Abstract
Hajdu-Cheney syndrome is a rare, autosomal dominant skeletal dysplasia marked by acro-osteolysis of the distal phalanges and severe osteoporosis. Although there are more than 60 reports published to date, proper treatment and subsequent outcome have been scarce. Herein, we report a progress of anti-resorptive therapy with zoledronic acid, in a woman with Hajdu-Cheney syndrome. Results suggest that anti-resorptive therapy may be important in delaying the progress of osteoporosis and preventing fractures, but not necessarily acro-osteolysis itself.
Keywords: Hajdu-Cheney Syndrome, osteoporosis, zoledronic acid
INTRODUCTION
Hajdu-Cheney syndrome (HCS) is a rare disorder, characterized by prominent skeletal dysplasia which includes craniofacial change, dental anomalies, short stature, and acro-osteolysis and generalized osteoporosis.1-3 Although the etiology and pathogenesis are unknown, inadequate bone mass acquisition, subsequent bone loss may contribute to advanced osteoporosis in this syndrome.4-6 The aim of the treatment for the osteoporosis in HCS is to prevent the frequent occurrence of fragility fracture. Herein, we report the progress of anti-resorptive therapy with zoledronic acid in a woman with HCS.
CASE REPORT
A 41-year-old, premenopausal woman was referred for evaluation of short stature and low bone mass. She went through surgical correction of the patent ductus arteriosus at the age of 28. She underwent complete dental extraction for poor root development. She had persistent pain on both hands and feet and gait disturbance for nearly 30 years. Her menstruation was normal, and there was no consanguinity in her family members, including her parents, two younger brothers, two older and one younger sister. The patient's 7-year-old daughter was phenotypically normal but had not been confirmed with radiologic examination.
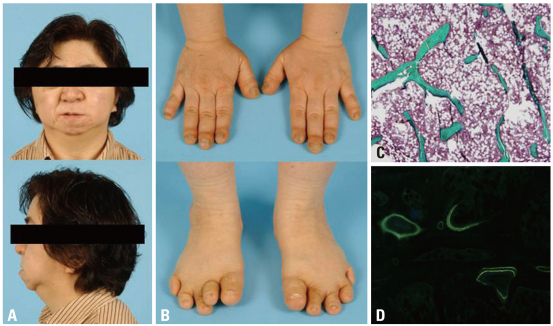
Physical examination revealed short stature (height, 139 cm; weight, 52 kg), coarse facial features with micrognathia, and short digits showed pseudo-clubbing (Fig. 1A and B) but otherwise unremarkable. The following hematological and biochemical results were normal: complete blood count, electrolytes, liver function, renal function, serum calcium, phosphate, alkaline phosphatase, thyroid stimulating hormone (TSH), estradiol, parathyroid hormone (PTH), and urine analysis. Initial 25-hydroxy-vitamin D was 27.12 ng/mL (≥30). Skeletal survey revealed loss of the terminal phalanges of all fingers bilaterally and osteolytic defect at the first metatarsal head of both feet (Fig. 2A and B). Lateral skull radiograph showed wormian bones with persistent patency of cranial sutures, bathrocephaly, and midfacial hypoplasia. Bone mineral density (BMD) of the lumbar (L) spine and the mean total hip by dual-energy X-ray absorptiometry were 0.678 and 0.958 g/cm2, respectively, corresponding to T scores of -3.0 and 0.9. Iliac crest bone biopsy showed low bone volume and thin and widely separated trabeculae compared to the data of normal Korean women7 (Fig. 1C), with normal incorporation of tetracycline label (Fig. 1D). Her chromosome analysis and mental development were normal.
Fig. 1.
Clinical photographs of the face (A) and hands and feet (B). Micrognathia is evident. Distal clubbing of the fingers and toes are noted. Iliac bone biopsy shows decreased bone volume and thin and widely separated trabeculae (C) with normal incorporation of tetracycline label (D).
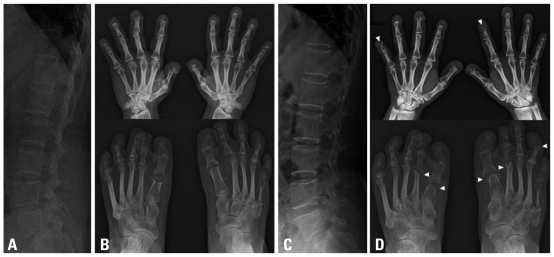
Fig. 2.
Initial and posttreatment radiographs of the lumbar spine and both hands and feet. Diffuse osteoporosis through the lumbar spine is noted (A). There is complete loss or scale-like residue of the distal phalanges in all fingers, and clubbing of the terminal tufts is seen (B). Subluxation of the metatarsophlangeal joint of the left second toe, osteolytic thinning of the left fifth metatarsal, and shortening of the proximal phalanges are seen (B). Follow-up radiographs 2 months after administration of the third dose of zoledronic acid shows nor osteoporosis or vertebral fracture, and dense end-plates represent the biphosphonate effect (C). Note progressive worsening of osteolysis affecting the middle phalanges of the fingers as well as the phalanges of the feet and peri-articular bone loss (arrowheads)(D).
