Abstract
Purpose
Transplant recipients in Asia appear to be at a higher risk for developing colorectal cancer (CRC). This study was performed to identify the clinicopathological features and oncologic outcomes of CRC in post-renal transplants in Korea.
Materials and Methods
We retrospectively reviewed the records of 17 patients with CRC out of 2,630 recipients who underwent renal transplantation between 1994 and 2007. These patients (transplant group) were compared with general CRC patients (n=170, control group) matched, based on the closest date of surgery to the transplant group.
Results
During 29.7 months of the median follow-up period, the recurrent and survival rates from recurrence were worse in the transplant group than in the control group (35.2% versus 15.2%; p=0.048 and p=0.025). The 2-year patient survival rate of the transplant group was significantly worse than the control group in advanced cancer (stages III-IV; 45.7% versus 71.6%; p=0.023). In early cancer (stages 0-I), there was no significant difference in 5-year patient survival rate between the two groups (100% versus 92.6%, respectively; p=0.406).
Conclusion
In spite of a poor prognosis of advanced CRC in the transplant group, the early stage CRC of the transplant group showed a comparable oncologic outcome compared with the control group. Regular screening and early detection of CRC are essential in the post-transplant setting.
Keywords: Colorectal cancer, post-transplant malignancy, oncologic outcome
INTRODUCTION
Improvements in immunosuppressive agents led to significantly enhanced graft survival.1 As a result, transplant recipients have greater accumulations and longer exposure to immunosuppressive agents than ever before. Unfortunately, however, long-term exposure to immunosuppressive agents can cause severe side effects, including a variety of malignancies that have become major obstacles for long-term survival in transplant recipients.2 Many reports regarding de novo malignancies after transplantation have presented the incidence of post-transplant malignancies only.3-6 A few reports about oncologic outcomes of solid organ cancer after transplantation showed more aggressive and poorer oncologic outcomes than the general population.7,8 However, the clinical research involving colorectal cancer (CRC) is very limited, although the incidence of CRC after renal transplantation is 1.5-2 times higher than the standard incidence.9-11 Accordingly, we evaluated CRC in a large group of post-renal transplant recipients at a single center in Korea over a 25-year period in which the incidence of post-transplant CRC is higher in the Asian population than in the Western population.12,13 We compared the distinctiveness and oncologic outcomes of patients who had de novo CRC after renal transplantation with the outcomes of the general CRC population at the same center.
MATERIALS AND METHODS
Patient selection
Between September 1994 and November 2007, 2,630 patients underwent renal transplantation at Severance Hospital of the Yonsei University Healthcare System. Malignancies developed in 190 patients, including 17 (8.9%) cases of CRC. None of the transplantation patients had solid organ cancer, as confirmed by a computed tomography (CT) scan prior to transplantation. We performed colonoscopy for patients over 50 years old, based on Korean cancer guideline, but routinely performed for all patients prior to transplantation. After transplantation, patients routinely underwent physical examination and had renal function tested in an outpatient clinic. If patients suffered gastrointestinal discomfort, esophagogastroscopy and colonoscopy were performed like general population group, although not routinely. We used maintenance immunosuppressive therapy with an azathioprine-based regimen from 1979-1984. Cyclosporine became the main immunosuppressive agent in 1984, followed by tacrolimus in 1998. We did not use induction immunosuppression therapy, such as anti-thymocyte globulin, anti-lymphocyte globulin, or muromonab-CD3, but started induction therapy with interleukin-2 receptor antibody (Basiliximab) for high-risk recipients in 1999.
Control group patient selection from the general CRC population
We selected control patients from the colorectal database, which contained about 6,000 cases recorded from 1994-2007 at Severance Hospital of the Yonsei University Healthcare System. This database was collected prospectively for a previous CRC study.14,15 Based on the closest date of surgery, we matched 10 patients to each kidney transplantation patient who subsequently developed de novo CRC.
