Nocon M, Beyer K, et al. Utility of the ratio of food-specific IgE/total IgE in predicting symptomatic food allergy in children. Allergy. 2005;60:1034–1039. doi: 10.1111/j.1398-9995.2005.00806.x. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein IL, Chan-Yeung M, Malo JL, Berstein D. Asthma in the workplace. 2nd ed. New York: Marcel Dekker Inc.; 1999. [Google Scholar]
- 22.Di Stefano F, Siriruttanapruk S, McCoach J, Di Gioacchino M, Burge PS. Occupational asthma in a highly industrialized region of UK: report from a local surveillance scheme. Eur Ann Allergy Clin Immunol. 2004;36:56–62. [PubMed] [Google Scholar]
- 23.McDonald JC, Keynes HL, Meredith SK. Reported incidence of occupational asthma in the United Kingdom, 1989-97. Occup Environ Med. 2000;57:823–829. doi: 10.1136/oem.57.12.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liggett SB. The pharmacogenetics of beta2-adrenergic receptors: relevance to asthma. J Allergy Clin Immunol. 2000;105:S487–S492. doi: 10.1016/s0091-6749(00)90048-4. [DOI] [PubMed] [Google Scholar]
- 25.D'amato M, Vitiani LR, Petrelli G, Ferrigno L, di Pietro A, Trezza R, et al. Association of persistent bronchial hyperresponsiveness with beta2-adrenoceptor (ADRB2) haplotypes. A population study. Am J Respir Crit Care Med. 1998;158:1968–1973. doi: 10.1164/ajrccm.158.6.9804126. [DOI] [PubMed] [Google Scholar]
- 26.Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, et al. The effect of polymorphisms of the beta(2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000;162:75–80. doi: 10.1164/ajrccm.162.1.9907092. [DOI] [PubMed] [Google Scholar]
- 27.Cho SH, Oh SY, Bahn JW, Choi JY, Chang YS, Kim YK, et al. Association between bronchodilating response to short-acting beta-agonist and non-synonymous single-nucleotide polymorphisms of beta-adrenoceptor gene. Clin Exp Allergy. 2005;35:1162–1167. doi: 10.1111/j.1365-2222.2005.02319.x. [DOI] [PubMed] [Google Scholar]
- 28.Choudhry S, Ung N, Avila PC, Ziv E, Nazario S, Casal J, et al. Pharmacogenetic differences in response to albuterol between Puerto Ricans and Mexicans with asthma. Am J Respir Crit Care Med. 2005;171:563–570. doi: 10.1164/rccm.200409-1286OC. [DOI] [PubMed] [Google Scholar]
- 29.Tsai HJ, Shaikh N, Kho JY, Battle N, Naqvi M, Navarro D, et al. Beta 2-adrenergic receptor polymorphisms: pharmacogenetic response to bronchodilator among African American asthmatics. Hum Genet. 2006;119:547–557. doi: 10.1007/s00439-006-0169-2. [DOI] [PubMed] [Google Scholar]
- 30.Silverman EK, Kwiatkowski DJ, Sylvia JS, Lazarus R, Drazen JM, Lange C, et al. Family-based association analysis of beta2-adrenergic receptor polymorphisms in the childhood asthma management program. J Allergy Clin Immunol. 2003;112:870–876. doi: 10.1016/s0091-6749(03)02023-2. [DOI] [PubMed] [Google Scholar]