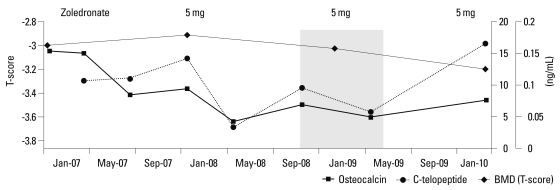
A diagnosis of a sporadic form of HCS was made based on the characteristic clinical, radiographic, and densitometric phenotypes. She started rehabilitation for gait disturbance and osteoporosis treatment. At first, she began on daily calcium carbonate 1,500 mg/day and cholecalciferol 4 mg/day. Then, once-yearly 5 mg intravenous zoledronic acid (diluted in 100 mL isotonic saline and infused over no less than 15 minutes) was started. The rheumatologist used empirical prednisolone 5 mg/day for her persistent arthralgia. However, they discontinued glucocorticoids 6 months later, because there was no definite evidence of rheumatoid arthritis. After administration of the second dose of zoledronic acid, BMD at L-spine decreased by 5.3% to 0.642 g/cm2 (T score -3.2) without any changes in femoral BMD. Follow-up radiographs of the hand and feet showed progressive acro-osteolysis (Fig. 2C and D). C-telopeptide was suppressed by 45.8%, 0.107 to 0.058 ng/mL (≤0.573) during the first 2 years but increased to 0.167 ng/mL over the recent 1 year, and osteocalcin was gradually decreased by 60.4% from 15.42 to 6.10 ng/mL (0.7-24.7) during the follow-up period (Fig. 3). We administered the third dose of zoledronic acid in March 2010, and she remained free of fracture this time. The pain in both hands and feet was also much relieved from visual analysis score 10 to 5.
Fig. 3.
Changes in bone turnover and bone mineral density (BMD) in response to zoledronic acid. L-spine BMD decreased by 5.3% from 0.678 to 0.642 g/cm2 over 3 years; C-telopeptide was suppressed by 45.8% from 0.107 to 0.058 ng/mL over the first 2 years but increased to 0.167 ng/mL over the recent 1 year; and osteocalcin gradually decreased by 60.4% from 15.42 to 6.10 ng/mL during the follow-up period after 3 years of treatment.
DISCUSSION
Since our patient was first diagnosed as HCS in Korea 3 years ago, another HCS case was recently reported in Korea.8 Brennan, et al. proposed diagnostic criteria based on the clinical and radiological characteristics at different stages of life.9-11 Our patient had all typical features of this syndrome.
Acro-osteolysis is a hallmark of the syndrome. Nunziata, et al.4 suggested that acro-osteolysis may be sustained by local acting factors stimulating osteoclastic resorption. Udell speculated a local increase in mast cells and elaboration of osteolytic cytokines could result in focal loss.6,12 Also, generalized osteoporosis commonly coexisted in HCS, which is frequently associated with atraumatic vertebral and nonvertevral fractures.6,12 The etiology, pathogenesis or the causative gene of this syndrome is still unknown, but a number of reports have suggested that low peak bone mass and high bone turnover may account for osteoporosis in HCS.12-14
Based on this speculation, there were two reports demonstrating the treatment outcome of acro-osteolysis and osteoporosis in HCS, one with bisphosphonate and the other with bisphosphonate combined with teriparatide.15-17 The L-spine BMD of the patient with alendronate had increased by 18.3% over 4 years, and that of the other patient with pamidronate and teriparatide increased by 24% in 9 months. They did not sustain any further fractures during treatment period. On the contrary, there was no effect on acro-osteolysis. In other acro-osteolysis syndrome, nodulosis, atorhtopathy and osteolysis syndrome, pamidronate therapy successfully decreased joint pain, improved functional ability, and significantly increased BMD.18
In our case, relatively low bone turnover in the beginning, reduced physical activity and use of glucocorticoid in between, might have attributed to less response in BMD. However, there was a worrisome result of progressive acro-osteolysis in this patient, too. Aggravated osteolysis without subsequent bone formation due to strong inhibition from zoledronic acid might have contributed in worsening of the process. Therefore, we can speculate that the pathogenesis could be different between aco-osteolysis and osteoporosis, and bisphosphonate therapy may help to prevent at least the occurrence of fractures and suppress bone resorption in HCS, but not necessarily acro-osteolysis itself. This further suggests that the causative gene of HCS should be identified to properly determine the direction of treatment.