Preoperative diagnosis and staging
Diagnostic evaluations included physical examination, colonoscopy, or abdominopelvic CT with double contrast, chest X-ray, complete blood cell count, liver function test, and measurements of serum carcinoembryonic antigen (CEA) levels. Standardized pathology analysis was performed on all radical rectal resection specimens. Rectal tumors were staged according to the 6th UICC TNM staging system. Resection specimens were evaluated for depth of tumor penetration, lymph node involvement, histologic type, and lymphovascular invasion.
Postoperative combined modality therapy
Stage III, IV patients and high risk stage II patients who had combined poorly differentiated tumor or lymphovascular invasion or neural invasion received a 5-flurouracil-leu-covorin (FL) regimen consisting of 425 mg/m2 of 5-fluorouracil plus 30 mg leucovorin for 5 days every 28 days for 12 cycles, according to tumor stage. Some of the patients who had undergone renal transplantation were treated with oral 5-fluorouracil-based chemotherapy; the remainder of those with advanced stage disease did not receive chemotherapy because of incompatibility with immunosuppressants.
Follow-up assessment
All patients were followed up every 3 months for the first 3 years after surgery, every 6 months for the next 2 years, and yearly thereafter. Follow-up examinations included clinical history, physical examination, serum CEA levels, chest X-ray, abdominopelvic CT or magnetic resolution imaging (MRI). Positron emission tomography (PET) scanning was used if available. Determination of recurrence was made by clinical and radiologic examinations, or by histologic confirmation.
Statistical analysis
Statistical evaluation was carried out using the SPSS statistical package for Windows (version 11.0; SPSS Inc., Chicago, IL, USA). Differences between the two groups were tested with Student's t-test, Fisher's exact test, and a Chi-squared test. The local and systemic recurrence rates were calculated with a Chi-squared test. The overall survival and disease-free survival rate were calculated with the Kaplan-Meier method. Differences between curves were evaluated with the log-rank test. A value of p<0.05 was considered statistically significant.
RESULTS
Characteristics of patients with de novo CRC after transplantation
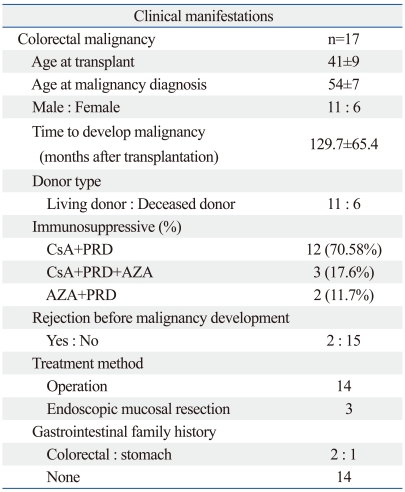
The characteristics of patients who developed de novo CRC subsequent to transplantation are summarized in Table 1. The mean follow-up duration was 195.3±11.5 months (range, 0-338 months). Malignancy occurred in 190 (7.2%) of 2,630 renal transplant recipients. Of the 190 patients, 17 (8.9%) developed CRC. The average age of the patients with CRC was 54±7 years, and the average time to develop cancer after kidney transplantation was 129.7±65.4 months. Twelve of 17 patients (70.58%) were treated with cyclosporine and steroids as an immunosuppressant regimen, 3 patients (17.6%) were treated with azathiopurine, cyclosporine, and steroids, and 2 patients (11.7%) were treated with azathiopurine and steroids. Two patients suffered rejection prior to the development of cancer. Of the 17 CRC patients, the three cases of adenocarcinoma confined to the submucosa were treated by endoscopic mucosal resection. Three patients had a family history of gastrointestinal cancer; of the three patients, two had a family history of colorectal cancer and one had a family history of gastric cancer.
Table 1.
Characteristics of Post-Renal Transplant Patients with de novo Colorectal Malignancy
CsA, cyclosporine; PRD, prednisolone; AZA, azathioprine.
Comparison of characteristics between CRC in the transplant and control groups
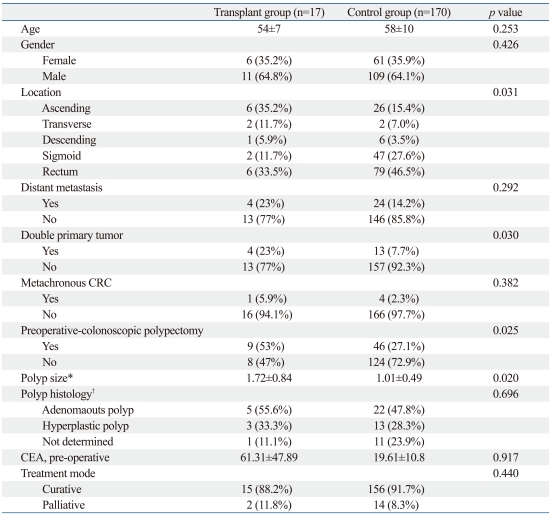
A summary of patient characteristics from the transplant CRC and control CRC group is presented in Table 2. There were no significant differences in age and gender between the two groups (p=0.253 and p=0.426, respectively). The combined rate of distant metastasis and the rate of curative resection also did not differ significantly between the two groups (p=0.292 and p=0.440, respectively). The rate of ascending colon cancer was significant higher, and the rate of rectal cancer was significantly lower in the transplant group than in the control group (35.2% versus 15.4% and 33.5% versus 46.5%, respectively, p=0.031). In the transplant group, the incidence of combined polypectomy during pre-operative colonoscopy was higher (p=0.025) and the polyp size was greater (p=0.02) than in the control group. The average initial CEA level was higher in the transplant group, but the difference was not significant (p=0.917). There was no statistical significance in combining the metachronous cases of CRC between the two groups (p=0.382), but double primary tumors were more common in the transplant group (p=0.030).
Table 2.
Patient Characteristics in Colorectal Cancer with Adenocarcinoma
CRC, colorectal cancer; CEA, carcinoembryonic antigen.
*Only polyp size.
†Polyp histology were compared in combined polypectomy patients.
Clinicopathologic characteristics
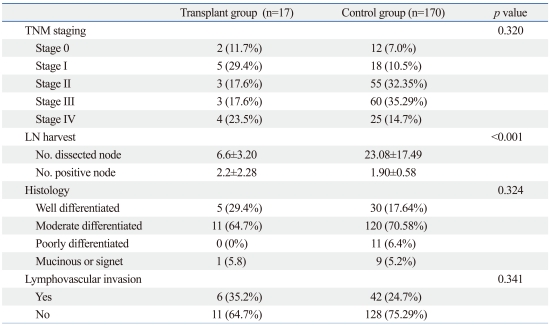
A summary of the clinicopathologic characteristics of the patients is presented in Table 3. We found no significant differences in staging, histologic type, or lymphovascular invasion between the two groups (p=0.320, p=0.324, and p=0.341, respectively). In the transplant group, the total number of harvested lymph nodes was significantly less than in control patients (6.6±3.20 versus 23.08±17.49, respectively; p<0.0001), but the number of positive lymph nodes did not differ significantly between the two groups (2.2±2.28 versus 1.90±0.58, respectively; p=0.324).
Table 3.
Clinicopathological Characteristics
LN, lymph node.