Bisphosphonate therapy in our patient was well tolerated and somewhat effective in pain relief and prevention of bone fractures. However, in order to provide appropriate treatment and prevent further acro-osteolysis, pathogenesis of focal osteolysis and generalized osteoporosis must be elucidated.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Hajdu N, Kauntze R. Cranio-skeletal dysplasia. Br J Radiol. 1948;21:42–48. doi: 10.1259/0007-1285-21-241-42. [DOI] [PubMed] [Google Scholar]
- 2.Cheney WD. Acro-osteolysis. Am J Roentgenol Radium Ther Nucl Med. 1965;94:595–607. [PubMed] [Google Scholar]
- 3.Diren HB, Kovanlikaya I, Süller A, Dicle O. The Hajdu-Cheney syndrome: a case report and review of the literature. Pediatr Radiol. 1990;20:568–569. doi: 10.1007/BF02011397. [DOI] [PubMed] [Google Scholar]
- 4.Nunziata V, di Giovanni G, Ballanti P, Bonucci E. High turnover osteoporosis in acro-osteolysis (Hajdu-Cheney syndrome) J Endocrinol Invest. 1990;13:251–255. doi: 10.1007/BF03349553. [DOI] [PubMed] [Google Scholar]
- 5.Leidig-Bruckner G, Pfeilschifter J, Penning N, Limberg B, Priemel M, Delling G, et al. Severe osteoporosis in familial Hajdu-Cheney syndrome: progression of acro-osteolysis and osteoporosis during long-term follow-up. J Bone Miner Res. 1999;14:2036–2041. doi: 10.1359/jbmr.1999.14.12.2036. [DOI] [PubMed] [Google Scholar]
- 6.Udell J, Schumacher HR, Jr, Kaplan F, Fallon MD. Idiopathic familial acroosteolysis: histomorphometric study of bone and literature review of the Hajdu-Cheney syndrome. Arthritis Rheum. 1986;29:1032–1038. doi: 10.1002/art.1780290815. [DOI] [PubMed] [Google Scholar]
- 7.Won YY, Chung YS, Park YK, Yoo VY. Correlations between microcomputed tomography and bone histomorphometry in Korean young females. Yonsei Med J. 2003;44:811–815. doi: 10.3349/ymj.2003.44.5.811. [DOI] [PubMed] [Google Scholar]
- 8.Han EJ, Mun JI, An SY, Jung YJ, Kim OH, Chung YS. A Case Report of Hajdu-Cheney Syndrome. Endocrinol Metab. 2010;25:152–156. [Google Scholar]
- 9.Kawamura J, Matsubayashi K, Ogawa M. Hadju-Cheney syndrome. Report of a non-familial case. Neuroradiology. 1981;21:295–301. doi: 10.1007/BF02100164. [DOI] [PubMed] [Google Scholar]
- 10.Brennan AM, Pauli RM. Hajdu--Cheney syndrome: evolution of phenotype and clinical problems. Am J Med Genet. 2001;100:292–310. doi: 10.1002/1096-8628(20010515)100:4<292::aid-ajmg1308>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Marik I, Kuklik M, Zemkowa D, Kozlowski K. Hajdu-Cheney syndrome: report of a family and a short literature review. Australas Radiol. 2006;50:534–538. doi: 10.1111/j.1440-1673.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 12.Brown DM, Bradford DS, Gorlin RJ, Desnick RJ, Langer LO, Jowsey J, et al. The acro-osteolysis syndrome: Morphologic and biochemical studies. J Pediatr. 1976;88:573–580. doi: 10.1016/s0022-3476(76)80009-1. [DOI] [PubMed] [Google Scholar]
- 13.Iwaya T, Taniguchi K, Watanabe J, Iinuma K, Hamazaki Y, Yoshikawa S. Hajdu-Cheney syndrome. Arch Orthop Trauma Surg. 1979;95:293–302. doi: 10.1007/BF00389701. [DOI] [PubMed] [Google Scholar]
- 14.Lifchus-Ascher RJ, Tucci JR. Hajdu-Cheney syndrome in a 19-year-old man. Endocr Pract. 2006;12:690–694. doi: 10.4158/EP.12.6.690. [DOI] [PubMed] [Google Scholar]
- 15.Drake WM, Hiorns MP, Kendler DL. Hadju-Cheney syndrome: response to therapy with bisphosphonates in two patients. J Bone Miner Res. 2003;18:131–133. doi: 10.1359/jbmr.2003.18.1.131. [DOI] [PubMed] [Google Scholar]
- 16.McKiernan FE. Integrated anti-remodeling and anabolic therapy for the osteoporosis of Hajdu-Cheney syndrome. Osteoporos Int. 2007;18:245–249. doi: 10.1007/s00198-006-0221-z. [DOI] [PubMed] [Google Scholar]
- 17.McKiernan FE. Integrated anti-remodeling and anabolic therapy for the osteoporosis of Hajdu-Cheney syndrome: 2-year follow-up. Osteoporos Int. 2008;19:379–380. doi: 10.1007/s00198-007-0461-6. [DOI] [PubMed] [Google Scholar]
- 18.Al-Mayouf SM, Madi SM, Bin-Abbas BS. Cyclic intravenous pamidronate treatment in children with nodulosis, arthropathy and osteolysis syndrome. Ann Rheum Dis. 2006;65:1672–1673. doi: 10.1136/ard.2005.035725. [DOI] [PMC free article] [PubMed] [Google Scholar]