Adjuvant treatment
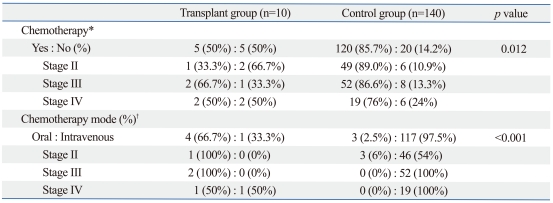
In the transplant group, patients with stage III and IV tumors were not adequately treated with adjuvant chemoradiotherapy compared to control patients (stage III: 66.7% versus 86.6%, stage IV: 50% versus 76%, respectively, p=0.012). Among chemotherapy patients, all 2 stage III transplant patients (100%) were treated with oral FL chemotherapy, whereas all 52 stage III control patients were treated with intravenous chemotherapy (100%). One of two stage IV transplant patients (50%) was treated with intravenous chemotherapy, whereas all 19 stage IV control patients were treated with intravenous chemotherapy (100%)(Table 4). Of 5 chemotherapy patients, 2 patients lost the graft within 1 year of adjuvant chemotherapy.
Table 4.
Adjuvant Chemotherapy
*Stage II, III, IV patients were selected and compared between transplant group (n=10) and control group (n=140).
†Patients were compared between two group who were treated with adjuvant chemotherapy.
Oncologic outcomes
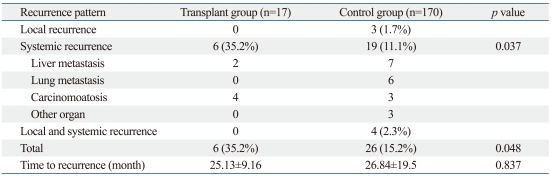
The median follow-up period was 29.69 months (range, 1-118 months). The incidence of local, systemic, and combined recurrence among transplant group patients was 0%, 35%, and 0%, and 1.7%, 11.1%, and 2.3% among control group patients, respectively. The overall rate of recurrence was significantly higher in the transplant group patients than in the control group patients (35.2% versus 15.2%, p=0.048) (Table 4). In systemic recurrence, carcinomatosis was significantly higher in the transplant group than in control group (p=0.037)
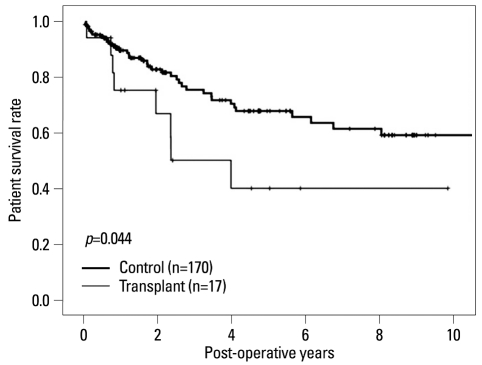
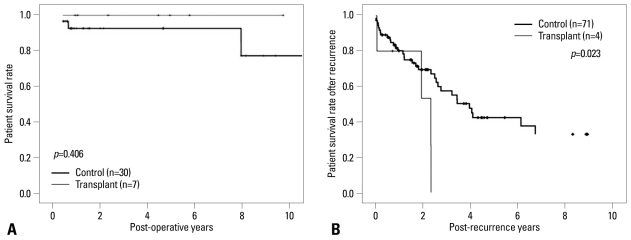
There were significant differences in the 5-year survival between the two groups (control, 67.8% versus transplant, 40.2%; p=0.044)(Fig. 1). However, the 5-year survival rate for those with early cancer (stages 0-I) was not significantly different between the two groups (100% versus 92.6%, respectively, p=0.406)(Fig. 2A). Although, there were significant differences in the 2-year survival rate between the transplant and control groups for patients with advanced cancer (stages III-IV; 45.7% versus 71.6%, respectively; p=0.023)(Fig. 2B).
Fig. 1.
Overall patient survival rate. Transplant group showed inferior survival rate compared with control group (p=0.044).
Fig. 2.
Survival rate by pathologic staging. Early staged transplant group showed survival rate comparable to control group (p=0.406)(A), but advanced staged transplant group showed extremely poor survival rate compared with control group (p=0.023)(B).
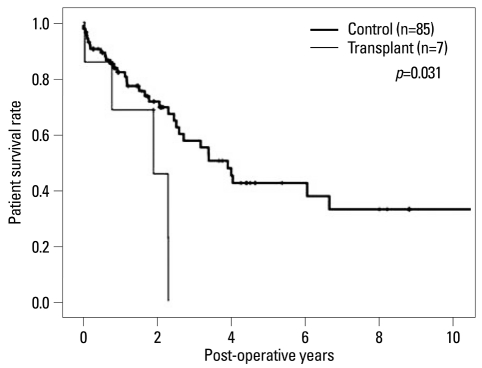
In subanalysis, stage III-IV CRC patients in the transplant group who were treated with adjuvant chemotherapy were compared with the advanced CRC in controls who were treated with adjuvant chemotherapy. 2-year survival rates were 69.5% in control group and 53.3% in transplant group. In the transplant group, all patients died within 2.5 years (p=0.031)(Fig. 3).
Fig. 3.
The advanced staged colorectal cancer (CRC) in transplant group which were treated with adjuvant chemotherapy also showed poor survival rate compared with advanced stage CRC in control group who were treated with adjuvant chemotherapy (p=0.031).
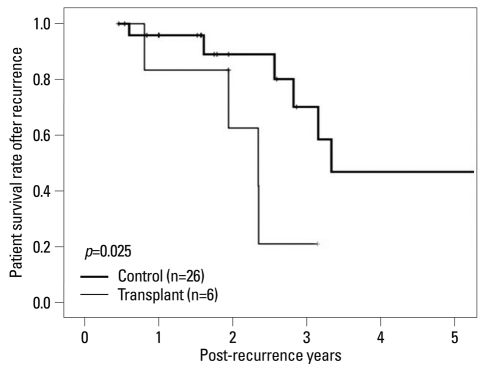
There was no significant difference in mean time to recurrence from surgery between groups (p=0.837)(Table 5), however, the survival rate from recurrence was significantly lower in the transplant group than control patients (p=0.025) (Fig. 4).
Table 5.
Recurrence Pattern According to Type of Cancer
Fig. 4.
Survival rate after tumor recurrence. Transplant group had poor survival rate after tumor recurrence compared with control group (p=0.025).
DISCUSSION
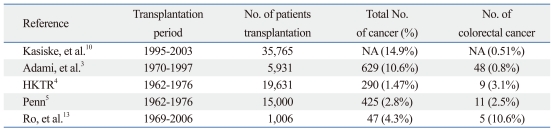
Recent evidence indicates that transplantation patients appear to be at a higher risk for developing CRC.9 Villeneuve, et al.11 described an incidence of CRC after renal transplantation, showing 1.49 times higher than the standard incidence. Furthermore, Kasiske, et al.10 reported the risk of colon cancer development in post-renal transplantation patients to be two-fold higher in the first post-transplantation year, and an additional 2.2-fold higher after the third post-transplantation year than with the general population (Table 6). However, less effort has been made to identify specific characteristics or oncologic outcomes in CRC in post-renal transplant recipients. In our study, we could not perform separate analysis of colon and rectum, because of not enough cases of rectal cancer, especially advanced stage rectal cancer in transplant recipients.
Table 6.
Incidence of Colorectal Cancer in Post-Transplant Recipients
NA, not applicable; HKTR, human kidney transplant registry.
We observed that CRC in post-renal transplantation patients displayed atypical characteristics in both tumor location, polyp size and occurrence. Our study showed that the rate of right CRC in the transplantation group was significantly higher than in the general cancer population group (35.2% versus 15.4%, respectively), while the rate of rectal cancer was significantly lower in the transplantation group (33.5% versus 46.5%; p=0.031). Similar to our data, Stewart, et al.16 postulated that transplantation reduces the incidence of rectal cancer. The results indicate that lymphoglandular complexes are most frequent in the rectum. Thus, in the rectum compared to the colon, there is an increased capacity for antigen transport into a dense population of lymph follicles with a higher percentage of germinal centers. Abolition of such weak immune promotion of oncogenesis by chronic immunosuppression would explain the highly significant reduction in the incidence of rectal cancer.16 However, we cannot be sure whether the transplant increased the incidence of right colon cancer and decreased the incidence of rectal cancer. Our data showed a tendency of more frequent right colon cancer and less frequent rectal cancer, compared to the general population. More observation or data are needed to conclude the higher incidence of right colon cancer and the lower incidence of rectal cancer. In terms of polyps, more and larger polyps were observed in preoperative colonoscopy of transplant patients. Although the result of Parikshak, et al.17 shows that the incidence of adenomatous polyps was not higher in post-renal transplant recipients compared with the general population, more combined polypectomies were performed at preoperative colonoscopy in our transplantation group (p=0.025). Furthermore, the polyps observed in our post-renal transplant recipients were significantly larger than in control patients (p=0.02).
Oncologic outcomes were poorer in transplant recipients than in the general CRC population (p=0.044). In particular, transplant recipients with advanced stage malignancies showed relatively poor oncologic outcome compared to controls. In our study, the 2-year survival rate of post-renal transplant patients with stage III-IV CRC was 45.7% compared to 71.6% in general CRC patients. The 5-year survival of stage III-IV patients in the transplant group was 0%. Furthermore, we compared the survival rate of stage III-IV CRC patients who were treated with adjuvant chemotherapy between the transplant and control groups. Although the 2-year survivals in the transplant group were not inferior to the control group (69.5% versus 53.3%), transplant recipients showed poor oncologic outcomes compared to controls. All recipients died within 2.5 years of surgery (p=0.031).
CRC seems to progress faster after diagnosed in an advanced stage following transplantation compared to the general population. We observed more frequent recurrence in transplant patients, compared to the general population group of CRC (35.2% versus 15.2%), and the recurrence pattern in post-renal transplant recipients was entirely systemic recurrence, especially carcinomatosis (p=0.037). Furthermore, when the tumor recurred, the survival period was less than 12 months after recurrence. The overall survival of stage IV patients in transplant was less than 10 months, and stage III patients in transplant were died within 25 months. Such faster progression in advanced stage suggest a role for immunosuppression included increased host susceptibility to tumorigenesis and cancer progression.18
It is highly likely that the decreased survival for advanced cancer were not only due to immunosuppressive drugs, but also through ineffective treatment of CRC by adjuvant chemotherapy. Although our sub-analysis (Fig. 3) provided an evidence for decreased survival in the adjuvant cases, however, the fact that only 4 transplant patients with CRC were included compared to 71 controls should be taken into account. Due to small numbers, it would be a possible limitation to conclude that advanced stage showed poorer survival compared to general population, regardless of adjuvant chemotherapy. We found that recipients with higher stage tumors did not receive adequate adjuvant chemotherapy due to incompatibility with immunosuppressants. Although surgical resection of the primary tumor with regional lymph nodes is the treatment of choice,19 combined treatment modalities are necessary in advanced stages.20 In our study, however, 42.8% of III-IV patients in the transplant group received chemotherapy compared with 83.5% of control patients who received chemotherapy. Some advanced stage patients in the transplant group refused chemotherapy due to their general condition or the risk of infection. Furthermore, concerns over graft failure with chemotherapy led to modified chemotherapy in advanced stage patients. Of 5 patients who received adjuvant chemotherapy in advanced malignancy, 2 patients rejected the graft within 1 year. However, we could not be certain whether the graft failure was related to adjuvant chemotherapy.
Conversely, the prognosis of early stage cancer after transplantation was comparable with the general colorectal adenocarcinoma population [5-year survival rate in early cancer (stages 0-I), 100% versus 92.6%, respectively; p=0.406]. Similar findings have been reported for stomach cancer. Choi, et al.8 reported that all patients with early stage stomach cancer were alive within 4 months of diagnosis without any evidence of recurrence, compared to 0% of stage IV cancer cases. Similarly, Buell, et al.7,8 described the oncologic outcome of stomach cancer that developed after transplantation, reporting that the survival rate of early stage cancer patients (stage I or II) was equivalent to that of the general population.
Regular screening tools, such as colonoscopy, are necessary for the early detection of CRC. Early detection is critical, as stage 0-I patient's survival rate in transplant group was comparable with those of the control group, while the advanced stage patients had poor oncologic outcomes and did not receive adequate adjuvant chemotherapy. Furthermore, larger, more frequent polyps were observed in post-transplant recipients, therefore, regular colonoscopy and polypectomy would be an another option to prevent CRC after transplant. Guidelines for cancer screening and prevention are needed for post-transplant recipients, suggested for CRC in inflammatory bowel disease or inherited diseases,21 as published in the Annals of Internal Medicine22 in 2002 and in the National Comprehensive Cancer Network.23 Based on our experience in the tumor screening schedules in post-transplant recipients, we recommend the same guidelines for the general population. Older age is the most important risk factor, therefore, screening is recommended after 55 years of age. Additionally, the mean exposure to the immunosuppressive agent is a significant risk factor in transplant recipients. Although a recent study showed that CRC in the transplant recipient developed at an earlier age compared to the general population,9,11 our data showed that age at diagnosis of the transplant recipient was not different from that of the control group. We do not regard younger age as an important risk factor, but long-term exposure to immunosuppression. Our present data showed the mean development time of malignancy was 129.7±65.4 months following transplantation, and our previous study showed that the relative ratio of CRC development compared to age-standardized incidence of malignancy in the Korean general population was 0.7 within 6-9 years, 2.1 within 9-12 years, 4.9 within 12-15 years, 8.9 within 15-18 years.24 Based on our study, a screening colonoscopy is recommended within 6-9 years after transplantation. Additionally, earlier screening should be considered, when there is an another risk factor, such as a family history of gastrointestinal cancer. Although not statistically significant, colon cancer developed at 45 years of age, 6 years after transplant, in a patient who had a family history of colon cancer.
Our study has another limitation; we did not analyze the risk based on the type of immunosuppressant. Actually, our data showed no transplant patients who developed CRC treated with tacrolimus, although tacrolimus was used from 1998. We enrolled patients for follow up more than 5 years after cancer surgery, however, there are very limited numbers of patients who used tacrolimus before 2004, and the use of tacrolimus took only 6 years since 2004. Therefore, it is highly likely that the duration exposed to tacrolimus is not enough to develop CRC in our study.
Conclusion, de novo CRC in renal transplant patients has a poor oncologic outcome, high recurrence rate, and poor survival rate when the cancer is advanced. Cancer detected at early stages, however, can be overcome. Regular and routine screening for CRC prevention and early detection are essential for post-transplantation recipients. Guidelines are needed for screening and prevention of CRC in renal transplant recipients.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605–612. doi: 10.1056/NEJM200003023420901. [DOI] [PubMed] [Google Scholar]
- 2.Kauffman HM, Cherikh WS, McBride MA, Cheng Y, Hanto DW. Post-transplant de novo malignancies in renal transplant recipients: the past and present. Transpl Int. 2006;19:607–620. doi: 10.1111/j.1432-2277.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- 3.Adami J, Gäbel H, Lindelöf B, Ekström K, Rydh B, Glimelius B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89:1221–1227. doi: 10.1038/sj.bjc.6601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Advisory committee to the renal transplant registry. The 13th report of the human renal transplant registry. Transplant Proc. 1977;9:9–26. [PubMed] [Google Scholar]
- 5.Penn I. Malignancies associated with immunosuppressive or cytotoxic therapy. Surgery. 1978;83:492–502. [PubMed] [Google Scholar]
- 6.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 7.Buell JF, Husted T, Hanaway MJ, Peddi VR, Trofe J, Gross TG, et al. Incidental diagnosis of gastric cancer in transplant recipients improves patient survival. Surgery. 2002;132:754–758. doi: 10.1067/msy.2002.127670. [DOI] [PubMed] [Google Scholar]
- 8.Choi DJ, Hyung WJ, Kwon KH, Kim SI, Kim YS, Noh SH, et al. Gastric Adenocarcinoma after Renal Transplantation. J Korean Surg Soc. 2003;64:201–205. [Google Scholar]
- 9.Kan M, Gill JS, Wiseman SM. Colon and rectal cancer after renal transplantation. Expert Rev Anticancer Ther. 2008;8:1339–1346. doi: 10.1586/14737140.8.8.1339. [DOI] [PubMed] [Google Scholar]
- 10.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905–913. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 11.Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. 2007;7:941–948. doi: 10.1111/j.1600-6143.2007.01736.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoshida Y, Aozasa K. Malignancies in organ transplant recipients. Pathol Int. 2004;54:649–658. doi: 10.1111/j.1440-1827.2004.01676.x. [DOI] [PubMed] [Google Scholar]
- 13.Ro H, Kim SM, Kim KW, Hwang YH, Yang JS, Oh KH, et al. Malignancy after kidney transplantation. J Korean Soc Transplant. 2006;20:187–192. [Google Scholar]
- 14.Kim NK. Anatomic basis of sharp pelvic dissection for curative resection of rectal cancer. Yonsei Med J. 2005;46:737–749. doi: 10.3349/ymj.2005.46.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim NK, Baik SH, Seong JS, Kim H, Roh JK, Lee KY, et al. Oncologic outcomes after neoadjuvant chemoradiation followed by curative resection with tumor-specific mesorectal excision for fixed locally advanced rectal cancer: Impact of postirradiated pathologic downstaging on local recurrence and survival. Ann Surg. 2006;244:1024–1030. doi: 10.1097/01.sla.0000225360.99257.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart T, Henderson R, Grayson H, Opelz G. Reduced incidence of rectal cancer, compared to gastric and colonic cancer, in a population of 73,076 men and women chronically immunosuppressed. Clin Cancer Res. 1997;3:51–55. [PubMed] [Google Scholar]
- 17.Parikshak M, Pawlak SE, Eggenberger JC, Lee CS, Szilagy EJ, Margolin DA. The role of endoscopic colon surveillance in the transplant population. Dis Colon Rectum. 2002;45:1655–1660. doi: 10.1007/s10350-004-7254-1. [DOI] [PubMed] [Google Scholar]
- 18.Feng S, Buell JF, Chari RS, DiMaio JM, Hanto DW. Tumors and transplantation: The 2003 Third Annual ASTS State-of-the-Art Winter Symposium. Am J Transplant. 2003;3:1481–1487. doi: 10.1046/j.1600-6143.2003.00245.x. [DOI] [PubMed] [Google Scholar]
- 19.Hawk ET, Umar A, Richmond E, Viner JL. Prevention and therapy of colorectal cancer. Med Clin North Am. 2005;89:85–110. doi: 10.1016/j.mcna.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Mano MS, Duhoux F. Colon cancer: update on adjuvant therapy. Clin Colorectal Cancer. 2008;7:178–183. doi: 10.3816/CCC.2008.n.023. [DOI] [PubMed] [Google Scholar]
- 21.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 22.Screening for breast cancer: recommendations and rationale. Ann Intern Med. 2002;137:344–346. doi: 10.7326/0003-4819-137-5_part_1-200209030-00011. [DOI] [PubMed] [Google Scholar]
- 23.The National Comprehensive Cancer Network (NCCN) Cancer Institute. http://www.glci.com/body.cfm?id=243.
- 24.Ju MK, Joo DJ, Kim SJ, Huh KH, Kim MS, Jeon KO, et al. Chronologically different incidences of post-transplant malignancies in renal transplant recipients: single center experience. Transpl Int. 2009;22:644–653. doi: 10.1111/j.1432-2277.2009.00846.x. [DOI] [PubMed] [Google Scholar]